Abstract
Volumetric muscle loss (VML) is defined as the loss of a critical mass of skeletal muscle that overwhelms the muscle’s natural healing mechanisms, leaving patients with permanent functional deficits and deformity. The treatment of these defects is complex, as skeletal muscle is a composite structure that relies closely on the action of supporting tissues such as tendons, vasculature, nerves, and bone. The gold standard of treatment for VML injuries, an autologous muscle flap transfer, suffers from many shortcomings but nevertheless remains the best clinically available avenue to restore function. This review will consider the use of composite tissue engineered constructs, with multiple components that act together to replicate the function of an intact muscle, as an alternative to autologous muscle flaps. We will discuss recent advances in the field of tissue engineering that enable skeletal muscle constructs to more closely reproduce the functionality of an autologous muscle flap by incorporating vasculature, promoting innervation, and reconstructing the muscle-tendon boundary. Additionally, our understanding of the cellular composition of skeletal muscle has evolved to recognize the importance of a diverse variety of cell types in muscle regeneration, including fibro/adipogenic progenitors and immune cells like macrophages and regulatory T cells. We will address recent advances in our understanding of how these cell types interact with, and can be incorporated into, implanted tissue engineered constructs.
Keywords: Volumetric Muscle Loss, Autologous Muscle Flap Transfer, Tissue Engineered Skeletal Muscle, Vascularization, Innervation, Myotendinous Junction
1. Background: The Need for Tissue Engineered Skeletal Muscle Flaps
Adult skeletal muscle is well-adapted to regeneration after small injuries, as a reserve of muscle satellite cells (SCs) proliferate and differentiate to efficiently replace damaged tissue [1,2]. However, the innate healing capacity of skeletal muscle is limited in addressing larger defects. Volumetric muscle loss (VML) is defined as a critical-sized skeletal muscle defect in which the natural healing mechanisms of the muscle are overwhelmed, and without intervention, loss of normal muscle structure and function persists over the lifetime of the individual [3,4]. VML most commonly arises from combat injury, tumor ablation, degenerative diseases, and other sources of physical trauma. While the incidence of VML in the general population is hard to estimate, it is known to be present in 92% of the muscle injuries sustained by military members who have retired for medical reasons [5]. Traumatic tissue damage by blunt force, explosions, laceration, or avulsion is often accompanied by necrosis, fibrosis and resorption of surrounding tissue due to a lack of blood supply, tissue denervation, or the harsh inflammatory environment [5,6]. In the case of tumor resection, eradication of disease tends to be prioritized with functional preservation being a secondary goal. A wide margin of healthy appearing tissue is often resected with a malignant tumor to minimize the risk of recurrence [7,8].
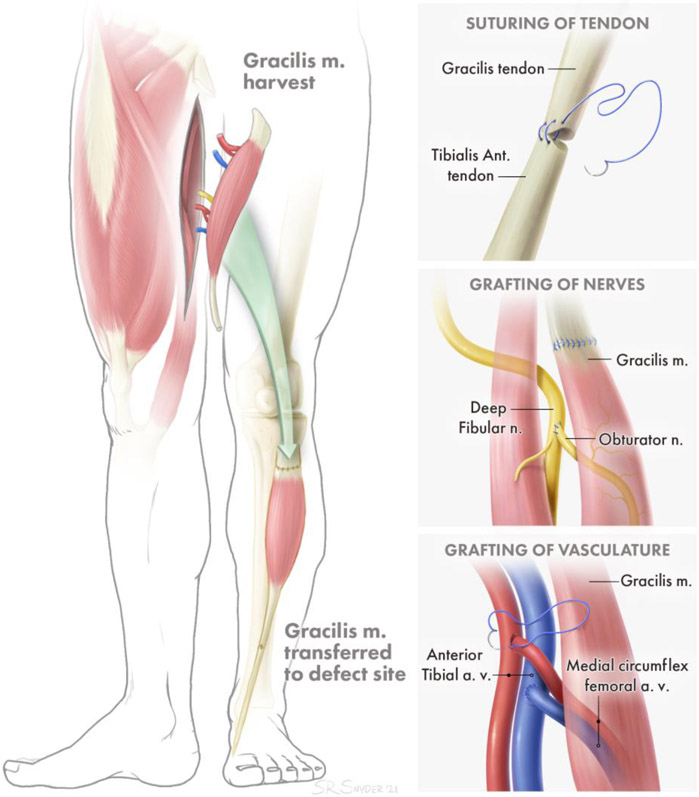
Whether arising from tumor resection or trauma, VML often necessitates surgical intervention to address the resulting functional deficits and deformity [9-11]. This typically involves resection of non-viable tissue and reconstruction of the resulting defect with autologous vascularized muscle tissue. Ideally, a nearby redundant muscle is utilized such that its origin and vascular supply remain intact, while its tendinous insertion point is relocated to recapitulate the action of the missing muscle [12]. Extensive rehabilitation is necessary following surgery to retrain the transferred muscle to perform a new function, making use of central plasticity. When no nearby redundant muscle is available, free functional muscle transfer (FFMT) is the clinical gold standard (Figure 1) [13,14]. This technically demanding surgery involves transplanting a distant muscle to the site of the defect, creating a new origin and insertion point. The clinical standard in FFMT is to keep the associated tendons of the transferred muscle intact. The tendons of the muscle flap are sutured to bone or remaining tendon at the site of the injury, in order to create a strong point of attachment and allow force transmission from the transplanted muscle to the bone (Fig. 1A).To restore contractile function to the transplanted muscle, a donor nerve at the recipient site is coapted to the nerve supplying the transplanted muscle, such that the axons from the donor nerve will regenerate across the coaptation and reinnervate the transplanted muscle (Fig 1B). The process of regeneration and reinnervation typically requires 6-12 months at a minimum, during which time denervation-associated atrophy of the transplanted muscle limits the extent of functional recovery that is ultimately achieved. Similarly, if muscle is transferred without a blood supply and is not quickly revascularized, it will deteriorate from a lack of oxygen and nutrients. Therefore, native vasculature in the FFMT (including arteries and veins, as well as smaller vessels such as arterioles, venules, and capillaries) is preserved and connected to local vasculature at the site of injury to provide rapid perfusion of the transferred muscle (Fig. 1C).
Figure 1: Example of Functional Free Muscle Transfer.
A. Attachment of transferred muscle tendon to remaining tendon for secure force transduction. B. Grafting of distal nerve stump of transferred muscle to proximal nerve of injured muscle for reinnervation. C. Attachment of arteries and veins to local artery and vein sources for perfusion of transferred muscle.
Complications in FFMT can arise at both the site of tissue transplantation and the donor site, and include hematoma or seroma formation, scarring, and infection [15,16]. At the recipient site, common complications include tendon adhesions (which may require corrective surgery), full or partial loss of the transferred muscle due to thrombosis of the vascular anastomoses or insufficient perfusion of the distal-most muscle, and inadequate recovery of muscle contractile force due to delayed or insufficient reinnervation. In 10 studies that used muscle flaps for treatment of both sarcoma excision and trauma, only 15.57% of 167 patients recovered normal muscle strength (Table 1). At the donor site, the patient may experience neuropathic pain due to neuroma formation, and measurable functional losses can occur despite the presence of other neighboring muscles serving a redundant function as the muscle that was transferred. Studies which measured strength of the donor site in comparison to the contralateral (healthy) side showed average weakness at the donor site that ranged from 11%-40% from various muscle donors (gracilis, rectus femoris, anterolateral thigh, and latissimus dorsi) [17-21]. The ongoing deficits in functional recovery in these procedures represents an opportunity for significant improvement in treatment.
Table 1: Functional Recovery After Muscle Flap Transfer.
Summary of muscle flap transfer outcomes measured by muscle power grade from M0 (no contraction) to M5 (maximum expected contraction) which was developed by the Medical Research Council.
| Reason for Muscle Flap |
Total # of Patients |
Final Muscle Power Grade | Publication | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | 0 | ?* | |||
| Sarcoma Excision | 17 | - | 10 | 5 | 2 | - | - | - | Doi et al. [22] |
| 23 | - | 15 | 6 | 2 | - | - | - | Ihara et al. [23] | |
| 22 | 9 | 8 | 1 | - | - | - | 4 | Grinsell et al. [24] | |
| Trauma | 3 | - | 2 | 1 | - | - | - | - | Fan et al. [25] |
| 14 | - | 12 | - | 2 | - | - | - | Lin et al. [26] | |
| 38 | - | 28 | 10 | - | - | - | Chuang et al. [27] | ||
| 23 | 12 | 4 | 5 | - | - | 2 | - | Barrie et al. [28] | |
| 2 | - | 2 | - | - | - | - | - | Wechselberger et al. [29] | |
| 7 | - | 5 | 1 | 1 | - | - | - | Cambon-Binder et al. [30] | |
| 18 | 5 | 6 | - | 3 | - | 4 | - | Lin et al. [31] | |
= Power Grade not reported
Over the past two decades, tissue engineered (TE) muscle constructs have emerged as a potential alternative treatment for VML injury. While a handful of TE scaffolds have been utilized for the treatment of VML injuries in clinical settings, they have consisted of relatively simple decellularized extracellular matrix (dECM) scaffolds that act by enabling passive force transmission and promoting a pro-regenerative environment, but fall short of reproducing the structure and functionality of an autograft [32-36]. Cell-based TE constructs have the potential to offer greater functionality but also face obstacles to clinical implementation. In particular, scaling up to an adequate size comparable to that of an adult human muscle has been a persistent barrier, inhibited by limitations in vascular perfusion. Recent advances have brought TE skeletal muscle grafts closer to replicating the composite tissues represented by autografts. This review focuses on these advances in engineering tissue complexity to enhance the functional capacity of engineered skeletal muscle, as well as the ongoing challenges to scaling up to the clinical gold standard of autologous muscle flap transfer.
2.1. A composite TE muscle graft approach to treating VML injury
The first clinical use of a TE muscle scaffold was documented in a 2010 case study of a 19-year old marine with VML of the quadricep muscles of the thigh [33]. In a novel procedure, a multi-layered sheet of dECM derived from porcine small intestine submucosa was transplanted into the defect site, and the surgery was followed by a renewed physical therapy regime conducted 3 times per week. The patient showed strength gains, which plateaued over time, and new tissue formation at the site of injury (as visualized by computed tomography). Other clinical trials with similar dECM constructs have followed [32,34-36]. The implantation of the dECM material is typically followed by a physical therapy regime, although the duration and types of exercise vary. To distinguish whether functional improvements are due to the implants rather than hypertrophy of the residual muscle, or compensation by neighboring muscles, the patients typically undergo a period of physical therapy until functional plateau prior to the implantation of the scaffolds. These studies have reported a spectrum of functional gains, with some patients responding to treatment and showing modest improvements in force production and the formation of new tissue at the site of the defect, while other patients did not show significant gains. However, any improvement in strength is typically ascribed to a combination of passive force transmission along the implanted construct, and the promotion of a pro-regenerative environment by the bioactive dECM material. Ultimately, acellular grafts lend themselves to clinical application due to their ease of use, but they do not recover the strength or complex functionality of the lost muscle. An ideal TE muscle construct would reliably recapitulate the structure and function of a composite tissue autograft, while retaining the practical simplicity of acellular grafts. The composite tissue engineered skeletal muscle would include the myotendinous junction, motor nerve innervation, and a vascular component. In this review, we will discuss recent advances have moved the field closer to achieving composite skeletal muscle tissues.
2.2. Cellular complexity of skeletal muscle
Skeletal muscle contains heterogenous cell populations and endothelial and perivascular cells along with neurons that directly signal to myoblasts during development and injury repair. Importantly, recent studies have shown that other non-myogenic cells are integral to the regenerative process. Following muscle injury, macrophages have been shown to regulate inflammation, myogenesis, and fibrosis [37-39]. Of noted interest is their regulation of proliferation and differentiation of muscle stem cells. Macrophage-derived inflammatory cytokines promote myoblast proliferation and inhibit differentiation, while macrophage anti-inflammatory cytokines inhibit myoblast proliferation and stimulate their differentiation and fusion [40]. Macrophages also interact with fibro/adipogenic progenitors (FAPs) to coordinate the regenerative process [39,41]. Release of TNF-α by macrophages induces apoptosis of FAPs and thereby prevents excessive deposition of extracellular matrix, while TGF-β release induces the fibrogenic differentiation of FAPs and blocks TNF-α induced apoptosis. The pro-myogenic effects of FAPs (stimulated by eosinophils) have been seen with FAPs derived from both the neural crest and mesoderm [42,43]. FAPs support myogenesis by facilitating the clearance of necrotic debris from the site of injury, but can also induce fatty replacement of muscle by adipogenic differentiation and infiltration. The release of IL-33 by FAPs increases the proliferation of regulatory T cells, promoting muscle repair [44]. A special population of regulatory T cells both mediates the response of other immune cells to muscle injury and interacts directly with SCs [45]. Regulatory T cells mediate the switch from pro- to an anti-inflammatory macrophages, which affects muscle regeneration. Additionally, Amphiregulin (which is produced by these regulatory T cells) has been shown to enhanced myogenic differentiation in cultures of SCs [46]. Incorporating these non-myogenic cells into TE skeletal muscle may enhance the function of new TE constructs, and choosing a scaffold for the TE construct can influence how these cells interact.
Recently, TE constructs that incorporate non-myogenic cells have been developed to enhance the regenerative response. Macrophages supported the proliferation and survival of muscle progenitor cells in an in vitro co-culture injury platform and, after implantation in vivo, they increased host vascular infiltration as well as muscle mass recovery and contractile function [47]. In vitro, FAPs have been shown to enhance the rate of differentiation of myogenic progenitor cells in co-culture experiments [48]. This effect was dose dependent, suggesting a potential mechanism for improving the efficiency of mature myotube generation in TE constructs. Quarta et al. [49] generated TE constructs with populations of bone marrow-derived stem cells and FAPs, as well as hematopoietic cells, endothelial cells, and fibroblast-like cells, and implanted them in a mouse tibialis anterior model of VML [49]. The addition of FAPs and other muscle-resident cells resulted in increases in muscle cross-sectional area, vascularization, and contractile force (when stimulated ex vivo), while fibrosis was reduced and donor-derived myofibers were generated. Furthermore, in vitro co-culture of satellite cells with regulatory T cells increased their proliferation and delayed differentiation [50]. These results could potentially be used to induce rapid expansion of satellite cells in vitro so that TE constructs can be made fast enough to be clinically relevant.
The presence of acellular scaffolds during muscle wound healing also influences which cells participate in the regenerative process. For example, dECM derived from bone and cardiac sources implanted into murine quadricep VML defects significantly increased the number of myeloid cells and lymphocytes present at the injury site at 1 and 3 weeks compared to saline treated controls and induced a pro-regenerative response through T helper 2 cells [51]. Different scaffold compositions elicited distinct immune profiles and these varied even further when implanted in (Rag1−/−) mice that lacked mature T and B cells [52]. This is important to consider, as the majority of current TE scaffolds are implanted and tested in animal models with a compromised/deficient immune system.
The profile of responding macrophages, dendritic cells, monocytes, and polymorphonuclear cells is different depending on scaffold composition: Implantation of urinary bladder matrix promoted tissue repair through T helper 2 cells, whereas polycaprolactone induced a foreign body response and fibrosis [53]. In a rat partial thickness abdominal wall defect, ECM coated polypropylene mesh devices decreased the presence of M1 macrophages around mesh fibers when compared to the uncoated mesh devices [54]. In this same defect model, Mehrban et al. [55] investigated how porcine small intestinal submucosa dECM, urinary bladder matrix dECM, hydro-gelating self-assembling fibers, and collagen affected the regenerative response and saw upregulation of myogenic differentiation markers and the expression of anti-inflammatory markers, indicating a pro-remodeling environment induced by the scaffolds [55].
2.3. Regenerating muscle-tendon connection:
The anatomical macrostructure and cellular composition of the myotendinous junction (MTJ) is tailored to promote force transmission during muscle contraction [56]. However, the MTJ is susceptible to injury due to the sharp difference in mechanical properties of muscles and tendons [57,58]. TE constructs have been developed to address combined tendon and skeletal muscle injuries. Turner et al. [59] had early success with a small intestine submucosa-derived scaffold in the treatment of a MTJ injury in a canine model, reporting the regeneration of a vascularized, innervated, and collagen-rich MTJ six months after the initial injury [59]. However, the authors were subsequently unable to extend these findings to an animal model of a complex injury involving bone, tendon, and muscle [60]. They hypothesized that the proximity of bone to the site of dECM implantation resulted in a distinct injury microenvironment that altered the dECM construct-mediated tissue remodeling. Subsequently, VanDusen et al. [61] developed a composite tissue construct that reproduced elements of skeletal muscle, tendon, and bone, building on previous work in which they developed a muscle-tendon composite tissue and implanted it for a week in a rat hindlimb [62,63], resulting in enhanced vascularization and innervation but limited force production. The authors created ‘bone anchors’ by isolating and expanding bone marrow from rats and cutting the bone into 5 mm segments. Primary rodent muscle cells were expanded into monolayers, detached and rolled into cylindrical constructs, pinned by the bone anchors at either end. Upon implantation into a rodent injury model in which 30% of the tibialis anterior was removed, the bone anchor on one end of the construct was inserted into a tunnel drilled into the tibia, while the opposing end of the construct was sutured onto the remaining distal tendon of the tibialis anterior (Fig. 2A). After 28 days post-implantation, the constructs continued to mature and immunohistochemical staining showed a structure resembling myotendinous junctions between the muscle portion of the construct and the bone anchor (Fig. 2B). The constructs failed to fully restore force production in the injured muscles, but nevertheless, this study is a promising step towards the development of a tissue engineered skeletal muscle with myotendinous regions.
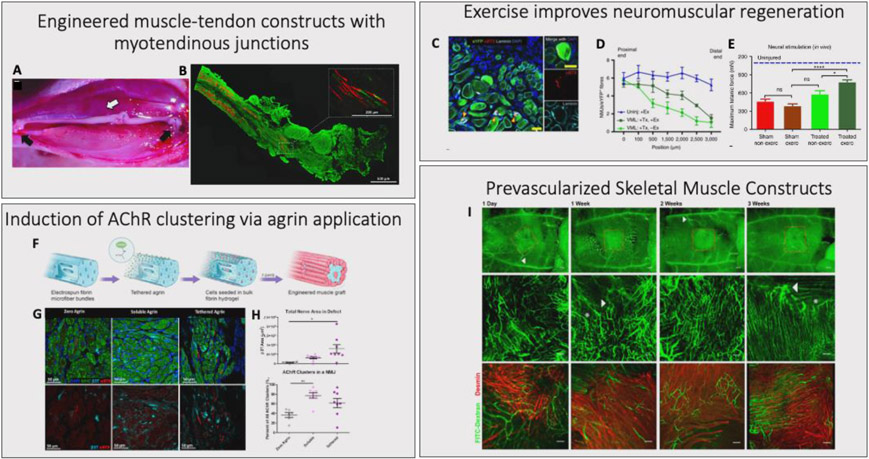
Figure 2. Advances in Composite TE Skeletal Muscle Grafts: Engineered muscle-tendon constructs with myotendinous Junctions.
A composite muscle-tendon-bone tissue engineered construct, composed of a muscle portion fixed at each end by bone anchors. A. The construct was implanted for a month into a rat tibialis anterior VML model, with the bone anchors secured into a hole in the tibial bone on one end, and the distal tendon of the tibialis anterior on the other end. B. The implantation resulted in increased functional maturation of the tissue, including the development of paxillin+ region resembling myotendinous junctions (Red: Myosin Heavy Chain, Green: Collagen type 1, Inset Green: Paxillin). Reproduced with permission [61]. Copyright 2020, Tissue Engineering. Exercise Improves neuromuscular regeneration. TE constructs with various combinations of mouse muscle stem cells and other muscle resident cells in a murine TA VML model. C. Groups that received treadmill training post-surgery showed improvements in innervation of the muscle construct (Green: eYFP, Red: αBTX, Teal: Laminin, Blue: DAPI, scale bars=50 μm). D. Increases in the number of neuromuscular junctions associated with the new myofibers. E. Improvements in in vivo force production. Reproduced with permission [49]. Copyright 2017, Nature Communications. Induction of AChR clustering via agrin applications. Incorporation of agrin into electrospun fibers, either by tethering or by incubating the fibers with a soluble form of agrin. These constructs were then seeded with C2C12 cells to create muscle grafts. The graft was transplanted into murine tibialis anterior VML defects for 4 weeks. F. Agrin tethering process. G. Tethered and soluble agrin both resulted in increased AChR clustering and nerve infiltration into the defect (Green: MHC, Red: αBTX, Teal: β3T, Blue: DAPI). H. Quantification of nerve area in defect and number of AChR clusters per NMJ, for zero agrin, soluble agrin, and tethered agrin groups. *: p < 0.05; **: p < 0.01. Reproduced with permission [78]. Copyright 2020, Biomaterials. Prevascularized skeletal muscle constructs. Endothelial cells, myoblasts, and fibroblasts, cultured on biodegradable scaffolds composed of pig jejunum ECM proteins, and then implanted into the abdominal wall of nude mice. I. After a 2 week implantation, the constructs remained viable and integrated with the host vasculature through both large vessels and microvessels (Green: FITC-Dextran, Red: Desmin, Bar-200 μm). Reproduced with permission [89]. Copyright 2011, PNAS.
Most recently, Laternser et al. [64] developed a novel 3D bioprinting method to generate skeletal muscle-tendon like tissues for the application of high-throughput drug testing. Bioink and cells (a combination of primary human skeletal muscle cells and primary rat tenocytes) were 3D printed in alternating layers to result in an overall dumbbell shape. The tenocytes and skeletal muscle cells were printed onto distinct areas of the construct, with the tenocytes being concentrated around the post areas and the skeletal muscle cells in the elongated region of the dumbbell. The results showed successful differentiation into skeletal muscle and tendon-like tissue, as well as functional contraction and Ca2+ signaling upon electrical stimulation. However, the myofibers in the construct remained thin, potentially inhibited from expansion by the bioink medium by which they were surrounded. Such a 3D printed design of a muscle-tendon tissue has yet to be evaluated for the treatment of VML.
2.4. Promoting motor innervation and functional NMJs in TE muscle
Numerous studies utilizing animal models of VML injury have documented the morphological changes that occur in both nerve and muscle after their connection has been disrupted. These have been reviewed extensively elsewhere [65]. Briefly, significant axotomy of motor neurons has been observed post-VML, with one study reporting that 70% of motor neurons lose connection with their target muscle by 21 days post injury [66]. After denervation, acetylcholine receptors (AChRs) at the postsynaptic end of the neuromuscular junction (NMJ) are degraded at higher rates, and the remaining AChRs are rearranged so they are no longer concentrated at the motor end plate [67]. NMJs in VML-injured muscle lose their characteristic ‘pretzel’ morphology and take on a fragmented appearance [68]. Other changes in denervated muscle include the loss of dense muscle fibers, infiltration of fat into the muscle, and in the case of chronic denervation, muscle atrophy [69-71]. These morphological changes compound the functional deficits resulting from the loss of contractile tissue. Indeed, it has been well documented in both human patients and animals that strength deficits in VML are disproportionately large compared to the volume of contractile tissue lost [4,72,73]. This has motivated the field to consider motor nerve input when engineering skeletal muscle, and a number of TE constructs that promote re-innervation of the injury site have been developed in recent years.
Attempts to enhance the ingrowth of nerve into TE skeletal muscle constructs have focused on two avenues: rehabilitative exercise, and the use of substrates like laminin or agrin to promote AChR clustering at the NMJ. Nakayama et al. [74] demonstrated an increase in the number of NMJs near an implanted scaffold after voluntary exercise in mice, but the scaffold implanted was acellular, and they observed no improvement in myofiber density [74]. Similarly, Quarta et al. [49] showed that transplantation of a bioconstruct seeded with mouse muscle stem cells in combination with exercise resulted in an increase in innervation and force production of the affected muscle, albeit not enough to fully rescue force production or the number and maturity of NMJs compared to the uninjured muscle (Fig. 2C-E). As these groups evaluated either murine cells or acellular scaffolds, the effects of rehabilitative exercise on human myogenic cells in the context of a VML injury have yet to be characterized.
Agrin and laminin have also been evaluated in the context of neuromuscular regeneration for their AChR cluster-inducing properties. Bruneau et al. [75] successfully induced AChR clusters in monolayer C2C12 in vitro culture via the addition of a laminin substrate and agrin in solution. Ko et al. [76] subcutaneously implanted agrin-treated C2C12s in a fibrin gel, embedded along with the common peroneal nerve to promote contact between the myofibers and host nerve tissue. They observed enhanced NMJ formation in agrin-treated constructs as compared to the untreated group. Scott et al. [77] embedded agrin-conjugated microspheres in a fibrin hydrogel, which was used as a culture platform for C2C12s or rat muscle-derived cells. When presented to C2C12s, the agrin-microspheres induced strong AChR clustering in the areas of cell-microsphere contact. However, these studies evaluated the AChR cluster-promoting capabilities of agrin and laminin either in vitro or via subcutaneous implantation. The VML defect is characterized by an inflammatory environment that impedes the engraftment of healthy tissue. Gilbert-Honick et al. [78] reported the first characterization of an agrin-treated muscle construct implanted into a VML defect. The authors tethered agrin to electrospun fibrin scaffolds, prior to seeding with C2C12s and implanting into a tibialis anterior VML injury model (Fig. 2F). After 4 weeks, an increase of NMJs within the defect site was observed, as well as ingrowth of nerves and vasculature and an increase in de novo myofiber formation (Fig. 2G-H). Notably, these studies of AChR cluster-inducing substrates in TE muscle have primarily used C2C12s. C2C12s are an immortalized mouse myogenic cell line that is highly robust and not representative of the challenges that human myogenic cell culture present. Therefore, further work needs to be done to characterize the effects of laminin and agrin treatment on a more clinically representative cell type.
In sum, these TE constructs have successfully improved outcomes in animal models in terms of enhanced nerve growth into the defect site and at least partial recovery of contractile force. However, when compared to the gold standard set by FFMT, it becomes clear that these constructs suffer from some limitations. An autograft comes ready-made with a system of nerves with well-established connections to neuromuscular junctions in the skeletal muscle tissue. All that is needed is to connect this nerve system to the proximal nerve stump in the defect, and re-train the nerves in their new role. As discussed previously, a regime of rehabilitative exercise post-transplantation is thought to aid in this process. Many TE constructs reviewed here simply promote the ingrowth of nerves from the host into the new tissue. This process could take a significant amount of time, during which the engineered tissue is subject to atrophy. This is less of a challenge in the small-animal models which have predominated in the field but becomes problematic when scaling up to the size of an adult human muscle.
Furthermore, the endogenous connections between nerve and muscle are well regulated, and subject to a system of pruning during embryonic development that ensures that only one motor nerve supplies each muscle, and that the nerve makes contact with the muscle at the motor end plate [65]. Less is understood about the organization of nerve-muscle connections that arise in the context of an implanted, TE muscle construct. These constructs may potentially promote disorganized or abnormal connections between nerve and muscle. In addition, it is important to distinguish between immature NMJ-like structures and mature, fully functional NMJs with all their necessary components. Mature NMJs have a complex, three-dimensional pretzel shaped morphology which has proved difficult to replicate in TE skeletal muscle constructs. Furthermore, the presence of Schwann cells in these constructs is rarely reported. Schwann cells are essential for normal maintenance of NMJs and the motor nerve terminal [79].
2.5. Engineering Vascularized Skeletal Muscle
Muscle is highly metabolically active and a dense network of blood vessels is essential to promote recovery after injury. In skeletal muscle, the microvasculature and myofibers are spatially arranged in highly organized microvascular units consisting of 3-4 adjacent myofibers in which 5-10 capillaries are located in between [80,81]. This ensures an active and rapid distribution of nutrients to each myofiber which is necessary for its survival and function.
In order to achieve faster perfusion of the implanted tissue, the vasculature of the transferred autologous muscle grafts is connected to the existing vasculature at the transfer site. An analogous strategy for TE muscle grafts has been to create ‘pre-vascularized’ engineered skeletal muscle by stimulating endothelial cells (often along with fibroblasts or stabilizing cell populations) to create capillary-like vascular networks in the constructs in vitro prior to implantation (Fig. 2G) [82-89]. The approach to vascularizing a TE construct [90] and specifically TE skeletal muscle [65] have been recently reviewed. For the purpose of this review, we want to briefly highlight the difference in size between the vasculature in FFMT (where arteries and veins are both a few millimeters in diameter) and the capillary-like vascular networks of current TE vascularized skeletal muscle (which are on the order of microns) and discuss how additional approaches are needed to achieve a TE option comparable to FFMT.
Pre-vascularized TE constructs create vessels with a diameter on the scale of ~5 – 26μm [86] and ~60 – 175μm [89] in vitro, and ~5 – 75μm [89] after implantation in vivo, and thus are still reliant on host vascular infiltration and anastomosis. As a result, perfusion remains a significant challenge for scale up of TE constructs. To address this issue, Shandalov et al. [88] implanted their pre-vascularized constructs around the femoral artery and veins for 1 week to allow for host vascular integration prior to transferring as an axial flap with its vascular pedicle to the site of injury [88]. This allowed larger host vessels to be integrated with the TE construct. However, for large defects in humans, the scale of the TE construct would be sufficiently different and keeping the cells in the construct viable for the time it would take for host vessels to infiltrate at the first site of implantation possess a great challenge. In order to scale up for clinical use, more work is still necessary to create TE constructs that have sufficient vasculature that they can be rapidly perfused and remain viable post implantation.
2.6. Sensory nerve deficits are often overlooked in VML injury
Movements of the body need to be precise in their timing and magnitude of force generation. Sensory feedback is therefore critical for accurate, coordinated movements of the limbs and trunk. Indeed, motor nerves constitute only a minority of the nerves that innervate any particular muscle [91]. The remainder are composed of free sensory nerve endings and sensory organs (muscle spindles, Golgi tendon organs) that transmit information about muscle length, the level of muscular contraction, and nociceptive stimuli to the central nervous system [92]. In particular, proprioceptive receptors and nerves relay essential information about the spatial orientation of muscles to the brain. Damage to these nerves can result in a lack of sensory feedback that manifests in movement disorders that persist for prolonged periods of time after the initial nerve injury [93-95]. Furthermore, peripheral damage to the sensory nerves can also result in persistent neuropathic pain [96].
Estimates of the incidence of long-term sensory nerve injury as a consequence of VML vary. Beltran et al. [97]. found that around 19% of patients with combat-related type II open tibial fracture are affected by sensory nerve injury. Rivera et al. [98] reviewed the records of soldiers medically discharged with upper limb injuries, and documented that 25% suffered from persistent sensory defects. Furthermore, the authors in Beltran et al. [97] noted that sensory nerve injuries in these individuals were less likely to show improvement over the time course of their study as compared to motor nerve injury, with partial or full recovery rates of 50% for motor nerves and only 27% for sensory nerves. Moradzadeh et al. [99] found similar outcomes in a comparison of tibial nerve repair with either motor or sensory nerve isografts. These results highlight the necessity of therapeutic intervention that addresses sensory nerve deficits caused by VML injury.
However, there is no standard intervention for promoting the re-innervation of sensory nerves during the transplantation of an autologous muscle flap. There has been limited evaluation of the incidence of sensory nerve pain or abnormal sensation post-autologous muscle flap transfer. This oversight carries over into the field of tissue engineered muscle grafts – few have incorporated sensory nerve recovery into their scaffolds, and even in vitro modeling platforms of the connections between skeletal muscle and sensory nerves are scarce [100]. There is a compelling argument that this is an area that could benefit from more research.
3. Conclusion:
Volumetric muscle loss is a significant burden on service members and civilians, leading to lifelong disability and impaired function in affected patients. A successful therapeutic strategy to replace the large quantity of skeletal muscle lost in VML, whether by autologous muscle flap or an engineered skeletal muscle construct, needs to account for the auxiliary tissues that provide support and functionality to the muscle. This includes the regeneration of healthy connections between the muscle and nerves, a system of vasculature to enable rapid perfusion of the tissue, and a tendinous connection point to secure the new muscle and ensure optimal force transmission to the bone. Autologous muscle flaps inherently have the advantage of many of these components but suffer from drawbacks such as donor site morbidity and failure to fully restore force output and range of motion of the affected limb.
The field of skeletal muscle tissue engineering has moved towards generating more complex constructs that incorporate elements of vasculature, nerve innervation, and in some cases, a myotendinous junction. Furthermore, as our understanding of the complex interactions between muscle tissue and other non-myogenic cell types such as macrophages, regulatory T cells, and fibro/adipo-progenitors grows, there is increasing interest in incorporating these cells into TE muscle constructs, and in more closely evaluating how these cell types interact with and signal to implanted TE muscle constructs. However, in many ways TE skeletal muscle constructs have yet to achieve the standard set by autologous muscle flaps. Ongoing limitations include the relative lack of focus on sensory nerve innervation and the generation of a tendon, and challenges in developing sufficient vasculature to support constructs approaching the size of healthy adult skeletal muscle.
Furthermore, the only tissue engineered constructs that have been evaluated clinically in human patients to date are acellular dECM scaffolds that do not approximate the functionality of an autologous muscle flap. The barriers to translating the more advanced, cell-seeded muscle constructs described here to clinical settings have been described extensively elsewhere [101-103]. Briefly, they include variability in cell batches, difficulty in sourcing the large quantities of myogenic cells needed, limitations in scale due to insufficient or delayed perfusion, and regulatory hurdles.
As a result of these obstacles, animal models have been the primary avenue to evaluate the efficacy of cell-seeded TE muscle scaffolds. However, most animal models do not account for all aspects of VML injuries that are typically seen in the clinic: they are conducted in sterile conditions, and with some exceptions [59,72], many disregard comorbid injuries to neighboring bone, tendon, and nerve that are typical in human patients [4]. The animals used are often immunocompromised, which limits our understanding of the complex host immune cell interactions with transplanted constructs. Furthermore, the importance of large animal models should be emphasized, as they better replicate the timing of reinnervation and re-vascularization after injury that are seen in human patients.
Acknowledgements:
Funding was provided by the NIH (NIAMS 1R01AR077581) to WLG. We would like to thank Shawna Snyder for providing original schematics for Figure 1.
Abbreviations:
- VML
Volumetric Muscle Loss
- SCs
Satellite Cells
- FFMT
Free Functional Muscle Transfer
- TE
Tissue Engineered
- dECM
decellularized Extracellular Matrix
- MuSCs
Muscle Stem Cells
- MRCs
Muscle Resident Cells
- AChR
Acetylcholine Receptors
- FAPs
Fibro/Adipogenic Progenitors
- MTJ
Myotendinous Junction
- NMJs
Neuromuscular Junctions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Chargé SBP & Rudnicki M. a. Cellular and molecular regu1. Chargé SBP, Rudnicki M a. Cellular and molecular regulation of muscle regeneration. Physiol. Rev 2004;84(1):209–38. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14715915.lation of muscle regeneration. Physiol. Rev. (2004). [DOI] [PubMed] [Google Scholar]
- 2.Hill M, Wernig A & Goldspink G Muscle satellite (stem) cell activation during local tissue injury and repair. J. Anat 203, 89–99 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correction to: A Murine Model of Volumetric Muscle Loss and a Regenerative Medicine Approach for Tissue Replacement by Sicari BM, Agrawal V, Siu BF, Medberry CJ, Dearth CL, Turner NJ, Badylak SF. Tissue Eng Part A 2012;18(19-20):1941–1948. DOI: 10.1089/ten.tea.2012.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tissue Engineering - Part A vol. 24 (2018). [Google Scholar]
- 4.Corona BT, Wenke JC & Ward CL Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs 202, (2016). [DOI] [PubMed] [Google Scholar]
- 5.Corona BT, Rivera JC, Owens JG, Wenke JC & Rathbone CR Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev 52, 785–792 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Grogan BF & Hsu JR Volumetric muscle loss. J. Am. Acad. Orthop. Surg 19 Suppl 1, S35–S37 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Endo M & Lin PP Surgical margins in the management of extremity soft tissue sarcoma. Chinese Clin. Oncol 7, 1–14 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Kandel R et al. Surgical margins and handling of soft-tissue sarcoma in extremities: A clinical practice guideline. Curr. Oncol 20, 247–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarewich CA & Hutchinson DT Tendon Transfers for Combined Peripheral Nerve Injuries. Hand Clin. 32, 377–387 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Stevanovic MV, Cuéllar VG, Ghiassi A & Sharpe F Single-stage reconstruction of elbow flexion associated with massive soft-tissue defect using the latissimus dorsi muscle bipolar rotational transfer. Plast. Reconstr. Surg. - Glob. Open 4, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinkenberg M et al. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast. Reconstr. Surg 131, 293–302 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Eckardt A & Fokas K Microsurgical reconstruction in the head and neck region: An 18-year experience with 500 consecutive cases. J. Cranio-Maxillofacial Surg 31, 197–201 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Bertelli JA & Ghizoni MF Nerve and Free Gracilis Muscle Transfers for Thumb and Finger Extension Reconstruction in Long-standing Tetraplegia. J. Hand Surg. Am 41, e411–e416 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Estrella EP & Montales TD Functioning free muscle transfer for the restoration of elbow flexion in brachial plexus injury patients. Injury 47, 2525–2533 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Collins J, Ayeni O & Thoma A A systematic review of anterolateral thigh flap donor site morbidity. Can. J. Plast. Surg 20, 17–23 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakhiani C, Defazio MV, Han K, Falola R & Evans K Donor-Site Morbidity Following Free Tissue Harvest from the Thigh: A Systematic Review and Pooled Analysis of Complications. J. Reconstr. Microsurg 32, 342–357 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Deutinger M et al. Donor-Site Morbidity of the Gracilis Flap. Plast. Reconstr. Surg 95, 1240–1244 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Caulfield WH, Curtsinger L, Powell G & Pederson WC Donor Leg Morbidity after Pedicled Rectus Femoris Muscle Flap Transfer for Abdominal Wall and Pelvic Reconstruction. Ann. Plast. Surg 32, 377–382 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Russell RC et al. Functional Evaluation of Latissimus Dorsi Donor Site. Plast. Reconstr. Surg 78, 336–344 (1986). [DOI] [PubMed] [Google Scholar]
- 20.Kuo YR et al. Versatility of the anterolateral thigh flap with vascularized fascia lata for reconstruction of complex soft-tissue defects: Clinical experience and functional assessment of the donor site. Plast. Reconstr. Surg 124, 171–180 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Daigeler A, Dodic T, Awiszus F, Schneider W & Fansa H Donor-site morbidity of the pedicled rectus femoris muscle flap. Plast. Reconstr. Surg 115, 786–792 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Doi K et al. Limb-sparing surgery with reinnervated free-muscle transfer following radical excision of soft-tissue sarcoma in the extremity. Plastic and Reconstructive Surgery vol. 104 1679–1687 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Ihara K, Shigetomi M, Kawai S, Doi K & Yamamoto M Functioning Muscle Tansplantation After Wide Excision of Sarcomas in the Extremity. Clin. Orthop. Relat. Res 358, 140–148 (1999). [PubMed] [Google Scholar]
- 24.Grinsell D, Di Bella C & Choong PFM Functional reconstruction of sarcoma defects utilising innervated free flaps. Sarcoma 2012, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan C et al. Functional Reconstruction of Traumatic Loss of Flexors in Forearm with Gastrocnemius Myocutaneous Flap Transfer. Microsurgery 28, 71–75 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Lin SH, Chuang DCC, Hattori Y & Chen HC Traumatic Major Muscle Loss in the Upper Extremity: Reconstruction Using Functioning Free Muscle Transplantation. J. Reconstr. Microsurg 20, 227–235 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Chuang DCC, Carver N & Wei FC Results of functioning free muscle transplantation for elbow flexion. J. Hand Surg. Am 21, 1071–1077 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Barrie KA, Steinmann SP, Shin AY, Spinner RJ & Bishop AT Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg. Focus 16, (2004). [DOI] [PubMed] [Google Scholar]
- 29.Wechselberger G, Ninkovic M, Pülzl P & Schoeller T Free functional rectus femoris muscle transfer for restoration of knee extension and defect coverage after trauma. J. Plast. Reconstr. Aesthetic Surg 59, 994–998 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Cambon-Binder A, Belkheyar Z, Durand S, Rantissi M & Oberlin C Elbow flexion restoration using pedicled latissimus dorsi transfer in seven cases Réanimation de la flexion du coude par transfert pédiculé de latissimus dorsi dans une série de sept cas. Chir. Main 31, 324–330 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Lin CH, Lin Y. Te, Yeh JT & Chen CT Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast. Reconstr. Surg 119, 2118–2126 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Sicari BM et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Scl. Transl. Med 6, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mase VJ et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, (2010). [DOI] [PubMed] [Google Scholar]
- 34.Han N et al. Electrodiagnostic Evaluation of Individuals Implanted With Extracellular Matrix for the Treatment of Volumetric Muscle Injury: Case Series. Phys. Ther 96, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dziki J et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. npj Regen. Med 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentile NE et al. Targeted Rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. Am. J. Phys. Med. Rehabil 93, (2014). [DOI] [PubMed] [Google Scholar]
- 37.Chazaud B Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 41, 481–492 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Pillon NJ, Bilan PJ, Fink LN & Klip A Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am. J. Physiol. Metab 304, E453–E465 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Dort J, Fabre P, Molina T & Dumont NA Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases. Stem Cells Int. 2019, 1–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold L et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med 204, 1057–1069 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biferali B et al. Fibro – Adipogenic Progenitors Cross-Talk in Skeletal Muscle : The Social Network. Front. Physiol 10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemos DR et al. Functionally Convergent White Adipogenic Progenitors of Different Lineages Participate in a Diffused System Supporting Tissue Regeneration. Stem Cells 30, 1152–1162 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Heredia JE et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuswanto W et al. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells Article Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation o. Immunity 44, 355–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiaffino S, Pereira MG, Ciciliot S & Rovere-querini P Regulatory T cells and skeletal muscle regeneration. Fed. Eur. Biochem. Soc 284, 517–524 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Burzyn D et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 155, 1282–1295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juhas M et al. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng 2, 942–954 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joe AW et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol 12, 153–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quarta M et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castiglioni A, Corna G & Rigamonti E FOXP3 + T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. 1–18 (2015) doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadtler K et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science (80-. ). 352, 366–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadtler K et al. The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Nontraumatic Applications. Tissue Eng. - Part A 23, 1044–1053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sommerfeld SD et al. Interleukin-36γ–producing macrophages drive IL-17–mediated fibrosis. Sci. Immunoloy 4, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faulk DM et al. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials 35, 8585–8595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehrban N et al. Host macrophage response to injectable hydrogels derived from ECM and α-helical peptides. Acta Biomater. 111, 141–152 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Trotter JA Structure-function considerations of muscle-tendon junctions. Comp. Biochem. Physiol. - Part A 133, 1127–1133 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Feng YN, Li YP, Liu CL & Zhang ZJ Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep 8, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noonan TJ, Best TM, Seaber AV & Garrett WE Identification of a Threshold for Skeletal Muscle Injury. Am. J. Sports Med 22, 257–261 (1994). [DOI] [PubMed] [Google Scholar]
- 59.Turner NJ et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng. - Part A 16, (2010). [DOI] [PubMed] [Google Scholar]
- 60.Turner NJ, Badylak JS, Weber DJ & Badylak SF Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J. Surg. Res 176, (2012). [DOI] [PubMed] [Google Scholar]
- 61.Vandusen KW, Syverud BC, Williams ML, Lee JD & Larkin LM Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng. - Part A 20, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larkin LM, Calve S, Kostrominova TY & Arruda EM Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 12, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams ML, Kostrominova TY, Arruda EM & Larkin LM Effect of implantation on engineered skeletal muscle constructs. J. Tissue Eng. Regen. Med 7, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laternser S et al. A Novel Microplate 3D Bioprinting Platform for the Engineering of Muscle and Tendon Tissues. SLAS Technol. 23, 599–613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert-Honick J & Grayson W Vascularized and Innervated Skeletal Muscle Tissue Engineering. Advanced Healthcare Materials vol. 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corona BT et al. Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle and Nerve 57, (2018). [DOI] [PubMed] [Google Scholar]
- 67.Stanley EF & Drachman DB Denervation accelerates the degradation of junctional acetylcholine receptors. Exp. Neurol 73, (1981). [DOI] [PubMed] [Google Scholar]
- 68.Anderson SE et al. Muscle Stem Cell Niche Dysregulation in Volumetric Muscle Loss Injury. bioRxiv (2018) doi: 10.1101/346395. [DOI] [Google Scholar]
- 69.Fleckenstein JL et al. Denervated human skeletal muscle: MR imaging evaluation. Radiology 187, (1993). [DOI] [PubMed] [Google Scholar]
- 70.Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H & Järvinen M Muscle injuries: Biology and treatment. American Journal of Sports Medicine vol. 33 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Dedkov EI, Kostrominova TY, Borisov AB & Carlson BM Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat. Rec 263, (2001). [DOI] [PubMed] [Google Scholar]
- 72.Willett NJ et al. Attenuated human bone morphogenetic protein-2-mediated bone regeneration in a rat model of composite bone and muscle injury. Tissue Eng. - Part C Methods 19, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corona BT et al. Autologous minced muscle grafts: A tissue engineering therapy for the volumetric loss of skeletal muscle. Am. J. Physiol. - Cell Physiol 305, (2013). [DOI] [PubMed] [Google Scholar]
- 74.Nakayama KH et al. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. npj Regen. Med 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruneau EG, Macpherson PC, Goldman D, Hume RI & Akaaboune M The effect of agrin and laminin on acetylcholine receptor dynamics in vitro. Dev. Biol 288, (2005). [DOI] [PubMed] [Google Scholar]
- 76.Ko IK et al. The effect of in vitro formation of acetylcholine receptor (AChR) clusters in engineered muscle fibers on subsequent innervation of constructs in vivo. Biomaterials 34, (2013). [DOI] [PubMed] [Google Scholar]
- 77.Scott JB et al. Achieving acetylcholine receptor clustering in tissue-engineered skeletal muscle constructs in vitro through a materials-directed agrin delivery approach. Front. Pharmacol 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert-Honick J et al. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials 255, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barik A, Li L, Sathyamurthy A, Xiong WC & Mei L Schwann cells in neuromuscular junction formation and maintenance. J. Neurosci. 36, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gitiaux C et al. Whole microvascular unit deletions in dermatomyositis. Ann. Rheum. Dis 72, 445–452 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Latroche C et al. Skeletal muscle microvasculature: A highly dynamic lifeline. Physiology 30, 417–427 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Jank BJ et al. Engineered Composite Tissue as a Bioartificial Limb Graft. Biomaterials 61, 246–256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carosio S et al. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET). Sci. Rep 3, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perry L, Flugelman MY & Levenberg S Elderly Patient-Derived Endothelial Cells for Vascularization of Engineered Muscle. Mol. Ther 25, 935–948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perry L, Landau S, Flugelman MY & Levenberg S Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun. Biol 1, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lesman A et al. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 32, 7856–7869 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Levenberg S et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Shandalov Y et al. An engineered muscle flap for reconstruction of large soft tissue defects. Proc. Natl. Acad. Sci 111, 6010–6015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koffler J et al. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc. Natl. Acad. Sci. U. S. A 108, 14789–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rouwkema J & Khademhosseini A Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 34, 733–745 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Barker D The Morphology of Muscle Receptors, in (1974). doi: 10.1007/978-3-642-65945-4_1. [DOI] [Google Scholar]
- 92.Roatta S & Passatore M Muscle Sensory Receptors. in Wiley Encyclopedia of Biomedical Engineering (2006). doi: 10.1002/9780471740360.ebs0809. [DOI] [Google Scholar]
- 93.Abelew TA, Miller MD, Cope TC & Nichols TR Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J. Neurophysiol 84, (2000). [DOI] [PubMed] [Google Scholar]
- 94.Chang YH, Auyang AG, Scholz JR & Richard Nichols T Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J. Exp. Biol 212, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer-Rasmussen T & Jensen PE Proprioceptive sensitivity and performance in anterior cruciate ligament-deficient knee joints. Scand. J. Med. Sci. Sport 10, (2000). [DOI] [PubMed] [Google Scholar]
- 96.Schaible HG & Richter F Pathophysiology of pain. Langenbeck’s Archives of Surgery vol. 389 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Beltran MJ et al. Fate of Combat Nerve Injury. J. Orthop. Trauma 26, (2012). [DOI] [PubMed] [Google Scholar]
- 98.Rivera JC, Glebus GP & Cho MS Disability following combat-sustained nerve injury of the upper limb. Bone Jt. J 96 B, (2014). [DOI] [PubMed] [Google Scholar]
- 99.Moradzadeh A et al. The impact of motor and sensory nerve architecture on nerve regeneration. Exp. Neurol 212, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo X et al. Tissue engineering the mechanosensory circuit of the stretch reflex arc with human stem cells: Sensory neuron innervation of intrafusal muscle fibers. Biomaterials 122, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffman T, Khademhosseini A & Langer R Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 25, 679–687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vranckx JJ & Hondt M. Den. Tissue engineering and surgery: From translational studies to human trials. Innovative Surgical Sciences vol. 2 189–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghaemi RV, Siang LC & Yadav VG Improving the Rate of Translation of Tissue Engineering Products. Adv. Healthc. Mater 8, 1900538 (2019). [DOI] [PubMed] [Google Scholar]