Abstract
Background
Since the beginning of the Coronavirus disease 2019 (COVID-19) pandemic, various complementary and alternative medicines (CAMs) have been used in clinical practice. In this overview, we summarized the evidence for CAM interventions in the treatment of COVID-19 patients.
Methods
For this overview, PubMed, Embase and Cochrane Library were searched from inception to October 2021. Systematic reviews (SRs) on the effectiveness and safety of CAM interventions for COVID-19 patients were located, and the MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) was used to evaluate the reporting quality of the included SRs. Keywords including COVID-19 and CAM interventions were used for locating SRs. For evidence mapping, we created a two-dimensional bubble plot that included the width and strength of the evidence for each CAM intervention and specific outcome.
Results
In this overview, we identified 24 SRs (21 for Traditional Chinese Medicine (TCM) medications, two for vitamin D and one for home-based activity). From the included SRs, TCM herbal medications were reported to show good results in decreasing the rate of disease progression (relative risk (RR) 0.30, 95% confidence intervals (CI) [0.20, 0.44]), time to the resolution of fever (standard mean difference (SMD) -0.98, 95% CI [-1.78, -0.17]) and rate of progression to severe COVID-19 cases (RR 0.34, 95% CI [0.18, 0.65]), but the evidence for other interventions did not show effectiveness with certainty. Gastric disturbance was a major adverse event of TCM medications.
Conclusion
There is evidence that TCM medications are effective in the symptom management of COVID-19 patients. However, evidence for the effectiveness of most CAM interventions still needs evaluation.
Keywords: Complementary and alternative medicine, COVID-19, Evidence mapping, Overview of systematic review, Traditional Chinese Medicine
1. Introduction
Since the first patient was diagnosed in November 2019 with Coronavirus disease 2019 (COVID-19), the disease has spread worldwide, and the pandemic has still continued till 2022. Due to active implementation of newly developed vaccines and the establishment of acute treatment strategies globally, confusion at the beginning of the outbreak has settled, but prediction about how long this situation will go on seems unclear.1 As the pandemic continues, in addition to the strategies that have focused only on the prevention of COVID infection and management of acute symptoms, there is a growing interest in long-term symptom management. In the case of underdeveloped countries, there is a possibility that internationally accepted treatment strategies may not be fully used due to insufficient medical resources. For these reasons, complementary and alternative medicine (CAM) interventions are being employed as alternative interventions for COVID-19 according to the medical situation of each country.2
However, with these increases in CAM use, it is important to consider the clinical evidence of these interventions. In the midst of the flood of information about the pandemic and viable remedies, it is necessary to find appropriate CAM treatments for COVID-19 patients based on the current best evidence. CAM treatments cannot be incorporated into an appropriate strategy to combat COVID-19 when the CAM interventions are influenced by false beliefs or cultural habits.3
Clinical trials involving COVID-19 patients have been planned and conducted actively, and a significant accumulation of information on the effectiveness and safety of CAM interventions has been achieved in a fairly short period of time.4,5 To establish the clinical evidence for individual CAM interventions is an important step; however, it is now time to summarize the evidence from different sources, to evaluate the level and quality of the evidence and to identify where a lack of evidence exists. The purpose of this study is to create an evidence map which includes information for the CAM interventions according to what those interventions have strong evidence and those that need further testing.
2. Methods
This study is an overview of SRs for assessing the current research status of CAM interventions for COVID-19 patients. In this overview, we summarize published systematic reviews (SRs) of CAM interventions, and suggest an evidence map. We searched published SRs and included studies for analysis based on the following selection criteria.
2.1. Inclusion criteira
2.1.1. Population
The study population included patients with acute COVID-19 infection and long COVID symptoms. Long COVID-19 was defined as a variety of symptoms that continued after the clearance of acute COVID-19 infection, such as fatigue, cognitive impairment, dyspnea, cardiac problems, sleep disturbances, pain and posttraumatic stress disorder.6 There is no confirmed definition but patients with recovered clinical symptoms, or no symptoms were diagnosed as clearance of acute COVID-19 infection when two consecutive reverse transcriptase polymerase chain reaction (RT-PCR) tests in a 24 h interval for respiratory specimens were all negative.
2.1.2. Intervention
Regarding interventions, we included any type of CAM intervention following the definitions provided by the United States National Institutes of Health.7 Nutritional, psychological, physical, combinations of psychological and physical, combinations of psychological and nutritional and manipulative or traditional medicine interventions were included. Combination therapy with conventional formal treatment for acute COVID-19 and long COVID were allowed if CAM interventions were offered to patients.
2.1.3. Outcomes
Outcomes were not limited. As the research purpose of this overview was to evaluate the current research on CAM interventions for COVID-19, we did not impose any restrictions on the outcomes in the included reviews. Clinical results and laboratory tests could be included.
2.1.4. Comparision
Studies that offered comparisons of interventions were not limited in any way. Conventional treatment for COVID-19 or no treatment could be included in this review.
2.1.5. Study design
Regarding our study design, we only included SRs that assessed the clinical evidence of CAM interventions for COVID-19 patients.
2.2. Searching, selection of studies and data extraction
PubMed, Embase and Cochrane Library were searched from inception to October 2021. Two authors (S-RJ and T-HK) conducted selection of the studies independently, and discussed the results until an agreement was reached. After selection of the studies, data extraction was conducted by two authors (S-RJ and T-HK) independently with a predefined extraction form, and disagreement was solved through discussion. Data including intervention types, review objectives, population, number of included studies, summary effect estimates and overall risk of bias were extracted from the original SRs. Search strategies were modified according to the specific features of the electronic databases (Supplementary file 1).
2.3. Assessment of the reporting quality of the included SRs
To assess the reporting quality of the included SRs, the MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) checklist was used for each included study. Sixteen items of the AMSRAR 2 checklist were assessed for each SR, and overall quality was also suggested, which we used as an item for evaluating the overall confidence of the included SRs. Overall quality was graded as “High” when no or noncritical weakness was present in all items, “Moderate” when one more noncritical weakness was present in the items, “Low” when one critical limitation was identified or “Critically low” when more than one critical limitation was observed. AMSTAR 2 was assessed by two authors (T-HK and S-RJ) and discussed if there was any disagreement. An arbiter (JWK) decided if the disagreement was not resolved.8
2.4. Evidence mapping
A bubble plot, which included information about the effectiveness of interventions and the confidence level of the included SRs, was drawn based on Hempel et al.’s methodology.9 In the bubble plot, each intervention had two-dimensional information, including the x-axis (overall risk of bias), y-axis (the number of included original studies in each SR) and bubble size (overall confidence level of the included SR assessed by AMSTAR 2 checklist). When an intervention had more than two SRs, we selected only one SR for the intervention, which was rated as the highest quality considering the number of included studies. The x-axis for each intervention was decided with the summary effect estimates with overall risk of bias in the selected SR, and it had three categories with “Effective” if statistically significant positive effect estimates with low overall risk of bias: “Potentially effective” if statistically significant positive with high risk of bias (or unclear risk of bias) or “Unidentified” if no significant positive effect with high risk of bias (or unclear risk of bias). The y-axis shows the number of included randomized controlled trials (RCTs) in the SR. The size of the bubble represents the confidence level for the evidence, which was decided with AMSTAR 2 assessment, and a larger circle suggests higher confidence. R software (ver 4.0.2) and the ‘ggplot2’ package were used for the evidence map.
3. Results
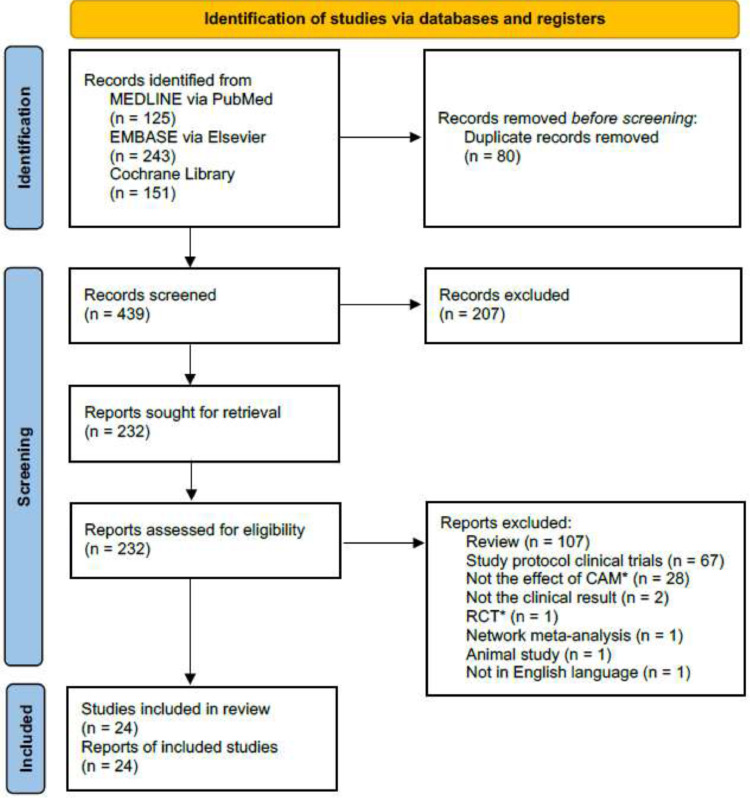
For this study, a total of 24 SRs were included through the database search (Fig. 1). Among the included SRs, TCM medications were evaluated in 21 studies,10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 home-based activity (n = 1)31 and vitamin D (n = 2)32,33 were assessed. Various types of TCM medications were included in most of the SRs, and LinghuaQingwen granules or capsules were tested in four SRs (Table 1).13,19,28,30 Most SRs did not include analysis of the herbal ingredients which were used in the included studies. However, some studies reported that Radix Glycyrrhizae, Herba Ephedrae, Radix Scutellariae Baicalensis, Rhizoma Pinelliae Tematae, Fructus Forsythiae Suspensae and Semen Armeniacae Amarum were most frequently used herbs (supplementary file 3).12,13,15,26,29,30 There was no RCTs on patients with long COVID. The overall confidence level of the included SRs was high to moderate when assessed with the AMSTAR 2 checklist (Supplementary file 2).
Fig. 1.
Study flow diagram.
Table 1.
Summary of the included systematic review of CAM for COVID-19
| Author (year) [ref] | Review objectives (quoted) | No. included studies | Intervention | Comparator | Main Outcomes | Direction of effect | Overall risk of bias (quoted) | Overall confidence (AMSTAR2) | Conclusion (quoted) |
|---|---|---|---|---|---|---|---|---|---|
| TCM | |||||||||
| Ang 202010 | “… to evaluate the effectiveness and adverse events of herbal medicines for the treatment of COVID-19” | 7 RCTs | HM plus WM | WM | 1) Effective rate 2) Hospital discharge rate 3) Symptom disappearance rate |
1), 2) + 3) cough: +; sputum: + |
Unclear | Moderate | “… the combined therapy of herbal medicine with Western medicine … revealed the potential role of herbal medicine in treating COVID-19” |
| Du 202111 | “… to evaluate the add-on effect of CHM in the treatment of mild to moderate COVID-19” | 12 RCTs | CHM plus WM | WM | 1) CT recovery rate 2) Effective rate |
1), 2) + |
Unclear | High | “CHM combined with conventional therapy may be effective and safe in the treatment of mild to moderate COVID-19” |
| Fan 202012 | “… summarized … CHM to treat COVID-19” | 7 RCTs | CHM | Standard care (WM or routine medical care) | 1) Symptom improvement score 2) CRP 3) CT recovery rate |
1)–3) + |
Unclear | High | “… CHM, as an adjunct treatment with standard care, … improve treatment outcomes in COVID-19 …” |
| Hu 202013 | “… to provide reliable assessments of …COVID-19 pneumonia” | 42 RCTs | LQ or LQ plus WM | WM (antibiotics, antiviral drugs) | 1) Flu-Like symptoms 2) Cure rate |
1), 2) + |
Unclear | Moderate | “Lianhua Qingwen combined with conventional drugs may be a promising therapy for treating … COVID-19 pneumonia” |
| Jiang 202114 | “… provides … efficacy … of treating COVID-19 with combined TCM and WM” | 19 RCTs and 16 OS | TCM (CHM and CPM) or TCM plus WM | WM | 1) Effective rate 2) Disease progression rate 3) VNA negative conversion rate |
1), 2) + 3) +/– |
Low | High | “TCM combined with CWM was a potential treatment option for … COVID-19 patients” |
| Li 202115 | “… to evaluate the supportive effects of …TCM for the treatment of COVID-19” | 8 studies | CHM plus WM | WM | 1) Effective rate 2) CT recovery rate 3) Aggravation or hospitalization |
1)–3) + |
NOS (score 4: 6studies; score 5: 1 study); Jadad (score 6: 1 study) | Moderate | “… TCM with Western medicine has significantly improved … COVID-19 …” |
| Liang 202016 | “To present the … therapeutic effects … of CHM … for COVID-19” | 10 RCTs, 1 NRS, 11 RS, 12 CS and 24 CR | CHM | WM or placebo | 1) Cure rate 2) Mortality rate 3) Aggravation rate |
1) +/- 2) + 3) +/– |
Low | Moderate | “… CHM plus conventional western therapy is superior to conventional western therapy alone” |
| Liang 202117 | “… to evaluate the therapeutic effects and safety of oral Chinese patent medicine for COVID-19” | 7 RCTs | CHM or CHM plus WM | WM | 1) Cure rate 2) Mortality rate 3) Aggravation rate |
1), 2) + 3) +/– |
low-certainty or very low-certainty | High | “… oral Chinese patent medicine may have …therapeutic effects for patients with non-serious COVID-19” |
| Liu 202018 | “… to assess the efficacy and safety of …Integrated Medicine to COVID-19” | 4 RCTs, 7CCS | CHM | WM | 1) Effective rate 2) Hospital stays |
1), 2) + |
Low | Moderate | “… Integrated Medicine had better effects … for COVID-19” |
| Liu 2021a19 | “… to assess the efficacy and safety of LQ in treating patients with COVID-19 …” | 3 RCTs, 3 CCS, 2 CS | LQ | WM | 1) Effective rate 2) CT recovery rate 3) Aggravation rate |
1)–3) + |
Low | Moderate | “Lianhuaqingwen combined with conventional treatment seems to be …effective for … mild or ordinary COVID-19” |
| Luo 202120 | “… to investigate the efficacy and safety of CHM or CHM combination therapy for COVID-19” | 19 (6 RCTs, 13 CCS) | CHMor CHM plus other treatments | Placebo or other therapy | 1) Effective rate 2) CT recovery rate 3) RT-PCR negative rate |
1)–3) + |
Unclear | High | “The combined treatment of COVID-19 with Chinese and Western medicine may be effective …” |
| Pang 202021 | “… to evaluate the efficacy and safety of CMD for COVID-19” | 11 RCTs | CHM | WM | 1) The rate of clinical change to sever or critical condition 2) All-cause death |
1) + 2) +/– |
Unclear | Moderate | “CMD may bring potential benefit to … COVID-19. ” |
| Shi 202122 | “… testing the efficacy of CHM on … COVID-19…” | 19 RCTs and 29 OS | CHM plus drug therapy | Drug therapy or drug therapy plus placebo | 1) Remission rate 2) Effective rate of returned normal level |
1) Nausea: +/–; anorexia: +; diarrhea: +/– 2) ALT: +/–; AST: +/– |
Unclear (RCT), High (OS) | High | “… CHM had some benefits in …of COVID-19 …” |
| Sun 202023 | “…to evaluate … Chinese medicine in the treatment of COVID-19 pneumonia” |
7 RCTs | CHM | WM | 1) Effective rate 2) VNA negative conversion rate 3) CT recovery rate |
1) + 2) +/– 3) + |
Unclear | Moderate | “… Chinese medicine has demonstrated clinical efficacy and safety on COVID-19 …” |
| Wang 2021a24 | “To evaluate …three medicines and three formulations… for COVID-19” | 13 studies (6 RCTs, 7 RS) | CHM | Usual care | Time to resolution of fever | + |
Low | High | “…three medicines and three formulations, … on top of usual care, may offer some relief for …mild or moderate COVID-19 …” |
| Wang 2021b25 | “… to summarize the latest evidence of TCM in COVID-19” | 7 RCTs | CHM or CHM plus other treatments | Placebo, WM, and usual care | 1) Cure rate 2) VNA negative conversion rate |
1) + 2) +/– |
Some concerns | Moderate | “… TCM plus routine treatment could promote a clinical cure …in patients with COVID-19” |
| Xiong 202026 | “… to evaluate …CHM for … COVID-19” | 11 RCTs | CHM | WM | 1) CT recovery rate 2) Clinical cure rate 3) Length of hospital stay |
1) + 2), 3) +/– |
Unclear | High | “… CHM group has improvements in several clinical parameters including lung CT” |
| Yan 202127 | “To explore the clinical efficacy of the combination of TCM and WM…for …COVID-19” | 9 RCTs | CHM and WM | WM | 1) Rate of progression to severe stage 2) Cure rate 3) Mortality rate |
1), 2) + 3) +/– |
Low | Moderate | “A combined treatment strategy is effective for COVID-19” |
| Zeng 202028 | “… LQ in the treatment of …COVID-19…” | 2 RCTs | LQ plus WM | WM | Disappearance rate |
Cardinal clinical symptoms: +/–; fever duration: + | Low | High | “… TCM … can be used as an effective therapy to improve …” |
| Zhou 202129 | “… to assess the effects of TCM … for COVID-19 …” | 10 RCTs | CHM | WM | 1) Cure rate 2) Relief rate |
1) + 2) fever: +/–; cough: + |
Unclear | Moderate | “TCM may be an effective auxiliary treatment for COVID-19 …” |
| Zhuang 202130 | “…evaluate the clinical efficacy of LQ on … COVID-19” | 1 RCT and 2RS | LQ granules or capsules | Symptomatic, nutritional support, antiviral and antibacterial, and other routine treatment | 1) The rate of clinical change to sever or critical condition 2) Fever duration 3) disappearance rate of fever |
1)–3) + |
Unclear | Moderate | “…Lianhua Qingwen on the treatment of COVID-19 has significant efficacy …” |
| Home based activities | |||||||||
| Puyat 202031 | “… home-based activities …were examined” | 16 RCTs | Exercise, yoga, and etc | No restrictions were placed on study comparators | Mental health outcomes | + | High | Moderate | “…home-based activities can promote mental wellness during the COVID-19 pandemic” |
| Vitamin D | |||||||||
| Bassatne 202132 | “… assesses the impact of vitamin D status … on COVID-19 …” | 3 RCTs and 31 OS | High serum vitamin D | Low serum vitamin D level | 1) Mortality rate 2) ICU admission rate 3) Hospitalization rate 4) Length of hospital stay |
1)–4) +/– | Unclear | High | “… none of the outcomes ... revealed …cause effect relationship of vitamin D status on COVID-19 …” |
| Rawat 202133 | “… to assess the impact of Vitamin-D … in …COVID-19” | 3 RCTs and 2 QES | Vitamin D plus standard therapy | No vitamin D | 1) Mortality rate 2) ICU admission rate |
1), 2) +/– | Low | Moderate | “No significant difference with vitamin-D supplementation on major health related outcomes in COVID-19” |
All the studies did not mention the publication bias.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CAM: complementary and alternative medicine; CCS: case control studies; CHM: Chinese herbal medicine; CI: confidence interval; COVID-19: Coronavirus disease 2019; CPM: Chinese patent medicine; CR: case reports; CRP: C-reactive protein; CS: case studies; CT: computed tomography; HM: herbal medicine; ICU: Intensive care unit; LQ: Lianhua Qingwen; NOS: Newcastle–Ottawa Scale; NRS: non-randomized studies; OR: odds ratio; OS: observational studies; QES: quasi experimental studies; RCT: randomized controlled trial; RT-PCR: Reverse Transcription Polymerase Chain Reaction; RR: risk ratio; RS: retrospective studies; TCM: traditional Chinese Medicine; VNA: Viral nucleic acid; WM: Western medicine; WMD: weighted mean difference; +: favorable to intervention; +/–: no difference.
3.1. Effectiveness and safety of Traditional Chinese Medicine for COVID-19 patients
The overall clinical effectiveness of TCM treatments in relative risk (RR) ranged from 1.15, 95% confidence intervals (CI) [1.04, 1.26]29 to 1.23, 95% CI [1.04, 1.38]17 (or odds ratio (OR) 2.5, 95% CI [1.46, 4.29]15 to 2.67, 95% CI [1.83, 3.89]),20 and these differences in summary effect estimates might be introduced from the status of the included RCTs in each SR. TCM herbal medications were reported to show good results in decreasing the rate of disease progression (RR 0.30, 95% CI [0.20, 0.44]),14 time to the resolution of fever (standard mean difference (SMD) −0.98, 95% CI [-1.78, -0.17])25 and rate of progression to severe COVID-19 cases (RR 0.34, 95% CI [0.18, 0.65]).27 Combination therapy with TCM herbal medication and conventional treatments was reported to be effective in the improvement of lung CT for mild to moderate COVID-19 patients. The estimated effect size for the improvement in lung CT was RR 1.26, 95% CI [1.15, 1.38].11,26 However, GI symptoms, including nausea, did not show significant improvement in the TCM medication group (RR 1.04, 95% CI [0.83, 1.30]).22 In particular, LianhuaQingwen showed significant improvement in overall clinical effectiveness (RR 1.16, 95% CI [1.04 to 1.30]),19 reduced flu-like symptoms (SMD -1.81, 95% CI [-1.99, -1.17])13 or main clinical symptoms (OR 3.34, 95% CI [2.06, 5.44])28 and protected conversion to severe or critical case rates (RR 0.38, 95% CI [0.17, 0.85]).30 Most studies reported any types of AEs related to the usage of TCM herbal medication. However, six studies reported no information of AEs.15,22,27,28,30 The reported main AEs of TCM medications were gastrointestinal reactions such as abdominal distention, abdominal pain, nausea, vomiting, diarrhea, belching, acid reflux or loss of appetite. In one study, overall RR of total adverse drug events was suggested to be RR = 1.13, 95% CI (0.45, 2.83), which represented that there was no significant difference in the overall risk of AEs between groups.11 In addition, there was no significantly different risk for specific types of AEs between the TCM medication group and the control group. About the assessment of causality and severity of AEs, there was no information in the included SRs (Table 2).
Table 2.
Summary of the adverse drug events.
| Study ID | Interventions | Types of adverse events | Causality | Severity | No. of studies (No. of patients) | Effect estimate, 95% CI |
| Ang 202010 | Chinese herbal medicine | N.R | N.R | Mild | 5 (30) | RD = 0.06, 95% CI (-0.04, 0.15), I2 = 95%, P = 0.24 |
| Du 202111 | Chinese herbal medicine | Nausea and vomiting | N.R | N.R | 2 (388) | RR = 1.09, 95% CI (0.49, 2.41), P = 0.83 |
| Diarrhea | N.R | N.R | 5 (759) | RR = 1.72, 95% CI (0.34, 8.67), P = 0.51 | ||
| Abnormal liver function | N.R | N.R | 2 (388) | RR = 0.41, 95% CI (0.05, 3.69), P = 0.43 | ||
| Poor appetite, renal dysfunction, headache | N.R | N.R | 1 (17) | N.R | ||
| Total cases of adverse drug events | N.R | N.R | 10 (1286) | RR = 1.13, 95% CI (0.45, 2.83), P = 0.79 | ||
| Fan 202012 | Chinese herbal medicine | Mild liver dysfunction | N.R | Mild | 1 (5) | N.R |
| Diarrhea | N.R | N.R | 1 (27) | N.R | ||
| Hu 202013 | Lianhua Qingwen | Diarrhea, rash, gastrointestinal reaction, headache, nausea, vomiting, abnormal liver function, and renal insufficiency | N.R | N.R | 18 (N.R) | OR = 0.74, 95% CI (0.56, 0.97), P = 0.029 |
| Jiang 202114 | Traditional Chinese Medicine | N.R | N.R | N.R | 19 (N.R) | RR = 0.77, 95% CI (0.53, 1.13), I2 = 28%, p = 0.18 |
| Li 202115 | Traditional Chinese Medicine | No description on AEs | N.R | N.R | N.R | N.R |
| Liang 202016 | Chinese herbal medicine | Mild abdominal pain, diarrhea, nausea, vomiting and drug allergy | N.R | N.R | 8 (N.R) | RR = 2.06, 95% CI (0.34, 12.38) |
| Liang 202117 | Oral Chinese patent medicine | Abnormal liver function, renal dysfunction, headache, nausea, vomiting, diarrhea, loss of appetite | N.R | N.R | 1 (65) | N.R |
| Liu 202018 | Traditional Chinese Medicine | Nausea and vomiting | N.R | N.R | 2 (10) | RR = 0.915, 95% CI (0.267, 3.138), P = 0.888 |
| Diarrhea | N.R | N.R | 2 (35) | RR = 5.598, 95% CI (0.267, 166.774), P = 0.320 | ||
| Liver damage | N.R | N.R | 2 (15) | RR = 0.281, 95% CI (0.046, 1.706), P = 0.168 | ||
| Liu 2021a19 | Lianhua Qingwen | Abnormal liver function, renal dysfunction, headache, nausea, vomiting, diarrhea, loss of appetite | N.R | N.R | 1 (N.R) | N.R |
| Luo 202120 | Chinese herbal medicine |
Nausea, abdominal pain, and diarrhea |
N.R | N.R | 8 (N.R) | OR = 1.21, 95% CI (0.48, 3.07), I2 = 43.5% |
| Pang 202021 | Chinese medical drugs | Gastrointestinal reaction(nausea, vomiting, diarrhea, loss of appetite), abnormal liver function, renal dysfunction, headache, and pruritus | N.R | N.R | 8 (1152) | RD = 0.03, 95% CI (−0.02, 0.08), I2 = 83%, p = 0.31 |
| Shi 202122 | Chinese herbal medicine | No description on AEs | N.R | N.R | N.R | N.R |
| Sun 202023 | Chinese medicine | N.R | N.R | N.R | 7 (681) | RR = 1.17, 95% CI (0.39, 3.52), p = 0.78 |
| Wang 2021a24 | Chinese herbal medicine | Abnormal liver function, renal dysfunction, headache, nausea, vomiting, diarrhea, loss of appetite | N.R | N.R | 1 (65) | RD = 0.84, 95% CI (0.67, 1.07) |
| Diarrhea | N.R | N.R | 1 (27) | N.R | ||
| Wang 2021b25 | Traditional Chinese Medicine | Allergic reaction, diarrhea | N.R | N.R | 17 (N.R) | RR = 0.85, 95% CI (0.71, 1.03) |
| Xiong 202026 | Chinese herbal medicine | Gastrointestinal reactions (abdominal distention, diarrhea, abdominal pain, nausea, vomiting, belching, acid reflux, poor appetite), headache, dizziness, drowsiness, abnormal liver function, renal dysfunction, and drug allergy | N.R | Mild | 9 (1069) | RR = 0.93, 95% CI (0.49–1.75), I2= 46%, C = 0.82 |
| Yan 202127 | Traditional Chinese and Western medicines | No description on AEs | N.R | N.R | N.R | N.R |
| Zeng 202028 | Lianhua Qingwen | No description on AEs | N.R | N.R | N.R | N.R |
| Zhou 202129 | Traditional Chinese Medicine | Abnormal liver function, diarrhea | N.R | N.R | 9 (212) | RR = 0.81, 95% CI (0.42, 1.57), I2 = 56%, P = 0.54 |
| Zhuang 202130 | Lianhua Qingwen | No description on AEs | N.R | N.R | N.R | N.R |
| Puyat 202031 | Home-based activity | No description on AEs | N.R | N.R | N.R | N.R |
| Bassatne 202132 | Vitamin D | No description on AEs | N.R | N.R | N.R | N.R |
| Rawat 202133 | Vitamin D | No description on AEs |
N.R | N.R | N.R | N.R |
AE: Adverse event; CI: Confidence interval; N.R: Not reported; RR: Relative risk; OR: Odds ratio; RD: Risk difference.
3.2. Effectiveness and safety of physical exercise and vitamin D for COVID-19 patients
Home-based activities, including exercise, yoga and muscle relaxation techniques, were reported to show improvement in mental wellness.31 However, vitamin D supplements did not result in the improvement of major health outcomes in COVID-19 patients (RR of mortality 0.55, 95% CI [0.22, 1.39]).33 However, there were no descriptions of AEs in the SRs for home-based activity31 and vitamin D (Table 2).32,33
3.3. Current evidence status of CAM interventions for COVID-19
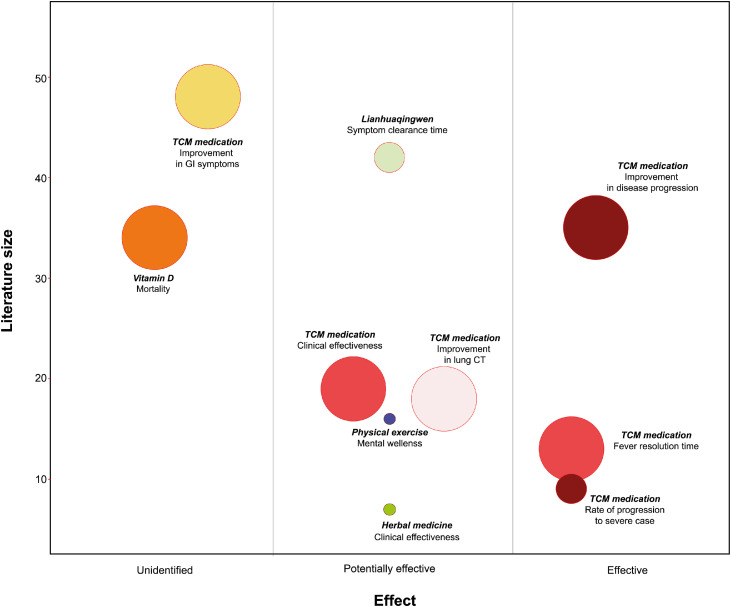
From this overview of SRs, we assessed the evidence status of CAM interventions for COVID-19 patients through evidence mapping methods. TCM medications seem to be effective in improving disease progression, reducing fever resolution time and reducing the rate of progression to severe cases, which was supported by evidence based on comparatively large numbers of RCTs. However, TCM medications did not seem to be related to the improvement of GI symptoms in COVID patients, as vitamin D was not effective in improving mortality. From the evidence map, there are still unclear areas of evidence for TCM medication, herbal medications and physical exercise in terms of symptom clearance time, overall clinical effectiveness, improvement in lung CT and mental wellness, which remain areas for future rigorous RCTs (Fig. 2).
Fig. 2.
Evidence mapping of complementary and alternative medicine interventions for COVID-19
In the bubble plot, each intervention had two-dimensional information including x-axis (overall risk of bias), y-axis (the number of included original studies in each systematic review (SR)) and bubble size (overall confidence level of the included SR assessed by AMSTAR 2 checklist). Bigger size represents more confidence of the assessed SR. The name of each bubble includes the name of intervention-symptom (or condition) of interests.
4. Discussion
From this study, we found that studies on TCM medications have been suggested actively for COVID-19 patients. Some medications appeared to be effective and safe for managing COVID-19 symptoms and disease progression, while others should be tested in future RCTs. Some CAM interventions seem to stray far from the established evidence. Vitamin D supplements did not seem to improve major health outcomes in COVID-19 patients, although there were some controversial results on the causal relationship between low serum levels of vitamin D and severity (or mortality) induced by COVID-19 infection.32,34, 35, 36 Although the evidence for safety cannot be guaranteed due to the limitation of reporting in SRs, administration of TCM medications does not seem to increase the incidence of AEs in COVID-19 patients, other than digestive discomfort.
Evidence mapping is a novel methodology for synthesizing research evidence that identifies the quantity and quality of established evidence in specific areas and can present gaps between current research and needs for future research.37 Although it has limitations due to the absence of a standardized methodology and the disadvantage of being unable to provide accurate and complete quantification, evidence mapping is an effective tool for integrating studies in a wide range of areas and presenting an overall picture of what evidence has accumulated.37,38 During the COVID-19 pandemic, various CAM interventions, including TCM, yoga, Ayurveda, homeopathy and dietary supplements, have been prescribed for COVID-19 patients and the general population with considerable usage prevalence.39, 40, 41, 42 However, from this overview study, we found that most of these interventions need appropriate evidence regarding their clinical effectiveness and safety. Some countries published recommended guidelines on the usage of CAM interventions,43,44 and some underdeveloped countries adopted CAM interventions for the management of the COVID-19 pandemic. If CAM interventions are to be used appropriately in response to the COVID-19 pandemic and contribute to improving the health situation of each country, evidence for the effectiveness and safety of these interventions that are assumed to be effective or are already effective in clinical practice should be presented to support the medical decision process.
This study has limitations. Due to the absence of standardized methodology for evidence mapping, there could be ambiguities in the process of analysis or research data and interpretation of the results. We did not analyze detailed interventions for each type of CAM therapy, and heterogeneity should be critical in the synthesized results. We only included SRs, and some CAM interventions had RCTs assessing the effectiveness and safety of interventions that were not evaluated in SRs. All these limitations urge the readers to interpret these study results carefully. Future updated overviews and mapping of evidence will be requisite. In addition to this, there should be new RCTs on the effectiveness and safety of CAM interventions for long COVID, which is expected more frequently.
In conclusion, there is evidence that TCM medications reduce the fever resolution time and the rate of progression to severe cases. However, evidence of the effectiveness of most CAM interventions still needs to be evaluated. Clinical evidence of various CAM interventions for acute COVID 19 infection and long COVID needs to be evaluated through rigorous RCTs in future.
Author contributions
Sae-Rom Jeon: Methodology, Investigation, Writing review & editing. Jung Won Kang: Validation, Formal analysis, Resources, Data curation, Writing review & editing. Lin Ang: Writing review & editing. Hye Won Lee: Writing review & editing. Myeong Soo Lee: Writing review & editing. Tae-Hun Kim: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Writing original draft, Visualization.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study is funded by Korea Institute of Oriental Medicine (KSN20214115).
Ethical statement
Not applicable.
Data availability
The datasets analyzed in this study are available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2022.100842.
Appendix. Supplementary materials
Reference
- 1.Charumilind S., Craven M., Lamb J., Sabow A., Wilson M. When will the Covid-19 pandemic end? an update: january; 2021.
- 2.Kretchy I.A., Boadu J.A., Kretchy J.P., et al. Utilization of complementary and alternative medicine for the prevention of COVID-19 infection in Ghana: a national cross-sectional online survey. Prev Med Rep. 2021;24 doi: 10.1016/j.pmedr.2021.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugavanam S.C., Natarajan B. Pseudoscientific beliefs and practices in the COVID-19 pandemic: a narrative review of unwanted experiments attributed to social media-based misinformation afflicting the public health. J Health Biol Sci. 2020;8:1–9. [Google Scholar]
- 4.Ganguly S., Bakhshi S. Traditional and complementary medicine during COVID-19 pandemic. Phytother Res. 2020;34:3083–3084. doi: 10.1002/ptr.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paudyal V., Sun S., Hussain R., Abutaleb M.H., Hedima E.W. Complementary and alternative medicines use in COVID-19: a global perspective on practice, policy and research. Res Soc Adm Pharm. 2021;18:2524–2528. doi: 10.1016/j.sapharm.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crook H., Raza S., Nowell J., Young M., Edison P. Long Covid—mechanisms, risk factors, and management. BMJ. 2021:374. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 7.NCCIH. "Complementary, Alternative, or Integrative Health: What’s In a Name?" Accessed on Noverber 4, 2021 from https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name.
- 8.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hempel S., Shekelle P.G., Taylor S.L., Solloway M.R. Evidence map of acupuncture. US Department of Veterans Affairs; Washington DC: 2014. [PubMed] [Google Scholar]
- 10.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9 doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du X., Shi L., Cao W., Zuo B., Zhou A. Add-on effect of Chinese herbal medicine in the treatment of mild to moderate COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0256429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan A.Y., Gu S., Alemi S.F. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J Integr Med. 2020;18:385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C., Liang M., Gong F., He B., Zhao D., Zhang G. Efficacy of Lianhua Qingwen compared with conventional drugs in the treatment of common pneumonia and COVID-19 pneumonia: a meta-analysis. Evid Based Complement Altern Med. 2020;2020 doi: 10.1155/2020/5157089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang F., Xu N., Zhou Y., et al. Contribution of Traditional Chinese Medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis. Phytother Res. 2021;35:5992–6009. doi: 10.1002/ptr.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F., Jiang Y., Yue B., Luan L. Use of Traditional Chinese Medicine as an adjunctive treatment for COVID-19: a systematic review and meta-analysis. Medicine. 2021;100:e26641. doi: 10.1097/MD.0000000000026641. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang S.B., Zhang Y.Y., Shen C., et al. Chinese herbal medicine used with or without conventional western therapy for COVID-19: an evidence review of clinical studies. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.583450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S.B., Fang M., Liang C.H., et al. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: a rapid systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M., Gao Y., Yuan Y., et al. Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M., Gao Y., Yuan Y., et al. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10 doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X., Ni X., Lin J., et al. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang W., Liu Z., Li N., et al. Chinese medical drugs for coronavirus disease 2019: a systematic review and meta-analysis. Integr Med Res. 2020;9 doi: 10.1016/j.imr.2020.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi S., Wang F., Li J., et al. The effect of Chinese herbal medicine on digestive system and liver functions should not be neglected in COVID-19: an updated systematic review and meta-analysis. IUBMB Life. 2021;73:739–760. doi: 10.1002/iub.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C.Y., Sun Y.L., Li X.M. The role of Chinese medicine in COVID-19 pneumonia: a systematic review and meta-analysis. Am J Emerg Med. 2020;38:2153–2159. doi: 10.1016/j.ajem.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Greenhalgh T., Wardle J. Chinese herbal medicine (“3 medicines and 3 formulations”) for COVID-19: rapid systematic review and meta-analysis. J Eval Clin Pract. 2021;28:13–32. doi: 10.1111/jep.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Xu B., Zhang Y., et al. Efficacy and safety of Traditional Chinese Medicine in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan L., Mao F., Cao Y., Xie M. Clinical effects of the combination of Traditional Chinese and Western Medicines on coronavirus disease 2019: a systematic review and meta-analysis. J Tradit Chin Med. 2021;41:499–506. doi: 10.19852/j.cnki.jtcm.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Zeng M., Li L., Wu Z. Traditional Chinese Medicine Lianhua Qingwen treating corona virus disease 2019 (COVID-19): meta-analysis of randomized controlled trials. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L.P., Wang J., Xie R.H., et al. The effects of Traditional Chinese Medicine as an auxiliary treatment for COVID-19: a systematic review and meta-analysis. J Altern Complement Med. 2021;27:225–237. doi: 10.1089/acm.2020.0310. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang J., Dai X., Wu Q., et al. A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19) Complement Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puyat J.H., Ahmad H., Avina-Galindo A.M., et al. A rapid review of home-based activities that can promote mental wellness during the COVID-19 pandemic. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassatne A., Basbous M., Chakhtoura M., El Zein O., Rahme M., El-Hajj Fuleihan G. The link between COVID-19 and vitamin D (VIVID): a systematic review and meta-analysis. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawat D., Roy A., Maitra S., Shankar V., Khanna P., Baidya D.K. Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrelli F., Luciani A., Perego G., Dognini G., Colombelli P.L., Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211 doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szarpak L., Rafique Z., Gasecka A., et al. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol J. 2021;28:647–654. doi: 10.5603/CJ.a2021.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miake-Lye I.M., Hempel S., Shanman R., Shekelle P.G. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:1–21. doi: 10.1186/s13643-016-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colquhoun H.L., Levac D., O'Brien K.K., et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67:1291–1294. doi: 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Alotiby A.A., Al-Harbi L.N. Prevalence of using herbs and natural products as a protective measure during the COVID-19 pandemic among the Saudi population: an online cross-sectional survey. Saudi Pharm J. 2021;29:410–417. doi: 10.1016/j.jsps.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charan J., Bhardwaj P., Dutta S., et al. Use of complementary and alternative medicine (CAM) and home remedies by COVID-19 patients: a telephonic survey. Indian J Clin Biochem. 2021;36:108–111. doi: 10.1007/s12291-020-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jethani B., Gupta M., Wadhwani P., et al. Clinical characteristics and remedy profiles of patients with COVID-19: a retrospective cohort study. Homeopathy. 2021;110:086–093. doi: 10.1055/s-0040-1718584. [DOI] [PubMed] [Google Scholar]
- 42.Lam C.S., Koon H.K., Chung V.C.H., Cheung Y.T. A public survey of traditional, complementary and integrative medicine use during the COVID-19 outbreak in Hong Kong. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang N., Ma Y., Wang J., et al. Traditional Chinese Medicine guidelines for coronavirus disease 2019. J Tradit Chin Med. 2020;40:891–896. doi: 10.19852/j.cnki.jtcm.20200902.001. Chung i tsa chih ying wen pan. [DOI] [PubMed] [Google Scholar]
- 44.Pandit R.D., Singh R.K. COVID-19 Ayurveda treatment protocol of governments of Nepal and India: a review and perspective. Appl Sci Technol Ann. 2020;1:72–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author on reasonable request.