Abstract
Lung, bronchial mucosa, and pleural tissue samples were obtained from 14 patients undergoing lung surgery 1 to 5 h after administration of 1 g of meropenem. The mean (range) peak concentrations of meropenem were 3.9 (0.2 to 8.2), 6.6 (3.0 to 13.3), and 2.8 (0.6 to 7.8) mg/kg of tissue, respectively, exceeding the MICs at which 90% of isolates are inhibited for most respiratory pathogens.
Meropenem, a carbapenem antimicrobial agent, has a broad spectrum of antibacterial activity which includes most respiratory pathogens, such as Streptococcus sp., methicillin-sensitive Staphylococcus aureus, Haemophilus sp., strains of the Enterobacteriaceae, and Pseudomonas aeruginosa, as well as anaerobes (9). The aim of this study was to assess the concentrations of meropenem in human lung, bronchial mucosa, and pleural tissues. Fourteen patients (13 males and 1 female) underwent lobectomy (12 patients) or pneumectomy (2 patients) for bronchial cancer. The mean (range) patient age was 58 (38 to 68) years and the mean (range) weight was 72 (56 to 98) kg. All patients had normal renal and hepatic functions. None presented any clinical or laboratory signs of infection or had received antibiotic treatment during at least the previous 7 days. The study received the approval of the Ethical Committee of our institution. All patients gave written consent. The patients were given a single dose of 1,000 mg of meropenem (Zeneca Laboratories, Destelbergen, Belgium) administered intravenously over 3 min. The drug was injected approximately 1 (six patients), 2 (four patients), and from 3 to 5 (four patients) h before the expected time of tissue sampling. Lung, bronchial mucosa, and pleural tissues were sampled simultaneously at the time of extraction of the pulmonary lobe or lung. Blood was collected at the same time as the tissue samples and was centrifuged after clotting. Tissue samples were gently blotted with absorbent paper to remove surface blood and were immediately placed in a small vial with a cap to prevent evaporation of water. Tissues were placed on ice before being weighed. The mean (range) weights of these samples were 0.953 (0.151 to 3.956), 0.024 (0.006 to 0.095), and 0.623 (0.183 to 2.241) g for lung, bronchial mucosa, and pleural tissues, respectively. Tissue samples and serum were stored within 30 min at −70°C until assay. The median variation of weight of the tissues after freezing and thawing compared to the initial weight was 6%. After thawing, each tissue sample was cut in small pieces and crushed in a glass-type tissue grinder with a known amount of buffer solution (pH 6.8). After centrifugation (1,000 × g for 10 min), the supernatant was stored at −70°C until assay. Meropenem concentrations in tissues and serum were determined by bioassay by using a microbiological plate diffusion technique with the indicator strain Klebsiella pneumoniae (ATTC 10031). Standards were diluted in human serum for the serum samples and in phosphate buffer (pH 7.0) for the tissue samples. The lower limits of sensitivity of the assays were 0.01 mg/liter for serum and 0.05 mg/kg for tissues. The interassay variation was less than 10%.
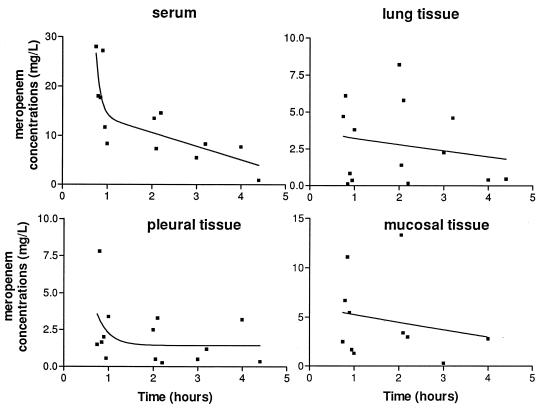
The serum and lung, bronchial mucosa, and pleural tissue meropenem concentrations are shown in Fig. 1. One hour after the single intravenous injection of meropenem, the mean concentration in serum was similar to those observed in other studies in which 1 g was injected over 5 or 30 min (8, 10, 14). Between 1 and 5 h after dosing, the mean concentrations of the drug in lung and bronchial mucosa were in the same range of magnitude. The mean concentrations in pleural tissues appeared somewhat lower. Until 5 h after the injection, the mean concentrations of meropenem in the sampled tissues were at least 1.3 mg/liter. The mean meropenem tissue penetration (i.e., the mean tissue/concomitant serum concentration) according to the delay after injection of meropenem ranged from 0.17 to 0.43, 0.20 to 0.55, and 0.18 to 0.26 for lung, bronchial mucosa, and pleural tissues, respectively.
FIG. 1.
Concentrations of meropenem in serum and lung, bronchial mucosa, and pleural tissues over time and corresponding estimated regression curves.
The penetration of carbapenems in respiratory tissues has been very little studied. In a previous study 1 h after intravenous injection of 1 g of imipenem, the concentration of the drug in human lung tissue was 9.1 mg/liter, and it fell to <1 mg/liter 2 h afterwards (4). To our knowledge, no study of the penetration of these drugs in bronchial mucosa or pleural tissue has been performed. The significance of antibiotic concentration in respiratory tissues has still not been fully assessed as far as the clinical efficacy of the drug is concerned (3, 5). However, for an efficient treatment of lower respiratory tract infections, it seems logical to try to obtain antibiotic levels equal or superior to the MIC for the pathogen in infected tissues or secretions. Recent animal data suggest that pharmacokinetic parameters of the drug in lung tissue could be important in the outcome of the treatment of experimental pneumonia. In the treatment of lung infection in rats, the better cure rates obtained with cefadroxil compared to cefalexin may be due to the higher drug concentration and greater area under the lung tissue concentration-time curve of cefadroxil compared to those of cefalexin (6). In mouse experimental pneumonia, survival rates and lung clearance of the pathogen are greater when the animals are treated with temafloxacin than when they are treated with ofloxacin or ciprofloxacin (2). This result could be related to the greater tissue concentration and longer half-life and maximum residence time over the MBC achieved in the infected lung with temafloxacin than with ciprofloxacin (12). Similarly, lung half-lives of various macrolides have been shown to be predictive of their efficacy in treating experimental respiratory tract infection (1, 13). From these experimental data, it appears that the pharmacokinetics of an antibiotic in pulmonary tissues can be important in the outcome of pulmonary infections. Therefore, such pharmacokinetic studies with humans can be of clinical interest. As far as the treatment of bronchial infections is concerned, the respective roles of sputum and bronchial mucosa antibiotic concentrations are still being debated (5, 7). Histological studies suggest that both sputum and bronchial mucosa may be the sites of infection in acute exacerbations of chronic bronchitis and bronchiectasis (3). Thus, antibiotic concentrations in these two sites might be of clinical importance. To our knowledge, the role of pleural tissue or pleural fluid antibiotic concentration in the treatment of pleural infection has not been investigated. In this study, the meropenem concentrations obtained in respiratory tissues are higher than the MICs at which 90% of isolates are inhibited for most of the sensitive pathogens responsible for lower respiratory tract infections. Moreover, it should be stressed that the whole-tissue antibiotic concentrations represent an average of different tissue compartments. Since the cellular penetration of meropenem, as with other beta-lactam drugs, is poor, the concentrations of the drug in the interstitial fluid of these tissues and in the epithelial lining fluid may be higher than that revealed by the concentration in homogenate tissues (11). The antibacterial spectrum and the penetration of meropenem in the respiratory tract make this drug a suitable agent for the treatment of bronchopulmonary infections.
Acknowledgments
We are grateful to Mr. Patrick Van den Abeele for technical assistance.
This work was supported by a grant from Zeneca, Destelbergen, Belgium.
REFERENCES
- 1.Azoulay-Dupuis E, Vallée E, Bedos J P, Muffat-Joly M, Pocidalo J J. Prophylactic and therapeutic activities of azithromycin in a mouse model of pneumococcal pneumonia. Antimicrob Agents Chemother. 1991;35:1024–1028. doi: 10.1128/aac.35.6.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis E, Bedos J P, Vallée E, Hardy D J, Swanson R N, Pocidalo J J. Antipneumococcal activity of ciprofloxacin, ofloxacin, and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J Infect Dis. 1991;163:319–324. doi: 10.1093/infdis/163.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin D R, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observation and clinical relevance. Antimicrob Agents Chemother. 1992;36:1176–1180. doi: 10.1128/aac.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoni G, Cuzzolin L, Bertrand C, Puchetti V, Velo G. Imipenem kinetics in serum, lung tissue and pericardial fluid in patients undergoing thoracotomy. J Antimicrob Chemother. 1987;20:725–728. doi: 10.1093/jac/20.5.725. [DOI] [PubMed] [Google Scholar]
- 5.Bergogne-Bérézin E. Predicting the efficacy of antimicrobial agents in respiratory infections—is tissue concentration a valid measure? J Antimicrob Chemother. 1995;35:363–371. doi: 10.1093/jac/35.3.363. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm D R, DeRegis R G, Behr D A. Therapeutic efficacy of cefadroxil and cephalexin for pneumonia in a rat test model. Antimicrob Agents Chemother. 1986;30:105–109. doi: 10.1128/aac.30.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies B I, Maesen F P V, Gubbelmans R. Azithromycin (CP-62,993) in acute exacerbations of chronic bronchitis: an open clinical, microbiological and pharmacokinetic study. J Antimicrob Chemother. 1989;23:743–751. doi: 10.1093/jac/23.5.743. [DOI] [PubMed] [Google Scholar]
- 8.Drusano G L, Hutchison M. The pharmacokinetics of meropenem. Scand J Infect Dis Suppl. 1995;96:11–16. [PubMed] [Google Scholar]
- 9.Edwards J R. Meropenem: a microbiological overview. J Antimicrob Chemother. 1995;36(Suppl. A):1–17. doi: 10.1093/jac/36.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 10.Kelly H C, Hutchison M, Haworth S J. A comparison of the pharmacokinetics of meropenem after administration by intravenous injection over 5 min and intravenous infusion over 30 min. J Antimicrob Chemother. 1995;36(Suppl. A):35–41. doi: 10.1093/jac/36.suppl_a.35. [DOI] [PubMed] [Google Scholar]
- 11.Nix D E, Goodwin S D, Peloquin C A, Rotella D L, Schentag J J. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother. 1991;35:1953–1959. doi: 10.1128/aac.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallée E, Azoulay-Dupuis E, Pocidalo J J, Bergogne-Bérézin E. Pharmacokinetics of four fluoroquinolones in an animal model of infected lung. J Antimicrob Chemother. 1991;28:39–44. doi: 10.1093/jac/28.suppl_c.39. [DOI] [PubMed] [Google Scholar]
- 13.Veber B, Vallée E, Desmont J M, Pocidalo J J, Azoulay-Dupuis E. Correlation between macrolide lung pharmacokinetics and therapeutic efficacy in a mouse model of pneumococcal pneumonia. J Antimicrob Chemother. 1993;32:473–482. doi: 10.1093/jac/32.3.473. [DOI] [PubMed] [Google Scholar]
- 14.Wise R, Logan M, Cooper M, Ashby J P, Andrews J M. Meropenem pharmacokinetics and penetration into an inflammatory exudate. Antimicrob Agents Chemother. 1990;34:1515–1517. doi: 10.1128/aac.34.8.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]