Abstract
Quiescent cells exploit an array of transcription factors to activate stress response machinery and maintain survival under nutrient limited conditions. Our recent findings reveal that these transcription factors also play an important role in the exit of quiescence and regrowth. By studying Saccharomyces cerevisiae under a continuous, nutrient-limited condition, we found that Msn2 and Msn4 function as master regulators of glycolytic genes in the quiescent-like phase. They control the timing of transition from quiescence to growth by regulating the accumulation rate of acetyl-CoA, a key metabolite that is downstream of glycolysis and drives growth. These findings suggest a model that Msn2/4 not only protect the cells from starvation but also facilitate their regrowth from quiescence. Thus, understanding the functions of stress response transcription factors in metabolic regulation will provide deeper insight into how quiescent cells manage the capacity of regrowth.
Keywords: Msn2, Msn4, glycolysis, acetyl-CoA, quiescence exit, regrowth
In nature, most cells live in quiescence, defined as temporary exit of normal cell cycles upon limited nutrients or various stresses (De Virgilio 2012, Gray, et al. 2004). To ensure survival, quiescent cells dramatically remodel the transcriptional, metabolic and cellular states to adapt to adverse conditions but maintain the capacity of re-proliferation (Broach 2012, Klosinska, et al. 2011, Miles and Breeden 2017, Soontorngun 2017, Zhang and Cao 2017). Taking Saccharomyces cerevisiae as an example, this yeast exploits a series of transcription factors (TFs) such as Msn2/4, Gis1, Mig1 and Adr1, to develop defensive mechanisms against starvation and other stresses (Broach 2012, De Virgilio 2012, Soontorngun 2017). When nutrients are replenished, yeast cells can quickly re-enter into a proliferative state (Broach 2012, Dechant and Peter 2008). Emerging evidence suggests that acetyl-CoA plays a key role during the transition from quiescence to growth by inducing histone acetylation and subsequent activation of growth and cell cycle genes (Cai, et al. 2011, Shi and Tu 2013). Recently, we discovered that stress response TFs Msn2/4 promote regrowth of quiescent yeast cells via glycolysis-dependent production of acetyl-CoA (Kuang, et al. 2017). Our findings reveal a previously unknown connection between exit of quiescence and stress response TFs through their functions in metabolic regulation.
We studied the metabolic regulations of stress response factors using an ultradian cycling system named the yeast metabolic cycle (YMC) (Tu, et al. 2005). When grown in a chemostat under continuous glucose-limited condition, prototrophic yeast cells of the appropriate genetic background are synchronized and exhibit respiratory oscillations with a period of 4~5 hours. The YMC is divided into three phases, OX/growth, RB/proliferation and RC/quiescence (Fig. 1), based on the phases of cycling transcripts and metabolites (Tu, et al. 2005, Tu, et al. 2007). Therefore, the continuously alternating phases in the YMC reflects the transitions of yeast cells between growth and quiescence when nutrient is constantly limited.
Fig. 1.
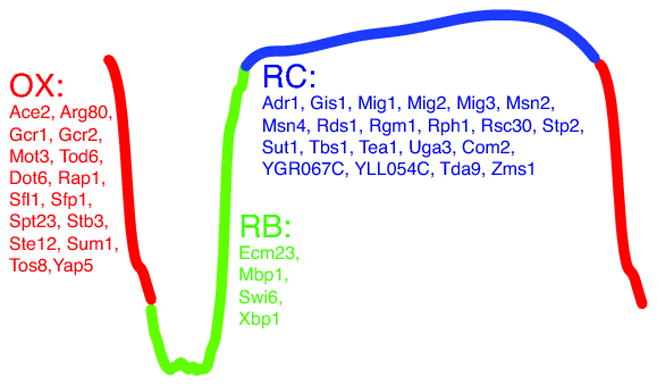
Transcription factors predicted to be important in the YMC by DynaMO. The YMC trace level in the medium. The YMC is divided into three phases: OX phase represents the dissolved O2 is red, RB phase is green and RC phase is blue. Transcription factors with binding sites enriched in each phase are listed.
To study the functions of TFs in the YMC, we first exploited a novel software package DynaMO that we developed to identify TFs specifically linked to each phase of the YMC (Kuang, et al. 2018). DynaMO takes TF motifs and time-course ChIP-seq datasets of histone modification to identify important TFs driving a dynamic process and predict the spatiotemporal binding patterns of these TFs. In this study, we took the motifs of 175 yeast TFs and a 16-time-point H3K9ac ChIP-seq dataset across one metabolic cycle and identified 41 TFs that were specifically associated with one of the three phases (Fig. 1). By examining mutants of a subset of these candidate TFs, we observed a much higher frequency of disrupted respiratory oscillation phenotypes than observed in the remaining TFs, suggesting that the 41 predicted TFs were more likely to be indispensable for driving the YMC.
One unusual phenotype we observed is the longer RC/quiescence phase in the msn2Δ mutant and the RC phase is further lengthened in the msn2Δ msn4Δ mutant (Kuang, et al. 2017). The lengthened RC phase suggests that the msn2Δ or msn2Δ msn4Δ mutant cells face certain roadblocks which prevent them from exiting of quiescence and entering into growth. Msn2 and Msn4 are well-characterized transcription factors that are activated in quiescent states and regulate the expression of genes in response to various stresses including starvation, heat shock and oxidative stress (Estruch 2000, Estruch and Carlson 1993, Martinez-Pastor, et al. 1996, Schmitt and McEntee 1996). Because the RC phase but not the OX or RB phase is altered in the msn2Δ msn4Δ mutant, we confirmed that Msn2/4 are activated in the RC phase from multiple lines of evidences. First, predicted Msn2/4 binding sites by DynaMO are significantly enriched in the RC phase. Second, although the expression of MSN2 is relatively constant across the YMC, MSN4 is highly expressed in the RC phase. Third, DNA binding of Msn2/4 across the genome is highly elevated in the RC phase.
How do Msn2/4 regulate the exit of quiescence and reentry into growth? We first applied cistromic analysis on the Msn2/4 ChIP-seq dataset to identify the genes targeted by Msn2/4. As expected, many stress response genes are targeted by Msn2/4 in the RC phase of the YMC, consistent with the known function of Msn2/4 in stress response. Intriguingly, many carbohydrate transport and metabolic genes are also bound by Msn2/4 in the RC/quiescence phase. Therefore, Msn2/4 could regulate the transition from quiescence to growth via either the stress response route or the metabolic route. Multiple studies from Tu lab have shown that acetyl-CoA is the key metabolite that drives the growth and proliferation program in the YMC (Cai, et al. 2011, Shi and Tu 2013). Cai et al., hypothesized that acetyl-CoA accumulates through the RC phase and when it reaches a threshold, it induces histone acetylation at growth genes via SAGA acetyltransferase. We found that adding acetate in the RC phase of the msn2Δ mutant can immediately induce growth and proliferation phases, suggesting that acetyl-CoA is the limiting factor in the msn2Δ mutant. Acetyl-CoA accumulation is significantly delayed in the msn2Δ msn4Δ mutant cells and only when it reaches the same level as that in the WT cells, the OX phase occurs. What genes are regulated by Msn2/4 and contribute the accumulation of acetyl-CoA? Previous studies show that both glycolysis and fatty acid oxidation genes are activated in the RC phase and both can generate acetyl-CoA (Kuang, et al. 2014, Tu, et al. 2005). We found that almost every glycolytic gene is bound by Msn2/4 and majority of them are downregulated in the RC phase of msn2Δ msn4Δ mutant. However, only 2 genes encoding fatty acid oxidation enzymes (FAA1 and POX1) show Msn2/4 binding signals and neither of them have altered expression in the msn2Δ msn4Δ mutant.
Together, these studies suggest a novel metabolic regulatory function of the yeast stress response factors Msn2/4 in promoting the exit of quiescence and reentry into growth under constant glucose-limited condition. By activating the glycolysis pathway, Msn2/4 enable the quiescent cells to utilize the very limited glucose from the environment and accumulate acetyl-CoA so that the cells can enter into another cycle of growth and proliferation (Fig. 2).
Fig. 2.

Model of Msn2/4-dependent glycolysis driving acetyl-CoA accumulation and regrowth of quiescent cells. In the RC quiescent phase of YMC, the yeast cells utilize the limited glucose and convert it to acetyl-CoA through Msn2/4-dependent glycolysis. Acetyl-CoA is accumulated through the RC phase and functions as a gauge/valve set that controls the transition from the RC/quiescence state to the OX/growth state.
Glucose plays an essential role during the exit of many quiescent states through both the nutrient sensing signaling and metabolic pathways, and glycolysis is one of the key components (Broach 2012, De Virgilio 2012, Dechant and Peter 2008, Laporte, et al. 2011). In the classical model in which stationary yeast cells are transferred back to rich medium, Laporte et al. found that many glycolytic gene mutants were unable to disassemble actin bodies, a marker of quiescence exit (Laporte, et al. 2011). Moreover, they found that ATP generation was not required for the disassembly of actin bodies. Therefore, their results suggest that energy production is not the main metabolic consequence of glycolysis. The findings in the YMC from us and the Tu lab suggest that acetyl-CoA is a key product of glycolysis that drives the exit of quiescence. Besides its role in generating ATP, acetyl-CoA may also signal the availability of biomass for a new round of growth and initiate a transcriptional growth program. Our findings further identified Msn2/4 as the transcriptional regulator of glycolysis and acetyl-CoA production in the RC phase of YMC.
Does the scenario of Msn2/4 in the YMC represent other quiescence exit conditions, such as transferring stationary phase cells to rich media? It is obvious that glucose is exhausted in the typical > 7 days stationary phase cells, which is different from the cells in the YMC in which glucose is continuously limited. Therefore, it is reasonable to hypothesize that the cells in the YMC can utilize the limited glucose to accumulate acetyl-CoA through the long RC phase via the Msn2/4-dependent glycolysis pathway. On the other hand, our findings suggest that Msn2/4-mediated glycolysis pathway may also contribute the quiescence exit of stationary phase cells. First, we found that yeast cells under various nutrient starvation or limitation conditions also express a lot of the glycolytic genes. Second, msn2Δ msn4Δ mutant cells showed a slightly delay of regrowth compared to the WT cells when they were transferred from stationary cultures to fresh YP + 2% glucose media. However, the abundant nutrient in the YPD media may accelerate the quiescence exit process and minimize the difference between WT and mutant cells given our model that Msn2/4 deletion only slows but not stops the accumulation of acetyl-CoA. It will be very interesting to carefully examine the role of Msn2/4-dependent glycolysis and acetyl-CoA production in different quiescence exit scenarios.
Do any of the other stress response TFs also regulate metabolic pathways to promote the exit of quiescence? From the DynaMO analysis, we found a couple of other TFs whose target sites were also enriched in the RC phase cluster (Fig. 1). Some of these, such as Gis1, Rph1 and Rgm1, have very similar predicted target genes to Msn2/4. Some other TFs also have target genes enriched in GO terms like carbohydrate metabolism (Kuang, et al. 2017). The facts that msn2Δ msn4Δ mutant cells are still capable of accumulating acetyl-CoA and entering growth suggest that there are alternative routes of acetyl-CoA production in RC/quiescent cells. It will be interesting to explore whether any of these other TFs are also required during the exit of quiescence by regulating the production of acetyl-CoA. It may also be worth testing whether any of the TFs is specific to certain quiescent state. By examining Msn2/4 and other stress response TFs in the YMC and other quiescence exit scenarios, we may gain deeper insights in metabolic regulation by stress response TFs and how quiescent cells enter into regrowth through transcriptional and metabolic mechanisms.
Acknowledgments
Supported by NIH grant 5P50GM107632-06 to H.J. and J.D.B and R01HG006282 to H.J..
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Broach JR. Nutritional control of growth and development in yeast. Genetics. 2012;192:73–105. doi: 10.1534/genetics.111.135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Molecular cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C. The essence of yeast quiescence. FEMS microbiology reviews. 2012;36:306–339. doi: 10.1111/j.1574-6976.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- Dechant R, Peter M. Nutrient signals driving cell growth. Current opinion in cell biology. 2008;20:678–687. doi: 10.1016/j.ceb.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Estruch F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS microbiology reviews. 2000;24:469–486. doi: 10.1111/j.1574-6976.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Estruch F, Carlson M. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Molecular and cellular biology. 1993;13:3872–3881. doi: 10.1128/mcb.13.7.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JV, Petsko GA, Johnston GC, Ringe D, Singer RA, Werner-Washburne M. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiology and molecular biology reviews: MMBR. 2004;68:187–206. doi: 10.1128/mmbr.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosinska MM, Crutchfield CA, Bradley PH, Rabinowitz JD, Broach JR. Yeast cells can access distinct quiescent states. Genes & development. 2011;25:336–349. doi: 10.1101/gad.2011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Cai L, Zhang X, Ji H, Tu BP, Boeke JD. High-temporal-resolution view of transcription and chromatin states across distinct metabolic states in budding yeast. Nature structural & molecular biology. 2014;21:854–863. doi: 10.1038/nsmb.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Ji Z, Boeke JD, Ji H. Dynamic motif occupancy (DynaMO) analysis identifies transcription factors and their binding sites driving dynamic biological processes. Nucleic acids research. 2018;46:e2. doi: 10.1093/nar/gkx905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Pinglay S, Ji H, Boeke JD. Msn2/4 regulate expression of glycolytic enzymes and control transition from quiescence to growth. eLife. 2017 doi: 10.7554/eLife.29938. doi:610.7554/eLife.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte D, Lebaudy A, Sahin A, Pinson B, Ceschin J, Daignan-Fornier B, Sagot I. Metabolic status rather than cell cycle signals control quiescence entry and exit. The Journal of cell biology. 2011;192:949–957. doi: 10.1083/jcb.201009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor MT, Marchler G, Schuller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) The EMBO journal. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Miles S, Breeden L. A common strategy for initiating the transition from proliferation to quiescence. Current genetics. 2017;63:179–186. doi: 10.1007/s00294-016-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AP, McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu BP. Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7318–7323. doi: 10.1073/pnas.1302490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontorngun N. Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Current genetics. 2017;63:1–7. doi: 10.1007/s00294-016-0609-z. [DOI] [PubMed] [Google Scholar]
- Tu BP, Kudlicki A, Rowicka M, McKnight SL. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL. Cyclic changes in metabolic state during the life of a yeast cell. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16886–16891. doi: 10.1073/pnas.0708365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Cao L. Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Current genetics. 2017;63:839–843. doi: 10.1007/s00294-017-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


