Abstract
Letter production relies on a tight coupling between motor movements and visual feedback—each stroke of the letter is visually experienced as it is produced. Experience with letter production leads to increases in functional connectivity, a measure of neural communication, among visual and motor brain systems and leads to gains in letter recognition in preliterate children. We hypothesized that the contingency between the motor and visual experiences of the written form during production would result in both effects. Twenty literate adults were trained on four sets of novel symbols over the course of one week. Each symbol set was trained through one of four training conditions: drawing with ink, drawing without ink, watching a handwritten symbol unfold as if being drawn, and watching a static handwritten symbol. Contingency of motor and visual experiences occurred in the drawing with ink condition. The motor and visual experiences were rendered non-contingent in each of the other three conditions by controlling for visual or motor experience. Participants were presented with the trained symbols during fMRI scanning at three time points: one pre-training, one post-training, and one after a week-long no-training delay. Recognition was tested after each training session and after the third scan. We found that the contingency between visual and motor experiences during production changed the pattern of functional connectivity among visual, motor, and auditory neural communities and resulted in better recognition performance at post-training than at pre-training. Recognition gains were maintained after the no-training delay, but the functional connections observed immediately after training returned to their pre-training baselines. Our results suggest that behaviors that couple sensory and motor systems result in temporary changes in neura communication during perception that may not directly support changes in recognition.
Keywords: fMRI, Learning, Functional connectivity, Handwriting, Letters, Perception, Recognition
Symbol Production Contributes to Short-term Changes in Functional Connectivity During Symbol Perception and Long-term Gains in Symbol Recognition
Symbol production—the production of written forms by hand—is a sensorimotor activity that requires a tight coupling between sensory and motor systems (Feder and Majnemer, 2007, Treiman and Kessler, 2014). The visual percepts of symbols are directly related to the motor movements used to create them, and this direct link may be important for learning. Writing letters of the alphabet by hand, for example, leads to faster and more accurate letter recognition than other training activities (e.g., typing or viewing) that do not emphasize the direct visual-motor relationship (Longcamp et al., 2008, Longcamp et al., 2006, Longcamp et al., 2005, Zemlock et al., 2018). Writing letters of the alphabet by hand also leads to greater functional connectivity—a measure of neural communication—among sensory and motor cortices during letter perception when compared to typing training (Vinci-Booher et al., 2016). These studies, together, suggest that the sensorimotor contingency, particularly the visual-motor contingency, that occurs during letter production may lead to the emergence of visual-motor functional connectivity during letter perception and, further, that this functional connectivity may be related to concurrent gains in letter recognition (Longcamp et al., 2008).
The developmental trajectory of the neural system supporting letter perception—the visual perception of an individual letter of the alphabet—suggests that the sensorimotor nature of letter production is important for the development of processes in sensorimotor and perceptual brain regions during letter perception (Polk and Farah, 1998). The perception of individual letters recruits sensorimotor regions of the brain in preliterate children more after letter production experience than after other motor experiences (e.g., typing) (James and Engelhardt, 2012) and activation in the fusiform gyri in perceptual regions only emerges after letter production training and not before (James, 2010). The literate adult response to letters typically includes activation in the fusiform gyri and similar sensorimotor regions (James and Gauthier, 2006, Longcamp et al., 2003, Longcamp et al., 2005). Letter production, therefore, leads to the onset of a neural response during letter perception in preliterate children that includes regions that are also engaged during literate letter perception in adults. This observation has led to the hypothesis that producing individual letters by hand contributes to the development of the neural system supporting adult-like letter perception (James, 2017, James and Engelhardt, 2012). The question of how letter production leads to the development of the neural system supporting adult-like letter perception is still an open question to which there have been two broad approaches.
1. Integration and segregation during learning and development
First, letter production may lead to the development of adult-like neural responses during letter perception through a process of integration. Some authors have suggested that letter production recruits a sensorimotor functional network that is reified with each letter production experience and increasingly integrates with visual-perceptual regions in ventral-temporal cortex (James and Gauthier, 2006, Longcamp et al., 2008, Vinci-Booher et al., 2016), a suggestion that is generally in line with theories of sensorimotor representation (McClelland et al., 1995, Versace et al., 2009). The neural systems that comprise this sensorimotor network become so integrated among themselves and with visual-perceptual processes through repeated letter production practice that the entire network is re-engaged if any of the neural systems receives stimulation. There is work to support this hypothesis: The same set of ventral-temporal and motor regions are active when literate adults visually perceive letters without any motor activity and when they produce letters without visual feedback (James and Gauthier, 2006) and functional connectivity between ventral-temporal and motor cortices is greater in preliterate children during the perception of letters trained through letter production compared to letters trained through typing (Vinci-Booher et al., 2016).
Second, letter production may lead to the development of adult-like neural responses during letter perception through a process of segregation. Some authors have suggested that if such a sensorimotor network existed during letter production, the long-term effect would be a refinement of the processes happening in each of the neural systems that comprise the network (Amit and Brunel, 1995, Freeman, 1995, Makino et al., 2016). The sensorimotor network is, then, more of a transient developmental mechanism that fine-tunes local processes that ultimately function relatively autonomously. There is also work that supports this hypothesis: Functional connectivity was greater when performing novel finger sequences compared to well-learned sequences (Sun et al., 2007) and became progressively less dense as participants’ performance on a novel piano sequence improved from novice to expert levels (Bassett et al., 2015).
There are two crucial differences between the integration and segregation views as they are presented here. First, the integration view is that connections increase somewhat permanently while the segregation view is that connections are transient (if the relevant connections exist at all). Second, the integration view is that the increase in connectivity is actually directly relevant for behavior (e.g., supports recognition) while the segregation view is that the connectivity is indirectly relevant for behavior (e.g., changes local processes that support recognition). Evidence in support of the integration view comes from work that has looked at training-induced changes in functional connectivity immediately after a short amount of training (e.g., Vinci-Booher et al., 2016); however, studies that have assessed functional connectivity after extensive amounts of training are more in line with the segregation view (e.g., Sun et al., 2007; Bassett et al., 2015). Additionally, evidence in support of the integration view comes from work that has looked at the effects of sensorimotor training on subsequent visual perception (e.g., Vinci-Booher et al., 2016) while studies that have assessed functional connectivity during the performance of the sensorimotor training task itself are more in line with the segregation view (e.g., Sun et al., 2007; Bassett et al., 2015).
Studies using resting functional connectivity, a measure of neural communication at rest, also support the segregation view. Resting functional connectivity studies have measured the temporal relationship between performance on a sensorimotor training task and resting functional connectivity. These studies have demonstrated that the timeline of changes in learning is different than the timeline of changes in resting functional connectivity. Phillip and Frey (Philip and Frey, 2016) trained participants on a tracing task in which the participants were instructed to prioritize accuracy in their drawings for 10 days and measured resting functional connectivity before training, after training, and again at a 6-month follow-up visit. Resting functional connectivity was greater after training than before training and positively correlated with the fluency of their drawings. Performance on the drawing task was maintained at the 6-month follow-up visit and, somewhat surprisingly, the resting functional connectivity was not. Indeed, other studies have reported similar time line differences between learning and resting functional connectivity: resting functional connectivity decreased monotonically after reading training ceased (Berns et al., 2013) and correlated with performance on a motor task until performance plateaued, at which time connectivity began to dissipate even as training continued (Ma et al., 2011).
2. Selectivity of training-induced changes in functional connectivity to the task
Training effects on functional connectivity are often related to connectivity among brain regions related to the modalities that would be expected to be relevant for the training task (i.e., visual, somatosensory, motor, etc.). Training on a novel finger sequence task affected functional connectivity among somatomotor and premotor regions (Sun et al., 2007), and training on a visually guided novel piano sequence affected functional connectivity among primary visual and motor brain regions (Bassett et al., 2015). When the task was purely visual, early learning was characterized by stronger functional connectivity among primary visual regions whether the training was focused on identifying affective stimuli (Damaraju et al., 2009) or visually presented textures (Schwartz et al., 2002). A similar specificity of training-induced changes in functional connectivity to connections that would be expected to be relevant to the training task has been found using measures of functional connectivity at rest. When training on a shape identification was restricted to one visual hemisphere, resting state functional connectivity positively correlated with task performance in the trained hemisphere but not in the non-trained hemisphere (Lewis et al., 2009). These studies suggest that the communication among neural systems that occurs during the performance of a task is likely related to the modalities required to perform that task.
3. This study
We were interested in understanding how letter production leads to changes in functional connectivity during letter perception and how those changes relate to increased letter recognition after production experience. Prior work has demonstrated that letter production leads to increased functional connectivity immediately after training and seems to suggest that this increase in functional connectivity directly supports increased recognition—supporting the integration view (James and Gauthier, 2006, Longcamp et al., 2008, Longcamp et al., 2006, VinciBooher et al., 2016, Zemlock et al., 2018, but see Longcamp et al., 2005). There is, however, other prior work that has demonstrated that functional connectivity is greater early in training compared to later in training even though task performance is greater later in training compared to early in training—supporting the segregation view (Bassett et al., 2015, Berns et al., 2013, Ma et al., 2011, Philip and Frey, 2016, Sun et al., 2007). Prior work supporting the integration view did not assess functional connectivity or recognition at any time-point after the immediate post-training assessment. We thought it was possible that the increased functional connectivity observed immediately after training was transient and not directly supportive of recognition (segregation)–because this would be consistent with both bodies of prior work. Such a finding would not only support the segregation view but would also bring into accord the prior work suggesting integration with the prior work suggesting segregation. We, therefore, explored the timeline of training-related changes in recognition and functional connectivity to better understand how letter production experience leads to changes in functional connectivity during letter perception and how those changes relate to increased recognition with the expectation that the timeline would be consistent with the segregation view.
We were also interested in understanding how the visual-motor coordination inherent to letter production might contribute to the increase in visual letter recognition and visual-motor functional connectivity that has been observed during letter perception (James and Gauthier, 2006, Longcamp et al., 2005, Longcamp et al., 2008, Longcamp et al., 2006, Vinci-Booher et al., 2016, Zemlock et al., 2018). To address these two aims we imposed a within-participants training manipulation that was designed to manipulate the contingency between the motor and visual experiences of a letter that occur during letter production. Twenty literate adults were trained over the course of two weeks on four sets of novel symbols. Each symbol set was trained through one of four conditions: drawing with ink (motor, dynamic visual), drawing without ink (motor, no dynamic visual), watching a handwritten symbol unfold (no motor, dynamic visual), and watching a static handwritten symbol (no motor, no dynamic visual). Participants underwent three task-based fMRI scanning sessions: one pre-training, one post-training, and one after a no-training delay. Training only occurred between the first and second scanning sessions and included four 45-min training sessions over the course of four days. During fMRI scanning, participants were presented with the symbols that they had learned in the different training conditions as well as a fifth set of untrained symbols. A symbol recognition test was administered after each training session and at the third scan to assess changes in their ability to visually recognize the practiced symbols.
We expected that (1) symbol recognition would be best for symbols that were learned through Draw Ink training and that (2) functional connectivity among visual and motor brain systems after training would be greater for these symbols compared with symbols learned through other forms of training. Our final prediction was related to the timelines of these training-induced increases in symbol recognition and functional connectivity. We expected that (3) the functional connections found immediately after training would not be present after the one-week no-training delay, but that recognition performance would remain elevated. These results would suggest that the contingency between the visual and motor experiences of a letter during letter production results in temporary increases in integration among sensory and motor brain systems during letter perception that may not directly support letter recognition.
4. Materials and methods
4.1. Participants
Twenty-two participants were recruited through word of mouth. Two participants did not begin the study due to MRI contraindications, leaving a total of twenty 19–25 year-old adults (10 females). All participants were native English speakers with no experience with logographic languages and had normal or corrected-to-normal vision. All participants were right-handed and screened for history of neurological illness or trauma. This study was approved by the Institutional Review Board at Indiana University. All participants provided written informed consent.
4.2. Stimuli
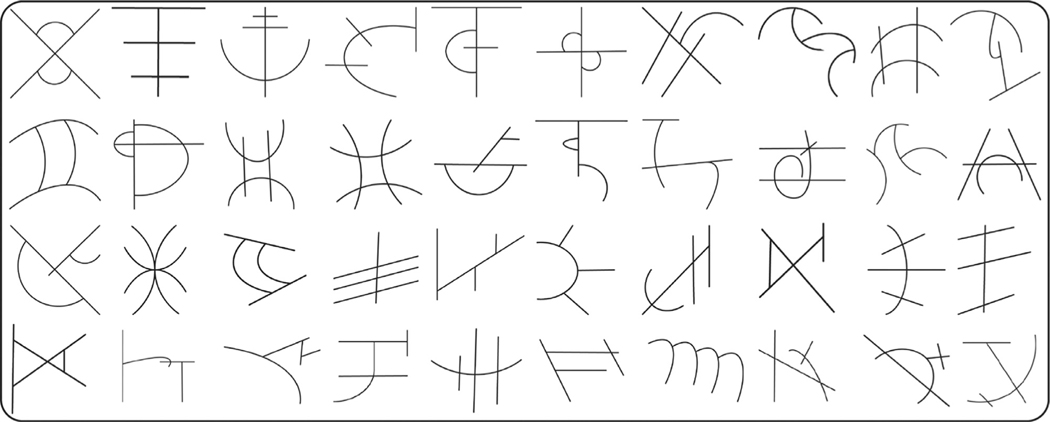
Stimuli included 400 novel symbols (see Fig. 1 for examples). Using novel, unfamiliar symbols is a well-documented approach that controls for individual differences in pretraining symbol knowledge (James and Atwood, 2009, Kersey and James, 2013, Longcamp et al., 2008, Longcamp et al., 2006) and allows for a cleaner manipulation of visual, auditory, and motor experience with those symbols. The training required 40 symbols while the testing and fMRI scanning required an additional 360 symbols that would not be learned. We initially generated 700 novel symbols and then removed symbols that too closely resembled known symbols as well as symbols that were reversals, mirror images, or rotations of other symbols. This filtering process resulted in 400 novel, unique symbols that each had four strokes, no circles, and each line connected to at least one other line. Adobe Illustrator was used to create typed versions of these novel symbols. All symbols were in black ink displayed on a white background at the center of the screen.
Fig. 1. Examples of novel pseudoletters.
Four hundred novel, unique symbols were constructed. Each symbol had four strokes, no circles, and each line of the symbol connected to at least one other line of the symbol. Forty of these symbols were selected as targets (symbols on which participants would receive training) and the remaining symbols were used as distractors.
Only 40 of the 400 novel symbols were used during training. These 40 were selected carefully to ensure that participants would be likely to spontaneously select the desired stroke order and stroke directions during the drawing conditions (see Supplemental Materials for more details). The 40 symbols selected for training were subsetted into groups of 10 symbols, one symbol set for each training condition. The other 360 symbols became novel symbols that were used as unlearned symbols for the functional neuroimaging or distractor symbols for the recognition tasks.
4.3. Procedure
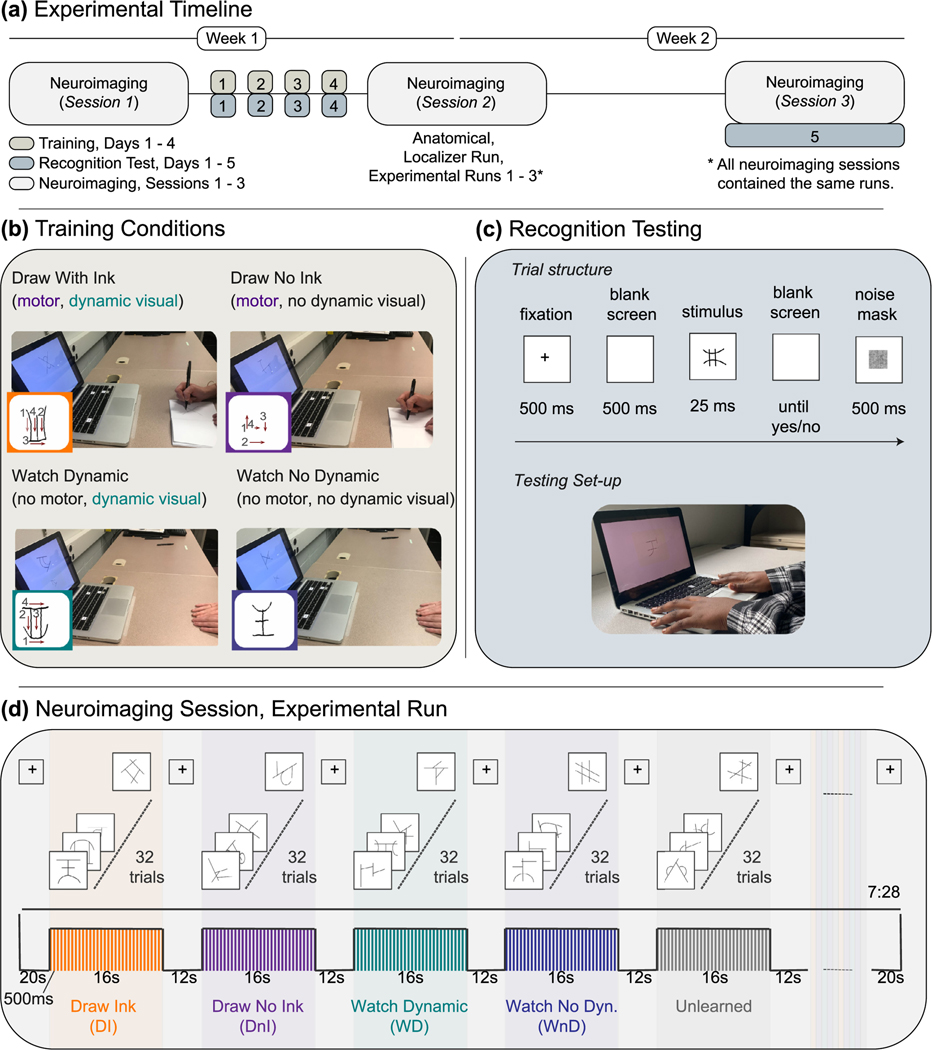
Participants completed three neuroimaging sessions and four training sessions (Fig. 2a). The first and second neuroimaging sessions occurred before and after training. The third neuroimaging session occurred approximately one week after the second neuroimaging session. Within the first week, participants completed four days of training with no more than one training session per day. Training days were not required to be consecutive, but all four training sessions were required to be completed within the 5–6-day period between the first and second neuroimaging session. No training occurred between the second and third neuroimaging session. Powerpoint was used to present all stimuli during training. Superlab was used to present stimuli and record accuracy and reaction time measurements during the recognition test.
Fig. 2. Experimental Procedures.
(a) Experimental Timelines. Participants completed three neuroimaging sessions, one before training, one after training, and one after a one-week no-training delay. Recognition testing occurred after every training session and at the third scan. (b) Training Conditions. The four training conditions manipulated the visual and motor components of letter production. A typed version of the symbol was provided on the computer screen in all conditions. Participants trained on novel symbol by either drawing them or not drawing them (motor factor) and/or by seeing the strokes of the letter unfold as if being written or presented statically (visual factor). (c) Recognition Testing. Symbol learning was assessed after each training session and at the final scanning session. Participants were presented with static, typed versions of the 40 learned symbols along with 40 novel distractors one at a time in random order and were asked to perform an old/new recognition judgement. (d) Neuroimaging: Task fMRI Paradigm. Functional neuroimaging was performed before training, after training, and after a no-training delay of approximately one week. Participants were presented with symbols, blocked by training condition, and were asked to perform one-back task to maintain attention. All symbols were presented in a typed format.
Training.
Each participant learned a set of ten novel symbols in each of four training conditions for a within-participants, between-symbols design. The four training conditions were designed to reflect the components of letter production (Fig. 2b). The Draw Ink (DI) condition included the motor and dynamic visual aspects of letter production (participants wrote each symbol and could see their own hand movements and production result); the Draw No Ink (DnI) condition only included the motor aspect (participants wrote symbol and could see their own hand movements, but could not see the strokes produced); the Watch Dynamic (WD) condition included only the dynamic visual presentation of the form (participants saw the production – the strokes of the symbol unfolding as if it were being produced – but their hands were held stationary on the desk); the Watch No Dynamic (WnD) condition included neither the dynamic visual nor the motor aspects of letter production (participants saw the final production result – the static image of the symbol – while their hands were held stationary on the desk). Only the Draw Ink condition resulted in a visual percept of each symbol that was coincident with the actions used to create it. Each participant, therefore, learned a set of ten symbols in the Draw Ink block, another ten in the Draw No Ink block, another ten in the Watch Dynamic block, and the last ten in the Watch No Dynamic block. The order of training blocks was randomized, within and across participants. The assignment of symbol sets to training condition was counterbalanced across participants.
At each training session, participants sat at a desk with a laptop computer in front of him/her and completed four blocks of training, one for each condition. For each training condition block, a PowerPoint slideshow presented each of the ten symbols for that condition one at a time, six times each, in random order. A typed version of the current symbol was displayed at the top and center of each PowerPoint slide for the entire trial in every condition (Figs. 1 and 2b). During Draw Ink training, participants were asked to copy the typed versions of the symbols that were displayed on the screen into a paper booklet with 4.15 × 5.5 inch white sheets using a pen with ink. The Draw No Ink condition was similar to the Draw Ink condition except that the participants wrote the symbols with a pen without ink. During the Watch Dynamic condition, participants placed their hands palm-down on the desk in front of them and were presented with a handwritten version of the symbol unfolding, as if being written, below the typed version of the same symbol. The Watch No Dynamic condition was similar to the Watch Dynamic condition except that the handwritten symbols shown on the screen were static and not dynamically unfolding. The dynamic handwritten symbols were created by a screen recording of an experimenter copying the symbols. The static handwritten symbols were created by taking the final frame of this screen recording that contained the completed handwritten symbol. Only one dynamic and one no-dynamic version were created for each symbol.
Each slide was timed and advanced to the next slide (i.e., symbol) after a certain amount of time had passed. The amount of time allotted for each slide at each training session was experimentally determined to account for practice-induced changes in speed that were observed during piloting and to equate, as much as possible, exposure times between the draw and watch conditions (see Supplemental Materials; Supplementary Table 1). The time allotted to each symbol in the draw conditions was 5.5–8.5 seconds with a 7.0 second average. The distribution was skewed to the longer times for the first training day and gradually moved to being skewed to the shorter times for the final training day. The time allotted to each symbol in the watch conditions was also between 5.5 – 8.5 seconds with a 7.0 second average but the distribution was skewed to the shorter times for all training days. This provided the best match in exposure times between the drawing and watch conditions while accounting for differences in processing times between the motor and visual systems.
Each training block lasted about 10 minutes and the entire training session lasted no more than 45 minutes.
Recognition Testing.
Participants were asked to perform an old/new recognition judgement immediately following each training session and at the final scanning session (Fig. 2c). During recognition testing, participants were presented with static, typed versions of the 40 learned symbols along with 40 unlearned distractor symbols one at a time in random order. Unlearned symbols that were presented during recognition testing were only seen once throughout the entire experiment. Each symbol was only presented once at each test, for a total of 80 trials at each test. For each symbol, participants were instructed to answer yes if they had learned the symbol or no if they had not by pressing the ‘yes’ or ‘no’ button on a computer keyboard. To counteract the possibility that participants may respond faster with one hand for a particular response than for the other hand (e.g., faster to reply “yes” when “yes” is assigned to the right hand than when it is assigned to the left hand), half of the participants always pressed yes with their right finger and the other half always pressed yes with their left finger. Before the first recognition task on the first training day, a practice task was administered that consisted of letters and keyboard symbols (e.g., $, &, %) and the participants were asked to press ‘yes’ for letters and ‘no’ for the keyboard symbols. The practice test helped orient participants to the testing context and was repeated until it was clear that they understood the task.
Each trial began with a 500 ms fixation cross, followed by a 500 ms blank screen, and then a 25 ms stimulus presentation during which a stationary symbol was displayed in the center of the screen (Fig. 2c). After the stimulus presentation ended, the symbol was replaced by a noise mask until the participant responded. The noise mask was followed by a 500 ms black screen after which a new trial would begin. If the participant responded before the symbol was replaced by the noise mask, the program advanced to the blank screen for 500 ms before moving on to the next trial. Reaction time and accuracy were measured. Procedures for the recognition test closely followed the procedures described in prior work (James and Atwood, 2009). The stimulus presentation time, however, was experimentally determined to ensure that our test was sensitive enough to detect a learning effect from the first to last training days (see Supplementary Materials).
Neuroimaging.
Functional neuroimaging was performed before training, after training, and after a no-training delay of approximately one week (Fig. 2a). Each functional neuroimaging session was required to be within 6–7 days of the previous neuroimaging session. Each scanning session included an anatomical scan followed by a functional localizer run and then three functional experimental runs. During the localizer and experimental runs, participants performed a one-back identification task to keep their attention focused on the experiment. The responses from the one-back task were not recorded; however, an experimenter kept watch to ensure that the participants were responding.
For the localizer runs, there were four block types: letters with thin lines, letters with thick lines, shapes with thin lines, and shapes with thick lines. There were 24 letters (the alphabet excluding I and O) with both thin and thick lines as well as 24 shapes with both thin and thick lines, for a total of 96 unique stimuli. Each block type was repeated three times over the course of the run, for a total of twelve blocks. Each block consisted of 32 stimuli, one presented in each of the 32 trials within a block. The order of the 32 stimuli within each block was pseudo-randomized to ensure that at least 1 and no more than 3 one-back matches occurred in each block. The number of one-back match trials per condition were counterbalanced to ensure that the number of one-back match trials in each block type was held constant. Each trial lasted for 500 ms and there were no gaps between trials, resulting in 16-second blocks. Each block was separated by a 12-s inter-block interval. During the inter-block interval, a fixation cross was presented in the center of the screen. The same fixation cross was presented for 20 s before the first block of each run and for 20 seconds following the last block of each run. Each localizer run, therefore, totaled 6:04 min.
For the experimental runs, there were five block types: symbols learned through Draw Ink (DI), symbols learned through Draw No Ink (DnI), symbols learned through Watch Dynamic (WD), symbols learned through Watch No Dynamic (WnD), and unlearned symbols (Fig. 2d). All symbols were presented in a typed format. Each block type was repeated three times over the course of each run, for a total of fifteen blocks of symbols. Each block contained 32 stimuli, one presented in each of the 32 trials within a block in random order with replacement. One-back match trials occurred randomly across blocks; the specific symbols displayed in each block were randomly selected from symbols suitable for that block type. For example, the symbols selected for the DI block were the 10 symbols that the participant had learned through DI training. For the unlearned blocks, 10 unlearned symbols were selected from the pool of 360 symbols set aside as unlearned symbols (see Materials and Methods: Stimuli). A new set of 10 unlearned symbols was selected for every task block so that a particular instance of an unlearned symbol was only used in one block. Once an unlearned symbol had been used, it was not used again throughout the entire experiment.
Each stimulus was shown for 500 ms, resulting in 16-s blocks. Each trial lasted for 500 ms and there were no gaps between trials, resulting in 16-s blocks. Each block was separated by a 12-s inter-block interval. During the inter-block interval, a fixation cross was presented in the center of the screen. The same fixation cross was presented for 20 s before the first block of each run and for 20 s following the last block of each run. Each experimental run, therefore, totaled 7:28 min.
Neuroimaging Parameters.
Neuroimaging was performed using a Siemens Prisma Fit 3-T whole-body MRI system housed in the Indiana University Imaging Research Facility. High-resolution T1-weighted anatomical volumes were acquired using a Turbo-flash 3-D sequence: TI = 900 ms, TE = 2.98 ms, TR = 2300 ms, flip angle = 9°, with 176 sagittal slices of 1.0 mm thickness, a field of view of 256 × 248 mm, producing an isometric voxel size of 1.0 mm3. For functional images, the field of view was 220 × 220 mm, with an in-plane resolution of 110 × 110 pixels, and 72 axial slices of 2.0 mm thickness per volume with 0% slice gap, producing an isometric voxel size of 2.0 mm3. Functional images were acquired using a gradient echo EPI whole-brain acquisition sequence with interleaved slice order: TE = 30 ms, TR = 1000 ms, flip angle = 52°, multi-band acceleration factor = 6 for blood-oxygen-level-dependent (BOLD) imaging. Localizer runs included 364 volumes. Experimental runs included 448 volumes.
Neuroimaging Preprocessing.
All preprocessing steps were performed in BrainVoyager 20.6. Individual anatomical volumes were normalized to Talairach space (Talairach and Tournoux, 1988). Preprocessing of functional data included slice scan time correction, 3-D motion correction using trilinear/sinc interpolation, and 3D Gaussian spatial blurring with a full-width-at-half-maximum of 6 mm. Temporal high-pass filtering was performed using a voxel-wise GLM with predictors that included a Fourier basis set with a cut-off value of 2 sine/cosine pairs and a linear trend predictor. Coregistration of functional volumes to anatomical volumes was performed using a rigid body transformation. All within-session functional runs were aligned to the first functional run within that session. The first functional runs of each session were then coregistered to the anatomical scan acquired in session one.
Neuroimaging Functional Connectivity Analysis.
All functional connectivity analyses were performed using in-house Matlab scripts (see Data and Code Availability Statement).
Parcellation.
Two-hundred and sixty-four regions of interest (ROIs) were selected based on a parcellation scheme constructed from a meta-analysis of task-based fMRI as well as a network-based community detection procedure using resting state functional connectivity analysis (Dosenbach et al., 2010, Power et al., 2011). The center x-,y-,z- coordinates for each of the 264 ROIs were dilated so that they included 2 voxels on either side of the center voxel. Preprocessed functional time courses were extracted from all voxels for each run. Average time courses for each ROI were created by averaging across voxels within the ROI.
Community Detection.
Each of the 264 ROIs were assigned to mutually exclusive communities (each ROI assigned to only one community) based on their correlations with one another during the localizer runs. We began with the resting-state-derived partition presented in Power et al. (2011) and applied a community detection process to optimize the partition for our data and our task (i.e., letter perception) (Dwyer et al., 2014, Hearne et al., 2017).
A group-averaged partial correlation matrix was constructed by taking the element-wise average of all participants’ correlation matrices for the localizer runs, controlling for motion parameters. We used the Generalized Louvain search algorithm to find the partition with the maxi- mum modularity (Blondel et al., 2008, Jeub et al., 2011). The resolution parameter, γ, was selected based on a normalized pair-counting algorithm applied to 1000 community partitions generated at 20 different values of γ linearly spaced between 0 and 1 (Traud et al., 2011). A consensus partition was created based on an iterative clustering procedure (the Generalized Louvain algorithm, as described above) performed on the agreement matrix for 1000 partitions produced at our selected γ value (Lancichinetti and Fortunato, 2012).
The gamma-detection procedure suggested two γ values, 0.4211 and 0.8421, with Z SR values of 108 and 163, respectively. We, therefore, selected a γ of 0.8421. This γ produced a consensus partition that included 13 communities with a Q value of 5.61 × 106. Most communities demonstrated a very high agreement among iterations, with the exception of community 12 and, to a lesser extent, with the exception of community 4. Whereas there was near perfect agreement between iterations for most communities, some of the ROIs that were assigned to communities 12 and 4 in the consensus partition were assigned to other communities in someiterations. In partitions where ROIs from communities 12 and 4 were not assigned to those communities, they were most often assigned to community 6 and, to a lesser extent, to communities 2, 3, and 7. Community 2 had two ROIs that were, in some partitions, assigned to community 1.
Functional Connectivity Analysis.
Our first step was to determine if any functional connections were related to visual-motor training that resulted in the production of a temporally coincident visual percept. We, therefore, evaluated the change in the functional connectivity associated with the interaction between the MOTOR and VISUAL factors between sessions, akin to evaluating the significance of a three-way interaction between MOTOR, VISUAL, and SESSION with a measurement of functional connectivity as the dependent measure. We used beta-weights from a psychophysiological interactions (PPI) analysis on the data collected during the experimental runs at each session as our measurement of functional connectivity (Friston et al., 1997, O’Reilly et al., 2012). Each community identified by our community detection algorithm was treated as a seed for PPI.
For each community and at each session, we constructed a PPI model that included a psychological predictor for the interaction between MOTOR and VISUAL factors (i.e., (DI>DnI)>(WD>WnD)), a physiological predictor representing the activity of a seed community, and a psychophysiological predictor representing the interaction between the psychological and physiological predictors, and a set of nuisance predictors. The psychological predictor was constructed by convolving the dummy-coded task predictor for the DI, DnI, WD, and WnD conditions with a single-gamma hemodynamic response function (HRF, Boynton et al., 1996); though Cole et al. (2019) suggests that using the FIR instead of the standard HRF may be an advantageous approach) and then combining these predictors to produce a single psychological predictor representing the interaction between MOTOR and VISUAL factors (i.e., (DI>DnI)>(WD>WnD)). The physiological predictor was constructed by averaging the activation time course across all ROIs within the community. The psychophysiological predictor was constructed by an element-wise multiplication between the psychological and physiological predictors. The nuisance predictors included the six rigid body motion regressors (Bullmore et al., 1999) and spike regressors for each time point at which the relative root mean squared (RMS) time course exceeded 0.5 mm (Satterthwaite et al., 2013). All independent and dependent variables were standardized.
Between-session differences in functional connectivity were evaluated for significance using permutation testing where the session labels were permuted. For each iteration, we subtracted the PPI beta-weights between two sessions of interest (e.g., Sessions 1 and 2, Sessions 2 and 3) for each subject and compared the distribution of the resulting values with a null distribution. The null distribution was constructed from 10,000 random selections where each selection was the difference between beta-weights for two sessions of interest from one of all possible permutations of the order of session. For each random selection, the pairing between the permutation selected and the subject selected was also allowed to vary randomly, thereby treating subjects as random effects. The null distribution was then estimated at the group-level and compared to the real distribution at the group-level to determine the likelihood of finding the real distribution by chance. The distance between the real distribution and the null distribution was quantified by a z-score for each community pair. Z-scores were considered significant if they passed a p < .05 threshold after an FDR adjustment based on the linear step up procedure with q < .10 (Benjamini and Hochberg, 1995). We selected a liberal q-value because this was a selection step. We wanted to select the community pairs that underwent any training-related change for further investigation. We selected q < .10, specifically, because it is the highest q-value that gives no significance when evaluating the interaction between MOTOR and VISUAL at the pre-training session, Session 1.
Community pairs that underwent significant changes in functional connectivity between sessions were further evaluated by assessing the strength of the interaction between MOTOR and VISUAL factors at each level of SESSION, akin to evaluating the component 2-way interaction effects after finding a significant three-way interaction. Similar to the between-session assessment, a null distribution was constructed from 10,000 random selections where each selection was the beta-weight associated with the interaction between MOTOR and VISUAL from one of all possible permutations of the order of condition in the interaction contrast (i.e., (DI > DnI) > (WD > WnD), (DnI > WD) > (WnD > DI), etc.). As with the between-session assessment, subjects were treated as random effects and the distance between the null and real distributions at the group level was subjected to the same significance criteria.
Community pairs that demonstrated a significant 2-way interaction effect at any one session were further evaluated by extracting the standardized beta-weights at each session from a generalized PPI model (McLaren et al., 2012, O’Reilly et al., 2012). A generalized PPI (gPPI) model was used for this comparison to evaluate functional connectivity associated with each condition because extracting condition-specific beta-weights is not possible with the standard PPI method when an interaction is tested. All conditions in an interaction must be combined into one psychophysiological predictor in the standard PPI method leaving no condition-specific beta-weights to be extracted. The gPPI model included four psychological predictors, one for each condition of interest: DI, DnI, WD, and WnD. Each of these predictors was constructed by convolving the dummy-coded task predictor for each condition with a single-gamma hemodynamic response function (Boynton et al., 1996). The physiological predictor was, again, the average activation time course across all ROIs within the community. The psychophysiological predictors were constructed by an element-wise multiplication of each psychological predictor with the physiological predictor, result in four psychophysiological predictors—one for each condition. The nuisance regressors used in the standard PPI model were also included in this model as predictors of no interest. All independent and dependent variables were standardized.
We then performed a series of ANOVA-based comparisons on the gPPI beta-weights for each significant community pair. Factors included MOTOR, VISUAL, and SESSION. MOTOR had 2 levels: draw, watch. VI- SUAL had 2 levels: dynamic visual, no dynamic visual. SESSION had 3 levels: session 1, session 2, and session 3. We first verified that the same three-way interaction and simple interaction effects with the standard PPI model and permutation tests were also found with the gPPI model and a standard ANOVA, given that gPPI has less power than standard PPI (McLaren et al., 2012, O’Reilly et al., 2012). The 2-way interaction effects were evaluated at each level of SESSION using the gPPI beta-weights; the 2-way interaction effects at each level of MOTOR and VISUAL were of no interest. Significant 2-way interaction effects at any level of SESSION were followed with planned paired t-tests: DI vs. DnI, DI vs. WD, and DI vs. WnD.
5. Results
5.1. Recognition testing
Two trials were omitted from both RT and accuracy analyses because the reaction time measure indicated that they had responded prematurely. Additionally, trials that were above or below 3 standard deviations of the within-condition, within-day mean for RT were considered outliers and removed from the RT data. We applied the same outlier removal procedure to the accuracy data. Trials that were above or below 3 standard deviations of the within-condition, within-day mean for accuracy were considered outliers and removed from the accuracy data. Details about trials removed in each condition and session can be found in Supplemental Table 2.
We performed two Three-way Repeated-measures ANOVAs, one for accuracy and one for reaction time. For both models, MOTOR, VISUAL, and DAY were entered as a within-participant factors. MOTOR had two levels, corresponding to the manipulation of the motor experience (Draw, Watch) and VISUAL had two levels, corresponding to the manipulation of the visual experience with the symbol (Dynamic, No Dynamic). DAY had three levels (Day 1, Day 4, Day 5), corresponding to the days on which a recognition tests was administered (Fig. 2a), immediately after the first training day (Day 1), after the training week (Day 4), and after the no-training delay (Day 5). The ANOVA with reaction time was calculated using only correct trials. Each omnibus ANOVA was followed by planned comparisons for DAY. Planned comparisons for DAY were one-tailed paired t-tests between Days 1 and 4 to assess the effect of the training week, between Days 4 and 5 to assess the effect of the no-training delay, and between Days 1 and 5 to assess learning over the entire experimental timeline.
Reaction time.
Mauchly’s test indicated a violation of sphericity for DAY, χ2 (2) = 25.593, p < .001, for the MOTOR*DAY interaction, χ2 (2) = 10.504, p = .005, for the VISUAL*DAY interaction, χ2 (2) = 17.560, p < .001, and for the three-way interaction, χ2 (2) = 22.217, p < .001. The Greenhouse-Geisser Epsilon values for all sphericity violations were less than 0.75. We, therefore, applied the Greenhouse-Geisser correction for DAY, MOTOR*DAY, VISUAL*DAY, and the three-way interaction.
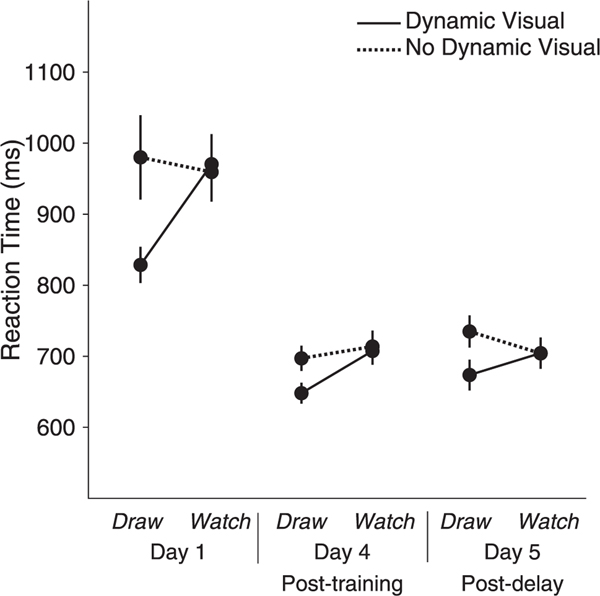
The three-way repeated-measures ANOVA for reaction time revealed main effects of MOTOR, F(1, 19) = 8.530, p = .009, ƞp 2 = .310, VISUAL, F(1, 19) = 13.186, p = .002, ƞp 2 = .410, and DAY, F(1.137, 21.607) = 47.840, p < .001, ƞp 2 = .716. Participants responded faster to symbols learned by drawing than symbols learned by watching and for symbols learned by watching the strokes of the symbol unfold than not watching the strokes unfold. The analysis also revealed a significant two-way interaction between MOTOR and VISUAL, F(1, 19) = 9.402, p = .006, ƞp 2 = .331. The interactions between DAY and MOTOR and between DAY and VISUAL were not significant, F(1.387, 26.351) = 2.438, p = .121, ƞp 2 = .114, and, F(1.232, 23.413) = 1.346, p = .272, ƞp 2 = .310, respectively. The three-way interaction was not significant, F(1.170, 22.236) = 3.389, p = .074, ƞp 2 = .151.
Post hoc comparisons for MOTOR*VISUAL revealed that participants responded faster when presented with symbols learned through Draw Ink (M = 716.77, SE = 15.382) than symbols learned through Draw No Ink (M = 804.000, SE = 26.837), p < .001, Watch Dynamic (M = 794.099, SE = 22.612), p < .001, and Watch No Dynamic (M = 792.381, SE = 19.852), p < .001, all Bonferroni corrected. There were no differences between Draw No Ink and Watch Dynamic, p = .58, between Draw No Ink and Watch No Dynamic, p = .59, or Watch Dynamic and Watch No Dynamic, p = .919 (Fig. 4).
Fig. 4. Reaction Time at Recognition Testing.
Participants responded faster when presented with symbols learned through Draw Ink than symbols learned through Draw No Ink, Watch Dynamic, and Watch No Dynamic. Participants responded faster after the last day of training and after a week-long no-training delay, compared to the first day of training. Error bars represent standard error.
Planned comparisons for DAY revealed that participants responded faster on day 4 (M = 691.571, SE = 16.250) compared to day 1(M = 934.607, SE = 34.563), p < .001, and on day 5 (M = 704.265, SE = 19.074) compared to day 1, p < .001. There was no difference between reaction time on day 4 compared to day 5, p = .116 (Fig. 4).
Accuracy.
Mauchly’s test indicated a violation of sphericity for the MOTOR*DAY interaction, χ2 (2) = 6.186, p = .045, and the VISUAL*DAY, χ2 (2) = 0.681, p = .003. The Greenhouse-Geisser Epsilon values for both were less than 0.75. We, therefore, applied the Greenhouse-Geisser correction for MOTOR*DAY and VISUAL*DAY.
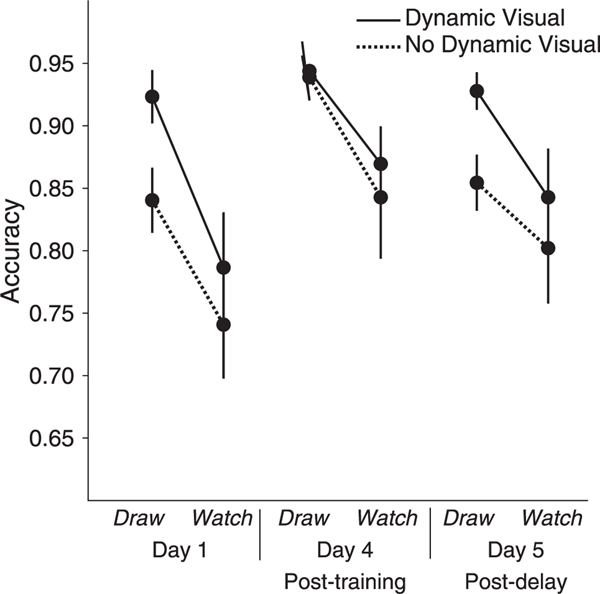
The three-way repeated-measures ANOVA for accuracy revealed main effects of MOTOR, F(1, 19) = 8.687, p = .008, ƞp 2 = .314, VISUAL, F(1, 19) = 10.880, p = .004, ƞp2 = .364, and DAY, F(2, 38) = 6.008, p = .005, ƞp 2 = .240. Participants responded more accurately to symbols learned by drawing than symbols learned by watching and to symbols learned by watching the strokes of the symbol unfold than not watching the strokes unfold. The interaction between MOTOR and VISUAL was not significant, F(1, 19) = 0.318, p = .579, ƞp 2 = .016. The interactions between DAY and MOTOR and between DAY and VISUAL were not significant, F(2, 38) = 0.917, p = .408, ƞp 2 = .046, and, F(2, 38) = 0.997, p = .379, ƞp 2 = .050, respectively. The three-way interaction was not significant, F(2, 38) = .756, p = .476, ƞp 2 = .038.
We performed post hoc comparisons for MOTOR*VISUAL even though two-way interaction did not reach significance as this was our main hypothesis. Post hoc comparisons revealed that participants responded more accurately when presented with symbols learned through Draw Ink (M = 0.931, SE = 0.012) than symbols learned through Draw No Ink (M = 0.878, SE = 0.013), p = .004, Watch Dynamic (M = 0.833, SE = 0.030), p = .006, and Watch No Dynamic (M = 0.795, SE = 0.038), p = .001, all Bonferroni corrected. There were no differences between Draw No Ink and Watch Dynamic, p = .188, or between Watch Dynamic and Watch No Dynamic, p = .118. The difference between Draw No Ink and Watch No Dynamic, p = .033, did not pass Bonferroni correction (Fig. 5).
Fig. 5. Accuracy at Recognition Testing.
Participants were more accurate in the drawing conditions compared to the watching conditions and in the dynamic visual conditions compared to the conditions with no dynamic visual conditions. Planned comparisons for DAY revealed that participants were more accurate after four days of training compared than on their first day of training and com- pared to after a week-long no-training delay. There was no significant difference in accuracy between their first day of training and after the no-training delay. Error bars represent standard error.
Participants were more accurate in the drawing conditions (M = .905, SE = .010) compared to the watching conditions (M = .814, SE = .032) and in the dynamic visual conditions (M = .882, SE = .017) compared to the conditions with no dynamic visual (M = .837, SE = .022).
Planned comparisons for DAY revealed that participants were more accurate on day 4 (M = .899, SE = .020) compared to day 1(M = .823, SE = .022), p = .001, and compared to day 5 (M = .857, SE = .023), p = .01. There was no difference between day 1 and day 5, p = .203 (Fig. 5).
5.2. Neuroimaging
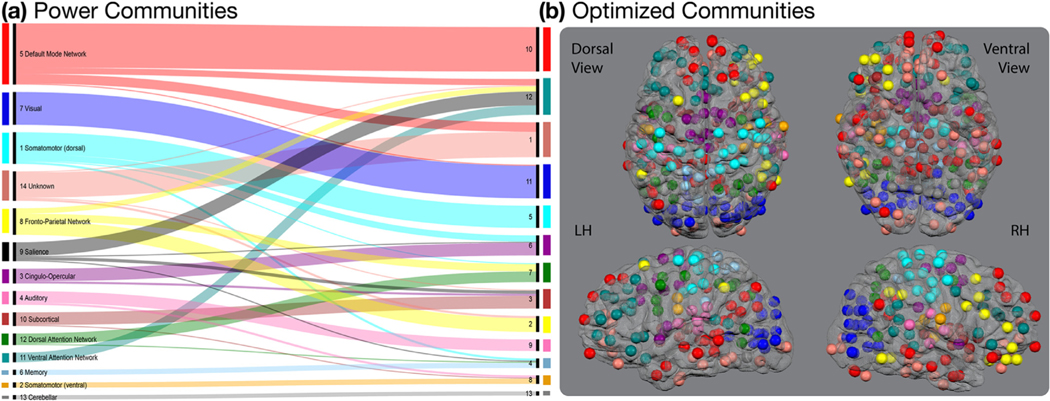
The division of ROIs into the communities presented in Power et al. (2011) was, in general, retained after the community detection procedure (Fig. 3). Exceptions were associated with the communities from the optimized partition that displayed the least agreement across iterations, communities 1 and 2 as well as communities 3, 4, 6, 7, 8, and 12. Fig. 3 displays the relationship between the original partition presented in Power et al. (2011) and the optimized partition used here. A list of the Talairach X-, Y-, and Z-coordinates for the optimized partition as well as the community to which that ROI was assigned in the original Power et al. (2011) partition is provided in Supplemental Table 3.
Fig. 3. Relationship Between Power Communities and Optimized Communities.
(a) A large portion of the original communities presented in Power et al. (2011) were retained after optimizing the partition for our data set and task. The most notable exceptions included the optimized communities 4 and 12 that were constructed of ROIs from several different communities in the Power et al. (Power et al., 2011) partition. The alluvial flow chart was produced using RAWGraphics Software at rawgraphs.io (Mauri et al., 2017). (b) The 264 ROIs are displayed on a glass brain and color coded for their community assignment in the optimized community. ROI sizes were reduced for display. The actual ROI radii were about twice as large as those displayed here.
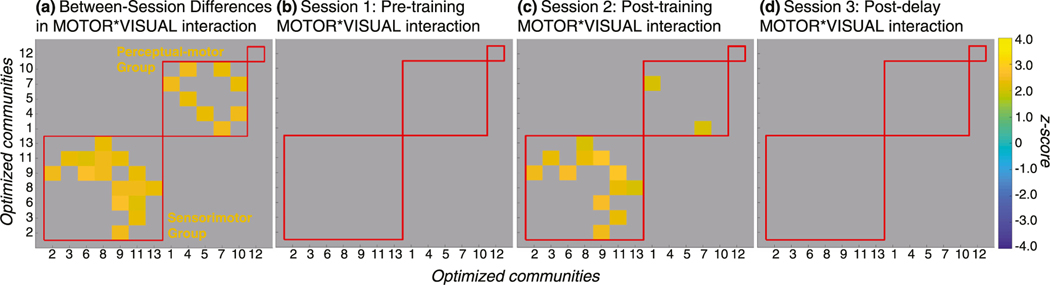
All communities demonstrated a three-way interaction for DAY, MOTOR, and VISUAL with at least one other community besides community 12. Matrices displaying the z-scores of significant community pairs are presented in Fig. 6. Community pairs that demonstrated a significant three-way interaction effect were separable into three non-overlapping groups based on their between-session changes in connectivity. The community pairs demonstrated obvious grouping (Fig. 6a); we did not apply a clustering algorithm. The first group included communities 2, 3, 6, 8, 9, 11, and 13. The second group included communities 1, 4, 5, 7, and 10.
Fig. 6. Significant Differences in Functional Connectivity Between Sessions.
Each matrix displays z-scores for community pairs that demonstrated significant changes in functional connectivity among sessions, thresholded at p < .05 with an FDR adjustment at q < .10. Red lines mark the boundaries of the three community groups. All communities demonstrated some change in connectivity, except for community 12. (a) The overall three-way comparison indicated that functional connectivity associated with the interaction between motor and visual factors was significantly different between sessions in two non-overlapping groups of community pairs. The first of these groups included communities 2, 3, 6, 8, 9, 11, and 13 with the majority of this group’s connections being associated with community 11. The second of these groups included communities 1, 4, 5, 7, and 10. (b) Community pairs that demonstrated a significant three-way interaction were evaluated for simple interaction effects between MOTOR (draw, watch) and VISUAL (dynamic visual, no dynamic visual) at each level of SESSION (pre-training, post-training, post-delay). There were no community pairs that demonstrated a significant simple interaction between MOTOR and VISUAL factors before training at Session 1. (c) Several community pairs demonstrated a significant simple interaction between MOTOR and VISUAL factors after training at Session 2. For the first group, these community pairs include 2–9, 6–9, 3–11, 8–11, 9–11, and 8–13. For the second group, only the 1–7 community pair demonstrated a significant simple interaction at Session 2. (d) There were no community pairs that demonstrated a significant simple interaction between MOTOR and VISUAL factors after a one-week no-training delay at Session 3.
All ANOVA-based analyses with the gPPI-estimated beta-weights were consistent with the results of the permutation tests using the beta-weights estimated from the standard PPI model. We, therefore, report on only the results of the permutation tests using the beta-weights estimated from the standard PPI model below as well as the planned paired t-tests using the gPPI-estimated beta-weights. Results are provided for each group separately.
Sensorimotor Group of Community Pairs.
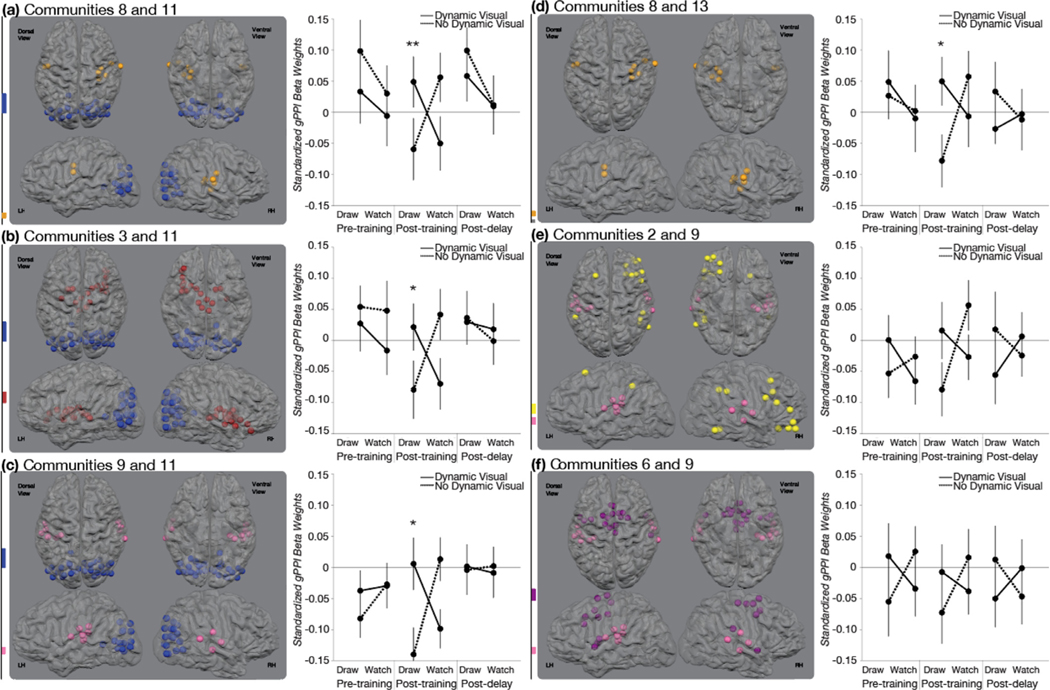
There were no significant functional connections associated with the VISUAL*MOTOR interaction before training at Session 1 or after the no-training delay at Session 3. There were, however, six community pairs that demonstrated a significant VISUAL*MOTOR interaction immediately after training at Session 2 in this group (Fig. 7). Communities included in this group were communities 2, 3, 6, 8, 9, 11, and 13. The backbone of this group included communities 8, 9, and 11. Each of these communities were directly relatable to original communities reported in Power et al. (2011): Somatomotor (Ventral), Auditory, and Visual.
Fig. 7. Training-related Changes in Functional Connectivity for the Sensorimotor Group.
The three-way interaction among MOTOR (draw, watch), VISUAL (dynamic visual, no dynamic visual), and SESSION (pre-training, post-training, post-delay) was significant for six community pairs that shared a community with at least one other significant community pair. Each community pair demonstrated a significant crossover interaction between MOTOR (draw, watch) and VISUAL (dynamic visual, no dynamic visual) at Session 2 that was not found at Session 1 or Session 3. Significant two-way interactions were followed with planned paired t-tests. (a, b, c) Three communities demonstrated significant differences in their functional relationship with community 11, a community comprised of nearly entirely visual ROIs. Communities 8, 3, and 9 are made of bilateral ventral somatomotor ROIs, subcortical ROIs, and bilateral auditory cortex. (c, e, f) Three communities demonstrated significant differences in their functional relationship with community 9, a community comprised of auditory ROIs. These include communities, 11, 2, and 9. Community 2 is comprised of frontal and parietal ROIs and is largely right-lateralized. Community 6 is comprised of primarily of bilateral frontal motor regions. (a, d) Two communities demonstrated differences in their functional relationship with community 8. These include communities 11 and 13. Community 13 is comprised of 4 cerebellar regions. Error bars represent standard error across subjects. * * p < .01, * p < .05.
The periphery of this group of community pairs included communities 2, 3, 6, and 13. Community 2 was comprised of a subset of the Fronto-Parietal community reported in Power et al. (2011). Community 3 was largely comprised of the Subcortical community originally reported in Power et al, (2011) with a few additional ROIs. Community 6 was comprised of two communities from the Power et al. (2011) parcellation: a subset of the Somatomotor (Dorsal) community and the entire Cingulo-Opercular community. Community 13 was exactly the same as the original Cerebellar community reported in Power et al. (2011).
Five community pairs in this group showed greater connectivity for DI than for DnI: subcortical and visual communities [3 and 11], ventral somatomotor and visual communities [8 and 11], ventral somatomotor and cerebellar communities [8 and 13], and auditory and visual communities [9 and 11]. One community pair in this group showed greater connectivity for DI than for WD: auditory and visual communities [9 and 11]. All other comparisons were not significant (see Supplemental Results for details).
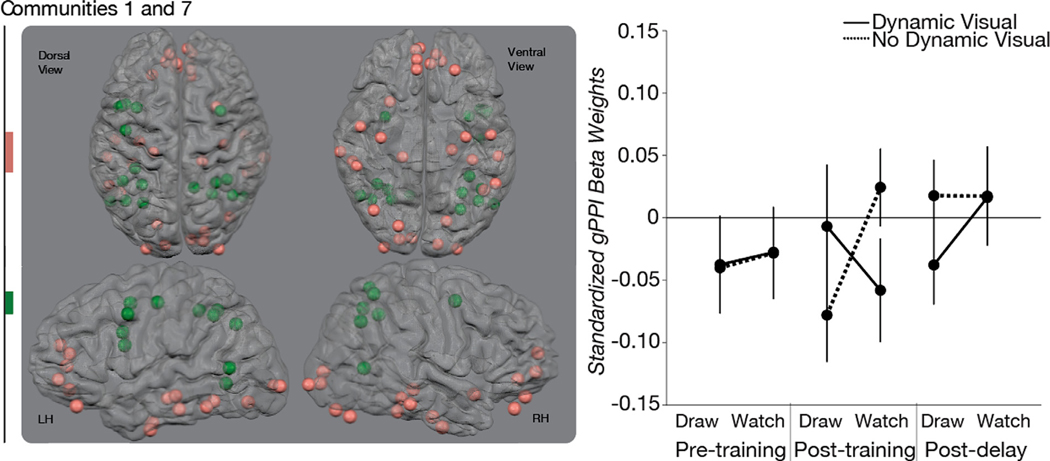
Perceptual-motor Group of Community Pairs.
There were no significant functional connections associated with the VISUAL*MOTOR interaction before training at Session 1 or after the no-training delay at Session 3. There was only one community pair that demonstrated a significant VISUAL*MOTOR interaction immediately after training in this group at Session 2: communities 1 and 7 (Fig. 8). Community 1 was comprised of the Unknown Power et al. (Power et al., 2011) community and a subset of ROIs from the Default Mode Network Power et al. (Power et al., 2011) community. Community 7 was comprised of the entire Dorsal Attention Network Power et al. (Power et al., 2011) community as well as a sub-set of ROIs from the Fronto-Parietal Network Power et al. (Power et al., 2011) community. All post hoc and planned comparisons were not significant (see Supplemental Results for more detail).
Fig. 8. Training-related Changes in Functional Connectivity for the Perceptual-motor Group.
The three-way interaction among MOTOR (draw, watch), VISUAL (dynamic visual, no dynamic visual), and SESSION (pre-training, post-training, post-delay) was significant for one community pair that did not share a community with any other significant community pair. This community pair demonstrated a significant crossover interaction between MOTOR (draw, watch) and VISUAL (dynamic visual, no dynamic visual) at Session 2 that was found at neither Session 1 nor Session 3. The significant two-way interaction was followed with planned paired t-tests. None of the planned paired t-tests were significant. Community 1 was comprised of ventral-temporal and prefrontal ROIs and was largely similar to the Unknown community in the original Power et al. (2011) partition. Community 7 was comprised of frontal and parietal ROIs. Error bars represent standard error across subjects.
6. Discussion
Letter production is a sensorimotor activity that relies on a tight coupling between motor movements and visual feedback in the early stages of letter learning. This study explored the importance of this visual-motor contingency to (1) gains in letter recognition and (2) the emergence of visual-motor functional connectivity during letter perception. This study also sought (3) to provide new evidence concerning the relationship between functional connectivity and recognition. We hypothesized that the spatiotemporal contingency among the motor and visual experiences of the letter during letter production would lead to gains in recognition and changes in visual-motor functional connectivity during visual perception. The results support these hypotheses. Participants recognized symbols learned by drawing them with ink faster than symbols learned in other conditions where motor and visual experiences were not coupled, such as drawing symbols without ink or simply watching them unfold as if they were being written. Participants also demonstrated changes in functional connectivity among visual, motor, and (surprisingly) auditory neural communities that were associated with the contingency between the visual and motor experiences during letter production. After a week-long no-training delay, participants were still faster and more accurate at recognizing symbols learned by drawing them with ink than symbols learned in the other conditions, but the functional connections observed immediately after training had returned to their pre-training baseline. Taken together, these results suggest that the visual-motor functional connectivity observed during perception after symbol production training was not directly related to the concurrent gains in recognition.
6.1. Recognition
Our hypothesis was that the contingency between the visual and motor experiences of a letter that occur during letter production is important for the gains in letter recognition—greater accuracy and faster reaction times—that often follow letter production training (Longcamp et al., 2005, Zemlock et al., 2018). We predicted that symbols learned through Draw Ink training would be recognized faster and more accurately than symbols learned in Draw No Ink, Watch Dynamic, and Watch No Dynamic training because Draw Ink training was the only condition that explicitly coupled the visual and motor experiences of the symbol. Participants recognized Draw Ink symbols faster and more accurately than symbols from the other conditions at all testing time points, thereby demonstrating training-related recognition gains for Draw Ink symbols relative to other symbols. We interpret these results to indicate that the contingency between the visual and motor experiences of a letter that occur during letter production is an important part of why letter production leads to gains in letter recognition.
Several other works have demonstrated similar recognition gains in both accuracy and reaction time after production practice for letters in preschool-aged children (Longcamp et al., 2005, Zemlock et al., 2018) and for novel symbols in adults (Longcamp et al., 2008, Longcamp et al., 2006). These studies have collectively demonstrated that production practice increases recognition for the practiced forms relative to typing and visual-only training, suggesting that the benefits of production on recognition were not simply due to the positive effects of motor actions (i.e., typing) on the orienting of attention or to visual exposure alone. The results of the current study extend these findings by demonstrating that one reason that production practice facilitates learning more than typing or visual-only training is because production requires a spatiotemporal contingency between the visual and motor experiences of the form produced. Indeed, positive effects of production experience on recognition performance are found in other sensory domains (speech and audition: for review see MacLeod and Bodner, 2017) and with other object categories (drawing and recognizing common objects: Fan et al., 2018, Wammes et al., 2019).
The current study also demonstrated that recognition gains were present immediately after training and were maintained to a certain degree after a one-week no-training delay. This result is in line with prior work demonstrating that recognition gains for symbols learned through production over typing experience were evident immediately after training ended and after a one-week no-training delay in preschool-aged children (Longcamp et al., 2005) and in literate adults (Longcamp et al., 2008). These results are also in line with prior work reporting that training-induced changes in recognition in adults were apparent one and three weeks after training ended (Longcamp et al., 2006).
6.2. Functional connectivity
Our hypothesis for functional connectivity was similar to our hypothesis for recognition: the contingency between the visual and motor experiences of a letter that occur during letter production is important for increases in functional connectivity between visual and motor brain systems during letter perception. This hypothesis was addressed with two primary predictions: (1) training will affect connectivity among visual and motor brain regions, specifically, and (2) functional connectivity will be more positive for Draw Ink training than all other training conditions. Each will be addressed in turn.
First, we predicted that Draw Ink training would change functional connectivity among visual and motor brain systems during symbol perception more than Draw No Ink, Watch Dynamic, or Watch No Dynamic training. We, therefore, looked for functional connections among neural communities that demonstrated an interaction at the post-training scan that was not present at the pre-training scan. We found that two groups of community pairs demonstrated an interaction at post-training that was not present at the pre-training scan. The first group was largely comprised of primary sensory regions (visual, auditory) as well as frontal motor regions, cerebellar, and subcortical regions (Fig. 7). The visual and auditory communities were both central to this group, both being associated with at least three other communities in the group. This group can, therefore, be summarized as a functional network comprised of primary sensory and motor-related regions, a sensorimotor network. The second group was comprised of ventral-temporal regions as well as parietal and frontal motor regions and can be summarized as a functional network comprised of ventral-temporal, parietal, and frontal motor regions (Fig. 8). These results, therefore, are generally in line with the prediction that the functional networks affected would be related to visual and motor brain regions, though other functional connections were also affected.
Second, we predicted that functional connectivity would be more positive for symbols trained through Draw Ink training than symbols trained through Draw No Ink, Watch Dynamic, and Watch No Dynamic training. Our results were not in line with this prediction. Functional connectivity was not more positive for symbols trained through Draw Ink training than symbols trained through Draw No Ink, Watch Dynamic, and Watch No Dynamic training. Symbols trained through Draw Ink and Watch No Dynamic training both resulted in similarly positive levels of functional connectivity in both groups and were often no different than zero (Figs. 7 and 8). The interactions were generally due to lower functional connectivity for symbols learned through Draw No Ink and Watch Dynamic training relative to Draw Ink and Watch No Dynamic training. This suggests that the interaction between visual and motor factors after training was due to a decoupling of neural systems during the perception of symbols learned through Draw No Ink and Watch Dynamic training. The results, therefore, were not in line with the prediction that Draw Ink training would increase functional connectivity during perception but do not necessarily invalidate the hypothesis that the visual-motor coordination inherent to letter production leads to the emergence of visual-motor functional connectivity during letter perception.
It is important to remember that this study was conducted with literate adults who have likely already established functional networks for learning through visual-motor activities. The sensorimotor and perceptual-motor groups that demonstrated training-induced changes are likely established functional networks that routinely support visual-motor activities and expect certain spatiotemporal contingencies. Interpreting the results with this in mind, the findings that Draw No Ink and Watch Dynamic training led to less functional connectivity among motor and primary sensory systems (sensorimotor group) than Draw Ink and Watch No Dynamic training suggests that training with de-coupled visual and motor experiences may facilitate a decoupling among sensory and motor systems that would, otherwise, have continued their general function. This would explain why we found no difference in functional connectivity between Draw Ink and Watch No Dynamic at the post-training scan and why the pre- to post-training changes from Draw Ink and Watch No Dynamic generally appeared small relative to the changes from Draw No Ink and Watch Dynamic (although this was not directly tested). This interpretation receives some support from stud- ies demonstrating that learning a new task utilizes pre-existing neural patterns, at least in cases where the new task is similar to a learned task (e.g., finger sequencing) (Sale et al., 2017). The Draw Ink and Watch No Dynamic training are both tasks that participants had likely experienced often and resulted in the ‘normal’ patterns of functional connectivity that would accompany symbol learning in adults through these tasks. Draw no ink and Watch Dynamic, however, were likely more novel tasks for adults and resulted in a disruption of the ‘normal’ patterns of connectivity. Future work should investigate changes in functional connectivity in young children to determine if these training conditions have different effects in young children who may still be learning about the expected spatiotemporal contingencies and, perhaps, learning how to learn from them.
6.3. Relationship between functional connectivity and recognition
Our results are consistent with the segregation view in that they suggest that functional connectivity does not directly support gains in recognition but leave open the possibility that functional connectivity indirectly translates to gains in recognition by facilitating the development of some other neural mechanism. Although this study could not address any causal relationship between recognition and the neural communication indexed by functional connectivity, it was able to characterize the time scale of training-induced changes in functional connectivity and recognition to provide evidence in support of the notion that training-induced changes in functional connectivity are not, in and of themselves, supporting training-induced changes in recognition. We predicted that any training-induced functional connections found at the post-training scan would not be present after the one-week no-training delay but that the recognition gains would be maintained. We found, as predicted, that the patterns of functional connections found at post-training were no longer present at post-delay and that changes in recognition were maintained to a certain degree over the no-training delay. The results, therefore, were in line with our predictions and provide support for the notion that training-induced changes in functional connectivity do not directly support changes in recognition.
An additional finding supports the notion that visual-motor functional connectivity at post-training did not directly support the gains in recognition that were observed at post-training. Training produced an ordinal interaction between the visual and motor factors for recognition while training produced a cross over interaction for functional connectivity. Recognition of symbols learned through Draw Ink training was faster and more accurate than recognition of symbols learned in any of the other three training conditions. Functional connectivity, however, was greater for Draw Ink training when compared to training with only the motor component (i.e., Draw No Ink) or with only the visual component (i.e., Watch Dynamic) but was no different than training with neither (i.e., Watch No Dynamic). This suggests that at least two mechanisms were at work and, in fact, provides further support for the hypothesis that training-induced patterns of functional connectivity during perception affect the development of some other neural mechanism that supports recognition because training conditions had different effects on functional connectivity than they did on recognition.
6.4. The potential role of the two groups of community pairs in perceptual learning from sensorimotor experiences
Spatiotemporal contingencies among sensory and motor experiences define all of our interactions with the world. There is hardly an action that does not elicit a sensory consequence—hardly a sensation that does not elicit an action. All of these sensorimotor pairings had to be learned—we did not always know the somatosensory and visual sensations that accompany drawing the letter “A” with a pen. After a history with these sensorimotor contingencies, sensorimotor cycles are built and within these cycles is an expectation that certain actions correspond to certain sensations and certain sensations correspond to certain actions. This study demonstrates that two functional networks were affected by experiences that violate what would be an expected contingency between visual and motor experiences during letter production. Neither Draw Ink nor Watch No Dynamic training would violate an expected contingency and neither condition appeared to affect the functional connectivity in these functional networks. We suggest that the state of these sensorimotor networks during the perception of symbols that had been learned through Draw Ink and Watch No Dynamic training was simply the continual functioning of a learning network that has two relatively independent learning mechanisms. In what follows, we speculate on the potential contribution of these two functional networks to symbol learning.
We suggest that the changes in the sensorimotor networks were related to the actual motor movements used during training while the changes in the perceptual-motor network were related to the visual percepts created during letter production. It is interesting to note that the major difference between the two groups of community pairs that were affected by training is that one group contained primary visual cortex (i.e., sensorimotor group) and the other contained ventral-temporal cortex (i.e., perceptual-motor group). The perceptual-motor group contained the only community pair that demonstrated a trend towards a unique effect on functional connectivity for the Draw Ink condition at either post-training or post-delay sessions. The perceptual-motor group is, therefore, the only group to demonstrate anything close to the same training effects for functional connectivity as for recognition. The perceptual-motor group demonstrated a trend toward an ordinal interaction for functional connectivity at the post-delay session, with similar, positive functional connectivity values for all conditions besides Draw Ink. Consider that no community pairs in the sensorimotor group resulted in anything trending towards an effect for Draw Ink compared to the other three conditions at either post-training or post-delay. The perceptual-motor group, however, demonstrated a trend towards this pattern at the post-delay scan. Given the abundance of research suggesting a role for ventral-temporal cortex on object perceptual processes and the possibility that it may function relatively autonomously during perception in later stages of learning (Gauthier, 2000, Gauthier et al., 1999, Grill-Spector and Weiner, 2014, Haxby et al., 2001, Malach et al., 2002, Milner and Goodale, 2006, Ungerleider and Mishkin, 1982), the perceptual-motor group may be related to perceptual processes—it may be best at perceptual processes when it isn’t coupled with the fronto-parietal system.
7. Conclusion
Our results demonstrate that the precision of the match between sensory and motor experiences during sensorimotor learning is important for perceptual learning and, simultaneously, contributes to the state of sensorimotor and perceptual-motor functional networks in the brain during visual perception. The results of the current study suggest, however, that training-related changes in functional connectivity during perception may not directly support training-related increases in recognition.
Data sharing statement
Data will be made available upon request until we finish additional planned analyses with these data. Deidentified MRI data will be shared privately upon request via brainlife.io, an online platform for open neuroscience. Deidentified behavioral data will be shared upon request via email. The data repository on brainlife.io will be made public when we have finished our planned analyses. Matlab code was developed in-house and is available at https://github.com/svincibo/VinciBooher-James-James_2020.
Supplementary Material
Acknowledgments
This research was funded by the Indiana University Office of the Vice Provost for Research Emerging Areas of Research Initiative, Learning: Brains, Machines, and Children and by the National Institute of Health, T32 Grant # HD 07475. The authors would like to thank Anastasia Nikoulina and Richard Betzel for their help in implementing the community detection algorithms. The authors would also like to thank Courtney DelaCuesta, Sarah Harris, and Hannah Marotta for their help with data collection.
Footnotes
Author statement
Sophia Vinci-Booher: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Project Administration. Thomas W. James: Methodology, Software, Validation, Formal Analysis, Resources, Writing – Review & Editing, Funding Acquisition. Karin H. James: Methodology, Resources, Data Curation, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2020.117520.
References
- Amit DJ, Brunel N, 1995. Global spontaneous activity and local structured (learned) delay activity in cortex. SCAN-9503265 Retrieved from http://www.cds.cern.ch/record/279089/files/SCAN-9503265.pdf. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Yang M., Wymbs NF, Grafton ST, 2015. Learning-induced autonomy of sensorimotor systems. Nat. Neurosci 18 (5), 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 289–300. [Google Scholar]
- Berns GS, Blaine K., Prietula MJ, Pye BE, 2013. Short- and long-term effects of a novel on connectivity in the brain. Brain Connect. 3 (6), 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E, 2008. Fast unfolding of commu- nities in large networks. J. Stat. Mech. Theory Exp P10008 2008CrossRef. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ, 1996. Linear systems analy- sis of functional magnetic resonance imaging in human V1. J. Neurosci 16 (13), 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe-Hesketh S., Curtis VA, Morris RG, Williams SCR, Sharma T., McGuire PK, 1999. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum. Brain Mapp 7 (1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Ito T., Schultz D., Mill R., Chen R., Cocuzza C., 2019. Task activations produce spurious but systematic inflation of task functional connectivity estimates. NeuroImage 189, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E., Huang Y-M, Barrett LF, Pessoa L., 2009. Affective learning enhances activity and functional connectivity in early visual cortex. Neuropsychologia 47 (12), 2480–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B., Cohen AL, Fair DA, Power JD, Church JA, Nel- son SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. , 2010. Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yücel M., Whittle S., Zalesky A., Pantelis C., … Fornito A., 2014. Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci.: Off. J. Soc. Neurosci 34 (42), 14096–14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JE, Yamins DLK, Turk-Browne NB, 2018. Common object representations for visual production and recognition. Cognit. Sci 42 (8), 2670–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder KP, Majnemer A, 2007. Handwriting development, competency, and in- tervention. Dev. Med. Child Neurol 49 (4), 312–317. doi: 10.1111/j.1469-8749.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, 1995. The Hebbian paradigm reintegrated: local reverberations as internal representations. Behav. Brain Sci 18 (4), 631. [Google Scholar]
- Friston KJ, Buechel C., Fink GR, Morris J., Rolls E., Dolan RJ, 1997. Psychophysi- ological and modulatory interactions in neuroimaging. Neuroimage 6 (3), 218–229. [DOI] [PubMed] [Google Scholar]
- Gauthier I, 2000. What constrains the organization of the ventral temporal cortex. Trends Cognit. Sci 4 (1), 1–2. doi: 10.1016/S1364-6613(99)01416-3. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC, 1999. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nat. Neurosci 2 (6), 568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Weiner KS, 2014. The functional architecture of the ventral tem- poral cortex and its role in categorization. Nat. Rev. Neurosci 15 (8), 536–548. doi: 10.1038/nrn3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P, 2001. Dis- tributed and overlapping representations of faces and objects in ventral temporal cor- tex. Science 293 (5539), 2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hearne LJ, Cocchi L., Zalesky A., Mattingley JB, 2017. Reconfiguration of brain net- work architectures between resting-state and complexity-dependent cognitive reason- ing. J. Neurosci.: Off. J. Soc. Neurosci 37 (35), 8399–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, 2010. Sensori-motor experience leads to changes in visual processing in the developing brain. Dev. Sci 13 (2), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, 2017. The importance of handwriting experience on the development of the literate brain. Curr. Direct. Psychol. Sci 26 (6), 502–508. [Google Scholar]
- James KH, Atwood TP, 2009. The role of sensorimotor learning in the perception of letter-like forms: tracking the causes of neural specialization for letters. Cognit. Neuropsychol 26 (1), 91–110. doi: 10.1080/02643290802425914. [DOI] [PubMed] [Google Scholar]
- James KH, Engelhardt L, 2012. The effects of handwriting experience on functional brain development in preliterate children. Trends Neurosci. Educ 1 (1), 32–42. doi: 10.1016/j.tine.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, Gauthier I., 2006. Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia 44 (14), 2937–2949. [DOI] [PubMed] [Google Scholar]
- Jeub LGS, Bazzi M, Jutla IS, & Mucha PJ (2011. –2017). A general- ized Louvain method for community detection implemented in MATLAB, http://netwiki.amath.unc.edu/GenLouvain. [Google Scholar]
- Kersey AJ, James KH, 2013. Brain activation patterns resulting from learning letter forms through active self-production and passive observation in young children. Front. Psychol 4. doi: 10.3389/fpsyg.2013.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancichinetti A., Fortunato S., 2012. Consensus clustering in complex networks. Sci. Rep 2, 336 CrossRef Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A., Committeri G., Romani GL, Corbetta M., 2009. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. USA 106 (41), 17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, Anton J-L, Roth M, Velay J-L, 2003. Visual presentation of single letters activates a premotor area involved in writing. NeuroImage 19 (4), 1492–1500. doi: 10.1016/S1053-8119(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Anton J-L, Roth M, Velay J-L, 2005. Premotor activations in response to visually presented single letters depend on the hand used to write: a study on left-handers. Neuropsychologia 43 (12), 1801–1809. doi: 10.1016/j.neuropsychologia.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Boucard C, Gilhodes J-C, Anton J-L, Roth M, Nazarian B, Velay JL, 2008. Learning through hand- or typewriting influences visual recognition of new graphic shapes: behavioral and functional imaging evidence. J. Cognit. Neurosci 20 (5), 802–815. doi: 10.1162/jocn.2008.20504. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Tanskanen T, Hari R, 2006. The imprint of action: motor cortex in- volvement in visual perception of handwritten letters. NeuroImage 33 (2), 681–688. doi: 10.1016/j.neuroimage.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Zerbato-Poudou M-T, Velay J-L, 2005. The influence of writing practice on letter recognition in preschool children: a comparison between handwriting and typing. Acta Psychol. 119 (1), 67–79. doi: 10.1016/j.actpsy.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Ma L., Narayana S., Robin DA, Fox PT, Xiong J., 2011. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage 58 (1), 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM, Bodner GE, 2017. The production effect in memory. Curr. Direct. Psy- chol. Sci 26 (4), 390–395. [Google Scholar]
- Makino H., Hwang EJ, Hedrick NG, Komiyama T., 2016. Circuit mechanisms of sen- sorimotor learning. Neuron 92 (4), 705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R., Levy I., Hasson U., 2002. The topography of high-order human object areas. Trends Cognit. Sci 6 (4), 176–184. [DOI] [PubMed] [Google Scholar]
- Mauri M, Elli T, Caviglia G, Uboldi G, Azzi M, 2017. RAWGraphs: a visualisa- tion platform to create open outputs. In: Proceedings of the 12th Biannual Con- ference on Italian SIGCHI Chapter, 28. ACM, New York, NY, USA, pp. 1–28. doi: 10.1145/3125571.3125585. [DOI] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC, 1995. Why there are complemen- tary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev 102 (3), 419–457. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC, 2012. A generalized form of context- dependent psychophysiological interactions (gPPI): a comparison to standard ap- proaches. NeuroImage 61 (4), 1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Goodale MA, 2006. The Visual Brain in Action, 2nd ed. Oxford University Press, Oxford, UK. [Google Scholar]
- O’Reilly Jill X., Woolrich Mark W., Behrens Timothy E.J., Smith Stephen M., Jo- hansen-Berg Heidi, 2012. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience 7 (5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip BA, Frey SH, 2016. Increased functional connectivity between cortical hand areas and praxis network associated with training-related improvements in non-dominant hand precision drawing. Neuropsychologia 87, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah MJ, 1998. The neural development and organization of letter recognition: evidence from functional neuroimaging, computational modeling, and behavioral studies. Proce. Natl. Acad. Sci. USA 95 (3), 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE, 2011. Functional network organization of the human brain. Neuron 72 (4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Reid LB, Cocchi L., Pagnozzi AM, Rose SE, Mattingley JB, 2017. Brain changes following four weeks of unimanual motor training: Evidence from behavior, neural stimulation, cortical thickness, and functional MRI. Hum. Brain Mapp 38 (9), 4773–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K., Loughead J., Calkins ME, Eickhoff SB, Hakonarson H., Gur RC, Gur RE, Wolf DH, 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting- state functional connectivity data. Neuroimage 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Maquet P., Frith C., 2002. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc. Natl. Acad. Sci 99 (26), 17137–17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, Esposito M., 2007. Functional connectivity of corti- cal networks involved in bimanual motor sequence learning. Cereb. Cortex 17 (5), 1227–1234. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P., 1988. Co-planar Stereotaxic Atlas of the Human Brain. Thieme, New York. [Google Scholar]
- Traud A., Kelsic E., Mucha P., Porter M., 2011. Comparing community structure to characteristics in online collegiate social networks. SIAM Rev. 53 (3), 526–543. [Google Scholar]
- Treiman R., Kessler B., 2014. How Children Learn to Write Words. Oxford University Press. [Google Scholar]
- Ungerleider LG, Mishkin M., 1982. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (Eds.), Analysis of Visual Behavior. The MIT Press, Cambridge, MA, pp. 549–586. [Google Scholar]
- Vinci-Booher S., James TW, James KH, 2016. Visual-motor functional connectivity in preschool children emerges after handwriting experience. Trends Neurosci. Educ 5 (3), 107–120. [Google Scholar]
- Versace R., Labeye É, Badard G., Rose M., 2009. The contents of long-term memory and the emergence of knowledge. Eur. J. Cognit. Psychol 21 (4), 522–560. [Google Scholar]
- Wammes JD, Jonker TR, Fernandes MA, 2019. Drawing improves memory: the im- portance of multimodal encoding context. Cognition 191, 103955. [DOI] [PubMed] [Google Scholar]
- Zemlock D., Vinci-Booher S., James KH, 2018. Visual–motor symbol production facil- itates letter recognition in young children. Read. Writ 31 (6), 1255–1271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.