Abstract
OBJECTIVE
Rapid loss of estimated glomerular filtration rate (eGFR) within its normal range has been proposed as a strong predictor of future kidney disease. We investigated this association of eGFR slope early in the course of type 1 diabetes with long-term incidence of kidney and cardiovascular complications.
RESEARCH DESIGN AND METHODS
The annual percentage change in eGFR (slope) was calculated during the Diabetes Control and Complications Trial (DCCT) for each of 1,441 participants over a mean of 6.5 years and dichotomized by the presence or absence of early rapid eGFR loss (slope ≤−3% per year) as the exposure of interest. Outcomes were incident reduced eGFR (eGFR <60 mL/min/1.73 m2), composite cardiovascular events, or major adverse cardiovascular events (MACE) during the subsequent 24 years post-DCCT closeout follow-up.
RESULTS
At DCCT closeout (the baseline for this analysis), diabetes duration was 12 ± 4.8 years, most participants (85.9%) had normoalbuminuria, mean eGFR was 117.0 ± 13.4 mL/min/1.73 m2, and 149 (10.4%) had experienced early rapid eGFR loss over the preceding trial phase. Over the 24-year subsequent follow-up, there were 187 reduced eGFR (6.3 per 1,000 person-years) and 113 MACE (3.6 per 1,000 person-years) events. Early rapid eGFR loss was associated with risk of reduced eGFR (hazard ratio [HR] 1.81, 95% CI 1.18–2.79, P = 0.0064), but not after adjustment for baseline eGFR level (HR 0.94, 95% CI 0.53–1.66, P = 0.84). There was no association with composite cardiovascular events or MACE.
CONCLUSIONS
In people with type 1 diabetes primarily with normal eGFR and normoalbuminuria, the preceding slope of eGFR confers no additional association with kidney or cardiovascular outcomes beyond knowledge of an individual’s current level.
Introduction
Despite advances in the management of glycemic control and other metabolic risk factors, the lifetime cumulative risk of kidney failure in type 1 diabetes (T1D) remains substantial, with contemporary population estimates even exceeding one-third (1). Diabetic kidney disease itself is defined by the development of albuminuria or by loss in glomerular filtration rate (GFR). Estimated GFR (eGFR) <60 mL/min/1.73 m2 (termed “reduced eGFR”) is seen as the clinically relevant stage of kidney disease because of the strong association with subsequent kidney failure. Reduced eGFR as well as the development of albuminuria are also independent risk factors that greatly augment the incidence of cardiovascular disease (CVD) and mortality in T1D. Of major concern, the clinical prediction of future reduced eGFR and CVD in those with T1D remains a pragmatic challenge (1).
The traditional clinical measures for diabetic kidney disease have centered on albuminuria (albumin excretion rate [AER] ≥30 mg/24 h) as the fundamental early prognostic variable that heralds later macroalbuminuria and reduced eGFR stages that ultimately may progress to kidney failure (2). However, as phenotypes for clinical prediction, micro- and macroalbuminuria have significant limitations in that they represent dynamic processes that frequently remit to normal levels, and may not demonstrate abnormal levels in those on antihypertensive therapies despite higher risk (3,4). Furthermore, a subset of individuals may have reduced eGFR without prior or concurrent microalbuminuria (3–7).
In light of these limitations, there has been great interest to examine as a predictor the trajectory of GFR decline (the slope), particularly in the stages in which GFR levels themselves are not yet reduced. Variably named in the literature as “early renal function decline,” “progressive early renal decline,” “early rapid GFR loss,” and in this report as “early rapid eGFR loss,” these terms are meant to emphasize the distinction from the slope during late-stage loss when eGFR is already reduced (<60 mL/min/1.73 m2). Studies, primarily conducted within the Joslin Kidney Cohorts, have found that the early rate of loss approximates the slope during more advanced chronic kidney disease stages. For example, an individual with a 7.5% annual loss in eGFR while the eGFR is 125 mL/min/1.73 m2 appears to have a longitudinal slope trend such that ∼7.5% annual loss continues even when the eGFR is <60 mL/min/1.73 m2 (8,9). This offers the opportunity to identify those at risk for long-term loss during a specific interval of time—typically measured over 4- or 5-year intervals (10–12)—at earlier stages when the eGFR level is normal, rather than at later stages when the opportunities for prediction and intervention are limited. Typically defined by a ≥3% annual loss in GFR (although in some studies as a 3 mL/min/1.73 m2 annual loss) (13), the definition of early rapid eGFR loss is based on the 2.5th percentile of the normal reference distribution of GFR slope measured by way of creatinine clearance over time in populations without diabetes (10,14). This definition has been used to better understand the natural history (10,11,15), identify risk factors (16–21), and select higher-risk patients for interventional trials (22,23). However, much of this work examined GFR slope trajectories at study population levels rather than for individual risk or did not consider the confounding association of the current level of GFR. The association of early rapid eGFR loss determined over a discrete time interval (as the exposure of interest) with the subsequent long-term incidence of reduced eGFR and CVD (as the outcomes) independent of the level of eGFR is not known with certainty in those with early duration T1D.
The Diabetes Control and Complications Trial (DCCT) and the subsequent Epidemiology of Diabetes Interventions and Complications (EDIC) posttrial observational study demonstrated the role of reducing glycemic exposure to prevent or delay the development of reduced eGFR (44% risk reduction) (24) and cardiovascular outcomes (30% risk reduction) (25) compared with conventional glycemic therapy (11,26–28). We aimed to determine the association of early rapid eGFR loss independent of the level of eGFR, measured during the DCCT, with incident reduced eGFR and cardiovascular complications evaluated during 24 years of posttrial follow-up in EDIC.
Research Design and Methods
Subjects
The methods of the DCCT and EDIC study have been described in detail (29,30). Briefly, aimed at testing whether reducing levels of glycemia decreased the risk of complications of diabetes, the DCCT (1983–1993) enrolled 1,441 patients with T1D and randomly assigned them to receive conventional diabetes therapy (n = 730) or intensive therapy (n = 711). Conventional therapy was aimed at preventing symptoms of hypo- or hyperglycemia but with no glucose targets, while intensive therapy was aimed at achieving glycemic control as close to the nondiabetic range as safely possible. The DCCT study cohort consisted of a primary prevention cohort (50.4% of the study cohort) with 1- to 5-year diabetes duration, no retinopathy based on fundus photography, and <40 mg of albuminuria per 24 h; and a secondary intervention cohort (49.6% of the study cohort) with 1- to 15-year duration, minimal to moderate nonproliferative retinopathy and <200 mg of albuminuria per 24 h. Additional exclusion criteria included neuropathy sufficiently severe to require therapy, hypertension (>140/90 mmHg or medication), and hyperlipidemia (LDL >130 mg/dL or medication) (29). At the end of the DCCT, after an average follow-up of 6.5 years, all participants were taught intensive therapy and were referred to their health care providers for subsequent diabetes care. Started in 1994, EDIC enrolled 98% of the surviving DCCT cohort, with 94% of the cohort survivors still actively participating after >30 years of combined DCCT/EDIC follow-up.
Definition of Early Rapid GFR Loss
Serum creatinine was measured annually in the DCCT for the determination of the annualized eGFR slope (a quantitative variable), calculated for each participant using a linear regression on the natural log scale for the eGFR values. DCCT baseline eGFR values were excluded from the calculation in order to eliminate changes in eGFR that occurred in the intensive treatment group owing to rapid improvement in glycemic control (27). The main exposure of interest, early rapid eGFR loss, was defined as an annualized eGFR decline of ≥3% during DCCT, according to established conventions in which slope is measured while GFR is in normal ranges, >60 mL/min/1.73 m2 (9,14,22). In sensitivity analyses, we also defined eGFR loss by alternate thresholds and examined the eGFR slope as a continuous variable. Given prior observations that the rate of loss of eGFR as a percentage appears to be fairly constant from the early stages of diabetes when eGFR is in normal ranges to later stages when eGFR is reduced (8,9), we planned to sample a period of time for exposure measurement that was as distant as possible from kidney outcomes. Furthermore, including eGFR measurements that are close in time to reduced eGFR events (eGFR <60 mL/min/1.73 m2) would be a biased approach because the data used both for the definition of the exposure (eGFR slope) and the outcome (reduced eGFR events) would be correlated. For these reasons, we made use of the DCCT/EDIC design to choose a natural delineation between exposure and outcome measurement. Specifically, we chose to operationalize the measurement of the exposure (eGFR slope) during the DCCT and the measurement of outcomes during EDIC.
Outcomes
Serum creatinine was measured annually in EDIC. Serum creatinine levels, age, sex, and race were used to calculate the eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (27). Kidney failure was defined as the initiation of maintenance dialysis or kidney transplantation assessed yearly by questionnaire and adjudicated centrally. Reduced eGFR was defined as eGFR <60 mL/min/1.73 m2 or kidney failure (5,26,27).
CVD events were ascertained using annual medical histories and electrocardiograms. All CVD events were adjudicated by a committee masked to DCCT treatment group and HbA1c levels. The composite CVD outcome (any CVD) was defined as time to the first occurrence of any of the following: CVD death, nonfatal myocardial infarction, nonfatal stroke, subclinical myocardial infarction on electrocardiograms, angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant coronary obstruction demonstrated by angiography, revascularization (with angioplasty or coronary artery bypass), or congestive heart failure (paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease). A secondary CVD outcome, major adverse cardiovascular events (MACE), was defined as a first occurrence of CVD death, nonfatal myocardial infarction, or nonfatal stroke (31).
Only reduced eGFR events and CVD events that occurred during EDIC were included as outcomes, and participants with these events during DCCT were excluded. This applied to 6 participants with reduced eGFR events, 16 with CVD events, and 7 with MACE events during DCCT. All events that occurred between DCCT closeout and up to and including EDIC year 23 (the year 2017 for most participants) were included in these analyses.
Other Clinical Variables
Risk factors were assessed by standardized methods at periodic visits during DCCT and EDIC. HbA1c was measured with a high-performance liquid chromatography method quarterly during DCCT and annually during EDIC. AER (in mg/dL) was measured from 4-h urine samples by fluoroimmunoassay from DCCT baseline through EDIC year 18 (2012) and then from random, untimed urine samples subsequently. AER evaluations were conducted annually during DCCT and every other year during EDIC (27,32). Fasting lipoprotein levels were measured annually during DCCT and every other year during EDIC. All laboratory measurements were performed in the DCCT/EDIC central biochemistry laboratory with standardized methods and long-term controls in place (33).
Statistical Analysis
The associations between early rapid eGFR loss and clinical variables were assessed using logistic regression models for early rapid eGFR loss variables (annualized eGFR decline ≥3% vs. <3%) and using linear regression models for the quantitative annualized eGFR slope represented as a continuous variable. For the outcomes of interest, Kaplan-Meier estimates describe the cumulative incidence of reduced eGFR and MACE. The associations between early rapid eGFR loss and the risk of outcomes were assessed using unadjusted and adjusted multivariable Cox proportional hazards models, with early rapid eGFR loss and the other clinical variables included as fixed covariates evaluated at DCCT closeout. These included adjustment for current age, current BMI, current duration, sex, mean updated HbA1c, trial assignment, and membership in the primary versus secondary prevention cohort. The Cox models were stratified by the number of DCCT evaluations to account for the different number of DCCT evaluations among participants. Participants with outcome events during DCCT were excluded from analyses for that particular outcome. Analyses were then conducted using the DCCT closeout eGFR instead of the early rapid eGFR loss. We then assessed whether the early rapid eGFR loss was more strongly associated with outcomes than the DCCT closeout eGFR by including them jointly in the multivariable Cox proportional hazards models for outcomes.
Two sensitivity analyses for the annualized eGFR decline variable were conducted, one using a cutoff of 2% for annual eGFR and the other examining the DCCT annual eGFR slope as a continuous variable. Finally, sensitivity analyses excluding adolescents and excluding short diabetes duration (<10 years at DCCT closeout) were also conducted, as changes in eGFR in the first 10 years of diabetes are more likely to result from resolution of hyperfiltration than in later years when the trajectory of eGFR may more likely reflect underlying chronic kidney disease trajectory, which was also investigated by excluding participants with baseline eGFR values in the hyperfiltration range (eGFR ≥140 mL/min/1.73 m2) (34).
Given the exploratory nature of these analyses, no adjustment for multiple testing was performed. Associations with P values ≤0.05 were considered significant.
Data and Resource Availability
Data collected for the DCCT/EDIC study through 30 June 2017 are available to the public through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository (https://repository.niddk.nih.gov/studies/edic/). Data collected in the current cycle (July 2017–June 2022) will be available within 2 years after the end of the funding cycle.
Results
Baseline Characteristics
There were 1,435 participants with at least 2 DCCT eGFR measurements after randomization into the DCCT, and their characteristics are reported in the first two columns of Table 1. At this analysis, baseline (DCCT closeout) microalbuminuria prevalence was 14.1%, and mean eGFR was 117.0 ± 13.4 mL/min/1.73 m2. As such, the majority of participants at the baseline analysis had levels >90 mL/min/1.73 m2 (the common lower threshold for “normal eGFR”) and <140 mL/min/1.73 m2 (the common lower threshold for “hyperfiltration”) (34). This represented a decrease in eGFR for the study population from a mean eGFR of 126.2 ± 14.2 mL/min/1.73 m2 at DCCT baseline. There were 149 participants (10.4%) with early rapid eGFR loss (annualized eGFR decline of ≥3% or more during DCCT, with distribution of slopes shown in Supplementary Figure 1). Table 1 also summarizes characteristics according to presence or absence of early rapid eGFR loss. The variables associated with higher odds of early rapid eGFR loss (an annual decline ≥3%) were shorter duration of T1D, membership in the primary prevention cohort (compared with the secondary intervention cohort), higher mean AER values during DCCT and at closeout, and lower DCCT closeout eGFR (Table 1). Additionally, in Table 1 we present the factors associated with the level of DCCT closeout eGFR. This level was highly associated with mean eGFR slope (2.6487 mL/min/1.73 m2 lower closeout eGFR for every 1% per year loss in eGFR, P < 0.0001). Supplementary Table 1 shows the numbers and proportions of subjects with percentage annual eGFR slope values at alternate threshold levels that were used in the sensitivity analyses. Subjects who were adolescents at DCCT baseline (n = 198) tended to have higher annual DCCT eGFR decline compared with adults. Specifically, 15.4% of the adolescents had early rapid eGFR loss compared with 9.6% of the adults.
Table 1.
Clinical characteristics of the overall study population and according to presence or absence of measures of early rapid eGFR loss during DCCT
| Mean (SD) | Mean value by early rapid eGFR loss (eGFR annual decline ≥3%) | Early rapid eGFR Loss | Closeout eGFR* | ||||
|---|---|---|---|---|---|---|---|
| Risk factor | Overall (N = 1,435) | Present (n = 149) | Absent (n = 1,286) | Odds ratio (95% CI) | P value | Estimate (SE) | P value |
| DCCT baseline age (years) | 26.8 (7.1) | 26.5 (7.8) | 26.8 (7.0) | 0.99 (0.97, 1.02) | 0.6407 | −0.9072 (0.04352) | <0.0001 |
| DCCT closeout age (years) | 33.0 (7.0) | 32.0 (7.6) | 33.1 (6.9) | 0.98 (0.95, 1.00) | 0.0658 | −0.9488 (0.0438) | <0.0001 |
| Male (vs. female) (%) | 52.7 | 50.3 | 53.0 | 0.90 (0.64, 1.26) | 0.5326 | −0.0799 (0.7075) | 0.9100 |
| DCCT baseline BMI (kg/m2) | 23.5 (2.8) | 23.5 (2.8) | 23.4 (2.8) | 0.99 (0.93, 1.05) | 0.6981 | −0.4474 (0.1263) | 0.0004 |
| DCCT closeout BMI (kg/m2) | 25.8 (3.8) | 25.6 (3.7) | 25.9 (3.8) | 0.98 (0.94, 1.03) | 0.4828 | −0.1912 (0.0935) | 0.0411 |
| DCCT baseline T1D duration (years) | 5.8 (4.1) | 5.0 (3.9) | 5.9 (4.2) | 0.94 (0.90, 0.99) | 0.0099 | −0.2522 (0.0851) | 0.0031 |
| DCCT closeout T1D duration (years) | 12.0 (4.8) | 10.4 (4.6) | 12.2 (4.8) | 0.92 (0.88, 0.96) | <0.0001 | −0.2152 (0.0729) | 0.0032 |
| Intensive (vs. conventional) (%) | 49.3 | 51.7 | 49.1 | 1.11 (0.79, 1.56) | 0.5463 | −1.9178 (0.7047) | 0.0066 |
| Cohort (primary vs. secondary) (%) | 50.4 | 61.1 | 49.1 | 1.62 (1.15, 2.30) | 0.0062 | 1.9767 (0.7045) | 0.0051 |
| DCCT closeout mean HbA1c (%) | 8.2 (1.4) | 8.4 (1.5) | 8.2 (1.4) | 1.11 (0.99, 1.25) | 0.0690 | 1.3351 (0.2418) | <0.0001 |
| DCCT baseline AER (mg/dL) | 15.9 (18.6) | 15.7 (19.4) | 15.9 (18.5) | 0.94 (0.75, 1.16) | 0.5462 | 1.0998 (0.4479) | 0.0142 |
| DCCT baseline microalbuminuria* (Y/N) (%) | 10.9 | 11.4 | 10.8 | 1.06 (0.62, 1.81) | 0.8236 | 0.8418 (1.1327) | 0.4575 |
| DCCT mean AER (mg/dL) | 33.3 (145.6) | 102.5 (388.2) | 25.2 (75.3) | 1.39 (1.18, 1.62) | <0.0001 | −0.0121 (0.4037) | 0.9761 |
| DCCT closeout AER (mg/dL) | 52.5 (341.0) | 202.1 (903.6) | 35.1 (181.3) | 1.22 (1.07, 1.38) | 0.0030 | 0.0875 (0.3080) | 0.7761 |
| DCCT closeout microalbuminuria (Y/N) | |||||||
| At closeout (%) | 14.1 | 19.4 | 13.5 | 1.54 (1.00, 2.39) | 0.0507 | 1.0505 (1.0102) | 0.2986 |
| Ever in DCCT (%) | 34.9 | 36.9 | 34.2 | 1.13 (0.79, 1.60) | 0.5121 | 2.9102 (0.7389) | <0.0001 |
| DCCT baseline eGFR (mL/min/1.73 m2) | 126.2 (14.2) | 124.5 (15.6) | 126.3 (14.1) | 0.99 (0.98, 1.00) | 0.1257 | 0.4901 (0.0213) | <0.0001 |
| DCCT closeout eGFR (mL/min/1.73 m2) | 117.0 (13.4) | 101.9 (17.9) | 118.8 (11.6) | 0.91 (0.89, 0.92) | <0.0001 | — | — |
| Mean eGFR slope (mL/min/1.73 m2 per year) | −1.24 (2.54) | −5.90 (3.18) | −0.71 (1.80) | 0.02 (0.01, 0.04) | <0.0001 | 2.1476 (0.1271) | <0.0001 |
| Mean eGFR slope (% per year) | −1.04 (2.33) | −5.26 (3.34) | −0.55 (1.57) | — | — | 2.6487 (0.1349) | <0.0001 |
The data are presented as mean (SD) unless indicated otherwise. The bold values are statistically significant.
Odds ratios and P values for 3% annual decline are obtained using logistic models. The β estimates and P values for quantitative decline and closeout eGFR are obtained from linear regression models.
The distribution of AER is highly skewed, and the odds ratios and estimates are reported per unit increase in log AER (i.e., per 2.72-fold change in AER).
Outcome Measures
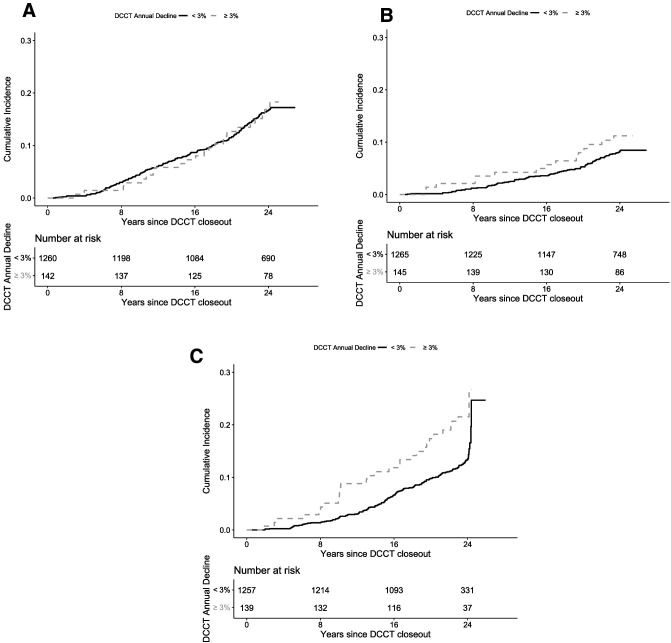
Over the 23 years of follow-up in EDIC, there were 187 reduced eGFR events (defined by eGFR<60 mL/min/1.73 m2 or kidney failure; incidence rate of 6.3 events per 1,000 person-years), 223 CVD events (incidence rate of 7.4 events per 1,000 person-years), and 113 MACE events (incidence rate of 3.6 events per 1,000 person-years). The number of reduced eGFR, CVD, and MACE events and their incidence rates are reported by presence of early rapid eGFR loss in Table 2. Figure 1A–C shows the cumulative incidences of CVD, MACE, and reduced eGFR, respectively, by the presence or absence of early rapid eGFR Loss. Compared with those without early rapid eGFR loss, participants with early rapid eGFR loss had similar risk for CVD (unadjusted hazard ratio [HR] 1.05, 95% CI 0.67–1.62, P = 0.8393) and MACE (1.54, 95% CI 0.87–2.72, P = 0.1408), while the risk was substantially higher for the outcome of reduced eGFR (HR 1.91, 95% CI 1.26–2.90, P = 0.0023) (Table 3).
Table 2.
Number of participants at risk, and the number of follow-up events and event rates* overall and by early rapid eGFR loss
| Early rapid eGFR loss | ||||
|---|---|---|---|---|
| Outcome | At risk (n) | Events (n) | Present | Absent |
| CVD | 1,402 | 223 (7.4) | 23 (7.5) | 200 (7.3) |
| MACE | 1,410 | 113 (3.6) | 15 (4.7) | 98 (3.5) |
| eGFR <60 mL/min/1.73 m2 | 1,396 | 187 (6.3) | 29 (10.1) | 158 (5.8) |
Numbers are shown as number of events (event rate per 1,000 patient-years).
Figure 1.
Cumulative incidence of CVD (P = 0.8393) (A), MACE (P = 0.1408) (B), and reduced eGFR (P = 0.0023) (C) during EDIC, separately by overall DCCT early rapid eGFR loss (i.e., ≥3% vs. <3% annual loss).
Table 3.
Association between annual DCCT early rapid eGFR loss and EDIC outcomes, using Cox proportional hazards models stratified by number of DCCT eGFR measurements
| Unadjusted | Adjusted* | Further adjusted* for adult/adolescent | Further adjusted* for DCCT closeout eGFR | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Early rapid eGFR loss | ||||||||
| CVD | 1.05 (0.67, 1.62) | 0.8393 | 1.04 (0.66, 1.62) | 0.8697 | 1.05 (0.67, 1.63) | 0.8462 | 1.03 (0.64, 1.67) | 0.9008 |
| MACE | 1.54 (0.87, 2.72) | 0.1408 | 1.60 (0.89, 2.87) | 0.1142 | 1.61 (0.90, 2.89) | 0.1068 | 1.51 (0.79, 2.90) | 0.2115 |
| eGFR <60 mL/min/1.73 m2 | 1.91 (1.26, 2.90) | 0.0023 | 1.84 (1.20, 2.81) | 0.0048 | 1.82 (1.18, 2.78) | 0.0062 | 0.94 (0.53, 1.66) | 0.8401 |
| DCCT closeout eGFR | ||||||||
| CVD | 0.99 (0.98, 0.99) | 0.0012 | 1.00 (0.99, 1.01) | 0.9007 | 1.00 (0.99, 1.01) | 0.9095 | 1.00 (0.99, 1.01) | 0.9514 |
| MACE | 0.98 (0.97, 0.99) | 0.0069 | 0.99 (0.97, 1.01) | 0.3062 | 0.99 (0.97, 1.01) | 0.3162 | 1.00 (0.98, 1.02) | 0.7107 |
| eGFR <60 mL/min/1.73 m2 | 0.97 (0.96, 0.98) | <0.0001 | 0.96 (0.95, 0.98) | <0.0001 | 0.96 (0.95, 0.98) | <0.0001 | 0.96 (0.95, 0.98) | <0.0001 |
Bold values are statistically significant.
Adjusted for current age, current BMI, current duration, sex, mean updated HbA1c, group, and cohort.
Table 3 further summarizes the adjusted HRs between early rapid eGFR loss and the risk of kidney and cardiovascular outcomes in the full cohort. Early rapid eGFR loss was not associated with risk for CVD or MACE events in any of the primary analysis models. The unadjusted HR for reduced eGFR was significant (HR 1.91, 95% CI 1.26–2.90, P = 0.0023), which retained significance in adjusted models except for the model that adjusted for DCCT closeout eGFR. The DCCT closeout eGFR retained significant association with incident reduced eGFR in adjusted models (Table 3, final row).
The results of sensitivity analyses for HRs according to annual DCCT eGFR decline ≥2% vs. <2% and using the slope as a continuous variable reveal similar findings to the primary analysis (Supplementary Table 2). As changes in eGFR in the earlier years of diabetes duration are more likely to result from resolution of hyperfiltration than in later years when the trajectory of eGFR may more likely reflect underlying chronic kidney disease trajectory, we conducted restricted analyses excluding adolescents (Supplementary Table 3), excluding participants with <10 years’ duration of T1D at DCCT closeout (Supplementary Table 4), and excluding participants with baseline eGFR levels in the hyperfiltration range (eGFR ≥140 mL/min/1.73 m2) (Supplementary Table 5). These results were similar to the primary analysis. However, we note that in models excluding participants with DCCT and closeout duration of <10 years, individuals with early rapid eGFR loss additionally had a higher risk of MACE in all unadjusted and adjusted models, even after adjustment for eGFR level at closeout (Supplementary Table 4). Finally, we examined for the influence of original DCCT therapy assignment (intensive and conventional therapy groups) and membership in the primary or secondary prevention cohorts. We found evidence for interaction by therapy assignment group but not by cohort. The association of early rapid eGFR loss was stronger in the participants assigned to conventional therapy, but, as in the primary analysis in the full cohort, the association was not significant in models adjusted for the DCCT closeout level of eGFR. In conclusion, the results of analyses for interaction by group did not influence the interpretation of overall findings.
Conclusions
Consistent with previous longitudinal reports of eGFR trajectory in those with T1D (8,9,11,35), we determined in a normotensive population with low prevalence of microalbuminuria and with normal levels of baseline eGFR that the presence of early rapid eGFR loss—defined by a rapid slope of eGFR decline over ∼5 years >3% per year—was associated with 23-year subsequent risk of reduced eGFR. However, we further report that this association was not independent of the baseline eGFR level, such that risk prediction could reasonably be accomplished by determination of a single level of eGFR in place of the determination of a 5-year eGFR trajectory. Except for a subgroup of participants with T1D duration >10 years, we additionally found no association—in unadjusted models or models adjusted for the achieved eGFR level—with future cardiovascular events.
Our findings may clarify previous knowledge about the trajectory of the eGFR slope in people with T1D and microalbuminuria, for which the eGFR slope was evaluated without consideration of the achieved eGFR level. Initially explored in the First Joslin Kidney Study, it was estimated that approximately one-third of patients with new-onset albuminuria begin a linear process of early rapid eGFR loss, measured by the slope of eGFR over time, that may even precede the onset of albuminuria (9,11,36). Later analysis in the Second Joslin Kidney Study identified that the initiation of this early rapid eGFR loss appeared to occur even prior to onset of microalbuminuria (11), serving potentially both as an earlier biomarker of the risk of progressive kidney disease and also a refinement of kidney disease risk among those with new-onset microalbuminuria, of which most do not appear to progress even over long-term follow-up to advanced stages of diabetic kidney disease (3,37). The work by the Joslin group focused on the use of cystatin C-based eGFR as studies had indicated greater accuracy of cystatin C estimates when GFR was normal or elevated (9,10,38,39).
The use of early rapid eGFR loss as a novel, early-stage kidney phenotype in T1D rather than the traditional early phenotype of microalbuminuria had several advantages. First, it led to the identification of novel putative mechanisms, such as those related to the renal handling of advanced glycation end products (20), uric acid (19), and inflammatory biomarkers (40,41). Second, as a proof-of-concept in T1D, it led to evaluation in those with type 2 diabetes and albuminuria (41,42). Finally, these novel findings led to implementation of evaluating eGFR change for inclusion in intervention trials to identify individuals likely at the highest risk of future kidney function loss (22,23). From this body of work it was evident that specific evaluation of slope may have offered advantages over simply examining the achieved level of eGFR for identifying mechanisms of renal function loss and predicting future kidney outcomes in those with albuminuria.
The use of slope in the Joslin Kidney Studies allowed the practical advantage of identifying those individuals, at variable duration of diabetes, who were actively losing renal function. There are practical limitations of examining only an individual’s achieved level of eGFR. First, eGFR levels are highly variable between individuals even without diabetes, while an individual’s specific trajectory over time has a known distribution (14). For those with diabetes, an identical level, such as 70 mL/min/1.73 m2, would intuitively be more concerning in an individual with shorter diabetes duration than someone with longer duration. In place of standardizing the assessment to a specific diabetes duration, the calculation of slope and the identification of an abnormal level of eGFR loss (≥3% annualized loss, termed early rapid eGFR loss) could help to identify that the individual with 70 mL/min/1.73 m2 at shorter diabetes duration carries a greater reduced eGFR risk (8,9). This is because the trajectory of loss would be greater than the individual with 70 mL/min/1.73 m2 at long diabetes duration, assuming a similar level of eGFR at the diagnosis of diabetes. However, from a mechanistic standpoint, we found in the current analysis that the trajectory of eGFR change offered no additional association with outcomes beyond knowledge of the current level of eGFR. For example, an individual at the end of the DCCT with an eGFR of 70 mL/min/1.73 m2 whose preceding 5-year eGFR trajectory was rapidly declining carried the same risk of future reduced eGFR as an individual with an eGFR of 70 mL/min/1.73 m2 whose preceding eGFR levels were stable. Although the practical advantages of measuring eGFR slope benefited prior research for identifying those at risk for reduced eGFR at early stages, from a mechanistic point of view, our findings demonstrate that the achieved level of eGFR is associated with long-term renal risk more so than eGFR trajectory.
While the Joslin studies examined the slope of eGFR to define early rapid eGFR loss at the time of or closely preceding the onset of albuminuria, the current study sought to determine whether measurement of trajectory earlier in the natural history of T1D and kidney disease would be associated with long-term reduced eGFR risk. The DCCT cohort was primarily normoalbuminuric, normotensive, and had relatively short (mean of 12 years) diabetes duration at the completion of the trial. In this context, our findings favoring the current eGFR level over eGFR slope trajectory may result from the phenomenon of normalization of renal function from hyperfiltration ranges observed at younger ages and in early T1D duration (34). In prior studies, the index time was at or prior to albuminuria onset, when diabetes duration was longer and the evaluation of slope was less likely to be complicated by the resolution of hyperfiltration (8,9). At this stage, slope trajectory may better represent a pathological loss of renal function than at the stage we examined in the current analysis.
Despite refinements in exposure definition, decades of follow-up in a highly phenotyped and large population with T1D, and substantial incidence of reduced eGFR and cardiovascular outcomes, the current study has limitations. First, this study aimed to examine an early renal function trajectory in T1D in primarily normoalbuminuric individuals. Consequently, this study population could not sufficiently determine the role of early rapid eGFR loss in those with albuminuria.
Second, the timing of the eGFR slope calculation was frequently early in the course of T1D when dynamic changes less reflective of a chronic course, such as recovery from hyperfiltration or from regression-dilution bias, may occur (34). The association of early rapid eGFR loss with MACE in the subset with longer diabetes duration implies that the trajectory of eGFR >10 years of diabetes duration may more likely reflect underlying chronic kidney disease trajectory than early in the course of diabetes where estimates of trajectory are affected in many individuals by the resolution of hyperfiltration (34).
Third, calculation of eGFR slope was based on the common, clinically available creatinine-based eGFR rather than endogenous markers that may have greater accuracy at normal or elevated GFR levels, such as cystatin C (10).
Finally, further work using diagnostic methods are required to determine whether a specific diabetes duration-standardized threshold of achieved eGFR in this early-duration, normoalbuminuric population could predict long-term kidney and cardiovascular events.
In patients with T1D primarily with normoalbuminuria, the presence of early rapid eGFR loss is associated with long-term kidney outcomes. However, this association is not independent of the achieved level of GFR, nor is it clearly associated with incident cardiovascular events. The slope of eGFR in early duration of T1D prior to the onset of albuminuria does not convey an advantage for the prediction of longterm renal outcomes, and from a mechanistic perspective it appears that the achieved level of eGFR—not its trajectory—best conveys risk for progressive kidney disease.
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2022), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, MD.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care, Animas, Bayer Diabetes Care, Becton Dickinson, Eli Lilly, Extend Nutrition, Insulet Corporation, LifeScan, Medtronic Diabetes, Nipro Home Diagnostics, Nova Diabetes Care, Omron, Perrigo Diabetes Care, Roche Diabetes Care, and Sanofi.
Duality of Interest. B.A.P. has received speaker honoraria from Abbott, Medtronic, Insulet, and Novo Nordisk, research support to his research institute from Boehringer Ingleheim, Novo Nordisk, and BMO (Bank of Montreal), and has served as a consultant to Boehringer Ingelheim, Abbott, Insulet, and Novo Nordisk. A.B.K. has received research support from the National Institutes of Health and JDRF, Kyowa Kirin Pharmaceutical Development, and Siemens Healthcare Diagnostics. M.E.M. has received research support from Bayer, Novartis, and Novo Nordisk and has served as a consultant to Novartis, Merck, Pfizer, and Janssen. I.H.d.B. served as a consultant to AstraZeneca, Bayer, Boehringer-Ingelheim, Cyclerion Therapeutics, George Clinical, Goldfinch Bio, and Ironwood, and received research equipment and supplies from DexCom. No other potential conflicts of interest relevant to this article were reported.
Authors Contributions. B.A.P. and I.B. wrote the initial draft of the manuscript. X.G. conducted the statistical analyses under the supervision of I.B. X.G., A.B.K., I.B.H, H.K., M.E.M., B.Z., J.M.L., and I.H.d.B. contributed revisions to the paper. All authors approved the final content. I.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
B.A.P. and I.B. contributed equally as primary authors.
Clinical trial reg. nos. NCT00360893 and NCT00360815, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.17076314.
A complete list of members in the DCCT/EDIC Research Group is presented in the supplementary material published online for the article in N Engl J Med 2017;376:1507–1516.
References
- 1. Costacou T, Orchard TJ. Cumulative kidney complication risk by 50 years of type 1 diabetes: the effects of sex, age, and calendar year at onset. Diabetes Care 2018;41:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams ME. Diabetic nephropathy: the proteinuria hypothesis. Am J Nephrol 2005;25:77–94 [DOI] [PubMed] [Google Scholar]
- 3. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 4. Dunger DB. Banting Memorial Lecture 2016 Reducing lifetime risk of complications in adolescents with Type 1 diabetes. Diabet Med 2017;34:460–466 [DOI] [PubMed] [Google Scholar]
- 5. de Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorn LM, Gordin D, Harjutsalo V, et al.; FinnDiane Study Group . The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2015;38:2128–2133 [DOI] [PubMed] [Google Scholar]
- 7. Molitch ME, Steffes M, Sun W, et al.; Epidemiology of Diabetes Interventions and Complications Study Group . Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkins BA, Krolewski AS. Early nephropathy in type 1 diabetes: the importance of early renal function decline. Curr Opin Nephrol Hypertens 2009;18:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 10. Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 2005;16:1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krolewski AS, Gohda T, Niewczas MA. Progressive renal decline as the major feature of diabetic nephropathy in type 1 diabetes. Clin Exp Nephrol 2014;18:571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Driver TH, Shlipak MG, Katz R, et al. Low serum bicarbonate and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2014;64:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorski M, Tin A, Garnaas M, et al. Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int 2015;87:1017–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985;33:278–285 [DOI] [PubMed] [Google Scholar]
- 15. Skupien J, Smiles AM, Valo E, et al. Variations in risk of end-stage renal disease and risk of mortality in an international study of patients with type 1 diabetes and advanced nephropathy. Diabetes Care 2019;42:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019;25:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012;23:516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 2008;3:706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 2010;33:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perkins BA, Rabbani N, Weston A, et al. Serum levels of advanced glycation endproducts and other markers of protein damage in early diabetic nephropathy in type 1 diabetes. PLoS One 2012;7:e35655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bjornstad P, Costacou T, Miller RG, et al. Predictors of early renal function decline in adults with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes and the Pittsburgh Epidemiology of Diabetes Complications studies. Diabet Med 2017;34:1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doria A, Galecki AT, Spino C, et al. PERL Study Group . Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020;382:2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afkarian M, Polsky S, Parsa A, et al.; PERL Study Group . Preventing Early Renal Loss in Diabetes (PERL) Study: a randomized double-blinded trial of allopurinol-rationale, design, and baseline data. Diabetes Care 2019;42:1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 25. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 26. de Boer IH; DCCT/EDIC Research Group . Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology Of Diabetes Interventions and Complications study. Diabetes Care 2014;37:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Boer IH, Sun W, Cleary PA, et al.; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Claeskens G, Hjort NL. Model Selection and Model Averaging. Cambridge, New York, Cambridge University Press, 2008 [Google Scholar]
- 29. The DCCT Research Group . The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 30. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bebu I, Schade D, Braffett B, et al.; DCCT/EDIC Research Group . Risk factors for first and subsequent CVD events in type 1 diabetes: the DCCT/EDIC Study. Diabetes Care 2020;43:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Younes N, Cleary PA, Steffes MW, et al.; DCCT/EDIC Research Group . Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010;5:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karger AB, Eckfeldt JH, Rynders GP, et al. Long-term longitudinal stability of kidney filtration marker measurements: implications for epidemiological studies and clinical care. Clin Chem 2021;67:425–433 [DOI] [PubMed] [Google Scholar]
- 34. Molitch ME, Gao X, Bebu I, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Early glomerular hyperfiltration and long-term kidney outcomes in type 1 diabetes: the DCCT/EDIC experience. Clin J Am Soc Nephrol 2019;14:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 2010;77:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perkins BA, Orszag A, Ngo M, Ng E, New P, Bril V. Prediction of incident diabetic neuropathy using the monofilament examination: a 4-year prospective study. Diabetes Care 2010;33:1549–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Boer IH, Gao X, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC Study. Clin J Am Soc Nephrol 2016;11:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cherney DZ, Sochett EB, Dekker MG, Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med 2010;27:1358–1365 [DOI] [PubMed] [Google Scholar]
- 39. de Boer IH, Sun W, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol 2014;25:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nowak N, Skupien J, Niewczas MA, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 2016;89:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ihara K, Skupien J, Krolewski B, et al. A profile of multiple circulating tumor necrosis factor receptors associated with early progressive kidney decline in type 1 diabetes is similar to profiles in autoimmune disorders. Kidney Int 2021;99:725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang X, You J, Liu D, Xia M, He L, Liu H. 5-hydroxyhexanoic acid predicts early renal functional decline in type 2 diabetes patients with microalbuminuria. Kidney Blood Press Res 2019;44:245–263 [DOI] [PubMed] [Google Scholar]