Abstract
Introduction
Age‐related neuropathology associated with sporadic Alzheimer's disease (AD) often develops well before the onset of symptoms. Given AD's long preclinical period, translational models are needed to identify early signatures of pathological decline.
Methods
Using structural magnetic resonance imaging and cognitive assessments, we examined the relationships among age, cognitive performance, and neuroanatomy in 48 vervet monkeys (Chlorocebus aethiops sabaeus) ranging from young adults to very old.
Results
We found negative associations of age with cortical gray matter volume (P = .003) and the temporal‐parietal cortical thickness meta‐region of interest (P = .001). Additionally, cortical gray matter volumes predicted working memory at approximately 1‐year follow‐up (correct trials at the 20s delay [P = .008]; correct responses after longer delays [P = .004]).
Discussion
Cortical gray matter diminishes with age in vervets in regions relevant to AD, which may increase risk of cognitive impairment. This study lays the groundwork for future investigations to test therapeutics to delay or slow pathological decline.
Keywords: Alzheimer's disease, magnetic resonance imaging, non‐human primate, vervet, working memory
1. BACKGROUND
Alzheimer's disease (AD) impacts >5 million adults in the United States, is the sixth leading cause of death, and has no cure. 1 The late‐onset (or sporadic) form of AD, which constitutes >95% of cases, is characterized by a decades‐long preclinical period in which patients accumulate neuropathologies but exhibit little‐to‐no cognitive deficits. 2 , 3 Exacerbating the difficulty for early detection and treatment even further, there is considerable heterogeneity in the presentation of AD‐related pathologies. 4 , 5 Recent efforts to delay the onset of cognitive impairment have focused on lifestyle interventions to treat conditions known to increase AD risk including type 2 diabetes, cardiovascular disease, depression, physical inactivity, sleep disturbance, smoking, social isolation, and Western diet. 6 , 7 While such programs show promise in extending individuals’ cognitive health spans, advances have been modest. 8 These results suggest that the development of effective clinical interventions requires a deeper understanding of the complex etiology of AD and full characterization of the transition from healthy to pathological aging.
Neuroimaging approaches have advanced efforts to characterize the pathophysiological mechanisms of AD 9 and have demonstrated functional and structural imaging patterns distinguishing healthy from pathological aging. For example, fluorodeoxyglucose–positron emission tomography (FDG‐PET) has been instrumental in detecting early changes in cerebral glucose metabolism that may reflect shifts in neuronal activity, whereas diffusion tensor imaging (DTI) has been used to determine microscopic changes underlying shifts in white matter integrity. 10 , 11 Structural magnetic resonance imaging (MRI) has been very helpful in examining age‐ and AD‐related changes in brain volumes 12 , 13 , 14 , 15 , 16 and relationships with AD‐related cognitive decline. Thus, structural MRI is a useful instrument for differentiating healthy versus AD‐related neuropathology across the life course.
However, key questions remain regarding the progression of AD‐like neuropathology, particularly during middle age. One factor contributing to this gap in knowledge may be the timing of many AD studies, as neuroimaging begins relatively later in life (after 50 years of age) in many clinical cohorts and may fail to capture the earliest stages of disease (e.g., Alzheimer's Disease Neuroimaging Initiative [ADNI] and Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability [FINGER]), but see Lupton et al. 17 Furthermore, repeated scans over long periods of time (10–20 years) are needed to fully characterize brain changes from healthy to neuropathology across the life course. While necessary for a full account of the disease course, such longitudinal cohort studies may extend beyond the time and resources of many funding mechanisms.
RESEARCH IN CONTEXT
Systematic review: The authors conducted a comprehensive review of the literature using resources such as PubMed and Google Scholar. Mounting neuropathological, physiological, and behavioral evidence suggests that vervet monkeys (a.k.a. African green monkeys [Chlorocebus aethiops sabaeus]) are useful models to study human aging and early Alzheimer's disease (AD) pathogenesis.
Interpretation: Our findings indicate that vervet monkeys exhibit aging‐related reductions in brain structures relevant to AD, and these patterns may represent a risk factor for cognitive impairment. This work also suggests that the vervet monkey is a relevant model in which to test early interventions on cognitive decline and AD‐like neuropathogenesis.
Future directions: This work may help future studies identify pathways through which some individuals evade pathological aging trajectories. Ultimately, such knowledge may improve efforts toward early identification of abnormal patterns of neurodegeneration and improve the ability to detect, track, and treat neuropathology.
Non‐human primates (NHPs) offer excellent opportunities in which to study the transitions from healthy to pathological brain aging. 18 , 19 , 20 , 21 NHPs are well established models of brain aging due to the many physiological, social, and anatomical characteristics shared with humans. 20 , 22 , 23 , 24 Accumulating data suggest that NHPs undergo reductions in brain volume reminiscent of human aging, 19 , 25 , 26 , 27 , 28 and several NHP models of AD indicate that elderly animals develop early AD‐like neuropathologies, including the accumulation of amyloid beta (Aβ) plaques and hyperphosphorylated tau. 29 Diminished cognitive abilities with aging have been demonstrated in several species, including marmosets, 30 , 31 rhesus macaques, 32 , 33 and chimpanzees. 34 , 35 Vervet monkeys (a.k.a. African green monkeys [Chlorocebus aethiops sabaeus]) represent another important model for aging and AD. 36 Members of this species exhibit myriad characteristics reminiscent of human aging and early AD, including age‐related cognitive impairment (diminished working memory and executive function), accumulation of cerebral Aβ deposits and hyperphosphorylated tau, decreased cerebrospinal fluid (CSF) Aβ1‐42 and Aβ1‐40, gait speed, and brain volumes with increasing Aβ burden, and cerebral glucose hypometabolism. 19 , 25 , 37 , 38 Thus, vervets are useful models in which to determine the relationships between brain volumes and cognitive performance from middle to very old age.
We previously reported coincident cognitive and physical impairment (slowing gait) associated with aging in a cohort of 30 middle to older aged female vervet monkeys (ages 8–29 years). 38 We found that physical and cognitive performance declined with age and that poorer cognitive performance was associated with slower gait, a well‐established translational biomarker of physical decline, morbidity, and mortality. 39 Here we investigated the relationships among age, working memory, and neuroanatomy. To maximize power to detect effects, we combined data from this aging cohort with data from a previous cross‐sectional study of age‐matched female monkeys from the same colony (see Methods). Our primary objective was to determine whether age‐related neuroanatomical patterns were similar to those observed in human aging and AD. Our secondary objective was to determine whether these global and regional cortical structures predicted cognitive performance at 1‐year follow‐up, a time that approximates 3 to 4 years in humans. 20 We found support for our hypotheses that age is negatively associated with changes in gray matter structures, like human aging, and that cortical gray matter volumes predicted working memory at 1‐year follow‐up. Taken together, this work suggests the vervet monkey is a relevant model in which to test early interventions on cognitive decline and AD‐like neuropathogenesis.
2. METHODS
2.1. Subjects and husbandry
To generate our complete sample (n = 48), we combined data from a previous cross‐sectional (N = 18; 8–23 years of age) and ongoing longitudinal (N = 30; 8–29 years of age) study of aging‐related phenotypes in socially housed female vervet monkeys (Chlorocebus aethiops sabaeus). These NHPs were born in the Vervet Research Colony (VRC) at the Wake Forest School of Medicine and were raised under a common husbandry protocol. The VRC includes approximately 300 individuals, housed in 16 social groups that recapitulate the natural social organization of free‐ranging vervets. All animals receive year‐round enrichment opportunities and are provided with water and LabDiet Monkey Chow ad libitum. 25 , 38 , 40 The selected age range corresponds roughly to early middle age to very old age in humans, a critical window for development of AD pathophysiology. 41 Sample and subject characteristics are shown in Table 1. All protocols described herein complied with state and federal regulations and with the approval of the Wake Forest School of Medicine Institutional Animal Care and Use Committee.
TABLE 1.
Sample characteristics
| N | Average age (years) | |||||
|---|---|---|---|---|---|---|
| Age class | Cross‐sectional cohort | Longitudinal cohort | Entire sample | Cross‐sectional cohort | Longitudinal cohort | Entire sample |
| 8–16 years | 8 | 10 | 18 | 10.58 | 11.73 | 11.27 |
| > 16 Years | 10 | 20 | 30 | 22.05 | 23.04 | 22.88 |
2.2. Neuroimage acquisitions
We conducted structural MRI to determine global brain volumes and temporoparietal cortical thicknesses relevant to human AD. For each scan, we transported animals from the VRC to the MRI Imaging Center at Wake Forest School of Medicine. At the MRI Imaging Center, monkeys were first sedated (ketamine HCl, 10–15 mg/kg body weight), and then anesthesia was maintained by isoflurane (3% induction, 1.5% maintenance) during the scanning procedure. We used a 3T Siemens Skyra scanner (Siemens) and a 3D volumetric magnetization‐prepared rapid gradient echo sequence (TR = 2700 ms; TE = 3.39 ms; TI = 880 ms; Flip Angle = 8°; 160 slices, voxel dimension = 0.5 × 0.5 × 0.5 mm3) to acquire scans. Given improvements in scanning technology over time, three head coils were used over the course of data collection, including two 32‐channel coils (longitudinal cohort) and an 8‐channel coil (earlier cross‐sectional cohort; Litzcage, Doty Scientific). Because we were interested in the anatomical segmentation of gray and white matter structures rather than functional measures, we did not expect that different head coils would produce different volumetric segmentations (personal communication Megan Lipford, WFSM).
2.3. Neuroimage processing
For brain extraction (“skull‐stripping”), we used a U‐net model specialized for NHPs 42 on the native‐spaced T1‐weighted images. We then rigidly aligned each native brain image to the INIA19 brain template 43 using the antsaffine.sh script provided by the Advanced Normalization Tools (ANTs, Penn Image Computing and Science Laboratory, University of Pennsylvania). Next, we denoised the aligned T1‐weighted images using the ANTs tool, DenoiseImage. For consistent analysis across projects, we created a study‐specific template (SST) using 79 of the aligned and denoised brain images using ANTs antsMultivariateTemplateConstruction.sh. We enabled bias field correction on each input image during the template creation process via ANTs N4BiasFieldCorrection. Next, we registered the INIA19 brain template, the UNC Template Brain, 44 and the NIMH Macaque Template (NMT version 2) 0.5 mm template brain 45 , 46 to the SST brain using ANTs nonlinear registration tool (antsRegistrationSyN.sh). We applied the transformations created during registration to the appropriate parcellation maps and tissue priors using the ANTs tool antsApplyTransforms. After parcellation, we used direct non‐linear registration of each of the rigidly aligned brains to the template brain using ANTs’ non‐linear registration tool, antsRegistrationSyN.sh. These transforms were created by the direct registration of the aligned native image to the template. Finally, we inversely transformed the Neuromaps parcellation map, UNC Cortical and Subcortical parcellation maps, and tissue priors to the realigned native brains.
We then used the ANTs segmentation tool (antsAtroposN4.sh) with the warped tissue priors (spatial weighting = 0.20) to generate labels for global brain volumes, including total gray matter (GM), cortical matter (cGM), deep gray matter (dGM), white matter (WM), and cerebrospinal fluid (CSF). The cortical GM region of interest (ROI) represents GM associated with the prefrontal, frontal, parietal, insular, cingulate, temporal, and occipital lobes. 44 The deep GM ROI represents GM associated with the hippocampus, amygdala, caudate, putamen, thalamus, hypothalamus, and cerebellum. The total GM was calculated as the sum of the cortical and deep GM ROIs. We also generated ROIs for total brain volume (TBV = GM + WM) and intracranial volume (ICV = GM + WM + CSF).
Cortical thickness maps were created by providing each cortical GM and WM tissue segmentation maps to the ANTs tool for a registration‐based estimate of cortical thickness, KellyKapowski. The thickness prior estimate was set to a vervet‐appropriate 1.5 mm. For each ROI, we warped the Neuromaps parcellation map (which had been transformed from the SST to the aligned native images) to generate the cortical thickness maps. The full parcellation consisted of separate definitions for the left and right hemisphere for the following cortical thicknesses: angular gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus, precuneus, fusiform gyrus, supramarginal gyrus, entorhinal area, and parahippocampal gyrus (Figure 1). In addition to the individual cortical thickness ROIs, we generated a temporal‐parietal thickness meta ROI. This meta ROI was a volume‐weighted average of the thicknesses of the individual ROIs, which allowed us to control for overall size of individual regions.
FIGURE 1.
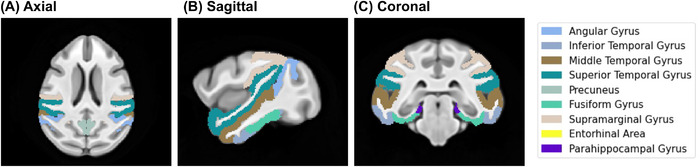
(A) Axial, (B) sagittal, and (C) coronal views of cortical thickness parcellation for temporo‐parietal regions of interest relevant to Alzheimer's disease. The parcellations consisted of separate definitions for the left and right hemisphere for the following cortical thicknesses regions of interest (ROIs): angular gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus, precuneus, fusiform gyrus, supramarginal gyrus, entorhinal area, and parahippocampal gyrus
2.4. Cognitive performance
We evaluated cognitive performance in a subset (N = 30) of our sample using two previously validated assessments—the Wake Forest Maze Task of executive function (WFMT) and the Delayed Response Task of working memory (DR Task). 38 The WFMT is a modified NHP puzzle feeder device (Primate Products), which consists of a series of increasingly difficult mazes through which the test subject must navigate a food reward. The WFMT consists of 38 maze configurations, half of which were mirror images to control for interindividual variation in directional preference. Easier mazes required the test subject to move the food reward horizontally, whereas more difficult mazes required both horizontal and vertical maneuvering of the food reward. After acclimation, each monkey was tested over the course of 5 days (30 minutes per day). Performance in the WFMT was assessed by the total number of puzzle levels solved by the end of the 5‐day testing period. For full description of the WFMT protocol and maze configurations, see Frye et al. 38
The DR Task is a modified Wisconsin General Test Apparatus, 47 and has been widely used to assess working memory in NHPs. 48 , 49 , 50 , 51 , 52 , 53 In this test, a food reward is randomly placed in one of the three opaque boxes that can be seen, but not accessed, by the animal. Next, after a fixed delay of time, the test subject is allowed to select one of the three boxes. Selecting the baited box constitutes a correct choice, whereas selecting an empty box constitutes an incorrect choice. The delays of time increase during the testing protocol—from 2.5 to 20 seconds—with longer delays indicating better working memory. We also tested animals at 30‐, 40‐, and 60‐second delays. Advancement from 20‐second to longer delays was performance‐dependent. That is, individuals were required to perform better than chance to advance to these three longest delays. Performance in the DR Task was assessed by the number of correct trials at the 20‐second delay (i.e., the longest delay all monkeys observed) as well as the longest delay achieved in the DR Task.
2.5. Statistical analyses
To determine the effects of age on global brain volumes and cortical thicknesses relevant to AD, we used general linear mixed models (GLMMs) with age and project (cross‐sectional or longitudinal) as fixed effects. We included individual ID as a random effect to account for non‐independence of data from repeated MRI scans. For all models examining cortical thickness, we included a fixed effect for the side of the brain. All models for volumetric ROIs were adjusted for interindividual variation in head size. We did not include a control variable for analyses of cortical thicknesses because this measure is independent of head size. 54 Relationships of age with brain volumes were assessed across the entire sample (8–29 years of age [yoa]) as well as within the middle‐ (8–16 yoa; N = 18) and older‐ (> 16 yoa; N = 30) aged subsets.
We next determined relationships between global brain volumes and cognitive performance. First, we assessed whether baseline ROIs predicted cognitive performance at 1‐year follow‐up via general linear models. This length of time equates to approximately a 3‐ to 4‐year follow‐up in humans. 20 We then included age as a fixed effect to control for age effects on cognitive performance. We designated statistical significance at P < .05.
3. RESULTS
3.1. Age effects on global brain volumes
Across the entire sample, age was negatively associated with GM volumes—total (β = –108.4, standard error [SE] = 25.17, t = –4.306, P < .001), cortical (β = –73.14, SE = 23.60; t = –3.100; P = .003), and deep (β = –29.26, SE = 7.81, t = –3.749, P < .001)—and was positively associated with CSF volumes (β = 74.42, SE = 25.16; t = 2.96; P = .005; Figure 2; Table 2). WM showed no significant relationship with age (β = 41.07, SE = 20.74, t = 1.98, P = .052). Among the older aged> subjects > 16 yoa), age was negatively associated with intracranial (β = –565.5, SE = –196.6, t = –2.876, P = .006), total brain (β = –425.5, SE = –145.1, t = –2.932, P = .005), and deep gray matter volumes (β = –57.93, SE = 17.38, t = –3.334, P = .002) in the younger‐aged animals.
FIGURE 2.
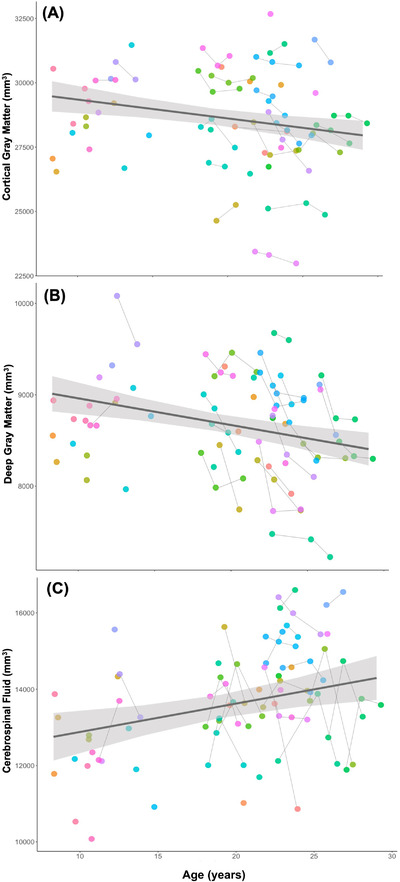
Age effects on (A) cortical gray matter, (B) deep gray matter, and (C) CSF volumes. Generalized linear mixed models showed that age was negatively associated with (A) cortical (β = –73.14, standard error [SE] = 23.60; t = –3.100; P = .003) and (B) deep gray matter volumes (β = –29.26, SE = 7.81, t = –3.749, P < .001). (C) There was a positive association between cerebrospinal fluid (CSF) volumes and age; CSF volumes were larger in older‐aged animals (β = 74.42, SE = 25.16; t = 2.96; P = .005). Each subject is represented by a distinct color, and the gray connecting lines indicate repeated scans
TABLE 2.
Age effects on global brain volumes and AD‐relevant temporal‐parietal regions of interest
| Effects of age (P values) | ||||
|---|---|---|---|---|
| ROI | ROI type | Middle‐aged (8–16 yoa) | Older‐aged (> 16 yoa) | Entire sample (8–29 yoa) |
| Intracranial volume | Volume | .243 | .006 | .945 |
| Total brain volume | Volume | .201 | .005 | .407 |
| Total gray matter | Volume | .412 | .047 | <.001 |
| Cortical gray matter | Volume | .412 | .356 | .003 |
| Deep gray matter | Volume | .857 | .002 | <.001 |
| White matter | Volume | .064 | .098 | .052 |
| Cerebrospinal fluid | Volume | .331 | .438 | .005 |
| Angular gyrus | Thickness | .939 | .033 | .103 |
| Inferior temporal gyrus | Thickness | .220 | <.001 | .007 |
| Middle temporal gyrus | Thickness | .557 | .005 | .273 |
| Superior temporal gyrus | Thickness | .761 | <.001 | <.001 |
| Precuneus | Thickness | .917 | .786 | .014 |
| Fusiform gyrus | Thickness | .445 | <.001 | <.001 |
| Supramarginal gyrus | Thickness | .802 | .803 | .074 |
| Entorhinal cortex | Thickness | .337 | .178 | .876 |
| Parahippocampus | Thickness | .562 | .012 | .194 |
| Temporal‐parietal meta‐ROI | Thickness | .436 | <.001 | .001 |
P values relate to main effects of age. P values < .05 is shown in bold.
Abbreviations: AD, Alzheimer's disease; ROI, region of interest.
3.2. Age effects on cortical thickness
Across the entire sample, age was negatively associated with several individual cortical thickness ROIs relevant to AD, including the inferior (β = –0.005, SE = 0.001, t = –2.815, P = .007) and superior (β = –0.005, SE = 0.001, t = –5.027, P < .001) temporal gyri, precuneus (β = –0.004, SE = 0.002, t = –2.524, P = .014), and fusiform gyrus (β = –0.008, SE = 0.002, t = –5.445, P < .001). Among the older aged subset, age was negatively associated with the following thicknesses: angular gyrus (β = –0.006, SE = 0.003, t = –2.163, P = .033), inferior (β = –0.020, SE = 0.003, t = –6.062, P < .001), middle (β = –0.006, SE = 0.002, t = –2.860, P = .005), and superior (β = –0.008, SE = 0.002, t = –4.365, P < .001) temporal gyri, fusiform gyrus (β = –0.017, SE = 0.003, t = –5.291, P < .001), and parahippocampus (β = –0.016, SE = 0.006, t = –2.592, P = .012; Table 2). There were no significant relationships between age and cortical thicknesses in the middle‐aged animals (P’s > .220). The temporal‐parietal thickness meta ROI was also negatively associated with age in both the whole sample (β = –2.983, SE = 0.918, t = –3.250, P = .001) and older‐aged subset (β = –5.784, SE = 1.151, t = –5.028, P < .001).
3.3. Temporal associations between brain structures and cognitive performance
WFMT Test: After controlling for the effects of age, none of the global brain volumes—ICV, TBV, GM, cGM, dGM, WM, nor CSF—significantly predicted performance in the WFMT at 1 year follow‐up (P’s > .447).
DR Task: Total GM predicted performance in the DR task 1 year later. Larger GM volumes predicted more correct trials at the 20‐second delay (β = 0.002, SE = 0.001, t = 2.618, P = .016), and correct responses after longer (more difficult) delays (β = 0.006, SE = 0.002, t = 2.668, P = .014). Of the two GM volumetric ROIs (cGM and dGM), only cGM volume was a significant predictor of these measures of working memory: more correct trials at the 20‐second delay (β = 0.002, SE < 0.001, t = 2.917, P = .008) and correct responses after longer (more difficult) delays (β = 0.007, SE = 0.002, t = 3.274, P = .004; Figure 3). In contrast, dGM volumes were not significant predictors of working memory (number of correct trials at 20s: β = –0.001, SE = 0.002, t = –0.353, P = .727; longest delay achieved: β = –0.010, SE = 0.009, t = –1.019, P = .320). WM volumes tended to be negatively associated with follow‐up DR Task performance; however, these relationships did not reach statistical significance (number of correct trials at 20 seconds: β = –0.002, SE = 0.001, t = –2.047, P = .053; longest delay achieved: β = –0.006, SE = 0.003, t = –1.689, P = .106). No individual cortical thickness ROI nor the temporal‐parietal thickness meta ROI predicted any cognitive performance metrics at 1‐year follow‐up (all P’s > .05).
FIGURE 3.
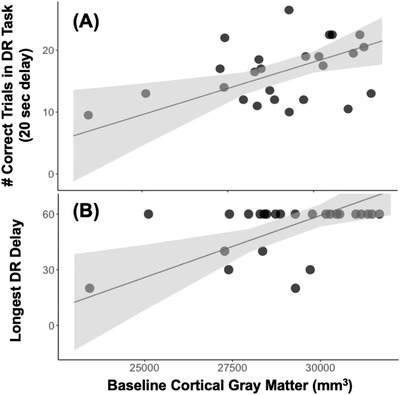
Temporal associations between cortical gray matter and working memory. Cortical gray matter (GM) was positively associated with performance in the delayed response (DR) task of working memory, with larger volumes being associated with (A) more correct trials at the 20‐second delay (β = 0.002, standard error [SE] = 0.001, t = 2.618, P = .016) and (B) advancement to the longer (i.e., more difficult) delays (β = 0.006, SE = 0.002, t = 2.668, P = .014). Individual points represent subjects
4. DISCUSSION
Cortical and deep GM volumes were negatively associated with age in vervet monkeys ranging from middle‐ to advanced age. We also observed negative relationships of age and intracranial and total brain volumes; these effects were only present in a subset of older‐aged animals (> 16 years old). Inverse relationships between age and cortical thicknesses were also observed in several temporal and parietal ROIs relevant to AD, and this relationship was maintained for the composite temporal‐parietal thickness meta ROI. In contrast to these negative relationships, CSF volumes were positively associated with age, while WM volumes were unrelated to age. Last, in addition to these age‐structure relationships, cGM volumes predicted performance in a working memory task, but not executive function, at approximately 1‐year follow‐up. Taken together, our findings demonstrate that vervet monkeys experience aging‐related declines in GM regions relevant to human AD, and such reductions in GM may predict cognitive impairment in domains associated with memory.
The age‐associated patterns we observed with regard to GM (negative associations) and CSF (positive associations) are consistent with GM atrophy and expansion of CSF‐filled cavities. These findings suggest that vervet monkeys may experience aging‐related changes to brain structures that are largely consistent with patterns observed in human aging. 55 , 56 , 57 , 58 Such volumetric reductions in the composite temporal‐parietal thickness meta ROI may be indicative of AD‐like pathophysiology. In humans, atrophy in the medial temporal lobe is a prominent feature of AD, 59 , 60 , 61 and early volumetric losses predict the development of the disease. 62 , 63 , 64 Of particular interest may be the age‐related loss of cortical thickness in the precuneus. Recent work has shown that diminished connectivity with the precuneus may distinguish healthy age‐matched controls from patients with AD. 65 Furthermore, early structural changes in the precuneus have been shown to predict progression to AD. 66 While additional longitudinal assessments will be needed to distinguish normal from pathological trajectories of volumetric decline, particularly in those changes associated with AD‐relevant ROIs, our findings bolster the utility of the vervet monkey as a model of aging and early AD‐like pathophysiology. 25
Upon inspection of middle‐ (< 16 years) and older‐aged (> 16 years) animals as distinct groupings, we discovered that many of these age‐dependent associations were present in the older‐aged individuals, but not in their younger counterparts. This difference across age groups may indicate that aging‐related declines in brain volumes may not become apparent until about 16 years of age in vervet monkeys, an age that equates to approximately 56‐ to 64‐year‐old humans. 20 Increasing evidence from clinical studies demonstrates that this age range represents a critical period in AD progression. Namely, individuals may begin accumulating signatures of AD pathology—for example, Aβ accumulation, cerebral hypometabolism, volumetric reductions in GM—during this time while remaining essentially cognitively normal. 3 Given the similarities between vervet and human aging, 19 the years preceding this age range—that is, before structural changes occur—might represent an intervention window during which therapies could be directed to delay or slow the rate of loss of GM structures and mitigate the risk for AD‐like neurodegeneration and cognitive impairment.
After controlling for age effects, diminished cGM in aging vervets predicted worse cognitive performance—working memory—at 1‐year follow‐up. Mounting evidence from longitudinal clinical studies suggests that accelerated atrophy in cGM in aging humans is associated with increased risk of developing mild cognitive impairment and clinical AD. 16 , 67 Such early structural changes may lead to a disruption or disorganization of neural networks supporting cognitive performance, 68 , 69 particularly declines in memory. 70 Previous studies in NHP models of AD have also demonstrated associations between GM volumes and cognitive performance. For example, a recent cross‐sectional neuroimaging study demonstrated that elderly chimpanzees with smaller GM volumes showed worse cognitive performance than their age‐matched counterparts with more GM. 27 Rhesus macaques, another important NHP model of aging and AD, 71 , 72 also show this relationship. 73 These results suggest that the preservation of GM may promote better working memory with aging in NHPs and humans alike. Future studies are needed to determine whether the rate of GM loss impacts the severity of cognitive decline as well as the mechanistic underpinnings of volumetric declines.
In contrast to our findings in the GM volumes, we did not observe significant effects of age on WM volumes, a pattern that differs from human aging. 55 These results add to a growing literature demonstrating that NHPs may differ from humans with respect to age‐related changes in WM, and these differences may well depend on sex. For example, Andersen et al. 28 determined that WM volumes increased with age in a sample of 31 female rhesus macaques. In chimpanzees, mixed‐sex samples have demonstrated that WM volumes remain stable with age. 74 , 75 Others have discovered negative associations between age and WM volumes in marmosets and macaques. 76 , 77 However, these significant patterns of decline have been demonstrated in either all‐male 77 or mixed‐sex cohorts. 76 In their survey of all‐female cohorts of rhesus macaques and chimpanzees, Chen et al. 26 reported no age‐dependent relationships in the WM volumes in macaques and a non‐significant negative association in chimpanzees (P = .08), with WM declines manifesting relatively later in the life than those observed in humans (using a scaling factor of 1.5 years in a chimpanzee's life to equate to 1 year of human life). Our sample consisted entirely of female monkeys; thus, a lack of WM reductions with age is consistent with previous reports in female macaques and chimpanzees, which share closer evolutionary relationships with humans than do marmosets.
One factor that may underlie the human–NHP difference is the relative timing of menopause. 20 In women, menopause occurs around the age of 51, after approximately 42% of the lifespan (maximum age = 122.5 years). 20 , 78 , 79 In contrast, menopause has not been observed in chimpanzees in the wild, 80 and captive females experience menopause near 50 years of age 81 after approximately 74% of max lifespan (maximum reported age = 68 years 82 ). Female macaques and vervets, who have menstrual cycles of similar length and hormonal fluctuations as women, also appear to undergo menopause later in life and closer to the time of natural death. Rhesus macaques undergo menopause around 27 yoa (maximum age = 40 [after 58% of maximum lifespan]), and vervet menopause occurs around the age of 23 (maximum lifespan = 30.8 [after 74% of maximum lifespan]). 40 , 78 , 83 , 84 , 85 , 86 In humans, menopause increases risk of AD. 87 , 88 Given that sex hormones, particularly estrogen, may affect WM integrity, 89 , 90 , 91 cognition, 92 and neurodegenerative disease risk, 93 , 94 differences in the relative timing of menopause may underlie human and NHP differences in age‐related patterns of WM degeneration.
The lack of WM volumetric decreases with age may underlie the lack of structural associations with executive function. Previously, we reported declines in executive function with age in the 30 female vervets in the ongoing longitudinal study (Frye et al. 38 ). In human studies, recent work suggests that executive function is associated with WM integrity, rather than volume. 95 , 96 In ongoing work, we are actively investigating WM integrity in association with cognitive function using DTI in these aging vervets.
There were some limitations of this study that should be considered. First, while we combined two datasets for the present work, our study may have been underpowered to detect small effects, particularly those associated with structure–function relationships. Combining datasets may also have contributed to variation in our sample. Given this potential confound, we controlled for interproject differences in our statistical analyses. Additionally, the animals from the cross‐sectional and longitudinal cohorts were raised under nearly identical husbandry regimes; therefore, we do not expect members of the same species, from the same breeding colony, from the same institution to exhibit differences in brain aging. In addition, the longitudinal portion of the study is ongoing. Repeated MRI scans and follow‐up cognitive assessments are planned for this longitudinal cohort and will be used to examine the temporal relationships more fully between structural brain changes and cognitive performance. Another limitation of this study is the absence of age‐matched males. A paucity of aging NHP males poses significant challenges to translational studies of sex differences in NHP aging and neurodegenerative disorders in the United States. 20 Studies are underway with DTI and functional imaging modalities in vervets that will shed light on relationships among age, microstructural and functional brain changes, and cognitive performance. In addition, the data reported here will be used to examine the relationships between brain structure, other AD biomarkers (e.g., CSF levels of Aβ1‐40, Aβ1‐42, phosphorylated tau181), and post mortem histopathology after animals reach natural end of life. Ultimately, this comprehensive, longitudinal approach will bolster our ability to distinguish healthy from pathological aging.
In conclusion, we have shown that vervet monkeys—an important NHP model of AD—exhibit aging‐related reductions in brain structures relevant to AD. In addition to recapitulating some of the large‐scale patterns typical of human brain aging, we also have shown that reduced GM volumes may represent a risk factor for cognitive impairment. In the future, this work may identify pathways through which some individuals evade pathological aging trajectories. Ultimately, such knowledge may improve efforts toward early identification of abnormal patterns of neurodegeneration and improve the ability to detect, track, and treat neuropathology. This work also suggests the vervet monkey is a relevant model in which to test early interventions on cognitive decline and AD‐like neuropathogenesis.
CONFLICTS OF INTEREST
THe authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the students, staff, and faculty at the Wake Forest School of Medicine who supported these studies and care for the vervets. This work was supported by National Institutes of Health (NIH) P30 AG049638, T32AG033534, P40‐OD010965, P30 AG021332, R24AG073199, UL1TR001420, and the Department of Pathology, Wake Forest School of Medicine.
Frye BM, Craft S, Register TC, et al. Early Alzheimer's disease‐like reductions in gray matter and cognitive function with aging in nonhuman primates. Alzheimer's Dement. 2022;8:e12284. 10.1002/trc2.12284
REFERENCES
- 1. 2020 Alzheimer's Disease Facts and Figures. Alzheimers Dement 2020;16:391+. [DOI] [PubMed] [Google Scholar]
- 2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritchie K, Ritchie CW, Yaffe K, Skoog I, Scarmeas N. Is late‐onset Alzheimer's disease really a disease of midlife?. Alzheimers Dement. 2015;1:122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira D, Verhagen C, Hernandez‐Cabrera JA, et al. Heterogeneity within Alzheimer's disease. Aging. 2017;10:3058‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devi G, Scheltens P. Heterogeneity of Alzheimer's disease: consequence for drug trials?. Alzheimers Res Ther. 2018;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edwards GA III, Gamez N, Escobedo G Jr, Calderon O, Moreno‐Gonzalez I. Modifiable risk factors for Alzheimer's disease. Front Aging Neurosci. 2019;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurol. 2014;13:788‐794. [DOI] [PubMed] [Google Scholar]
- 8. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653‐666. [DOI] [PubMed] [Google Scholar]
- 9. Marquez F, Yassa MA. Neuroimaging biomarkers for Alzheimer's disease. Mol Neurodegener. 2019;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer FU, Wolf D, Scheurich A, Fellgiebel A, Alzheimer's Disease Neuroimaging, I . Altered whole‐brain white matter networks in preclinical Alzheimer's disease. Neuroimage Clin. 2015;8:660‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubicki M, Baxi M, Pasternak O, et al. Lifespan trajectories of white matter changes in rhesus monkeys. Cereb Cortex. 2019;29:1584‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer's disease, and reserve. JAMA. 2008;65:113‐120. [DOI] [PubMed] [Google Scholar]
- 13. Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longituindal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989‐994. [DOI] [PubMed] [Google Scholar]
- 14. Blinkouskaya Y, Weickenmeier J. Brain shape changes associated with cerebral atrophy in healthy aging and Alzheimer's disease. Front Mech Eng. 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coupe P, Manjon JV, Lanuza E, Catheline G. Lifespan changes of the human brain In Alzheimer's disease. Sci Rep. 2019;9:3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eskildsen SF, Coupe P, Garcia‐Lorenzo D, Fonov V, Pruessner JC, Collins DL, Alzheimer's Disease Neuroimaging, I . Prediction of Alzheimer's disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. Neuroimage. 2013;65:511‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lupton MK, Robinson GA, Adam RJ, et al. A prospective cohort study of prodromal Alzheimer's disease: prospective Imaging Study of Ageing: genes, Brain and Behaviour (PISA). Neuroimage Clin. 2021;29:102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Upright NA, Baxter MG. Prefrontal cortex and cognitive aging in macaque monkeys. Am J Primatol. 2021:e23250. [DOI] [PubMed] [Google Scholar]
- 19. Frye BM, Craft S, Latimer CS, Keene CD, et al. Aging‐related Alzheimer's disease‐like neuropathology and functional decline in captive vervet monkeys (Chlorocebus aethiops sabaeus). Am J Primatol. 2021:e23260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shively CA, Lacreuse A, Frye BM, Rothwell ES, Moro M. Nonhuman primates at the intersection of aging biology, chronic disease, and health: an introduction to the American journal of primatology special issue on aging, cognitive decline, and neuropathology in nonhuman primates. Am J Primatol. 2021:e23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacreuse A, Raz N, Schmidtke D, Hopkins WD, Herndon JG. Age‐related decline in executive function as a hallmark of cognitive ageing in primates: an overview of cognitive and neurobiological studies. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verdier JM, Acquatella I, Lautier C, et al. Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Front Neurosci. 2015;9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baxter MG. Age‐Related Effects on Prefrontal Cortical Systems: translating Between Rodents, Primates, and Humans. In: Bizon JL and Woods AG, eds. Animal Models of Human Cognitive Aging. Humana Press; 2009:59‐72. [Google Scholar]
- 24. Lacreuse A, Herdon JG. Nonhuman Primat Moddels of Cognitive Aging. In: Bizon JL and Woods AG, eds. Animal Models of Human Cognitive Aging. Humana Press; 2009:29‐58. [Google Scholar]
- 25. Latimer CS, Shively CA, Keene CD, et al. A nonhuman primate model of early Alzheimer's disease pathologic change: implications for disease pathogenesis. Alzheimers Dement. 2019;15:93‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X, Errangi B, Li L, et al. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro‐ and microstructural changes. Neurobiol Aging. 2013;34:2248‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulholland MM, Sherwood CC, Schapiro SJ, Raghanti MA, Hopkins WD. Age‐ and cognition‐related differences in the gray matter volume of the chimpanzee brain (Pan troglodytes): a voxel‐based morphometry and conjunction analysis. Am J Primatol. 2021:e23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersen AH, Zhang Z, Zhang M, Gash DM, Avison MJ. Age‐associated changes in rhesus CNS composition identified by MRI. Brain Res. 1999;829:90‐98. [DOI] [PubMed] [Google Scholar]
- 29. Freire‐Cobo C, Edler MK, Varghese M, et al. Comparative neuropathology in aging primates: a perspective. Am J Primatol. 2021:e23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Workman KP, Healey B, Carlotto A, Lacreuse A. One‐year change in cognitive flexibility and fine motor function in middle‐aged male and female marmosets (Callithrix jacchus). Am J Primatol. 2019;81:e22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sadoun A, Rosito M, Fonta C, Girard P. Key periods of cognitive decline in a nonhuman primate model of cognitive aging, the common marmoset (Callithrix jacchus). Neurobiol Aging. 2019;74:1‐14. [DOI] [PubMed] [Google Scholar]
- 32. Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9:495‐502. [DOI] [PubMed] [Google Scholar]
- 33. Herdon JG, Moss MB, Rosene DL, Killany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25‐34. [DOI] [PubMed] [Google Scholar]
- 34. Lacreuse A, Parr L, Chennareddi L, erndon JG. Age‐related decline in cognitive flexibility in female chimpanzees. Neurobiol Aging. 2018;72:83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkins WD, Mareno MC, Neal Webb SJ, Schapiro SJ, Raghanti MA, Sherwood CC. Age‐related changes in chimpanzee (Pan troglodytes) cognition: cross‐sectional and longitudinal analyses. Am J Primatol. 2021;83:e23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neff EP. Animal models of Alzheimer's disease embrace diversity. Lab Anim (NY). 2019;48:255‐259. [DOI] [PubMed] [Google Scholar]
- 37. Chen JA, Fears SC, Jasinska AJ, et al. Neurodegenerative disease biomarkers Aβ1‐40, Aβ1‐42, tau, and p‐tau181in the vervet monkey cerebrospinal fluid: relation to normal aging, genetic influences, and cerebral amyloid angiopathy. Brain and Behavior. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frye BM, Valure PM & Craft S et al. Temporal emergence of age‐associated changes in cognitive and physical function in vervets (Chlorocebus aethiops sabaeus). Geroscience; 2021. [DOI] [PMC free article] [PubMed]
- 39. Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol A Biol Sci Med Sci. 2016;71:1243‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atkins HM, Willson CJ, Silverstein M, et al. Characterization of ovarian aging and reproductive senescence in vervet monkeys (Chlorocebus aethiops sabaeus). Comp Med. 2014;64:55‐62. [PMC free article] [PubMed] [Google Scholar]
- 41. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Li XH, Cho JW, et al. U‐net model for brain extraction: trained on humans for transfer to non‐human primates. Neuroimage. 2021;235:118001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohlfing T, Kroenke CD, Sullivan EV, et al. The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform. 2012;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Styner MM, Knickmeyer R, Joshi S, Coe C, Short SJ, Gilmore J. Automatic brain segmentation in rhesus monkeys. Proceedings SPIE Medical Imaging. 2007;6512. [Google Scholar]
- 45. Jung B, Taylor PA, Seidlitz J, et al. A comprehensive macaque fMRI pipeline and hierarchical atlas. Neuroimage. 2021;235:117997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seidlitz J, Sponheim C, Glen D, et al. A population MRI brain template and analysis tools for the macaque. Neuroimage. 2018;170:121‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts A. Wisconsin General Test Apparatus. In: Stolerman IP, ed. Encyclopedia of Psychopharmacology. Springer; 2010. [Google Scholar]
- 48. James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27:14358‐14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baxter MG, Santistevan AC, Bliss‐Moreau E, Morrison JH. Timing of cyclic estradiol treatment differentially affects cognition in aged female rhesus monkeys. Behav Neurosci. 2018;132:213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Primates M, Altschul DM, Beran MJ, et al. Establishing an infrastructure for collaboration in primate cognition research. PLoS One. 2019;14:e0223675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jacobsen CF. Functions of frontal association areas in primates. Arch Neurol Psychiatry. 1935;33:358‐369. [Google Scholar]
- 52. Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed‐alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Physiol Psychol. 1971;77:212‐220. [DOI] [PubMed] [Google Scholar]
- 53. Goldman‐Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325‐335. [DOI] [PubMed] [Google Scholar]
- 54. Schwarz CG, Gunter JL, Wiste HJ, et al. A large‐scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 2016;11:802‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676‐1689. [DOI] [PubMed] [Google Scholar]
- 57. Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle‐aged and older adults: regional and individual differences. Neuroimage. 2010;51:501‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shaw ME, Sachdev PS, Anstey KJ, Cherbuin N. Age‐related cortical thinning in cognitively healthy individuals in their 60s: the PATH Through Life study. Neurobiol Aging. 2016;39:202‐209. [DOI] [PubMed] [Google Scholar]
- 59. Guo X, Wang Z, Li K, et al. Voxel‐based assessment of gray and white matter volumes in Alzheimer's disease. Neurosci Lett. 2010;468:146‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moller C, Vrenken H, Jiskoot L, et al. Different patterns of gray matter atrophy in early‐ and late‐onset Alzheimer's disease. Neurobiol Aging. 2013;34:2014‐2022. [DOI] [PubMed] [Google Scholar]
- 61. Dicks E, Vermunt L, van der Flier WM, et al, Alzheimer's Disease Neuroimaging, I . Modeling grey matter atrophy as a function of time, aging or cognitive decline show different anatomical patterns in Alzheimer's disease. Neuroimage Clin. 2019;22:101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anstey KJ, Maller JJ. The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment Health. 2003;7:238‐250. [DOI] [PubMed] [Google Scholar]
- 63. Villa C, Lavitrano M, Salvatore E, Combi R. Molecular and imaging biomarkers in Alzheimer's disease: a focus on recent insights. J Person Med. 2020:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Varghese T, Sheelakumari R, James JS, Mathuranath PS. A review of neuroimaging biomarkers of Alzheimer's disease. Neurology Asia. 2013;18:239‐248. [PMC free article] [PubMed] [Google Scholar]
- 65. Klaassens BL, van Gerven JMA, van der Grond J, de Vos F, Moller C, Rombouts S. Diminished posterior precuneus connectivity with the default mode network differentiates normal aging from Alzheimer's disease. Front Aging Neurosci. 2017;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee S, Lee H, Kim KW, Alzheimer's Disease Neuroimaging, I . Magnetic resonance imaging texture predicts progression to dementia due to Alzheimer's disease earlier than hippocampal volume. J Psychiatry Neurosci. 2020;45:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pacheco J, Goh JO, Kraut MA, Ferrucci L, Resnick SM. Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiol Aging. 2015;36:903‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age‐related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci U S A. 2004;101:15846‐15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373‐4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dicks E, Tijms BM, Ten Kate M, et al. Gray matter network measures are associated with cognitive decline in mild cognitive impairment. Neurobiol Aging. 2018;61:198‐206. [DOI] [PubMed] [Google Scholar]
- 71. Gray DT, Barnes CA. Experiments in macaque monkeys provide critical insights into age‐associated changes in cognitive and sensory function. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arnsten AFT, Datta D, Preuss TM. Studies of aging nonhuman primates illuminate the etiology of early‐stage Alzheimer's‐like neuropathology: an evolutionary perspective. Am J Primatol. 2021:e23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alexander GE, Chen K, Aschenbrenner M, et al. Age‐related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28:2710‐2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherwood CC, Gordon AD, Allen JS, et al. Aging of the cerebral cortex differs between humans and chimpanzees. Proc Natl Acad Sci. 2011;108:13029‐13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hopkins WD, Phillips KA. Cross‐sectional analysis of the association between age and corpus callosum size in chimpanzees (Pan troglodytes). Dev Psychobiol. 2010;52:133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu JV, Bock NA, Silva AC. Rapid high‐resolution three‐dimensional mapping of T1 and age‐dependent variations in the non‐human primate brain using magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence. Neuroimage. 2011;56:1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wisco JJ, Killiany RJ, Guttmann CR, Warfield SK, Moss MB, Rosene DL. An MRI study of age‐related white and gray matter volume changes in the rhesus monkey. Neurobiol Aging. 2008;29:1563‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Allard M, Lèbre V, Robine J‐M, Calment J. Jeanne Calment: from Van Gogh's time to ours, 122 extraordinary years. WH Freeman & Company; 1998. [Google Scholar]
- 79. Gold E. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Emery Thompson M, Jones JH, Pusey AE, et al. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150‐2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Herndon JG, Paredes J, Wilson ME, Bloomsmith MA, Chennareddi L, Walker ML. Menopause occurs late in life in the captive chimpanzee (Pan troglodytes). Age (Dordr). 2012;34:1145‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Havercamp K, Watanuki K, Tomonaga M, Matsuzawa T, Hirata S. Longevity and mortality of captive chimpanzees in Japan from 1921 to 2018. Primates. 2019;60:525‐535. [DOI] [PubMed] [Google Scholar]
- 83. Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gynecologists, A.C.o.O.a . ACOG Committee Opinion No. 565: hormone therapy and heart disease. J Obstet Gynecol 2013;121:1407‐1410. [DOI] [PubMed] [Google Scholar]
- 85. Walker ML. Menopause in female rhesus monkeys. Am J Primatol. 1995;35:59‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weigl R. Longevity of mammals in captivity; from the Living Collections of the world: A list of mammalian longevity in captivity. Kleine Senckenberg‐Reihe; 2005. [Google Scholar]
- 87. Mosconi L, Rahman A, Diaz I, et al. Increased Alzheimer's risk during the menopause transition: a 3‐year longitudinal brain imaging study. PLoS One. 2018;13:e0207885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Scheyer O, Rahman A, Hristov H, et al. Female sex and Alzheimer's risk: the menopause connection. The journal of prevention of Alzheimer's disease. 2018;5:225‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ha DM, Xu JJ, Janowsky JS. Preliminary evidence that long‐term estrogen use reduces white matter loss in aging. Neurobiol Aging. 2007;28:1936‐2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Crawford DK, Mangiardi M, Song B, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999‐3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ryan J, Artero S, Carriere I, et al. Brain volumes in late life: gender, hormone treatment, and estrogen receptor variants. Neurobiol Aging. 2014;35:645‐654. [DOI] [PubMed] [Google Scholar]
- 92. Georgakis MK, Kalogirou EI, Diamantaras AA, et al. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2016;73:224‐243. [DOI] [PubMed] [Google Scholar]
- 93. Song YJ, Li SR, Li XW, et al. The effect of estrogen replacement therapy on Alzheimer's disease and Parkinson's disease in postmenopausal women: a meta‐analysis. Front Neurosci. 2020;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33:85‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hoagey DA, Lazarus LTT, Rodrigue KM, Kennedy KM. The effect of vascular health factors on white matter microstructure mediates age‐related differences in executive function performance. Cortex. 2021;141:403‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Webb CE, Rodrigue KM, Hoagey DA, Foster CM, Kennedy KM. Contributions of white matter connectivity and BOLD modulation to cognitive aging: a lifespan structure‐function association study. Cereb Cortex. 2020;30:1649‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


