Abstract
Introduction
In an effort to identify improvement opportunities for earlier dementia detection and care within a large, integrated health care system serving diverse Medicare Advantage (MA) beneficiaries, we examined where, when, and by whom Alzheimer's disease and related dementias (ADRD) diagnoses are recorded as well as downstream health care utilization and life care planning.
Methods
Patients 65 years and older, continuously enrolled in the Kaiser Foundation health plan for at least 2 years, and with a first ADRD diagnosis between January 1, 2015, and December 31, 2018, comprised the incident cohort. Electronic health record data were used to identify site and source of the initial diagnosis (clinic vs hospital‐based, provider type), health care utilization in the year before and after diagnosis, and end‐of‐life care.
Results
ADRD prevalence was 5.5%. A total of 25,278 individuals had an incident ADRD code (rate: 1.2%) over the study period—nearly half during a hospital‐based encounter. Hospital‐diagnosed patients had higher comorbidities, acute care use before and after diagnosis, and 1‐year mortality than clinic‐diagnosed individuals (36% vs 11%). Many decedents (58%‐72%) received palliative care or hospice. Of the 55% diagnosed as outpatients, nearly two‐thirds were diagnosed by dementia specialists; when used, standardized cognitive assessments indicated moderate stage ADRD. Despite increases in advance care planning and visits to dementia specialists in the year after diagnosis, acute care use also increased for both clinic‐ and hospital‐diagnosed cohorts.
Discussion
Similar to other MA plans, ADRD is under‐diagnosed in this health system, compared to traditional Medicare, and diagnosed well beyond the early stages, when opportunities to improve overall outcomes are presumed to be better. Dementia specialists function primarily as consultants whose care does not appear to mitigate acute care use. Strategic targets for ADRD care improvement could focus on generating pragmatic evidence on the value of proactive detection and tracking, care planning, and the role of specialists in chronic care management.
Keywords: dementia, diagnosis, integrated delivery system, Medicare Advantage, utilization
1. BACKGROUND
Alzheimer's disease and related dementias (ADRD) will reach nearly 14 million cases in the United States by 2050. 1 Concerns over persistent underdiagnosis 2 and quality of care 3 , 4 , 5 and the emergence of promising care management models 6 , 7 , 8 , 9 strengthen the case for population‐based approaches. 10 , 11 , 12 Medicare benefits have expanded to encourage the detection of cognitive impairment (Annual Wellness Visit, 2011) and comprehensive cognitive assessment and care planning (2017), and increase risk‐adjusted payments for the care of ADRD beneficiaries within Medicare Advantage (MA) plans (2020). These new benefits, coupled with funding to the Centers for Disease Control and Prevention (CDC; 2018) 13 to support three national Public Health Centers of Excellence in Dementia Detection, Prevention, and Caregiving, 14 could propel health systems toward systematizing ADRD diagnosis and care.
Nonetheless, the heterogeneity of payment models (eg, fee‐for‐service and various value‐based designs) and organization of care delivery across diverse health systems argues for the importance of comparative systems data. Recent reports on MA enrollees 15 , 16 , 17 , 18 consistently find lower ADRD prevalence rates (5.5‐6%) than in traditional fee‐for‐service Medicare (≈12%), 19 differences unlikely to be attributable to out‐of‐pocket costs because most MA plans have no or low co‐payments for provider visits that could present a barrier for ADRD diagnosis and care. Park et al. 17 found lower outpatient and hospital utilization but similar satisfaction with care among MA beneficiaries with ADRD compared to traditional Medicare, although the comparability of the two samples has been questioned. 20 Teno et al. 21 reported that MA beneficiaries dying with ADRD were less likely to receive certain burdensome and costly acute care at the end of life. In sum, ADRD detection may be poorer within MA plans, but end‐of‐life care for decedents may be better than in traditional Medicare. These first insights into ADRD diagnosis and utilization patterns for MA beneficiaries provide provisional benchmarks for health systems seeking to inform the development of pathways for earlier diagnosis and expectations for patient care and outcomes.
We offer a case study from one large integrated health care system, aimed at understanding its own patterns of care for people with ADRD, starting with capture of first ADRD diagnoses (where, by whom, and at what stage) and the downstream health care utilization and life care planning for identified patients, as a means to inform a more systematic, population‐based plan for dementia detection and care.
2. METHODS
2.1. Design and population
Patients for this retrospective cohort study were drawn from Kaiser Permanente Southern California (KPSC), serving mostly MA plan patients in Southern California. For the primary incident cohort, patients 65 years and older were included if they were enrolled in the health plan for at least 2 years prior to January 1, 2015, and had a first ADRD code during the study period, January 1, 2015 to December 31, 2018. Utilization was assessed over the 12 months before and immediately following the first diagnosis, through December 31, 2019, or until death or disenrollment due to other reasons, to avoid disruptions in care related to the coronavirus disease 2019 (COVID‐19) pandemic (Table S1). The study was approved by the KPSC (#12565) Institutional Review Board with a waiver of written informed consent.
2.2. Data collection
ADRD diagnoses were determined by International Classification of Diseases Clinical Modification codes obtained from electronic health records (EHRs), administrative and claims data: ICD‐9‐CM (290.40, 290.41, 294.10, 294.11, 294.20, 290.XX, 294.21, 331.0, 331.11, 331.19, and 331.82) and ICD‐10‐CM (F01.50, F01.51, F02.80, F02.81, F03.90, F03.91, G30.0, G30.1, G30.8, G30.9, G31.01, G31.09, and G31.83). Dementia subtypes included unspecified dementia, Alzheimer's disease (AD), and other non‐AD dementia (Table S2). We also examined codes for memory loss, mild cognitive impairment (MCI), and delirium during the 2 years prior to the first ADRD diagnosis to capture clinical identification of cognitive impairments that may precede a later ADRD diagnosis.
For ADRD codes, place of service included ambulatory care (outpatient/clinic) and hospital‐based (emergency department, observation stays, and inpatient) settings. Clinicians classified as dementia specialists included geriatricians, neurologists, and psychiatrists; all other providers were considered non‐dementia specialists and mostly represented primary care. Diagnoses made in other settings (n = 770; home, skilled nursing facility), non‐billable encounters (n = 1987; phone, email), and others (n = 514) were excluded from the analytical cohort.
Sociodemographic data (age, sex, marital status, race/ethnicity, spoken language, census‐based education and household income, and insurance type), missed visits, indicators of life care planning (advance directives or physician order for life sustaining treatment [POLST] forms), and selected clinical characteristics available in structured fields or easily mined through keyword searches (cognitive assessments at diagnosis [Mini‐Mental Status Exam (MMSE), 22 Montreal Cognitive Assessment (MoCA), 23 St Louis University Mental Status Exam (SLUMS) 24 ], code status, and Elixhauser comorbidities 25 ), were extracted from administrative, membership, and clinical records at the time of incident diagnosis or within 12 months prior.
Health care utilization data were extracted from the EHRs or derived from claims in the 12 months before and after the incident diagnosis, and these included primary and specialty clinic care (office visits, phone visits, video visits, nurse advice calls), urgent care, all‐cause acute care use (emergency department, observation stays, and inpatient), post‐acute care (skilled nursing facility and referrals to home health), and end‐of‐life care (home‐based palliative care 26 and hospice). All‐cause acute care days were calculated with emergency department (ED) visits as half days and observation and inpatient stays according to length of stay. 27 Vital status and place of death were obtained from membership files and the National and State Death Indices.
2.3. Statistical analyses
Incidence rate was calculated as the proportion of patients in the at‐risk population who received a new ADRD code; prevalence rates were calculated based on the cross‐section of patients with an ADRD code of all patients enrolled in the health plan for each sampling year. Our primary comparisons were between the clinic‐ and hospital‐diagnosed cohorts: (1) socio‐demographic, clinical, and utilization characteristics in the year before the incident ADRD code for all patients; (2) changes in utilization characteristics pre‐ and post‐diagnosis for patients who survived at least 1 year after diagnosis; and (3) end‐of‐life care for patients who died within the first year after diagnosis. Although we present descriptive data by what clinic‐based discipline assigned the first ADRD code, we did not perform statistical analyses on these subgroups due to the sheer volume of comparisons. The a priori threshold for statistical significance was a two‐sided P value < .05. All analyses were conducted using SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA).
RESEARCH IN CONTEXT
Systematic Review: Nearly 40% of Medicare beneficiaries are enrolled in Medicare Advantage plans. However, little is known about their pattern of Alzheimer's disease and related dementias (ADRD) diagnosis and health care utilization, especially within an integrated health system.
Interpretation: ADRD was under‐diagnosed in this health system and diagnosed well beyond the early stages, when opportunities to improve overall outcomes are presumed to be better. Nearly half (45%) of the incident diagnoses originated from a hospital encounter. Although there was greater use of dementia specialists for diagnosis and greater involvement of these specialists in the year after diagnosis, the sustained high volume of outpatient visits coupled with increases in acute care use present opportunities for better care coordination and earlier integration of palliative care principles.
Future directions: Efforts to improve ADRD detection and care in this health system and elsewhere must be grounded in generating the much‐needed real‐world evidence regarding the value of earlier diagnosis for multiple stakeholders.
3. RESULTS
3.1. Characteristics of sample
Among patients 65 and older without an ADRD diagnosis code before 2015, the annual ADRD incidence rate was 1.2% (Table S3) from 2015 to 2018 and overall prevalence was 5.5% (Table S4). Of a total of 25,278 incident diagnoses (Table 1), over half originated in ambulatory care (n = 13,962, 55%) and were made, as expected, by clinicians (primary care providers, 34%; geriatricians, 38%; neurologists, 24%; or psychiatrists, 3%). The remaining incident ADRD codes were from acute care encounters (n = 11,316, 45%), including ED visits (30%) and observation or inpatient stays (70%). Nearly all hospital‐based (91%) were assigned by administrative‐professional billing coders, not by clinicians (9%); notably, such codes appear in billing records but not in clinical records (eg, problem lists). Approximately one‐third of the cohort had a diagnosis code for memory loss, MCI, or delirium in the 2 years preceding the incident ADRD code; their sociodemographic, clinical, and utilization characteristics were not different from those without a prior cognitive disorder code (data not shown).
TABLE 1.
Baseline characteristics across clinic‐ and hospital‐based incident Alzheimer's disease and related dementias (ADRD) diagnosis from 2015 to 2018
| Clinic‐Diagnosed by Clinician Discipline | |||||
|---|---|---|---|---|---|
|
Primary Care (n = 4756) |
Geriatrics (n = 5322) |
Neuro/Psych (n = 3399) |
All Clinic a (n = 13962) |
Hospital‐Diagnosed a (n = 11,316) |
|
| Socio‐demographics | |||||
| Age | 82.4 (7.36) | 81.8 (7.03) | 79.2 (7.18) | 81.3 (7.31) | 82.7 (7.83) |
| 65‐75 | 908 (19%) | 1039 (20%) | 1093 (32%) | 3165 (23%) | 2232 (20%) |
| 76‐85 | 2185 (46%) | 2581 (48%) | 1600 (47%) | 6591 (47%) | 4737 (42%) |
| >85 | 1663 (35%) | 1702 (32%) | 706 (21%) | 4206 (30%) | 4347 (38%) |
| Female | 2864 (60%) | 3293 (62%) | 1862 (55%) | 8281 (59%) | 6221 (55%) |
| Marital Status: Partnered | 2204 (46%) | 2382 (45%) | 1905 (56%) | 6717 (48%) | 4715 (42%) |
| Race/Ethnicity | |||||
| White | 2606 (55%) | 2487 (47%) | 1955 (58%) | 7333 (53%) | 6651 (59%) |
| Hispanic | 1206 (25%) | 1344 (25%) | 847 (25%) | 3496 (25%) | 2226 (20%) |
| Black | 502 (11%) | 975 (18%) | 242 (7%) | 1769 (13%) | 1559 (14%) |
| Asian | 370 (8%) | 436 (8%) | 309 (9%) | 1151 (8%) | 725 (6%) |
| Pacific Islander | 27 (1%) | 32 (1%) | 18 (1%) | 79 (1%) | 48 (0%) |
| American Indian/ Alaska Native | 12 (0%) | 12 (0%) | 8 (0%) | 33 (0%) | 25 (0%) |
| Multi‐Race | 5 (0%) | 5 (0%) | 2 (0%) | 14 (0%) | 15 (0%) |
| Others/Unknown | 28 (1%) | 31 (0%) | 18 (0%) | 87 (1%) | 33 (1%) |
| Spoken language: Non‐English, interpreter needed | 567 (12%) | 719 (14%) | 391 (12%) | 1716 (12%) | 813 (7%) |
| Education (census‐based), < College | 3330 (70%) | 3709 (70%) | 2267 (67%) | 9642 (69%) | 7894 (70%) |
| Household annual median income (census), <$50,000 | 1811 (38%) | 2052 (39%) | 1197 (35%) | 5248 (38%) | 4428 (39%) |
| Insurance Status | |||||
| Medicare | 4121 (87%) | 4537 (85%) | 2963 (87%) | 12023 (86%) | 9678 (86%) |
| Medicaid | 34 (1%) | 35 (1%) | 24 (1%) | 100 (1%) | 62 (1%) |
| Medicare‐Medicaid | 321 (7%) | 459 (9%) | 190 (6%) | 1005 (7%) | 777 (7%) |
| Commercial (via employer)/private pay | 280 (6%) | 291 (5%) | 218 (6%) | 830 (6%) | 796 (7%) |
| Cognitive status and history | |||||
| ADRD Diagnosis Subtype | |||||
| Unspecified dementia | 4124 (87%) | 3153 (59%) | 2222 (65%) | 9869 (71%) | 9405 (83%) |
| Alzheimer's dementia | 359 (8%) | 1732 (33%) | 733 (22%) | 2881 (21%) | 634 (6%) |
| Other, non‐AD dementia | 273 (6%) | 437 (8%) | 444 (13%) | 1212 (9%) | 1277 (11%) |
| Any memory loss, MCI, or delirium code in prior 24 mo | 1620 (34%) | 1348 (25%) | 1186 (35%) | 4342 (31%) | 4119 (36%) |
| Any cognitive assessment with dx (MOCA, SLUMS, MMSE) | 681 (14%) | 3319 (62%) | 1138 (33%) | 5160 (37%) | 230 (2%) |
| MOCA | 151 (3%) | 1353 (25%) | 371 (11%) | 1882 (13%) | 43 (0%) |
| 15.0 (5.71) | 16.1 (5.16) | 15.3 (6.12) | 15.8 (5.44) | 12.1 (8.42) | |
| SLUMS | 5 (0%) | 314 (6%) | 62 (2%) | 384 (3%) | 2 (0%) |
| 14.0 (4.06) | 13.5 (5.20) | 14.6 (4.90) | 13.7 (5.15) | 8.0 (1.41) | |
| MMSE | 526 (11%) | 1930 (36%) | 782 (23%) | 3250 (23%) | 190 (2%) |
| 19.4 (5.88) | 20.5 (5.58) | 20.4 (5.61) | 20.3 (5.65) | 18.5 (8.01) | |
| Elixhauser Comorbidity Index | 6.8 (3.42) | 7.0 (3.41) | 7.1 (3.38) | 7.0 (3.43) | 8.7 (3.78) |
| Health care utilization in 12 months prior to incident dx | |||||
| % missed appointments | 10.4 (15.94) | 9.7 (12.28) | 8.3 (11.08) | 9.7 (13.47) | 13.2 (17.35) |
| No clinic encounters | 226 (5%) | 16 (0%) | 17 (1%) | 283 (2%) | 632 (6%) |
| Primary care visits | 4.6 (4.78) | 4.7 (4.31) | 5.7 (5.08) | 4.9 (4.74) | 5.1 (6.02) |
| Specialty care visits (all) | 5.6 (7.71) | 7.3 (8.51) | 8.4 (9.90) | 7.1 (8.79) | 7.8 (9.98) |
| Geriatrics | 0.1 (0.53) | 0.7 (1.35) | 0.2 (0.72) | 0.3 (1.01) | 0.2 (0.74) |
| 1 visit | 192 (4%) | 909 (17%) | 128 (4%) | 1267 (9%) | 576 (5%) |
| 2+ visits | 99 (2%) | 753 (14%) | 107 (3%) | 990 (7%) | 380 (3%) |
| Neurology/Psychiatry | 0.4 (1.73) | 0.5 (1.77) | 1.6 (3.66) | 0.7 (2.43) | 0.7 (2.66) |
| 1 visit | 334 (7%) | 393 (7%) | 614 (18%) | 1383 (10%) | 1022 (9%) |
| 2+ visits | 362 (8%) | 501 (9%) | 997 (29%) | 1928 (14%) | 1384 (12%) |
| Virtual encounters | |||||
| Telephone | 1344 (28%) | 2029 (38%) | 1343 (40%) | 4919 (35%) | 4366 (39%) |
| 1973 (41%) | 2274 (43%) | 1789 (53%) | 6280 (45%) | 4677 (41%) | |
| Video | 10 (0%) | 14 (0%) | 23 (1%) | 49 (0%) | 46 (0%) |
| Urgent care | 1383 (29%) | 1986 (37%) | 1073 (32%) | 4619 (33%) | 3914 (35%) |
| 0.5 (1.23) | 0.8 (1.67) | 0.6 (1.27) | 0.7 (1.45) | 0.8 (1.59) | |
| Nurse advice calls | 1131 (24%) | 1327 (25%) | 1028 (30%) | 3642 (26%) | 3606 (32%) |
| 0.4 (0.92) | 0.4 (0.92) | 0.5 (1.06) | 0.4 (0.96) | 0.6 (1.15) | |
| Referrals for home health | 1904 (40%) | 2119 (40%) | 1368 (40%) | 5609 (40%) | 6855 (61%) |
| All‐cause observation (Obs) or inpatient stay (IP), n% | 1070 (22%) | 1228 (23%) | 855 (25%) | 3319 (24%) | 5026 (44%) |
| Mean (SD) | 0.4 (0.82) | 0.4 (0.86) | 0.4 (0.97) | 0.4 (0.89) | 0.9 (1.53) |
| 2+ observation or inpatient stays, n% | 380 (8%) | 432 (8%) | 331 (10%) | 1225 (9%) | 2463 (22%) |
| All‐cause emergency department (ED) visits, n% | 1879 (40%) | 2121 (40%) | 1549 (46%) | 5812 (42%) | 6787 (60%) |
| Mean (SD) | 0.8 (1.41) | 0.8 (1.44) | 0.9 (1.54) | 0.8 (1.48) | 1.5 (2.26) |
| 2+ emergency department visits, n% | 867 (18%) | 951 (18%) | 709 (21%) | 2668 (19%) | 3902 (34%) |
| Any hospital stay (ED/Obs/IP), n% | 2166 (46%) | 2467 (46%) | 1783 (52%) | 6702 (48%) | 7774 (69%) |
| Mean (SD) | 1.1 (1.92) | 1.2 (2.00) | 1.3 (2.17) | 1.2 (2.05) | 2.4 (3.26) |
| 2+ hospital stays (ED/Obs/IP), n% | 1243 (26%) | 1394 (26%) | 1027 (30%) | 3862 (28%) | 5564 (49%) |
| Hospital days (ED/OBS/IP) per 100 patients | 171.2 (442.31) | 167.5 (404.23) | 208.5 (568.43) | 184.5 (471.42) | 501.1 (1010.00) |
| Any skilled nursing facility stay | 320 (7%) | 292 (5%) | 212 (6%) | 867 (6%) | 1972 (17%) |
| SNF days for those with any stay | 21.1 (23.94) | 22.5 (27.84) | 24.5 (32.30) | 23.4 (31.94) | 38.4 (64.55) |
n = 25,278.
Data are presented as n (%) or mean (SD).
Abbreviations: ED, emergency department visit; IP, inpatient hospitalization; MCI, mild cognitive impairment; MMSE , Mini‐Mental Status Exam; MoCA , Montreal Cognitive Assessment; Obs, observation stay; SLUMS , St Louis University Mental Status Exam; SNF, skilled nursing facility.
Differences between clinic‐ vs hospital‐diagnosed cohorts were significant for all variables (P < .01), except for SLUMS scores, where no statistical tests were performed due to sparse data.
3.2. Patient characteristics by incident ADRD diagnosis place of service and clinician type
Among the cohort diagnosed in ambulatory care, socio‐demographic characteristics were similar across clinician type, except for those diagnosed by neurologists/psychiatrists being slightly younger compared to the geriatrics‐ or primary care–diagnosed cohorts (Table 1 and Table S5). Few individuals (14%) diagnosed by primary care providers had a documented cognitive assessment at the time of the ADRD diagnosis, contrasting with 33% for neurologists/psychiatrists and 62% for geriatricians. For patients with any recorded cognitive assessment, mean scores (MoCA or MMSE) were consistent with moderate stage dementia at diagnosis and was similar across clinical disciplines.
Compared to the clinic‐diagnosed cohort, the hospital‐diagnosed cohort was somewhat older, more likely to be white and speak English, and had more missed appointments. They also had a higher rate of documented advance directive or POLST and a higher burden of comorbidity (all P’s < .01). Very few (2%) had a documented cognitive assessment at the time of the hospital diagnosis. All‐cause acute and post‐acute care use for the clinic‐diagnosed cohort was significantly lower than for the hospital‐diagnosed cohort (P < .01), consistent with their slightly lower burden of comorbidity.
3.3. Changes in health care utilization from the year before and after incident diagnosis by place of service and clinician type for patients surviving at least 1 year
A total of 19,659 patients (78%) survived at least 1 year following an incident ADRD diagnostic code and remained enrolled in the health plan. Although nearly all patients in the clinic‐diagnosed cohort had a second encounter that included an ADRD code within 1 year of the initial diagnosis (84%‐89%), far fewer hospital‐diagnosed patients had a second ADRD code (46%‐61%) (Table 2 and Table S6). Advance care planning increased across all cohorts, with the highest rate of completed advance directive or POLST forms for patients diagnosed by geriatricians (59%) or in the hospital (62%) (Figure 1); the hospital‐diagnosed cohort had more documented do not resuscitate or modified code orders.
TABLE 2.
Changes in life care planning and health care utilization from 1 year before and after incident ADRD diagnosis for patients who survived for at least 1‐year post diagnosis
| Clinic‐Diagnosed by Clinician Discipline | Hospital‐Diagnosed (n = 7224) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary Care (n = 4174) | Geriatric (n = 4832) | Neuro/Psych (n = 3049) | All Clinic (n = 12,435) a | |||||||
| Pre‐Dx | Post‐Dx | Pre‐Dx | Post‐Dx | Pre‐Dx | Post‐Dx | Pre‐Dx | Post‐Dx | Pre‐Dx | Post‐Dx | |
| 1+ ADRD code from outpatient encounter | – | 3243 (78%) | – | 4315 (89%) | – | 2626 (86%) | – | 10413 (84%) | – | 3322 (46%) |
| 1+ ADRD code from any encounter (outpatient or hospital) | – | 3515 (84%) | – | 4502 (93%) | – | 2744 (90%) | – | 11043 (89%) | – | 4427 (61%) |
| Life care planning | ||||||||||
| Advance directive or POLST in EHR | 1556 (37%) | 2050 (49%) | 2048 (42%) | 2836 (59%) | 1113 (37%) | 1512 (50%) | 4882 (39%) | 6604 (53%) | 3228 (45%) | 4485 (62%) |
| DNR or modified code status b | 263 (6%) | 491 (12%) | 307 (6%) | 549 (11%) | 171 (6%) | 331 (11%) | 763 (6%) | 1411 (11%) | 828 (11%) | 1849 (26%) |
| Health care Utilization | ||||||||||
| No clinic encounters | 204 (5%) | 152 (4%) | 13 (0%) | 123 (3%) | 14 (0%) | 50 (2%) | 252 (2%) | 364 (3%) | 358 (5%) | 516 (7%) |
| Primary care visits | 4.5 (4.59) | 4.5 (4.67) | 4.6 (4.16) | 3.8 (3.90) | 5.6 (4.94) | 4.8 (4.56) | 4.8 (4.59) | 4.3 (4.43) | 5.0 (5.56) | 4.7 (5.75) |
| Specialty care visits (all) | 5.5 (7.36) | 6.1 (9.61) | 7.1 (8.13) | 6.7 (7.25) | 8.3 (9.82) | 8.1 (8.99) | 6.9 (8.46) | 6.9 (8.72) | 7.7 (9.64) | 8.2 (9.95) |
| Geriatrics | 0.1 (0.25) | 0.5 (1.14) | 0.3 (0.47) | 1.0 (1.25) | 0.1 (0.26) | 0.3 (0.90) | 0.2 (0.38) | 0.6 (1.17) | 0.1 (0.31) | 0.4 (1.11) |
| 1+ visit | 261 (6%) | 973 (23%) | 1543 (32%) | 2662 (55%) | 209 (7%) | 362 (12%) | 2069 (17%) | 4094 (33%) | 673 (9%) | 1317 (18%) |
| Neurology/Psychiatry | 0.1 (0.29) | 0.4 (0.83) | 0.1 (0.31) | 0.2 (0.57) | 0.4 (0.49) | 1.0 (1.23) | 0.2 (0.38) | 0.4 (0.93) | 0.2 (0.39) | 0.4 (1.02) |
| 1+ visit | 382 (9%) | 963 (23%) | 477 (10%) | 485 (10%) | 1101 (36%) | 1621 (53%) | 2025 (16%) | 3171 (26%) | 1207 (17%) | 1600 (22%) |
| Any ED/Obs/IP, n% | 1819 (44%) | 2058 (49%) | 2151 (45%) | 2361 (49%) | 1549 (51%) | 1611 (53%) | 5725 (46%) | 6250 (50%) | 4680 (65%) | 5006 (69%) |
| Mean (SD) | 1.0 (1.84) | 1.3 (2.05) | 1.1 (1.78) | 1.3 (2.11) | 1.2 (2.07) | 1.5 (2.26) | 1.1 (1.90) | 1.3 (2.16) | 2.2 (3.05) | 2.7 (3.65) |
| 2+ ED/Obs/IP | 1006 (24%) | 1236 (30%) | 1179 (24%) | 1390 (29%) | 869 (29%) | 1010 (33%) | 3185 (26%) | 3775 (30%) | 3213 (44%) | 3646 (50%) |
| Hospital days (ED/Obs/IP) per 100 patients | 156.1 (421.0) | 213.3 (525.7) | 150.5 (370.3) | 197.9 (538.7) | 190.8 (543.2) | 231.5 (556.3) | 165.4 (441.2) | 213.8 (540.2) | 403.3 (883.6) | 544.5 (1187.7) |
n = 19,659.
Data are presented as n (%) or mean (SD).
Bold font indicates significant within‐group, pre‐post differences (P < .01).
Abbreviations: AD, advance directive; DNR, do not resuscitate; ED, emergency department visit; EHR, electronic health record; IP, inpatient hospitalization; Obs, observation stay; POLST, physician order for life‐sustaining treatments.
Between‐group, clinic vs hospital, significant differences in pre‐post change scores for all variables (P < .01), except for mean number of visits to neurology/psychiatry, which is italicized.
Code status Pre‐Dx was value closest to dx date and Post‐Dx was closest to end of 12‐month follow‐up.
FIGURE 1.

Changes in life care planning from year before and after incident Alzheimer's disease and related dementias (ADRD) diagnosis (n = 19,659)
In the post‐diagnosis year, there were small, albeit statistically significant, decreases in primary care visits and increases in dementia specialist visits across the clinic and hospital‐diagnosed cohorts (Figure 2), although only a minority of patients had two or more visits to geriatrics (15%, n = 1821) or neurology/psychiatry (19%, n = 2408). All‐cause acute care use increased at a similar rate for both clinic (46‐50%) and hospital (65‐69%) cohorts in the year post‐diagnosis. The hospital cohort continued to have the highest acute (Figure 3) and post‐acute care use.
FIGURE 2.
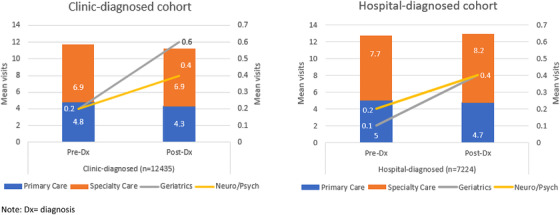
Changes in the mean number of primary and specialty (including visits to dementia specialists) care visits from year before and after incident Alzheimer's disease and related dementias (ADRD) diagnosis (n = 19,659)
FIGURE 3.

Changes in acute care use from year before and after incident Alzheimer's disease and related dementias (ADRD) diagnosis (n = 19,659)
3.4. End‐of‐life care for patients who died within 1 year of incident ADRD diagnosis by place of service and clinician type
The 1‐year mortality rate after diagnosis was 22% for the overall cohort (clinic‐diagnosed: 11%; hospital‐diagnosed: 36%). Decedents were generally older and had more co‐morbidities, and substantially higher outpatient, acute, and post‐acute care utilization in the year prior to diagnosis compared to patients surviving at least 1 year after diagnosis (Table S7). Median time from diagnosis to death was 87 days for the overall cohort (Table 3).
TABLE 3.
End‐of‐life characteristics for patients who died within 1 year after incident ADRD diagnosis
| Clinic‐Diagnosed by Clinician Discipline | ||||||
|---|---|---|---|---|---|---|
| All deaths (n = 5619) | Primary Care (n = 582) |
Geriatrics (n = 490) |
Neuro/Psych (n = 350) |
All clinic (n = 1521) a |
Hospital‐Diagnosed (n = 4092) a |
|
| Days from diagnosis to death | 87 (19, 205) | 173 (76, 270) | 199 (105, 284) | 185 (94, 266) | 183 (90, 271) | 53 (10, 162) |
| Life care planning | ||||||
| Advance directive or POLST in EHR | 4435 (79%) | 448 (77%) | 412 (84%) | 264 (75%) | 1202 (79%) | 3233 (79%) |
| Advance directive | 3534 (63%) | 350 (60%) | 306 (62%) | 192 (55%) | 914 (60%) | 2620 (64%) |
| POLST | 2643 (47%) | 262 (45%) | 304 (62%) | 174 (50%) | 790 (52%) | 1853 (45%) |
| Code status | ||||||
| Do not resuscitate/modified Code | 3730 (66%) | 354 (61%) | 289 (59%) | 205 (59%) | 901 (59%) | 2829 (69%) |
| Full Code | 1404 (25%) | 148 (25%) | 159 (32%) | 103 (29%) | 449 (29%) | 955 (23%) |
| Missing | 485 (9%) | 80 (14%) | 42 (9%) | 42 (12%) | 177 (12%) | 308 (8%) |
| End‐of‐life care | ||||||
| Received hospice | 3085 (55%) | 341 (59%) | 278 (57%) | 233 (67%) | 916 (60%) | 2169 (53%) |
| Days from diagnosis to hospice | 55 (10, 162) | 135 (50, 218) | 161 (79, 259) | 149 (55, 237) | 140 (56, 237) | 29 (3, 113) |
| Days in hospice | 15 (5, 61) | 17 (6, 58) | 12 (5, 41) | 19 (5, 68) | 15 (5, 57) | 15 (5, 64) |
| Received HBPC | 876 (16%) | 95 (16%) | 102 (21%) | 65 (19%) | 282 (18%) | 594 (15%) |
| Received hospice or HBPC | 3370 (60%) | 366 (63%) | 312 (64%) | 253 (72%) | 1001 (66%) | 2369 (58%) |
| Place of death | ||||||
| Home | 2022 (36%) | 254 (44%) | 227 (46%) | 165 (47%) | 692 (45%) | 1330 (33%) |
| Hospital | 1575 (28%) | 113 (19%) | 118 (24%) | 70 (20%) | 327 (21%) | 1248 (30%) |
| Intensive care unit | 202 (4%) | 14 (2%) | 19 (4%) | 6 (2%) | 42 (3%) | 160 (4%) |
| Nursing home/long‐term care facility | 1000 (18%) | 102 (18%) | 50 (10%) | 66 (19%) | 237 (16%) | 763 (19%) |
| Emergency dept/outpatient | 188 (3%) | 23 (4%) | 14 (3%) | 11 (3%) | 48 (3%) | 140 (3%) |
| Hospice facility | 81 (1%) | 9 (2%) | 5 (1%) | 7 (2%) | 23 (2%) | 58 (1%) |
| Other | 753 (13%) | 81 (14%) | 76 (16%) | 31 (9%) | 200 (13%) | 553 (14%) |
n = 5619.
Data are presented as n (%) or median (IQR); code status was closest to death date.
Abbreviations: EHR, electronic health record; HBPC , home‐based palliative care; POLST, physician order for life‐sustaining treatments.
Differences in clinic vs hospital, for all variables (P < .01), except for advance directive or POLST in EHR and median days in hospice as italicized.
Between diagnosis and death, the proportion of patients with an advance directive or POLST increased from 56% to 79%. More than half (60%) of the decedents received either hospice (55%) or home palliative care (16%). There were more home deaths in the clinic‐ vs hospital‐diagnosed cohort (45% vs 33%).
4. DISCUSSION
In this study of diverse older adults from a large integrated health delivery system serving mostly Medicare Advantage (MA) beneficiaries, we found that similar to previous studies of MA plans, 15 , 16 , 17 , 18 ADRD was under‐diagnosed relative to population estimates of about 10% 1 and diagnosed well beyond early stages, when opportunities to improve overall outcomes are presumed to be better.
Several noteworthy findings either diverge from or have not been sufficiently addressed in prior studies. Nearly half of the incident ADRD diagnostic codes originated from a hospital‐based encounter, and 90% were assigned by administrative‐professional coders, not clinicians. Patients first diagnosed in the hospital setting had higher overall illness burden and acute care use, and they were three times more likely to die in the year following diagnosis than those first diagnosed as outpatients, arguing for the need to stratify patients by the site of first ADRD diagnosis in future studies that rely on claims and/or EHR data.
Our findings also differ from those of previous studies in that we found a higher proportion of patients first diagnosed by dementia specialists, compared to prior reports on fee‐for‐service Medicare beneficiaries (35% vs 15% 28 ), and differences in specialty diagnoses by race/ethnicity or spoken language were not observed in our study. Although there were modest decreases in primary care visits and increases in dementia specialist visits, the sustained high overall volume of clinic visits (mean: 11‐13 visits/year) in the post‐diagnosis year, coupled with increased acute care encounters, suggest that care is both intensifying and potentially more fragmented at a time when consolidation could be highly desirable. Yet, among decedents, documentation of an advance directive or POLST (75%‐84%) and enrollment in home‐based palliative care or hospice (58%‐72%) were both high compared to prior studies, 29 , 30 likely driven by the health system's increased attention to end‐of‐life care during the study period.
The observation that 45% of all incident ADRD diagnostic codes came from a hospital‐based encounter may not be surprising, since previous studies have reported the prevalence of cognitive impairment in hospitalized older adults ranging from 13% to 63% depending on the sampling and assessment methodology 31 , 32 , 33 , 34 and that many patients with ADRD had not been previously diagnosed as outpatients. 31 , 32 , 33 Our data further suggest that planning for ADRD‐focused follow‐up was likely limited for the hospital‐diagnosed cohort because only 46% had a second outpatient ADRD code within 1 year of diagnosis vs 84% in the clinic‐diagnosed cohort. Because nearly all the hospital‐based codes originated with administrative coders and are not entered on the patient's problem list, outpatient clinicians may not be aware of the diagnosis, or other medical problems may simply dominate their attention. Our experience is not unique, as a recent European study showed that a national mandate to screen patients 75 and older for cognitive impairment during unplanned admissions was not associated with increased referrals for further workup or primary care follow‐up after discharge. 35 , 36
A majority of clinic‐diagnosed patients had a second ADRD code in the subsequent year, indicating that clinicians remain mindful of its presence, yet it is unclear how an ADRD diagnosis should inform ambulatory patient care in the context of advanced old age, multimorbidity, and frailty. All are markers of care complexity near the end of life and might signal futility, 37 , 38 , 39 shifting care toward symptom management, patient and family support, and overall palliation (regardless of an individual's eligibility for enrollment in a formal palliative care or hospice program). Among decedents, documentation of advance care planning was uniformly high (79%) compared to prior studies (46%), 29 regardless of where the initial diagnosis was made, and use of home palliative care or hospice by clinic‐diagnosed decedents (66%) was comparable to that of an academic dementia care management program that intentionally worked to increase base rates of end‐of‐life care (69%). 30 The high performance on these end‐of‐life quality care indicators reflects the effects of this health system's multi‐year investments in proactive life care planning and serious illness care for the older population. However, there is room for further improvement in identifying individuals who are diagnosed with ADRD during a rising trajectory of acute care use but are not “flagged” as needing attention for more coordinated, less burdensome care such as that offered in formal palliative care programs.
The role of specialists in this integrated system deserves comment. Although Drabo et al. 28 found that only 15% of Medicare fee‐for‐service patients were seen by a dementia specialist, we observed a rate more than twice that (35%), and for strictly clinic‐ rather than hospital‐based diagnoses, the specialist diagnosis rate was 62%. We also found little evidence of racial/ethnic differences by site of diagnosis. The relatively high percentage of patients diagnosed by dementia specialists may be attributable to the fact that they are enrolled in a health system that offers easier access to specialist consultations, and to state regulations that require managed care organizations to provide timely specialist appointments (within 15 days).
Reducing hospitalization rates, especially admissions considered potentially avoidable with proactive ambulatory care, is a goal of Healthy People 2030. 40 ADRD is associated with higher all‐cause and potentially preventable admissions, 41 , 42 rates (and associated costs 43 ) that are mitigated by high continuity of care in fee‐for‐service Medicare. In a universally insured population in Quebec, high primary care continuity is associated with lower all‐cause and potentially preventable admissions and emergency department visits. 44 Our data indicate that for clinic‐diagnosed patients who survived the first year after an incident ADRD diagnosis, acute care use increased similarly regardless of whether a primary care provider or dementia specialist made the diagnosis. Although dementia specialist care increased slightly following diagnosis, it was mostly consultative rather than longitudinal in nature, and by increasing visits, it could unintentionally impose even greater fragmentation of care and burden on an already fragile population. The role of dementia specialist care, in the diagnostic process and beyond, is an important focus for future research on patient and health system outcomes.
How best to coordinate care across primary and specialist providers to maximize the well‐being of older adults with ADRD and multi‐morbidities should be a high research priority, as reports of well‐publicized dementia care management programs, 6 , 45 and, similarly, our recent evaluation of a small home‐based primary care program within this health system, 46 have generally found limited if any effects of such care models on mitigating acute care use, an outcome of importance to health systems and payers, and for most patients and families.
Finally, we did not find consistent evidence of differences in socio‐demographic and clinical characteristics or care patterns between individuals who were and were not recognized as having a cognitive disorder (eg, MCI, memory loss, delirium) in the 2 years prior to the first ADRD code, suggesting that without systematic follow‐up, such “early signals” may not be acted upon. Although we did not observe racial/ethnic differences in the assignment of these cognitive disorder codes, a recent study of California fee‐for‐service Medicare beneficiaries found that Asian, Black, and Hispanic beneficiaries were less likely than White beneficiaries to receive a diagnosis of MCI prior to one of ADRD, considered by the authors to signify early detection of ADRD. 47
Diagnosing ADRD “early,” that is,, close to the time when symptoms are first apparent, and providing individuals and families with proactive, comprehensive post‐diagnostic medical and psychosocial care, are values that have gained momentum since the pre‐COVID data window we used in this study. It remains to be determined if such prevailing forces are sufficient to drive this and other health systems to alter the deeply entrenched, widespread patterns of late diagnosis over the next several years given the continued insufficient evidence‐base for benefits of earlier diagnosis. 2
4.1. Strengths and limitations
Our well‐characterized older, racially diverse cohort represents the most contemporary population‐based description of incident ADRD diagnosis and utilization pattern in a large integrated setting with low rates of health plan disenrollment (< 4%) 48 by place of diagnosis and clinician type. Our work provides data that can serve as a comparator for future studies in other health systems and deeper inquiry into the content of clinical care for people living with ADRD. In addition, we analyzed the hospital‐diagnosed cohort separately from the clinic‐diagnosed cohort and showed that prognosis varies importantly between the two groups, suggesting that future studies of ADRD prognosis consider diagnosis setting as a relevant factor.
Our study has several important methodological limitations, including the use of a single encounter code for ADRD to identify the cohort, which increases sensitivity at the cost of specificity. 49 However, requiring repeat codes over longer periods of time would have eliminated individuals who died in the first year after diagnosis and resulted in a less‐complete picture of diagnosis patterns. We recognize that the 1‐year post‐diagnosis follow‐up period is relatively short, considering the long ADRD illness trajectory. We were limited to the discrete data available from the EHR, limiting assessment of domains of care quality, 50 and did not have information on caregiver characteristics 51 that might be associated with utilization. Moreover, we were not able to conduct multivariable modeling to meaningfully compare outcomes across patient cohorts due to incomplete data on key variables, for example, ADRD severity, type, and functional impairment. For hospice utilization, we did not have access to the qualifying diagnosis. In addition, we did not evaluate variations in diagnosis or utilization across KPSC's 15 service areas, which can differ in practice structure, availability of specialists, and patient characteristics, and we could not assess variations in specialist referral decisions, clinical practices, or the content of care. We described utilization, not care costs, since cost accounting in a capitated system is complex; nonetheless, acute, hospital‐based care generally contributes to most of Medicare's expenses regardless of payment model. 52
5. CONCLUSION
In this integrated health care system, which serves mostly MA beneficiaries, we observed the same, well‐known pattern of delay and under‐diagnosis of ADRD that has been established in both fee‐for‐service and other MA populations. 15 , 16 , 17 , 18 Dementia diagnosis tended to occur in the context of rising multimorbidity, likely when cognitive impairment had become so obvious it could no longer be ignored. However, indicators of quality end‐of‐life care for decedents, such as advance care planning and palliative care/hospice enrollment, were comparable to or exceeded published reports. Finally, although there was greater use of dementia specialists for diagnosis and greater involvement of these specialists in the year after diagnosis, the sustained high volume of outpatient visits coupled with increases in acute care use present opportunities for better care coordination and integration of palliative care principles earlier in the illness trajectory. 53 Efforts to improve ADRD detection and care in this health system and elsewhere must be grounded in generating the much‐needed real‐world evidence regarding the value of earlier diagnosis for multiple stakeholders.
CONFLICTS OF INTEREST
Dr. Nguyen receives funding from the National Institutes of Health (NIH) and Patient‐Centered Outcomes Research Institute (PCORI)
Dr. Borson receives funding from the NIH, the Centers for Disease Control and Prevention (CDC), and University of Wyoming ECHO Project, and consulting fees from Biogen and Genentech
Dr. Langer‐Gould receives funding from the NIH, the National Multiple Sclerosis Society, and the California Technology Assessment Forum‐Institute for Clinical and Economic Review
Dr. Wang receives funding from the KPSC Care Improvement Research Team and PCORI
Drs. Khang and Carrol and Ms. Lee have no disclosures relevant to the content of this submission
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGEMENTS
This work is supported by funding from the Kaiser Permanente Southern California Care Improvement Research Team to Dr. Nguyen. Clinical leaders within the health system and who are also co‐authors, participated in the design and conduct of the study, interpretation of the data, preparation, review, approval, and decision to submit the manuscript for publication. The authors thank the patients of Kaiser Permanente for helping to improve care through the use of information collected through our electronic health record system.
Nguyen HQ, Borson S, Khang P, et al. Dementia diagnosis and utilization patterns in a racially diverse population within an integrated health care delivery system. Alzheimer's Dement. 2022;8:e12279. 10.1002/trc2.12279
REFERENCES
- 1. 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16:391–460. [DOI] [PubMed] [Google Scholar]
- 2. Owens DK, Davidson KW, et al. Screening for cognitive impairment in older adults: uS Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(8):757–763. [DOI] [PubMed] [Google Scholar]
- 3. Chodosh J, Mittman BS, Connor KI, et al. Caring for patients with dementia: how good is the quality of care? Results from three health systems. J Am Geriatr Soc. 2007;55(8):1260–1268. [DOI] [PubMed] [Google Scholar]
- 4. Odenheimer G, Borson S, Sanders AE, et al. Quality improvement in neurology: dementia management quality measures. J Am Geriatr Soc. 2014;62(3):558–561. [DOI] [PubMed] [Google Scholar]
- 5. Jennings LA, Tan Z, Wenger NS, et al. Quality of care provided by a comprehensive dementia care Comanagement Program. J Am Geriatr Soc. 2016;64(8):1724–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. [DOI] [PubMed] [Google Scholar]
- 7. Clark PA, Bass DM, Looman WJ, McCarthy CA, Eckert S. Outcomes for patients with dementia from the Cleveland Alzheimer's Managed Care Demonstration. Aging Ment Health. 2004;8(1):40–51. [DOI] [PubMed] [Google Scholar]
- 8. Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–726. [DOI] [PubMed] [Google Scholar]
- 9. Reuben DB, Tan ZS, Romero T, Wenger NS, Keeler E, Jennings LA. Patient and caregiver benefit from a comprehensive dementia care program: 1‐year results from the UCLA Alzheimer's and Dementia Care Program. J Am Geriatr Soc. 2019;67(11):2267–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forester B, Heintz H, Epstein‐Lubow G. The Urgency to Implement Comprehensive, Chronic Disease Management Models of Dementia Care While Pursuing Further Evidence for Clinical Effectiveness and Health Care Cost Reduction. Am J Geriatr Psychiatry. 2020;28(3):337–338. [DOI] [PubMed] [Google Scholar]
- 11. Borson S, Chodosh J. Developing dementia‐capable health care systems: a 12‐step program. Clin Geriatr Med. 2014;30(3):395–420. [DOI] [PubMed] [Google Scholar]
- 12. Lees Haggerty K, Epstein‐Lubow G, Spragens LH, et al. Recommendations to Improve Payment Policies for Comprehensive Dementia Care. J Am Geriatr Soc. 2020;68(11):2478–2485. [DOI] [PubMed] [Google Scholar]
- 13. Building Our Largest Dementia Infrastructure for Alzheimer's Act. 2018;https://uscode.house.gov/statutes/pl/115/406.pdf
- 14. BOLD Public Health Centers of Excellence . 2020;https://www.cdc.gov/aging/funding/phc/index.html
- 15. Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer's disease and related dementias in Medicare Advantage plans. Alzheimers Dementia (Amsterdam, Netherlands). 2020;12(1):e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyers DJ, Rahman M, Rivera‐Hernandez M, Trivedi AN, Mor V. Plan switching among Medicare Advantage beneficiaries with Alzheimer's disease and other dementias. Alzheimers Dementia (New York, N Y). 2021;7(1):e12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park S, White L, Fishman P, Larson EB, Coe NB. Health Care Utilization, Care Satisfaction, and Health Status for Medicare Advantage and Traditional Medicare Beneficiaries With and Without Alzheimer Disease and Related Dementias. JAMA Netw Open. 2020;3(3):e201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powers BW, Antol DD, Zhao Y, et al. Association Between Primary Care Payment Model and Telemedicine Use for Medicare Advantage Enrollees During the COVID‐19 Pandemic. JAMA Health Forum. 2021;2(7):e211597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Y, Chen Y, Crimmins EM, Zissimopoulos JM. Sex, Race, and Age Differences in Prevalence of Dementia in Medicare Claims and Survey Data. J Gerontol B Psychol Sci Soc Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dosa DM, Trivedi AN, Mor V. Implications of Medicare Advantage for Patients With Alzheimer Disease and Related Dementias. JAMA Netw Open. 2020;3(3):e201853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teno JM, Keohane LM, Mitchell SL, et al. Dying with dementia in Medicare Advantage, Accountable Care Organizations, or traditional Medicare. J Am Geriatr Soc. 2021;69(10):2802–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 24. Tariq SH, Tumosa N, Chibnall JT, Perry MH 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini‐mental state examination for detecting dementia and mild neurocognitive disorder–a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900–910. [DOI] [PubMed] [Google Scholar]
- 25. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 26. Wang SE, Liu IA, Lee JS, et al. End‐of‐Life Care in Patients Exposed to Home‐Based Palliative Care vs Hospice Only. J Am Geriatr Soc. 2019;67(6):1226–1233. [DOI] [PubMed] [Google Scholar]
- 27. Center for Medicare and Medicaid Services . Excess Days in Acute Care (EDAC) Measures Methodology. 2020. https://qualitynet.cms.gov/inpatient/measures/edac/methodology
- 28. Drabo EF, Barthold D, Joyce G, Ferido P, Chang Chui H, Zissimopoulos J. Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimers Dement. 2019;15(11):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Portanova J, Ailshire J, Perez C, Rahman A, Enguidanos S. Ethnic Differences in Advance Directive Completion and Care Preferences: what Has Changed in a Decade?. J Am Geriatr Soc. 2017;65(6):1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jennings LA, Turner M, Keebler C, et al. The Effect of a Comprehensive Dementia Care Management Program on End‐of‐Life Care. J Am Geriatr Soc. 2019;67(3):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boustani M, Baker MS, Campbell N, et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Timmons S, Manning E, Barrett A, et al. Dementia in older people admitted to hospital: a regional multi‐hospital observational study of prevalence, associations and case recognition. Age Ageing. 2015;44(6):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195(1):61–66. [DOI] [PubMed] [Google Scholar]
- 34. Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355. [DOI] [PubMed] [Google Scholar]
- 35. Burn AM, Bunn F, Fleming J, et al. Case finding for dementia during acute hospital admissions: a mixed‐methods study exploring the impacts on patient care after discharge and costs for the English National Health Service. BMJ Open. 2019;9(6):e026927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burn AM, Fleming J, Brayne C, Fox C, Bunn F. Dementia case‐finding in hospitals: a qualitative study exploring the views of healthcare professionals in English primary care and secondary care. BMJ Open. 2018;8(3):e020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis MA, Nallamothu BK, Banerjee M, Bynum JP. Identification of four unique spending patterns among older adults in the last year of life challenges standard assumptions. Health Aff (Millwood). 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aldridge MD, Bradley EH. Epidemiology and patterns of care at the end of life: rising complexity, shifts in care patterns and sites of death. Health Aff (Millwood). 2017;36(7):1175–1183. [DOI] [PubMed] [Google Scholar]
- 39. Wang SY, Aldridge MD, Gross CP, Canavan M, Cherlin E, Bradley E. End‐of‐life care transition patterns of medicare beneficiaries. J Am Geriatr Soc. 2017;65(7):1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Healthy People 2030. https://health.gov/healthypeople/objectives‐and‐data/browse‐objectives/dementias/reduce‐proportion‐preventable‐hospitalizations‐older‐adults‐dementia‐dia‐02
- 41. Anderson TS, Marcantonio ER, McCarthy EP, Herzig SJ. National trends in potentially preventable hospitalizations of older adults with dementia. J Am Geriatr Soc. 2020;68(10):2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amjad H, Carmichael D, Austin AM, Chang CH, Bynum JP. Continuity of care and health care utilization in older adults with dementia in fee‐for‐service medicare. JAMA Intern Med. 2016;176(9):1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Godard‐Sebillotte C, Strumpf E, Sourial N, Rochette L, Pelletier E, Vedel I. Primary care continuity and potentially avoidable hospitalization in persons with dementia. J Am Geriatr Soc. 2021;69(5):1208–1220. [DOI] [PubMed] [Google Scholar]
- 45. Jennings LA, Laffan AM, Schlissel AC, et al. Health care utilization and cost outcomes of a comprehensive dementia care program for medicare beneficiaries. JAMA Intern Med. 2019;179(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen HQ, Vallejo JD, Macias M, et al. A mixed‐methods evaluation of home‐based primary care in dementia within an integrated system. J Am Geriatr Soc. 2021. [DOI] [PubMed] [Google Scholar]
- 47. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of Racial/Ethnic Disparities in Timeliness and Comprehensiveness of Dementia Diagnosis in California. JAMA Neurol. 2021;78(6):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borson S, Chen A, Wang SE, Nguyen HQ. Patterns of incident dementia codes during the COVID‐19 pandemic at an integrated healthcare system. J Am Geriatr Soc. 2021;69(12):3389–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harding BN, Floyd JS, Scherrer JF, et al. Methods to identify dementia in the electronic health record: comparing cognitive test scores with dementia algorithms. Healthcare (Amsterdam, Netherlands). 2020;8(2):100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wenger NS, Solomon DH, Amin A, et al. Application of assessing care of vulnerable elders‐3 quality indicators to patients with advanced dementia and poor prognosis. J Am Geriatr Soc. 2007;55(Suppl 2):S457–463. [DOI] [PubMed] [Google Scholar]
- 51. Amjad H, Mulcahy J, Kasper JD, et al. Do Caregiving Factors Affect Hospitalization Risk Among Disabled Older Adults?. J Am Geriatr Soc. 2021;69(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fishman P, Coe NB, White L, et al. Cost of dementia in Medicare managed care: a systematic literature review. Am J Manag Care. 2019;25(8):e247–e253. [PMC free article] [PubMed] [Google Scholar]
- 53. Mataqi M, Aslanpour Z. Factors influencing palliative care in advanced dementia: a systematic review. BMJ Support Palliat Care. 2020;10(2):145–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


