Abstract
Introduction
The SARS-CoV-2 pandemic necessitated better understanding of the impact of disease-modifying therapies on COVID-19 outcomes and vaccination. We report characteristics of COVID-19 cases and vaccination status in ofatumumab-treated relapsing multiple sclerosis (RMS) patients.
Methods
COVID-19 data analyzed were from the ongoing, open-label, long-term extension phase 3b ALITHIOS study from December 2019 (pandemic start) and post-marketing cases from August 2020 (ofatumumab first approval) up to 25 September 2021. COVID-19 cases, severity, seriousness, outcomes, vaccination status, and breakthrough infection were evaluated.
Results
As of 25 September 2021, 245 of 1703 patients (14.4%) enrolled in ALITHIOS receiving ofatumumab (median exposure: 2.45 years) reported COVID-19 (confirmed: 210; suspected: 35). Most COVID-19 was of mild (44.1%) or moderate (46.5%) severity, but 9% had severe/life-threatening COVID-19. There were 24 serious cases (9.8%) with 23 patients hospitalized; 22 recovered and 2 died. At study cut-off, 241 patients (98.4%) had recovered or were recovering or had recovered with sequelae and 2 (0.8%) had not recovered. Ofatumumab was temporarily interrupted in 39 (15.9%) patients. Before COVID-19 onset, IgG levels were within the normal range in all COVID-19–affected patients, while IgM was < 0.4 g/l in 23 (9.4%) patients. No patient had a reinfection. Overall, 559 patients were vaccinated (full, 476; partial, 74; unspecified, 9). Breakthrough infection was reported in 1.5% (7/476) patients, and 11 reported COVID-19 after partial vaccination. As of 25 September 2021, the Novartis Safety Database (~ 4713 patient-treatment years) recorded 90 confirmed COVID-19 cases receiving ofatumumab. Most cases were non-serious (n = 80), and ten were serious (1 medically significant, 9 hospitalized, 0 deaths). Among 36 of 90 cases with outcomes reported, 30 recovered and 6 did not recover.
Conclusion
COVID-19 in RMS patients on ofatumumab was primarily of mild/moderate severity and non-serious in these observational data. Most recovered from COVID-19 without treatment interruption. Two people died with COVID-19. Breakthrough COVID-19 despite being fully/partially vaccinated was uncommon.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00341-z.
Keywords: ALITHIOS, Anti-CD20 therapy, B-cell therapy, COVID-19, Ofatumumab, Post-marketing, Relapsing multiple sclerosis, SARS-CoV-2, Vaccination
Key Summary Points
| Why carry out this study? |
| With the growing knowledge about COVID-19 and the launch of COVID-19 vaccinations, more evidence from clinical studies and real-world settings is needed to understand the impact of COVID-19 and vaccination status in multiple sclerosis (MS) patients receiving disease-modifying therapies, especially B-cell–depleting therapies. |
| We analyzed the characteristics of COVID-19 cases, outcomes, vaccination status, and breakthrough infections of relapsing MS (RMS) patients receiving ofatumumab in the open-label long-term phase 3b ALITHIOS study and Novartis post-marketing safety database as of September 25, 2021. |
| What was learned from the study? |
| Most COVID-19 cases in MS patients treated with ofatumumab were mild or moderate in severity and characterized as non-serious. |
| A small proportion of patients had COVID-19 despite being fully or partially vaccinated, from which all recovered. |
| These observational data provide relevant information to help healthcare professionals better understand the severity and outcomes of COVID-19 in patients with RMS receiving ofatumumab, a B-cell–depleting therapy. |
Introduction
The global pandemic outbreak of coronavirus disease (COVID-19) impacted the well-being of both healthy individuals and people with underlying health conditions [1]. More than 231 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in more than 4.7 million deaths (2.1%) as of September 25, 2021, the data cut-off for the current study. As of January 17, 2022, 326 million cases and 5.5 million deaths worldwide have been reported [2]. Vaccine breakthrough COVID-19 constitutes a small percentage (up to 1.5%) of all fully vaccinated cases [3, 4] but is expected to rise as COVID-19 vaccine effectiveness is waning over time and new SARS-CoV-2 variants emerge.
People with multiple sclerosis (MS) are at an increased risk of serious infections due to concomitant comorbidities or immunosuppressive treatments [5–8]. A recently published pooled analysis reported a 24% increased risk of death from COVID-19 in patients with MS after age standardization as compared with the general population [9]. The hospitalization rate reported due to COVID-19 in people with MS (general/with or without disease modifying therapy [DMT]) varied between 15.5% to 36.5% and the mortality rate from 1.97% to 10.3% [1, 9–13]. Several publications suggest that there is no increased risk of contracting COVID-19 in people with MS compared with the general population [13]. However, several factors such as older age, presence of comorbidities, elevated body mass index (BMI), MS-related disability, progressive MS phenotype, type and duration of DMT, and recent corticosteroid treatment may affect the severity and outcome of COVID-19 in patients with MS [1, 9, 10, 14, 15].
Anti-CD20 monoclonal antibodies (mAbs) deplete B cells, an action that may compromise immune responses and lead to a higher risk of severe COVID-19 [16]. Opinions were published during the COVID-19 pandemic recommending delayed dosing for B-cell–depleting therapies based on a perception of increased risk of COVID-19 [14, 17, 18]. In addition, some studies have observed that B-cell therapies impact humoral responses to COVID-19 vaccination [19–21]. Although much was learned since the start of the pandemic, healthcare professionals (HCPs) continue to face uncertainties in the management of patients with MS in routine clinical practice and are concerned with potential reinfections, waning effect of vaccination, and vaccine breakthrough infections. More evidence from clinical studies and post-marketing settings regarding the impact of COVID-19 in people with MS treated with B-cell–depleting therapies is needed [13].
Ofatumumab is a fully human anti-CD20 mAb that induces B-cell depletion and is approved for the treatment of relapsing MS (RMS). Ofatumumab received marketing authorization in the US on August 20, 2020, which was in the midst of the COVID-19 pandemic—declared by the World Health Organization (WHO) on March 11, 2020 [22]. The present analyses aimed to describe the characteristics of COVID-19 cases, COVID-19 vaccination status, and breakthrough infections in people with RMS receiving ofatumumab in the ongoing open-label ALITHIOS study and from post-marketing reports reported spontaneously to the Novartis Global Safety Database.
Methods
Open-Label Extension Study
Data Collection
ALITHIOS (NCT03650114) is an ongoing, open-label, single-arm, long-term extension phase 3b study designed to assess the safety, tolerability, and effectiveness of ofatumumab 20 mg administered subcutaneously every 4 weeks for up to 5 years in people with RMS [23, 24]. The study enrolled patients from the APLIOS [25] (NCT03560739), APOLITOS [26] (NCT03249714), and ASCLEPIOS I/II [27] (NCT02792218/NCT02792231) phase 2 and 3 trials who were treated with ofatumumab in the core studies and continued with the same treatment and patients who received teriflunomide in the core and switched to ofatumumab in the extension study. Data from ofatumumab-treated ALITHIOS patients and their reported COVID-19 status from December 2019 to September 25, 2021 (data cut-off) were analyzed.
COVID-19 Diagnosis
Cases were considered as confirmed COVID-19 if there was a positive laboratory test result including real-time polymerase chain reaction, antigen detection, or serological evidence of infection, as reported by the investigator. Cases were considered as suspected COVID-19 in the absence of a positive SARS-CoV-2 laboratory test result but with signs and symptoms consistent with COVID-19 (Fig. S1).
Study Outcomes and Assessments
Demographics, key baseline disease characteristics and history were summarized for patients who entered the ALITHIOS study, for patients with confirmed/suspected COVID-19, and for hospitalized COVID-19 patients. A Wilcoxon rank sum test was also performed between hospitalized and non-hospitalized MS patients for selected potential risk factors including age and BMI. Exposure to ofatumumab was expressed as the individual’s time at risk, which was defined as time from the first dose of ofatumumab until 100 days after the last dose of ofatumumab.
COVID-19 cases, seriousness and severity category, COVID-19 outcomes, reinfections, COVID-19 vaccination status, and vaccine breakthrough infection with associated outcomes were assessed. COVID-19 seriousness category was based on the regulatory reporting definition established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) [28] guidelines E2A [29]. Severity assessment was provided by the reporting investigator based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE [v5.0]) grading system [30], as described in the protocol; adverse events (AEs) were reported as mild (Grade 1), moderate (Grade 2), severe (Grade 3), or life-threatening (Grade 4). COVID-19 outcomes were reported by the investigator as recovered or recovering or recovered with sequelae, not recovered, or fatal (Fig. S1). Per the Centers for Disease Control and Prevention, reinfection was defined as a second confirmed infection after 90 days from the end date of the recovered first COVID-19. Vaccine breakthrough COVID-19 was defined as confirmed COVID-19 after being fully vaccinated. Fully vaccinated means at least 14 days after receiving all recommended doses of a COVID-19 vaccine (excluding booster), and partially vaccinated means either not received all recommended doses or it was < 14 days after receiving all recommended doses of a COVID-19 vaccine [31].
The last assessed serum immunoglobulin (IgG and IgM) levels before COVID-19 onset and in relation to COVID-19 seriousness were evaluated. For serious COVID-19 cases, age, BMI, Expanded Disability Status Scale (EDSS), and other potential risk factors were collected. Additional risk factor modeling analysis was not performed for these data, which will be in scope for future publications.
Ethics Statement
Ethics approval for the ALITHIOS study (NCT03650114) was obtained from all ethics committees or institutional review board at each site and the study is in accordance with the ethical code of the ethics committee or institutional review board for each study site and the Declaration of Helsinki. After approval from the ethics committee, written informed consent was obtained from all subjects before commencing the trial-related procedures and allowed the results to be part of a publication.
Post-marketing Reports
Data Collection
The analysis included post-marketing COVID-19 cases in RMS patients from the Novartis Global Safety Database received from the time of ofatumumab's first approval in August 2020 up to September 25, 2021. The database captures AEs reported to Novartis by HCPs, patients, and other sources.
COVID-19 Diagnosis
COVID-19 cases were classified as confirmed if a SARS-CoV-2–positive test result was available or if the patient was reported to have been diagnosed with COVID-19. Cases without a positive test result or a definitive diagnosis were classified as suspected (Fig. S1).
Outcomes and Assessments
Patient demographics, COVID-19 cases, severity, seriousness (including hospitalization), outcomes, and COVID-19 vaccination status were summarized based on reported information. Cases were considered “serious” based on the ICH regulatory reporting definition. Severity assessment of COVID-19 cases was adjudicated independently by 2 experts (BJW and BACC) using the US Food and Drug Administration and WHO COVID-19 severity scales where data were available. Cases were classified as asymptomatic, mild, moderate, severe, or critical (Fig. S1).
If available, the outcome status of each COVID-19–affected individual was reported as recovered/recovering/recovered with sequelae, condition unchanged, condition deteriorated, or fatal.
Results
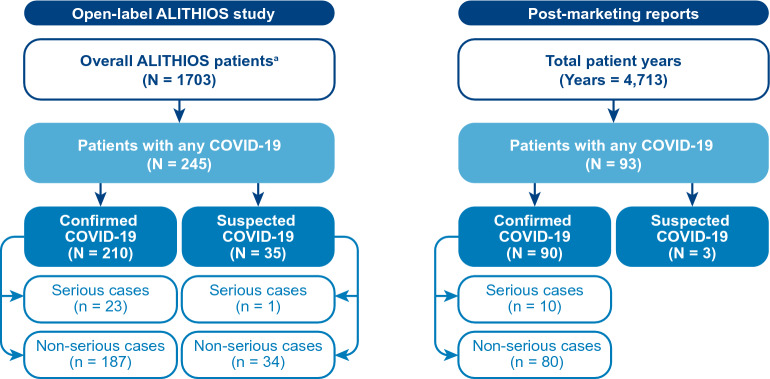
As of September 25, 2021, COVID-19 was reported in 245 patients with RMS receiving ofatumumab in the long-term open-label extension ALITHIOS study and 90 patients with RMS in the post-marketing setting (Fig. 1).
Fig. 1.
Patient designation in the open-label ALITHIOS study and post-marketing setting. a Patients in the open-label ALITHIOS study (N = 1703) rolled-over from the APOLITOS (n = 57), APLIOS (n = 279), and ASCLEPIOS I/II (n = 1367) studies
ALITHIOS Long-Term Open-Label Extension Study
As of the cut-off, 1703 people with RMS had received at least one dose of ofatumumab in the ALITHIOS study who were rolled over from previously completed studies, namely, APOLITOS (n = 57), APLIOS (n = 279), and ASCLEPIOS I/II (n = 1367). Demographics, baseline characteristics, and disease history of the patients who reported any COVID-19–related AE were comparable with those of the overall ALITHIOS population (Table 1).
Table 1.
Patient demographics and disease characteristics in the open-label ALITHIOS study
| Characteristics | OMB overall 20 mg N = 1703a |
Any COVID–19 related AE | |||
|---|---|---|---|---|---|
| Overall COVID-19 n = 245 |
Confirmed COVID-19 n = 210 |
Suspected COVID-19 n = 35 |
Hospitalized overall COVID-19 n = 23 |
||
| Age (years), mean ± SD | 38.6 ± 9.06 | 37.9 ± 8.75 | 38 ± 8.79 | 37.5 ± 8.58 | 41.7 ± 7.5 |
| Age > 41 years, n (%) | 725 (42.6) | 98 (40.0) | 85 (40.5) | 13 (37.1) | 16 (69.6) |
| Female, n (%) | 1186 (69.6) | 171 (69.8) | 147 (70.0) | 24 (68.6) | 13 (56.5) |
| Race, n (%) | |||||
| White | 1525 (89.5) | 224 (91.4) | 191 (91.0) | 33 (94.3) | 22 (95.7) |
| Asian | 75 (4.4) | 7 (2.9) | 7 (3.3) | 0 | 1 (4.3) |
| Black or African American | 39 (2.3) | 6 (2.4) | 5 (2.4) | 1 (2.9) | 0 |
| Countryb, n (%) | |||||
| Russian Federation | 386 (22.7) | 71 (29.0) | 60 (28.6) | 11 (31.4) | 7 (30.4) |
| US | 275 (16.1) | 36 (14.7) | 29 (13.8) | 7 (20.0) | 3 (13.0) |
| Poland | 213 (12.5) | 35 (14.3) | 30 (14.3) | 5 (14.3) | 5 (21.7) |
| Spain | 117 (6.9) | 18 (7.3) | 15 (7.1) | 3 (8.6) | 3 (13.0) |
| Czech Republic | 108 (6.3) | 19 (7.8) | 19 (9.0) | 0 | 0 |
| BMI categories, n (%) | |||||
| Overweight: BMI 25 to < 30 kg/m2 | 427 (25.1) | 62 (25.3) | 52 (24.8) | 10 (28.6) | 9 (39.1) |
| Obese: BMI ≥ 30 kg/m2 | 307 (18.0) | 45 (18.4) | 40 (19.0) | 5 (14.3) | 7 (30.4) |
| BMI (kg/m2), mean ± SD | 25.42 ± 5.92 | 25.42 ± 5.941 | 25.49 ± 6.022 | 25 ± 5.489 | 27.32 ± 5.316 |
| EDSS > 3.5, n (%) | 430 (25.2) | 44 (18.0) | 40 (19.0) | 4 (11.4) | 3 (13.0) |
| EDSS, mean ± SD | 2.84 ± 1.381 | 2.63 ± 1.205 | 2.65 ± 1.226 | 2.49 ± 1.074 | 2.67 ± 1.104 |
| Type of MS, n (%) | |||||
| RRMS | 1621 (95.2) | 239 (97.6) | 204 (97.1) | 35 (100) | 22 (95.7) |
| SPMS | 82 (4.8) | 6 (2.4) | 6 (2.9) | 0 | 1 (4.3) |
| Selected AEs prior to COVID-19 onsetc, n (%) | 60 (3.5) | 60 (24.5) | 52 (24.8) | 8 (22.9) | 3 (13.0) |
| Cardiac disorders | 9 (0.5) | 9 (3.7) | 8 (3.8) | 1 (2.9) | 0 |
| Metabolism and nutrition disorders | 14 (0.8) | 14 (5.7) | 13 (6.2) | 1 (2.9) | 1 (4.3) |
| Respiratory, thoracic and mediastinal disorders | 28 (1.6) | 28 (11.4) | 25 (11.9) | 3 (8.6) | 1 (4.3) |
| Vascular disorders | 18 (1.1) | 18 (7.3) | 15 (7.1) | 3 (8.6) | 2 (8.7) |
For age, BMI, and EDSS, baseline was the value at or the last non-missing assessment prior to the first dose of ofatumumab. Type of MS was assessed at the screening of core studies. Cardiac disorders include tachycardia, angina pectoris, atrial fibrillation, bradycardia, nodal arrhythmia supraventricular extrasystoles, Wolff-Parkinson-White syndrome, and cardiomyopathy. Metabolism and nutrition disorders include hypertriglyceridemia, vitamin D deficiency, hyperglycemia, hypercholesterolemia, iron deficiency, decreased appetite, diabetes mellitus, hypovitaminosis, and vitamin B complex deficiency. Respiratory, thoracic, and mediastinal disorders include cough, oropharyngeal pain, rhinitis allergic, nasal congestion, asthma, bronchial hyperreactivity, catarrh, chronic obstructive pulmonary disease, dyspnea, nasal discomfort, pharyngeal inflammation, respiratory disorder, rhinorrhea, and throat irritation. Vascular disorders include hypertension, hematoma, extremity necrosis, orthostatic hypertension, peripheral venous disease, superficial vein thrombosis, thrombophlebitis, and hypertensive crisis
AE, adverse event; BMI, body mass index; COVID-19, coronavirus disease; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; OMB, ofatumumab; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis
aN = 1703 represents the enrolled population in the ALITHIOS study
bOverall COVID-19 rate of > 5%
cThe selection of prior AEs was based on the following MedDRA System Organ Classes (SOCs) Cardiac disorders,’ ‘Metabolism and nutrition disorders,’ ‘Respiratory, thoracic and mediastinal disorders,’ and ‘Vascular disorders’
COVID-19 Cases Clinical Severity, Seriousness, and Reinfection
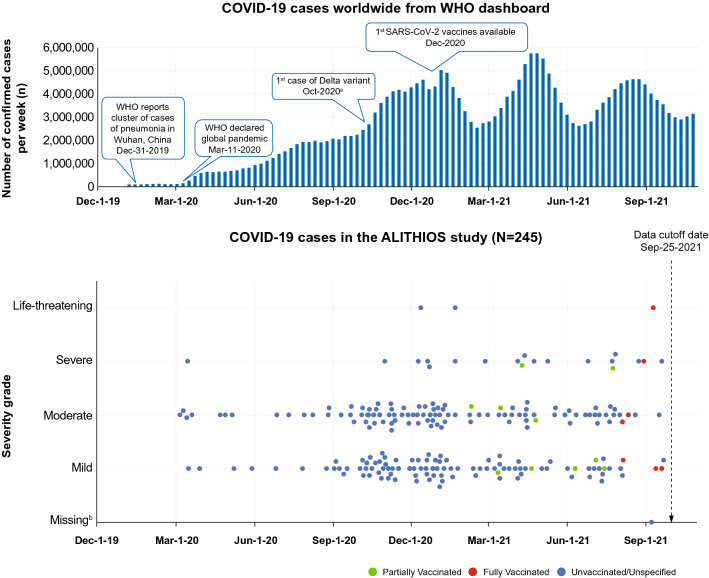
Of the 1703 patients, 245 (14.4%) had reported a COVID-19–related AE, including 210 (85.7%) confirmed COVID-19 cases and 35 (14.3%) suspected COVID-19 cases (Fig. 1). Most COVID-19 cases occurred in the timeframe of October 2020 to January 2021 (Fig. 2). Among all reported cases, the majority (90.6%) had infection of mild (108 [44.1%]) or moderate (114 [46.5%]) severity, while 19 had severe (7.8%) and 3 had life-threatening (1.2%) COVID-19; CTCAE grading was missing for 1 (0.4%) case. A total of 24 serious COVID-19 cases were reported; all but 1 patient were hospitalized, 2 patients required (non-invasive) mechanical ventilation, and 2 patients died. No patient had reinfection.
Fig. 2.
COVID-19 cases over time in the open-label ALITHIOS study by severity and vaccine status. aFirst case of Omicron variant reported after data cut-off: November 24, 2021. bSeverity grade for one patient was not yet reported at the time of data cut-off, as ALITHIOS is an ongoing study, and the data are subject to change. COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WHO, World Health Organization
Demographics and Disease Characteristics of Overall and Hospitalized COVID-19 Cases
Patient demographics and disease characteristics of COVID-19 patients are summarized in Table 1. COVID-19–affected patients had a mean ± standard deviation (SD) age of 37.9 ± 8.75 years at first initiation of ofatumumab, while hospitalized (n = 23) patients were older (41.7 ± 7.5 years; Wilcoxon rank-sum text of hospitalized vs non-hospitalized p = 0.026). Moreover, 69.8% of all COVID-19 patients were female, while 56.5% of hospitalized patients were female. The percentage of patients who were overweight or obese (BMI: ≥ 25 kg/m2) was 43.7% among overall COVID-19–affected patients and higher among those hospitalized (69.6%; Wilcoxon rank-sum text on BMI of hospitalized vs. non-hospitalized p = 0.038). The mean EDSS at first dose of ofatumumab was comparable between COVID-19–affected patients (2.63 ± 1.2) and those hospitalized (2.67 ± 1.1). Overall, 24.5% of patients with COVID-19 had relevant previously reported AEs prior to COVID-19 onset compared to 13% of hospitalized patients.
The median (range) ofatumumab exposure duration (expressed as “time at risk”) of the 245 patients with COVID-19 was 2.45 [0.1–4.98] years. The median onset time of COVID-19 since the first dose of ofatumumab was 2.08 years. Among COVID-19–affected patients, ofatumumab treatment interruption was reported in 39 (15.9%) patients, none of whom permanently discontinued treatment, except for two people who had a fatal COVID-19 outcome (Table 2).
Table 2.
Summary of COVID-19 cases in the open-label ALITHIOS study
| Characteristics | Any COVID–19 related AE | |||
|---|---|---|---|---|
| Overall COVID-19 N = 245 |
Confirmed COVID-19 n = 210 |
Suspected COVID-19 n = 35 |
Hospitalized overall COVID-19 n = 23 |
|
| COVID-19 seriousness, n (%) | ||||
| Non-serious | 221 (90.2) | 187 (89.0) | 34 (97.1) | 0 |
| Serious | 24 (9.8) | 23 (11.0) | 1 (2.9) | 23 (100) |
| Hospitalized | 23 (9.4) | 22 (10.5) | 1 (2.9) | 23 (100) |
| COVID-19 maximum severity, n (%) | ||||
| Mild | 108 (44.1) | 90 (42.9) | 18 (51.4) | 1 (4.3) |
| Moderate | 114 (46.5) | 99 (47.1) | 15 (42.9) | 5 (21.7) |
| Severe | 19 (7.8) | 17 (8.1) | 2 (5.7) | 14 (60.9) |
| Life-threatening | 3 (1.2) | 3 (1.4) | 0 | 3 (13.0) |
| Missing CTCAE grading | 1 (0.4) | 1 (0.5) | 0 | 0 |
| COVID-19 outcome, n (%) | ||||
| Recovered/recovered with sequalae/recovering | 241 (98.4) | 206 (98.1) | 35 (100) | 22 (95.7) |
| Not recovered | 2 (0.8) | 2 (1.0) | 0 | 0 |
| Fatal | 2 (0.8) | 2 (1.0) | 0 | 1a (4.3) |
| COVID-19 duration in days, median (range) | 15 (1–216) | 15 (1–216) | 14 (3–47) | 14 (4–57) |
| COVID-19 onset time since the first dose of ofatumumab, years, mean ± SD | 2.32 ± 0.999 | 2.38 ± 1 | 1.91 ± 0.903 | 2.52 ± 0.86 |
| AE led to ofatumumab interruption, n (%) | 39 (15.9) | 34 (16.2) | 5 (14.3) | 9 (39.1) |
| AE led to ofatumumab discontinuation, n (%) | 2 (0.8) | 2 (1.0) | 0 | 1 (4.3) |
COVID-19 duration could not be calculated for 2 patients because of missing AE end date. Please refer to supplementary material (Fig. S1) for severity and seriousness definitions
AE adverse event; COVID-19 coronavirus disease; CTCAE Common Terminology Criteria for Adverse Events; SD standard deviation
aOne patient with a fatal outcome was not admitted to the hospital owing to personal circumstances and financial reasons
COVID-19 Outcomes and Duration
At the time of data cut-off (Table 2), the majority (241, 98.4%) of COVID-19 cases had either recovered or recovered with sequelae, or were recovering, while two (0.8%) had not yet recovered, and two cases (0.8%) had a fatal outcome (Fig. S2).
Of the 24 serious cases, 22 recovered and 2 were fatal. The first fatal COVID-19 case was a female patient in her 5th decade of life from Europe who was on ofatumumab for 3.7 years. The patient was slightly overweight and had experienced a MS relapse 2.5 months before the onset of COVID-19, which was treated with methylprednisolone 1 g intravenously daily for 4 days. Her latest reported EDSS was 3.5. The patient was hospitalized with COVID-19 pneumonia and required prolonged mechanical ventilation; 33 days after the start of COVID-19, the patient died because of COVID-19 pneumonia. The second fatal COVID-19 case was a male patient in his 4th decade of life from Asia who was on ofatumumab treatment for 1.5 years. Reported comorbidities for this case were diabetes and hypertension. This patient was diagnosed with COVID-19 but was not hospitalized. Ten days after the start of COVID-19, the patient died because of COVID-19. Neither of the fatal cases were vaccinated against COVID-19; their serum IgG and IgM levels were within the normal range throughout the study.
The overall median (range) COVID-19 disease duration was 15 (1 to 216) days in 243 patients with non-missing end date (Table 2).
Immunoglobulin Levels and Their Association with COVID-19 Occurrence and Seriousness
Of the 245 COVID-19 cases, no COVID-19–affected participant had IgG levels below the lower limit of normal (LLN; 5.65 g/l) before COVID-19 onset. Twenty-three participants (9.4%) (confirmed: n = 19; suspected: n = 4) had IgM levels below the LLN (0.4 g/l) before COVID-19 onset, 22 of whom had non-serious COVID-19, and one 56-year-old male patient who was hospitalized with pneumonia and had an IgM level of 0.34 g/l 3 months before his diagnosis of COVID-19. The 22 non-serious cases were of mild-to-moderate severity, and all patients recovered with or without sequelae. Low IgG/IgM levels were not associated with any serious SARS-CoV-2 infections.
Vaccination Status, Vaccine Breakthrough Infections, and Their Severity and Outcomes
In total, 559 ALITHIOS patients were vaccinated of whom 476 were fully vaccinated (of these 27 patients received a booster vaccination), 74 were partially vaccinated, and 9 received an unspecified COVID-19 vaccination (Table S1). Among 550 fully or partially vaccinated patients, messenger ribonucleic acid (mRNA)-based vaccine (78.9%) was the most commonly received, followed by viral-vector-based (non-replicating) vaccine (16.9%; Fig. S3). Among 476 fully vaccinated patients, 7 (1.5%) had breakthrough COVID-19, of which 5 were mild/moderate or non-serious, 1 was severe, and 1 was life-threatening, and all 7 patients recovered. Among vaccinated patients, 11 (1.97%) had confirmed COVID-19 after partial vaccination, of which 9 were mild/moderate or non-serious and 2 were severe, and all 11 patients recovered. No cases of COVID-19 were reported after COVID-19 booster vaccination as per the data cut-off.
Post-marketing Setting
As of September 25, 2021, 90 confirmed and 3 suspected COVID-19 cases were reported to the Novartis Safety Database from the post-marketing setting for patients with RMS on ofatumumab subcutaneous 20 mg dose (Fig. 1). The cumulative post-marketing patient exposure since the first launch of ofatumumab up to data cut-off is estimated to be approximately 4713 patient-treatment years. The patient exposure data are derived from sales data and are therefore estimates.
COVID-19 Post-marketing Patient Characteristics
For the 90 confirmed cases, information on age was available for 62 patients, and the mean (range) age was 45 (19–66) years; 60 patients were female, 22 were male, and gender was not reported for the remainder. Many of the post-marketing cases had limited information regarding prior MS DMTs, comorbidities, MS duration, time of exposure to ofatumumab, EDSS/disability status, COVID-19 symptoms, and outcome, and some patients were lost to follow-up. All of the patients reported COVID-19 from the US, with the exception of one from France (diagnosed with neuromyelitis optica - spectrum disorder and aquaporine+) [32].
COVID-19 Cases, Seriousness, Severity, and Outcomes
Of the 90 confirmed cases (Table 3), 10 were serious in nature, including 1 medically significant case and 9 hospitalizations. No fatalities or life-threatening COVID-19 cases were reported in the post-marketing setting.
Table 3.
Summary of confirmed COVID-19 cases in the post-marketing setting
| Confirmed COVID-19 N = 90 |
|
|---|---|
| Reporter type, n (%) | |
| HCP | 12 (13.3) |
| Non-HCP | 78 (86.7) |
| COVID-19 seriousnessa, n (%) | |
| Non-serious | 80 (88.9) |
| Seriousb | 10 (11.1) |
| Fatal | 0 |
| Hospitalization | 9 (0.1) |
| Life-threatening | 0 |
| Medically significant | 1 (1.11) |
| COVID-19 maximum severityc, n | |
| Mild | 32 |
| Moderate | 7 |
| Severe | 3 |
| Critical | 1 |
| COVID-19 worst outcome, n | |
| Recovered/recovered with sequalae/recovering | 30 |
| Condition unchanged | 6 |
| Fatal | 0 |
| Not reported | 54 |
Severity was assessed using the WHO and FDA guidelines
COVID-19 coronavirus disease; HCP healthcare professional; US FDA United States Food and Drug Administration; WHO World Health Organization
aPercentages are calculated based on available information for seriousness (N = 90)
bNo information regarding comorbidities was provided; in the post-marketing setting serious cases are likely to be reported more frequently than non-serious cases
cPercentages are calculated based on available information for severity (N = 90)
Of the 90 confirmed COVID-19 cases, severity of COVID-19 was assessed as mild in 32 patients, moderate in 7 patients, severe in 3 patients, and critical in 1 patient by independent adjudication. In the remaining 47 cases, severity could not be assessed because of the insufficient information provided. Limited information was provided for the 9 hospitalized patients. Age was included in four of the nine cases (29, 42, 60, and 64 years of age). There was no information regarding comorbidities or MS severity. Outcome was provided in one of the nine cases, and the patient’s condition was improving; outcome was not provided in the remaining eight cases.
Thirty of the post-marketing patients on ofatumumab with COVID-19 recovered or were recovering, 6 patients had no change in condition at the time of the report, and outcomes were not available for the remaining 54 patients (Fig. S4).
Vaccine Breakthrough Infections and Their Severity and Outcomes
Limited information regarding vaccination status was available. There were four post-marketing cases in which patients developed COVID-19 post-vaccination; however, with limited information on vaccination dates, only one of these cases can be determined as breakthrough COVID-19 (recovered) while the other 3 (2 cases with outcome unknown and 1 case with condition unchanged) are considered as COVID-19 post full/partial unspecified vaccination.
Discussion
The data presented herein are reassuring in the sense that most MS patients with COVID-19 reported in the open-label ALITHIOS study and the post-marketing setting had non-serious COVID-19 disease and the majority recovered despite being treated with ofatumumab, which depletes circulating B cells.
Of 245 patients recorded as COVID-19 cases in ALITHIOS, fewer than 10% were serious or resulted in hospitalization with only two deaths. Fatal outcomes (2/245 or 0.8%) due to COVID-19 in ofatumumab-treated patients were lower than those reported in the general population (2.1%) [2], overall MS general population (1.97%) [9], and MS patients with DMTs (1.4–5.9%) [1, 11, 12] and without DMTs (10.3%) [12]. The two ALITHIOS patients with a fatal outcome were unvaccinated and had underlying comorbidities of diabetes and hypertension in one patient and slightly overweight (BMI of 28.3 kg/m2) in another patient, which are known risk factors for severe COVID-19 [33]. The hospitalization rate due to COVID-19 observed in the ALITHIOS study (9.4%) was also lower than that previously reported for the general MS population (15.5–20.9%) [1, 9, 12, 13, 34] as well as in patients with MS treated with DMTs (11.5–32.6%) [1, 10–12] and without DMTs (16.2–42.9%) [10, 12, 13, 34]. The findings of clinical severity and outcomes of COVID-19 cases in ofatumumab-treated patients are consistent with COVID-19 data presented previously with a data cut-off of January 29, 2021 [35]. The present data analysis does not suggest any evidence of an increased risk of severe COVID-19 or fatal outcomes in MS patients treated with ofatumumab compared with the hospitalization and fatality rates as reported in published literature for the general and MS populations [9, 13].
These comparisons should not be over-interpreted. Several factors may contribute to differences in the reported COVID-19 severity and outcomes in different MS populations. There may be relevant differences in the prevalence of known risk factors such as older age, type of MS, level of MS-related disability, and use of different types of DMTs. In addition, COVID-19 cases reported in MS registries are subject to ascertainment bias and may be skewed toward more severe cases being reported. It should be noted that the patients in ALITHIOS rolled over from earlier clinical trials that enrolled people up to 55 years of age and excluded those with certain comorbid conditions [25–27]. Thus, they may not fully represent the real-world population of people with MS.
In the recently published retrospective report on the combined Italian and French registries of confirmed COVID-19 in patients with MS, worse COVID-19 outcomes in people with MS on two other B-cell–depleting agents (ocrelizumab and rituximab) were noted compared to other DMTs. Additionally, patients reported in the registries taking rituximab did worse than those treated with ocrelizumab, and the study did not report on COVID-19 cases in patients taking ofatumumab [33]. Worse outcomes on rituximab versus ocrelizumab were also noted in the North American COViMS Registry data [10]. Salter and colleagues reported three cases of COVID-19 in people taking ofatumumab, with one hospitalization and no deaths. Sormani and colleagues hypothesized that the worse outcomes in patients treated with rituximab compared with ocrelizumab were related to longer exposure to the drug [33].
One concern with long-term B-cell depletion is the potential reduction in Ig production. Earlier studies found an increased risk of serious infections in patients with low IgG levels taking ocrelizumab [36]. In previously reported findings on the ALITHIOS trial [37] and the ASCLEPIOS I/II trials [38], no association between infection and lower IgG/IgM levels was observed in the overall RMS population treated with ofatumumab for up to 3.5 years [37, 38] and for up to 2 years [38], respectively. Consistent with previous findings, the present analysis of data from the ALITHIOS trial showed no apparent association between IgG/IgM levels and COVID-19 occurrence or its severity or outcome. Among all 245 COVID-19 cases in the present analysis, all patients had IgG levels above the LLN, while 23 had IgM below the LLN. The mean IgG/IgM levels remained above the LLN for up to 168 weeks in people with COVID-19, although mean IgM levels declined during this time, consistent with the mean IgM/ IgG trend in the overall ofatumumab safety population (data not shown). Prior registry studies have not reported data on IgG or IgM levels [10, 33, 39].
Data on the humoral and T-cell responses to COVID-19 in people taking ofatumumab are scarce. In an independent assessment of IgG antibodies during SARS-CoV-2 infection (funded by Novartis), four COVID-19 cases from the ALITHIOS study were examined, all of whom had mild COVID-19 and recovered. Three of the four patients had normal levels of IgM and IgG without measurable counts of CD19 cells prior to COVID-19. One patient had ofatumumab dosing temporarily interrupted because of low IgM. None of the three patients without circulating B cells developed detectable antibodies to SARS-CoV-2 [40]. Conversely, all three patients tested for SARS-CoV-2 had T-cell responses detected by interferon-γ ELISpot assay. Further investigation of both humoral and T-cell responses in a larger cohort of ofatumumab-treated patients contracting COVID-19 is warranted.
The Novartis Safety Database might be more representative of real-world experience of people living with RMS. As of September 25, 2021, 90 confirmed COVID-19 post-marketing cases on ofatumumab had been reported in the Novartis Safety Database. This is a voluntary post-marketing database, and all but one case came from the US, making the data potentially subject to bias. Similar to the results from ALITHIOS, most of the confirmed COVID-19 cases were considered non-serious in nature except ten serious COVID-19 cases, including nine patients who were hospitalized. No fatal or life-threatening cases were reported. It has been suggested that in a general post-marketing setting, serious cases are reported more frequently than non-serious cases [41].
Limitations
ALITHIOS is an open-label, single-arm, Phase 3b extension study. The inherent design of study lacks a control group. The relatively small number of patients in the hospitalized subgroup may hamper the risk factor and subgroup analyses.
The post-marketing surveillance includes the relatively small number of reported COVID-19 cases, which likely reflects the limited overall post-marketing exposure to ofatumumab, as it received marketing authorization in August 2020. Moreover, reporting of post-marketing cases is voluntary or spontaneous in nature, and a large proportion of cases had incomplete data or incomplete follow-up. The post-marketing setting also includes literature reports of AEs, but cases gleaned from the scientific literature are typically delayed in acquisition. Therefore, interpretation of these post-marketing reports should be made with caution. Although both the ALITHIOS clinical trial and post-marketing surveillance did not capture information on the SARS-CoV-2 variants causing COVID-19 in the ofatumumab-treated patients, most cases were likely caused by either the alpha or delta variant as these variants were the most dominant variants up to the data cut-off of September 25, 2021.
To date (December 2021), this is one of the largest studies in patients with RMS treated with a B-cell depleting therapy to report on COVID-19 cases post-vaccination. A small number of vaccinated patients in the ALITHIOS study and post-marketing setting reported breakthrough infections of which most cases were of mild-to-moderate severity and had a non-serious nature; all recovered. The immunization response to COVID-19 vaccinations is complex involving an interplay of both cellular and humoral immunity [42], both of which could be impaired because of B-cell depletion [19–21] Studies that further evaluate immunization responses to COVID-19 vaccination in patients on ofatumumab therapy are in progress. A COVID-19 vaccination sub-study embedded in the ALITHIOS open-label extension is underway to investigate the effect of ofatumumab on humoral and T-cell responses following selected COVID-19 vaccines. In addition, three phase 4 studies are underway to evaluate the immune response to the COVID-19 mRNA vaccine, two being conducted in the US (clinicaltrials.gov NCT04878211 and NCT04847596) and one in Germany (clinicaltrials.gov NCT04869358).
Evidence-based guidelines for treatment decisions for MS in the current COVID-19 pandemic are starting to emerge. However, much of the current guidelines continue to derive from expert opinions. At this point, treatment should be tailored in consideration of the individual patient’s history, existing comorbid conditions, and severity of MS [1, 14]. Initiation or continuation of ofatumumab treatment should be decided based on the benefit-risk assessment of each individual.
Conclusions
The current study included comprehensive data from the ongoing open-label extension study (ALITHIOS) and post-marketing reports of ofatumumab-treated RMS patients with confirmed or suspected COVID-19. These observational data were reassuring in that most COVID-19 cases were mild or moderate in severity and most patients recovered from COVID-19 despite being on ofatumumab, a B-cell-depleting therapy. Two fatal cases of COVID-19 were reported in ALITHIOS. There were no cases of reinfection, and a small proportion of patients had COVID-19 despite being fully or partially vaccinated, from which all patients recovered.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
The study sponsor (Novartis Pharma AG, Basel, Switzerland) participated in the design and conduct of the study, data collection, data management, data analysis and data interpretation; preparation, review, and approval of the manuscript, as well as writing of the report and decision to submit the paper for publication. All authors had full access to all study data and took final responsibility for the decision to submit the manuscript for publication. The journal rapid service fee for this publication was funded by Novartis Pharma AG, Basel, Switzerland.
Medical Writing and Other Assistance
We thank Swetha Sirimalla, Anuja Shah and Vaishnavi Khandal (Novartis Healthcare Ltd, Hyderabad, India) for providing medical writing support, which encompassed a literature search, writing of the manuscript, formatting, referencing, preparation of tables and figures per the journal guidelines, and incorporating the authors’ revisions and finalizing the draft for submission, all under the direction of the authors. The assistance of manuscript development and submission was funded by Novartis Pharma AG. Basel, Switzerland.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval to the version to be published. All authors are responsible for intellectual content and data accuracy.
Author Contributions
Anne H. Cross and Brian J. Ward contributed to writing and critical revisions of the manuscript. Silvia Delgado, Mario Habek, Maria Davydovskaya, Natalia Totolyan and Xavier Montalban contributed to critical revisions of the manuscript. Bruce A.C. Cree and Kevin Winthrop contributed to data analysis and critical revisions of the manuscript. Ratnakar Pingili, Linda Mancione, Xixi Hu, Roseanne Sullivan, Wendy Su, Ronald Zielman, Ayan Das Gupta, contributed to critical revisions of the manuscript.
Disclosures
Anne H. Cross has received consulting fees, research support, and honoraria from Biogen, Celgene, Bristol Myers Squibb, EMD Serono, Merck, Genentech, Roche, Greenwich Biosciences, Horizon, Janssen (subsidiary of Johnson & Johnson), Novartis, TG Therapeutics, Academic CME, Projects in Knowledge, CME Outfitters, WebMD, Conrad N. Hilton Foundation, The Potomac Center for Medical Education, Consortium of Multiple Sclerosis Centers, and ACTRIMS; serves on the scientific advisory board for ASCLEPIOS I/II for Novartis; has received grants from the National Institutes of Health, the Department of Defense, USA; has held an elected office (secretary) on the Board of Governors of the Consortium of Multiple Sclerosis Centers; was a member of the scientific advisory board of Race to Erase MS, program committee (chair) of ACTRIMS, member of the COVID-19 advisory committee of the National Multiple Sclerosis Society USA and National Multiple Sclerosis Society representative on the Progressive MS Alliance; and has received a patent for “Yablonskiy DA, Sukstansky AL, Wen J, Cross AH. Methods for simultaneous multi-angular relaxometry of tissue using magnetic resonance imaging. Patent 15060-630 (015875).” Silvia Delgado has received fees as a consultant on scientific advisory boards for Novartis and research grants through the University of Miami from EMD Serono, MAPI Pharma, NIH/NINDS, NMSS, and Novartis. Mario Habek participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, and TG Pharmaceuticals. Maria Davydovskaya received grants for consulting or speaking fees from Biogen Idec, Celgene/Receptos, Janssen/Actelion, Merck/EMD Serono, Novartis, Roche Sanofi Genzyme, and TG Pharmaceuticals; participated on the advisory board of Biogen Idec, Merck/EMD Serono and served as a president of Medicine Association of Professional and MS centers. Brian J. Ward serves on a scientific advisory board for Novartis and reports personal fees from Novartis for this activity. He is also medical officer for Medicago Inc and holds parts of patents for vaccines targeting influenza, Clostridioides difficile and Schistosoma mansoni. In the last 5 years, he has held academic industry awards with Medicago, MIT Canada, and Aviex Technologies. Bruce A.C. Cree has received personal compensation for consulting from Alexion, Atara Biotherapeutics, Autobahn, Avotres, Biogen, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini and received research support from Genentech. Natalia Totolyan has received fees for advisory boards or speaking for Merck and Novartis and institutional grants for conducting clinical trials for Alexion, BIOCAD, Janssen, MAPI Pharma, Merck, Novartis, Receptos, Roche, Sanofi, and TG Therapeutics. Ratnakar Pingili, Linda Mancione, Xixi Hu, Roseanne Sullivan, Wendy Su, Ronald Zielman, and Ayan Das Gupta are employees of Novartis. Roseanne Sullivan is employee of Novartis and has Novartis stock ownership. Xavier Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Alexion, Bayer, Biogen, Celgene, EMD Serono, Genzyme, Hoffmann-La Roche, Immunic, Medday, Merck, Mylan, NervGen, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, EXCEMED, MSIF, and NMSS. Kevin Winthrop has received consulting fees from Novartis, Roche, and Genentech.
Prior Presentation
Some results from this study were summarized in one research abstract and conference posters, presented at the ECTRIMS 2021 Virtual Congress (October 13–15, 2021).
Compliance with Ethics Guidelines
The ALITHIOS (NCT03650114) study was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice [43], local regulatory requirements, and the principles of the Declaration of Helsinki [44]. The study was approved by ethics committees or institutional review board for each study site, and all patients provided written informed consent before commencing trial-related procedures.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The original online version of this article was revised: to correct few values in the Abstract, Figures 1 and 2
Change history
5/3/2022
A Correction to this paper has been published: 10.1007/s40120-022-00346-8
References
- 1.Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/. Accessed 17 Jan 2022.
- 3.Gazit. Comparing SARS-CoV-2 Natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. 2021. https://ncrc.jhsph.edu/research/comparing-sars-cov-2-natural-immunity-to-vaccine-induced-immunity-reinfections-versus-breakthrough-infections/. Accessed 13 Jan 2022.
- 4.Naleway AL, Groom HC, Crawford PM, Salas SB, Henninger ML, Donald JL, et al. Incidence of SARS-CoV-2 infection, emergency department visits, and hospitalizations because of COVID-19 among persons aged ≥12 Years, by COVID-19 vaccination status - Oregon and Washington, July 4-September 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1608–1612. doi: 10.15585/mmwr.mm7046a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijnands JM, Kingwell E, Zhu F, Zhao Y, Fisk JD, Evans C, et al. Infection-related health care utilization among people with and without multiple sclerosis. Mult Scler. 2017;23(11):1506–1516. doi: 10.1177/1352458516681198. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217–233. doi: 10.1038/nrneurol.2016.21. [DOI] [PubMed] [Google Scholar]
- 7.Druyan A, Lidar M, Brodavka M, Levy I, Barzilai A, Pavlotsky F. The risk for severe COVID 19 in patients with autoimmune and/or inflammatory diseases: first wave lessons. Dermatol Ther. 2021;34(1):e14627. doi: 10.1111/dth.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhry F, Jageka C, Levy PD, Cerghet M, Lisak RP. Review of the COVID-19 risk in multiple sclerosis. J Cell Immunol. 2021;3(2):68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prosperini L, Tortorella C, Haggiag S, Ruggieri S, Galgani S, Gasperini C. Increased risk of death from COVID-19 in multiple sclerosis: a pooled analysis of observational studies. J Neurol. 2021 doi: 10.1016/j.jns.2021.117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter A, Fox RJ, Newsome SD, Halper J, Li DKB, Kanellis P, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes R, Whitley L, Fitovski K, Schneble HM, Muros E, Sauter A, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2021;49:102725. doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021 doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barzegar M, Mirmosayyeb O, Gajarzadeh M, Afshari-Safavi A, Nehzat N, Vaheb S, et al. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakolwiboon S, Zhao-Fleming H, Pan J, Scott JK, Shoji E, Sohn G, et al. Disease-modifying therapies during the COVID-19 outbreak: a narrative review of international and national recommendations. Int J MS Care. 2020;22(4):151–157. doi: 10.7224/1537-2073.2020-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garjani A, Evangelou N, Das Nair R, Hunter R, Tuite-Dalton K, Coles A, et al. COVID-19 in people with MS: a large community-based study of the UK MS register. Mult Scler J. 2020;26(3_Suppl):46–47. [Google Scholar]
- 16.Louapre C, Ibrahim M, Maillart E, Abdi B, Papeix C, Stankoff B, et al. Anti-CD20 therapies decrease humoral immune response to SARS-CoV-2 in patients with multiple sclerosis or neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-326904. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- 18.Rolfes L, Pawlitzki M, Pfeuffer S, Nelke C, Lux A, Pul R, et al. Ocrelizumab Extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadani SP, Reyes-Mantilla M, Jank L, Harris S, Douglas M, Smith MD, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73:103636. doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak F, Nilsson AC, Nielsen C, Holm DK, Østergaard K, Bystrup A, et al. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103251. doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Archived: WHO timeline-COVID-19. 2022. https://www.who.int/news/item/27-04-2020-who-timeline---covid-19. Accessed 13 Jan 2022.
- 23.Fox E, Mayer L, Aungst A, Mancione L, Rennie N, Roustan A, Stoneman D, Su W, Gupta A, Zalesak M, Ziehn M, Robertson D, Cohen J. Long-term safety, compliance, and effectiveness of ofatumumab in patients with relapsing multiple sclerosis: The ALITHIOS Phase 3b study. Jt ACTRIMS-ECTRIMS Meet (Virtual). 2020;0218:223–659. [Google Scholar]
- 24.Medicine USNLO. Long-term safety, tolerability and effectiveness study of ofatumumab in patients with relapsing MS (ALITHIOS). 2022. https://clinicaltrials.gov/ct2/show/NCT03650114. Accessed 17 Dec 2021.
- 25.Bar-Or A, Wiendl H, Montalban X, Alvarez E, Davydovskaya M, Delgado SR, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler. 2021 doi: 10.1177/13524585211044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kira JI, Nakahara J, Sazonov DV, Kurosawa T, Tsumiyama I, Willi R, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: Phase 2 APOLITOS study. Mult Scler. 2021 doi: 10.1177/13524585211055934. [DOI] [PubMed] [Google Scholar]
- 27.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, Cross AH, de Seze J, Leppert D, Montalban X, Selmaj K, Wiendl H, Kerloeguen C, Willi R, Li B, Kakarieka A, Tomic D, Goodyear A, Pingili R, Häring DA, Ramanathan K, Merschhemke M, Kappos L, ASCLEPIOS I and ASCLEPIOS II Trial Groups Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 28.European Medicines Agency (EMA). ICH guideline E2B (R3) on electronic transmission of individual case safety reports (ICSRs)-data elements and message specification-implementation guide. 2022. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-4.pdf. Accessed 17 Dec 2021.
- 29.E2A I. ICH harmonised tripartite guideline clinical safety data management: definitions and standards for expedited reporting E2A. 2022. https://database.ich.org/sites/default/files/E2A_Guideline.pdf. Accessed 17 Dec 2021.
- 30.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 17 Dec 2021.
- 31.COVID-19 CVf. Vaccines for COVID-19. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html. Accessed 17 Dec 2021.
- 32.Maillart E, Papeix C, Lubetzki C, Roux T, Pourcher V, Louapre C. Beyond COVID-19: DO MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies? Mult Scler Relat Disord. 2020;46:102482. doi: 10.1016/j.msard.2020.102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sormani MP, Salvetti M, Labauge P, Schiavetti I, Zephir H, Carmisciano L, et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohn N, Konen FF, Pul R, Kleinschnitz C, Pruss H, Witte T, et al. Experience in multiple sclerosis patients with covid-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross AH, Delgado S, Habek M, Davydovskaya M, Ward BJ, Cree BAC, Totolyan N, Pingili R, Mancione L, Sullivan R, Su W, Zielman R, Das Gupta A, Montalban X, Winthrop K. Outcomes of COVID-19 in patients with relapsing multiple sclerosis receiving ofatumumab: data from the ALITHIOS study and post marketing surveillance. In: European Committee for Treatment and Research in Multiple Sclerosis-37th Congress; 2021.
- 36.Hauser SL, Kappos L, Montalban X, et al. Safety of Ocrelizumab in Patients With Relapsing and Primary Progressive Multiple Sclerosis Neurology. 2021;97(16):e1546–e1559. doi: 10.1212/WNL.0000000000012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasińska E, Habek M, Wynn D, Dunphy S, Mancione L, Rennie N, Su W, Zielman R, Delgado S. Impact of ofatumumab on immune responses post-vaccination in RMS patients: ALITHIOS vaccination substudy design. In: Presented at 7th Congress of the European Academy of Neurology, (Virtual); June 19–22, 2021, OPR-207.
- 38.Wiendl H, Seze J, Bar-Or A, Correale J, Cross A, Kappos L, Selmaj K, Gupta A, Häring D, Jehl V, Kerloeguen C, Pingili R, Su W, Sullivan R, Zalesak M, Hauser S. Serum immunoglobulin levels and infection risk in the Phase 3 trials of ofatumumab in relapsing multiple sclerosis. Jt ACTRIMS-ECTRIMS Meet (Virtual) 2020;P0236:233–659. [Google Scholar]
- 39.Alshamrani F, Alnajashi H, AlJumah M, Almuaigel M, Almalik Y, Makkawi S, et al. Registry of patients with multiple sclerosis and COVID-19 infection in Saudi Arabia. Mult Scler Relat Disord. 2021;52:103004. doi: 10.1016/j.msard.2021.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamec I, Rogić D, Penz MG, Braun C, Habek M. Humoral and cellular immunity in convalescent COVID-19 people with multiple sclerosis treated with ofatumumab. J Neuroimmunol. 2022;362:577788. doi: 10.1016/j.jneuroim.2021.577788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. 2020;11:2042098620938595. doi: 10.1177/2042098620938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Medicines Agency. International Conference on Harmonisation: Guideline for good clinical practice E6(R2). 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf. Accessed 13 Jan 2022.
- 44.World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. 2018. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 13 Jan 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.