Abstract
Introduction
Recent studies highlighted the role of olive polyphenols in disrupting the ordered structure of highly cytotoxic amyloid beta protofibrils and the efficacy of a derivatized form of glutathione to counteract neuronal oxidative stress affecting specific brain regions at early stages of Alzheimer's disease (AD) pathogenesis. We performed a randomized cross‐over clinical trial to evaluate their potential benefits in mild AD.
Methods
Oleuropein and S‐acetyl glutathione were administered as dietary supplement for 6 months to 18 patients diagnosed for probable mild AD according to International Working Group 2 criteria. Patients underwent an extensive cognitive and behavioral neuropsychological test battery at the beginning and end of the study to evaluate cognitive deterioration, memory, visuospatial abilities, attention, language, executive functions, and behavioral disorders. We compared patients receiving treatment to patients receiving no treatment.
Results
All the measured neurocognitive parameters stabilized or improved after the treatment in all patients.
Discussion
Dietary supplement with olive polyphenols and bioavailable glutathione could be useful for patients diagnosed with mild AD.
Keywords: Alzheimer disease, glutathione, olive polyphenols
1. INTRODUCTION
According to Alzheimer's Disease International, Alzheimer's disease (AD) is characterized by the development of amyloid beta (Aβ) plaques and tau neurofibrillary tangles in the brain. 1 The great majority of clinical trials of potential disease‐modifying therapies for AD (including statins, non‐steroidal anti‐inflammatory drugs, monoclonal antibodies, estrogens, and nerve growth factor) have yielded substantially negative results over the past 20 years. Furthermore, in spite of genetic and molecular evidence pointing to Aβ as a key player in AD pathogenesis, 2 most trials with anti‐amyloid therapies have failed, possibly due to over‐simplistic pathogenic constructs unfit to cope with the complexity and variety of the pathological modifications favoring the expression of the clinical phenotype, including neuronal loss. 3 Numerous longitudinal studies using several AD biomarkers indicated that the AD pathology develops decades before symptom appearance, 4 suggesting the usefulness of preventive multi‐target treatments aimed at hindering or delaying disease onset.
Recent data indicate that deglycated oleuropein (Ole), the most abundant polyphenol in virgin olive oil, interferes with amyloid precursor protein and tau protein processing, preventing toxic oligomer growth both in vitro, in a transgenic strain of Aβ‐expressing C. elegans, and in CRND8 transgenic mice, a model of Aß plaque deposition. 5 In particular, transgenic mice fed a normal diet supplemented with Ole showed a dose‐dependent protection against cognitive deterioration compared to normally fed littermates. At the tissue level, these mice displayed a significant improvement of synaptic function, plaque load, neurogenesis, and neuroinflammation. 6
Glutathione, the main antioxidant defense in the body, is a metabolite with one of the highest concentrations in mammalian tissue cells. Its concentration (between 1 and 10 mM depending on the cell type) decreases with age and in specific central nervous system regions in AD patients. 7
When taken orally, glutathione as such is unable to cross the alimentary canal intact as it undergoes a hydrolytic cleavage catalyzed by digestive enzymes, in particular by pancreatic peptidase and the intestinal wall. 8 In recent years, S‐acetyl glutathione (SAG) and glutathione derivatives conjugated with long‐chain fatty acid conjugates have been shown to be highly bioavailable from preclinical and clinical studies. These glutathione derivatives have proven to be capable of significantly increasing the intracellular concentration of the tripeptide even when supplied at concentration levels of orders of magnitude lower than those reached within the cells. 9 This effect is believed to be the result of a trapping process by cells, which do not allow the release of glutathione penetrated as thioesters and subsequently hydrolyzed by intracellular thioesterases. In particular, glutathione derivatives with omega‐3 fatty acids such as S‐linolenoyl glutathione have proven particularly effective in counteracting oxidative stress in the neurons of patients with AD and in preventing cell death in cultures of human neurons exposed to oxidative stress induced by hydrogen peroxide and Aβ42. 9
The association with a bioavailable form of glutathione is therefore expected to further ameliorate Ole neuroprotection by counteracting oxidative stress, a dishomeostasis not directly relieved by Ole in TgCRND8 mice. 6 Even if the possible therapeutic role of many antioxidants in AD has been deeply investigated, no literature yet ascribes the potential benefits of these two molecules for patients who have already developed AD. 7
On the basis of the above‐reported findings from preclinical studies, we performed a randomized cross‐over clinical trial by enrolling patients with mild AD diagnosis to which a nutraceutical formulation containing Ole, SAG, and a low‐dose combination of other well‐known inhibitors of oxidative stress (vitamins B6, B12, E, and D3; piperine; bacopa) was administered daily, to assess protection against neuropsychological deterioration.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Several recent publications have suggested a potential role of olive polyphenols and bioavailable glutathione in contrasting amyloid beta cytotoxicity and neuronal oxidative stress affecting specific brain regions at early stages of Alzheimer's disease (AD).
Interpretation: Our findings suggested a potential role of olive polyphenols and bioavailable glutathione in slowing AD progression.
Future Directions: If further research confirms our observation the described treatment could be proficiently adopted for patients diagnosed with mild AD as well as a preventive tool for those at risk of developing dementia.
2. METHODS
The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and was approved by the Italian Regional Ethics Committee. It provided for up to 40 patients diagnosed with mild AD according to the International Working Group‐2 (IWG‐2) criteria to be enrolled, previous obtainment of informed consent, in a 12‐month trial and randomly assigned to two groups of equal sizes: Group 1, receiving treatment in the first 6 months and no treatment for the second 6 months, and Group 2, receiving no treatment for the first 6 months, and treatment for the second 6 months. Treatment consisted of a nutraceutical formulation comprising 50 mg/cps SAG and 80 mg/cps oleuropein associated with vitamin B6 1 mg/cps, vitamin B12 3mcg/cps, vitamin E 15 IU cps, vitamin D3 4 mcg/cps, piperine 3 mg/cps, bacopa dry extract 100 mg/cps administered b.i.d. 2 Exclusion criteria included previous phenomena of intolerance/allergy to one of the components present in the formulation. All the patients should have undergone a complete set of neuropsychological tests to evaluate cognitive functions and behavioral disturbances at the moment of the enrollment (T0), after 6 months (T1), and after 12 months (T2); cognitive deterioration, memory, visuospatial abilities, attention, language, executive functions, and behavioral disorders were included in the study as outcome variables using 14 indicators (Table 1). 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Given the nature and size of the study, a difference‐in‐difference (DID) approach was used to evaluate outcome variables. DID is a quasi‐experimental design that makes use of longitudinal data from treatment and control groups to obtain an appropriate counterfactual to estimate a causal effect.
TABLE 1.
Groups’ demographic data and effect of treatment on outcomes variables
| Group 1 (Treatment) | Group 2 (Control) | |
|---|---|---|
| Sex | 5 M 5F | 5 M 3F |
| Age | 67±5.84 | 67.8±4.41 |
| Education | 11.4±3.1 | 11.6±3.2 |
| AChEIs use | 100% | 100% |
| Family history of dementia | None | None |
| Test |
Baseline mean (SD) |
6 months mean (SD) |
Difference |
Baseline mean (SD) |
6 months mean (SD) |
Difference | Diff‐in‐diff | P | SE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive deterioration | MMSE (0–30) | 21.20 (1.03) | 22.90(1.20) | 1.70 (+8%) | 22.13 (0.83) | 22.13(0.83) | 0.00 (+0%) | 1.70 | .0008 | 0.4125 |
| CDT (0–9) | 5.40 (1.84) | 6.20 (1.69) | 0.80 (+15%) | 4.75 (1.28) | 4.25 (1.04) | 0.50 (–11%) | 1.30 | .0135 | 0.4684 | |
| Memory |
RAVLT IR (0–75) |
25.20 (5.32) | 26.90 (4.68) | 1.70 (+7%) | 24.75 (2.12) | 22.25 (2.25) | 2.50 (–10%) | 4.20 | .0014 | 1.0875 |
|
RAVLT DR (0–15) |
0.30 (0.67) | 1.20 (1.23) | 0.90 (+300%) | 0.63 (0.92) | 0.13 (0.35) | 0.50 (–80%) | 1.40 | .0046 | 0.4259 | |
| RCF IR (0–36) | 2.40 (2.80) | 4.60 (3.66) | 2.20 (+92%) | 1.50 (2.33) | 0.75 (2.12) | 0.75 (–50%) | 2.95 | .0016 | 0.7785 | |
| Visuospatial abilities | RCF C (0–36) | 23.30 (6.45) | 26.50 (5.15) | 3.20 (+14%) | 24.63 (4.50) | 24.50(4.72) | 0.13 (–1%) | 3.33 | .0603 | 1.6452 |
| Attention | MA (0–60) | 32.00 (6.32) | 32.90 (5.95) | 0.90 (+3%) | 38.25 (7.69) | 35.00(5.95) | 3.25 (–8%) | 4.15 | .0028 | 1.1763 |
| Language and speech | AAT (0– 120) | 100.60 (5.46) | 104.40 (3.92) | 3.80 (+4%) | 103.38 (10.39) | 103.38(10.39) | 0.00 (+0%) | 3.80 | .0142 | 1.3809 |
| Executive functions | FAB (0–18) | 11.80 (1.93) | 15.10 (0.99) | 3.30 (+28%) | 10.88 (2.23) | 9.25 (1.83) | 1.63 (–15%) | 4.93 | <.0000 | 0.5806 |
| STEP (0–40) | 21.20 (5.59) | 24.90 (4.43) | 3.70 (+17%) | 24.00 (4.31) | 21.75 (4.83) | 2.25 (–9%) | 5.95 | <.0000 | 0.9605 | |
| SVF (0–∞ ) | 32.50 (5.06) | 35.80 (4.18) | 3.30 (+10%) | 31.38 (5.10) | 29.50 (3.93) | 1.88 (–6%) | 5.18 | <.0000 | 0.9107 | |
| PVF (0 ‐ ∞) | 24.90 (5.78) | 30.50 (6.70) | 5.60 (+22%) | 27.75 (5.52) | 23.63 (4.90) | 4.13 (–15%) | 9.73 | <.0000 | 1.4194 | |
| Behavioral disorders | NPI (0–144) | 10.70 (5.58) | 5.80 (3.94) | 4.90 (–46%) | 12.00 (7.09) | 12.50 (6.65) | 0.50 (+4%) | –5.40 | .0001 | 1.0797 |
| AES (0–72) | 40.70 (10.72) | 33.60 (8.58) | 7.10 (–17%) | 33.63 (16.49) | 36.13 (15.68) | 2.50 (+7%) | –9.60 | <.0000 | 1.7221 |
Abbreviations: AAT, Aachener Aphasie Test 15 ; AChEI, acetylcholinesterase inhibitor; AES, Apathy Evaluation Scale 17 ; CDT, Clock Drawing Test 11 ; FAB, Frontal Assessment Battery 14 ; MA, Attentive Matrices 13 ; MMSE, Mini Mental State Examination 10 ; NPI, Neuropsychiatric Inventory 18 ; PVF, Phonological Verbal Fluency Test 12 ; RAVLT DR, Rey Auditory Verbal Learning TestDelayed Recall; RAVLT IR, Rey Auditory Verbal Learning TestImmediate Recall; RCF C, Rey Complex FigureCopy; RCF IR, Rey Complex FigureImmediate Recall 12 , 13 ; SD, standard deviation; SE, standard error; STEP, Estimation of Time and Weights Test 16 ; SVF, Semantic Verbal Fluency Test 13 . Or maybe it would be better just to put at the end of the whole text 15 , 16 , 17 , 18 .
The effect of the treatment (having taken the food supplement for 6 months) was calculated by comparing the average change in the outcome variables for the treatment group to the average change for the control group. We used a t test of the difference between T0 and T1 for the two groups (intergroup comparison). We did not use intragroup tests.
3. RESULTS
Due to the difficulties related to the COVID‐19 pandemic emergency, only 10 patients in Group 1 and 8 in Group 2 could be enrolled. These patients could be evaluated only at T0 and T1. Results of the study are summarized in Table 1. The effect of the treatment is statistically significant for all 14 outcome variables, and 11 of them are significant at the 1% level. These results appear surprisingly robust on a statistical basis, given the small sample size. In particular, the treatment seems to have a very strong and significant effect on:
(1) Cognitive deterioration: Strong reduction of cognitive deterioration for treated group versus control, with Mini‐Mental State Examination (MMSE) improvements of 8% for treatment group (vs. a 0% change for control group), significant at the 1% level and very near to the 2 points reported as the MMSE – minimal clinically important difference (MCID) 19 ; and a Clock Drawing Test improvement of 15% for treatment group (vs. a 11% reduction for control group), with a P‐value of .0135.
(2) Improving memory: All three indicators are significant at 1% level; Rey Auditory Verbal Learning Test–Immediate Recall, Rey Auditory Verbal Learning Test–Delayed Recall, and Rey Complex Figure–Immediate Recall show an improvement of 7%, 300%, and 92%, respectively, for treatment group, compared to a deterioration of 10%, 80%, and 50% for control group (Figure 1).
(3) Spatial and visual abilities: Improvement of 14% in Rey Complex Figure–Copy exam for treatment group (vs. a reduction of 1% for control group), with a P‐value of .0603.
(4) Improving attention: Patients in the treatment group show an improvement of 3% in the Attentive Matrices, compared to a reduction of 8% in control group; this is significant at the 1% level.
(5) Language and speech: An improvement of 4% in the Aachener Aphasie Test for treatment group (vs. a 0% change for the control group), with a P‐value of .0143.
(6) Improving executive functions: All indicators are significant at the 1% level. Improvements for treatment group are substantial and vary from a 10% improvement in Semantic Verbal Fluency Test (vs. 6% reduction in control group), to a 28% improvement in the Frontal Assessment Battery for the treatment group (vs. 15% decrease in control group)
(7) Diminishing behavioral disorders: Strong reduction in treatment group compared to control group. Treatment group exhibits a reduction of 46% in the Neuropsychiatric Inventory (vs. an increase of 4% in control), and a reduction of 17% in the Apathy Evaluation Scale (vs. an increase of 7% in control). Both outcome variables are statistically significant at the 1% level.
FIGURE 1.
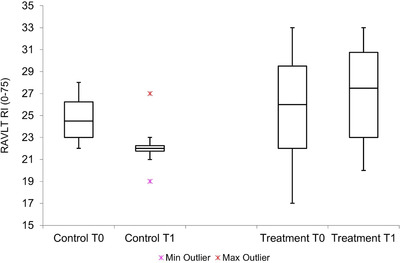
Box plot for the Rey Auditory Verbal Learning Test–Immediate Recall showing difference between treatment and control group
4. DISCUSSION
Overall, the treatment seems to successfully diminish the effect of neurodegeneration in all neuropsychological outcome variables observed. Even if the number of patients is very small and there is no placebo control, these relevant effects are unexpected and are tentatively ascribed by authors to a synergistic effect of the active compounds of the nutraceutical formulation toward neurotoxic activity of amyloid protofibrils and neuronal oxidative stress, two hallmarks in early stages of AD pathogenesis. Reviewing literature about this topic, we considered the SAG and Ole interaction as primarily responsible for the proposed metabolic synergy. 20
Despite the “proof‐of‐concept” character of this study, the authors believe that the results are impactful considering the lack of therapeutic perspectives for AD. We are planning to perform a double‐blind randomized controlled trial with a larger number of patients including neuropsychological measures and other validated AD biomarkers. At the moment, there is encouraging preliminary evidence that the described treatment could be proficiently adopted for patients with mild cognitive impairment or mild AD.
CONFLICTS OF INTEREST
Massimo Marianetti, Silvia Pinna, Angelo Venuti, and Gianfranco Liguri did not receive support for the submitted work nor receive any support outside the submitted work in the past 36 months. Massimo Marianetti, Silvia Pinna, and Angelo Venuti do not have any relationships/activities/interests to disclose. Gianfranco Liguri has been in the past 36 months a shareholder and unpaid scientific advisor for Innbiotec Pharma Srl, which developed the nutraceutical formulation and he has no other relationships/activities/interests to disclose. All the authors have nothing else to disclose according to the ICMJE Disclosure Form.
ACKNOWLEDGMENTS
The authors thank Enrico Calvanese, MPA/ID 2020 and Luca Giani, MPP candidate–Harvard University, Kennedy School, for their skilled advising and support in processing data for statistical analysis; Innbiotec Pharma s.r.l., for developing the nutraceutical formulation; and Igea Pharma NV for supplying the product (commercialized as Alz1 Tab/Euvitase). This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Marianetti M, Pinna S, Venuti A, Liguri G. Olive polyphenols and bioavailable glutathione: Promising results in patients diagnosed with mild Alzheimer's Disease. Alzheimer's Dement. 2022;8:e12278. 10.1002/trc2.12278
REFERENCES
- 1. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug‐development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espay AJ, Vizcarra JA, Marsili L, et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019;92:329‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS. Alzheimer's Disease Neuroimaging Initiative. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317:2305‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kostomoiri M, Fragkouli A, Sagnou M, et al. Oleuropein, an anti‐oxidant polyphenol constituent of olive promotes α‐secretase cleavage of the amyloid precursor protein (AαPP). Cell Mol Neurobiol. 2013;33:147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grossi C, Rigacci S, Ambrosini S, et al. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aβ plaque pathology. PLoS One. 2013;8:e71702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zandi PP, Anthony CC, Khachaturian AS, et al. for the Cache County Study Group (2004) Reduced Risk of Alzheimer Disease in Users of Antioxidant Vitamin Supplements. Arch Neurol. 61(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 8. Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43(6):667‐669. [DOI] [PubMed] [Google Scholar]
- 9. Pensalfini A, Cecchi C, Zampagni M, et al. Protective effect of new S‐acylglutathione derivatives against amyloid‐induced oxidative stress. Free Rad Biol Med. 2008;44:1624‐1636. [DOI] [PubMed] [Google Scholar]
- 10. Folstein MF, Folstein SE, McHugh PR. Mini Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 11. Thalmann B, Monsch AU, Ermini‐Fünfschilling D, Improved screening for dementia: combining the clock drawing test and the Mini‐Mental State Examination Presented at «The 4th International Nice/Springfield Alzheimer Symposium» Nice. April 10‐14th 1996; 1996.
- 12. Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36(6):378‐384. [DOI] [PubMed] [Google Scholar]
- 13. Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci. 1987;8:1‐120. [Suppl]. [PubMed] [Google Scholar]
- 14. Appollonio I, Leone M, Isella V, et al. The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci. 2002;26(2):108‐116. [DOI] [PubMed] [Google Scholar]
- 15. Luzzati C, Wilmes K, Bleser D, AAT Aachner Aphasie Test (Edizione italiana). Firenze: Giunti OS; 1996.
- 16. Nichelli P, Leone M, Caronna A, et al. Normative values of a new cognitive estimation task: the weight and time estimation test. Nuova rivista di Neurologia. 2002;12(2):37‐42. [Google Scholar]
- 17. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research. 1991;38(2):143‐162. [DOI] [PubMed] [Google Scholar]
- 18. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
- 19. Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement (N Y). 2019;5:354‐363. Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visioli F, Wolfram R, Richard D, Abdullah MI, Crea R. Olive phenolics increase glutathione levels in healthy volunteers. J Agric Food Chem. 2009;57(5):1793‐1796. [DOI] [PubMed] [Google Scholar]


