Abstract
Aim
The aim of this study was to assess the prevalence and clinical characterization of bocavirus infection in patients admitted with respiratory symptoms to a specialized children’s hospital in Riyadh, Saudi Arabia.
Methods
This is a retrospective cross-sectional study that included children aged 0-14 years and was conducted over a two-year period (2017-2019). All data were gathered from an electronic information recording system, which included patients’ demographics, comorbidities, clinical presentation, complication, and duration of hospitalization.
Results
Among all patients (11,709) admitted to King Abdullah Specialized Children’s Hospital with predominant respiratory symptoms during the study period, 193 (1.6%) patients had bocavirus infections. Most of the patients were diagnosed in winter months. Cough was the primary presenting symptom (91.7%) followed by fever (83.4%). Gastrointestinal symptoms were also common (anorexia in 62% and vomiting in 39%). In 80% (n=154/193) of cases, bocavirus co-existed with other viruses, namely, human rhinovirus (45.8%), human adenovirus (31.2%), and respiratory syncytial virus type A (17.5%). Moreover, those who required oxygen supply stayed longer in the hospital (p<0.001) and were more likely to receive multiple medications such as bronchodilators (p<0.001), corticosteroids (p<0.001), and nebulized racemic epinephrine (p>0.05). Children infected with bocavirus and co-existing viruses were less likely to require oxygen supply (p<0.050).
Conclusion
Bocavirus infection is more common during winter months and predominantly affects respiratory and gastrointestinal systems in children. More studies are needed to evaluate the global impact of this recently recognized infection.
Keywords: bocavirus, saudi arabia, kasch, respiratory, length of stay
Introduction
Bocavirus is a recently described virus that mainly affects lower respiratory and gastrointestinal tracts of children [1]. Bocavirus is a type of parvovirus family and is known to have a small size of 20 nm [1]. The name “bocavirus” is derived from a combination of bovine parvovirus and canine minute virus, which have similar genetic and amino acid structures [2]. Human bocavirus (HBoV) is non-enveloped with a single-stranded negative DNA virus [2]. Moreover, four strains of this virus have recently been identified: HBoV1, HBoV2, HBoV3, and HBoV4 [3]. HBoV1 has mainly been identified in respiratory specimens. Other types of bocavirus have been identified in stool specimens [3]. Moreover, bocavirus was also detected in urine, saliva, and blood, and had been identified in sewage and river water [2].
Bocavirus usually affects infants and children from six months to two years of age, although some cases have been found in children older than five years of age [1]. It can be detected alone or, more commonly, in conjunction with other viruses that cause respiratory or gastrointestinal infections such as human rhinovirus, adenovirus, norovirus, and rotavirus [4]. In particular, co-infection with respiratory syncytial virus (RSV) has been found in 89% of cases [5]. Therefore, it was thought that HBoV might be a harmless passenger rather than a true pathogen [5,6]. Bocavirus presents with non-specific symptoms such as cough, wheeze, pneumonia, and fever [3]. Also, especially with HBoV2 and HBoV3, it can present with acute gastroenteritis [3].
Rhinorrhea, asthma exacerbation, and bronchiolitis were detected in some young patients in conjunction with a progressive bocavirus infection [7]. Two of the rare and life-threatening conditions that bocavirus-infected children can develop are pneumomediastinum and bilateral pneumothorax [7]. HBoV is exclusively detected by molecular detection methods [8]. Currently, laboratories use either in-house polymerase chain reaction (PCR) or real-time PCR assays in order to detect the virus [8]. Moreover, the PCR method targets NP-1, NS-1, or VP1/2 genes, which are proteins or genes found on this virus [8,9]. Recently, some multiplexing assays have been developed, which are the RespiFinder assay (PathoFinder, Maastricht, the Netherlands) and the Luminex xTAG Respiratory Viral Panel (RVP) FAST v2 assay (Luminex Molecular Diagnostics, Toronto, ON, Canada) [1,9]. The latter has been approved by the U.S. Food and Drug Administration (FDA) [1,9]. So far, there is no antiviral medication known to treat HBoV [10]. Similar to many other viruses, supportive therapy to control symptoms remains the mainstay of treatment for HBoV [10]. This includes providing oxygen supply for hypoxia and the use of bronchodilators for patients with wheezes [1,10].
The first isolation of this virus was found in 2005 in upper respiratory secretions in acutely ill patients in Sweden [4]. The estimated worldwide rate of HBoV is 2-20%, but the true comparable rate cannot be detected due to lack of studies in different populations [1]. Locally, one study conducted in Al-Taif, a city in the western region of Saudi Arabia, determined the prevalence of bocavirus in 80 samples of nasopharyngeal swabs [11]. In this study, bocavirus had been detected in 22% (18/80), mainly in young children between five months and two years of age [11]. Our study aimed to examine the prevalence and clinical characterization of bocavirus infection among children in Saudi Arabia to raise awareness about the disease among the health personnel in public health care authorities.
Materials and methods
This is a retrospective cross-sectional study at King Abdullah Specialized Children’s Hospital (KASCH) in Riyadh, Saudi Arabia. We included all patients aged 0-14 years admitted as inpatients in KASCH with bocavirus infection who had respiratory, gastrointestinal, or other symptoms from November 2017 until November 2019. Data included all patients who tested positive for bocavirus, isolated or with co-infection. We excluded admissions in the emergency room and daycare unit during the study period. The estimated sample size was 660 patients using the online Raosoft sample size calculator. This was based on a precision of 10 percentage points of the true prevalence, with a confidence level of 95% and an expected prevalence of 22% [11]. However, we included all patients who met the inclusion criteria. The data were collected from an electronic health record system known as BestCare. The data collection sheet was designed to capture different variables including clinical and laboratory findings. Information about the level of baseline clinical comorbidities, duration of oxygen therapy, length of hospital stay (LOS), complications, and, lastly, mortality (if any) was also planned to be reported. The study was reviewed and approved by the Institutional Review Board of King Abdullah International Medical Research Center (KAIMRC).
Statistical analysis
The data were analyzed using SPSS (IBM Corp., Armonk, NY, USA). Data were presented as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. The chi-square test or Fisher’s exact test was used to compare categorical data. A logistic regression was constructed to determine independent significant predictors of an extended hospitalization, defined as an LOS above the mean of 5.3 days. Odds ratios (OR) with 95% confidence intervals (CI) were expressed relative to a reference baseline category. A p-value of 0.05 was used as a cut-off for statistical significance.
Results
Among all patients (11,709) admitted to King Abdullah Specialist Children Hospital with respiratory illness during the study period, we included 193 (1.6%) patients who had bocavirus infections (Table 1). The frequency of reported symptoms and signs varied between patients with a mean of 6 ± 2 complaints and clinical findings per admitted child with bocavirus infection (Table 2). Patients were treated using variable medications according to their clinical presentation (Table 2). Analysis showed that there were at least 224 co-viral infections in 154 (80%) of children admitted with bocavirus infection, with the highest association with human rhinovirus (45%) and human adenovirus (30%). Other viruses with less co-infection association with bocavirus in our cohort include RSV (7%) and less than 5% for each of the following viruses: influenza and parainfluenza viruses, human coronavirus OC43 and NL63, Epstein Barr virus, and rotavirus. Of these patients with co-infections, 100 had one co-infection, 41 had two co-infections, 10 had three co-infections, and three patients had four co-infections.
Table 1. Characteristics of study subjects (N=193).
BMI, body mass index; LOS, length of hospital stay; SD, standard deviation
| Frequency | Percentage | |
| Sex | ||
| Male | 102 | 52.8 |
| Female | 91 | 47.2 |
| Age (months), mean (SD) | 23.04 (22.5) | |
| Infant (0-11.9 months) | 68 | 38.6 |
| Toddler (1-3 years) | 75 | 42.6 |
| Pre-school aged (3.1-5 years) | 23 | 13.1 |
| School aged (5.1-12 years) | 8 | 4.5 |
| Adolescent (12.1-14 years) | 2 | 1.1 |
| Weight (kg), mean (SD) | 10.68 (5.4) | |
| Height (cm), mean (SD) | 80.27 (16.6) | |
| Body mass index, mean (SD) | 15.73 (2.68) | |
| Age-adjusted BMI classification | ||
| Underweight for age ≤ 5th percentile | 43 | 22.3 |
| Normal weight (>5th-90th percentile) | 117 | 60.6 |
| Over-weight >90% percentile | 33 | 17.1 |
| Length of hospital stay (days), mean (SD) | ||
| LOS≤5.3 days | 147 | 76.2 |
| LOS>5.3 days | 46 | 23.8 |
| Comorbidity | ||
| No | 118 | 61.1 |
| Yes | 75 | 38.9 |
| Year of admission | ||
| 2017 | 17 | 8.8 |
| 2018 | 111 | 57.5 |
| 2019 | 65 | 33.7 |
| Admission quarter of the year | ||
| 1st quarter | 78 | 40.4 |
| 2nd quarter | 44 | 22.8 |
| 3rd quarter | 25 | 13 |
| 4th quarter | 46 | 23.8 |
Table 2. Clinical characterization of bocavirus -infected children, symptoms and signs, and received medical treatment.
NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation
| Frequency | Percentage | |
| Presenting respiratory manifestations | ||
| Cough | 177 | 91.7 |
| Shortness of breath | 140 | 72.5 |
| Nasal discharge | 87 | 45.5 |
| Wheezing | 81 | 42 |
| Congested throat | 80 | 41.5 |
| Chest retractions using accessory muscles | 58 | 30.1 |
| Stridor | 7 | 3.6 |
| Cyanosis | 1 | 0.5 |
| Presenting gastrointestinal manifestations | ||
| Loss of appetite | 124 | 64 |
| Vomiting | 76 | 39.4 |
| Diarrhea | 25 | 13 |
| Abdominal pain | 1 | 0.5 |
| Splenomegaly | 1 | 0.5 |
| Presenting immune system manifestations | ||
| Fever | 161 | 83.4 |
| Fatigue | 112 | 58 |
| Tonsillitis | 8 | 4.1 |
| Lymph node enlargement | 6 | 3.1 |
| Convulsions | 5 | 2.6 |
| Other body systems’ presenting manifestations | ||
| Ear congestion | 12 | 6.2 |
| Conjunctivitis | 10 | 5.2 |
| Skin rash | 9 | 4.7 |
| Eye discharge | 3 | 1.6 |
| Ear discharge | 2 | 1 |
| Photophobia | 1 | 0.5 |
| Total symptoms and signs (severity), mean (SD) | 6.15 (1.84) | |
| Medical management | ||
| Bronchodilators | 148 | 77.5 |
| O2 supplement (0.2-1.5 L/minute) | 113 | 59.2 |
| Antibiotics | 111 | 58.1 |
| Antipyretics | 96 | 50.3 |
| Corticosteroids | 91 | 47.6 |
| Pain killers (NSAIDs) | 36 | 18.8 |
| Other medications | 28 | 14.7 |
| Racemic epinephrine (nebulized) | 22 | 11.5 |
| Duration (hours) of supplemental O2, mean (SD) | 10.74 (6.4) | |
| Other interventions | ||
| Blood cultures | 1 | 0.5 |
| Urine culture | 4 | 2.1 |
| Respiratory culture | 2 | 1 |
Laboratory results indicate that the mean leukocytes count for the patients upon admission was normal (4.0 - 11.0 x109/L) in 73.6% of cases compared to 8.8% of children admitted with lower than (4.0 x109/L) white blood cell count. Moreover, the mean hemoglobin level (gm/L) was normal for age in 82.4% of cases. Additionally, serum glucose levels were higher than the normal range for age in the majority (89.6%) of children, but only 10.4% of them had normal blood glucose and none of them had low serum glucose level. The majority of children with bocavirus infection (76.2%) showed signs of hyponatremia with serum Na < 135 mmol/L. A smaller number of them had a normal serum Na+ level (135-145 mmol/L), and none of these patients had hypernatremia. Moreover, most of the children (64.8%) had a normal serum potassium level (K+) upon admission and 27.5% presented with hypokalemia. Few (7.8%) of these patients had hyperkalemia.
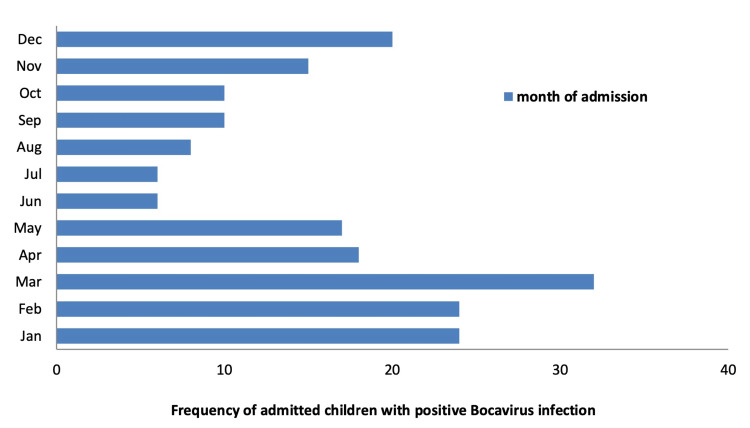
An overall average LOS for patients was 5.31 + 7 days, with 75% of children stayed for five or less days in the hospital. Based on the mean score (LOS=5.3), we divided patients based on their LOS into two groups of short LOS (≤5.31 days) and extended LOS (>5.3 days). Furthermore, we described the quarterly admission of the children with respiratory complaints who were diagnosed with a positive bocavirus infection in order to identify any seasonal variations in the numbers of admissions per year, month, and quarter (Figure 1 and Table 1). Most of these cases appeared to occur during the first three months (i.e., the winter quarter) of the year, and fewer cases were seen during the summer quarter (June-August). The number climbed again during the fall quarter (i.e., between September and December) (Figure 1). The most common reported complication with bocavirus infection in our cohort was respiratory distress (Table 3).
Table 3. Measured complications of hospitalized children with bocavirus infection (N=79).
| Complication | Frequency | Percentage |
| Respiratory distress | 75 | 94.9 |
| Dehydration | 3 | 3.6 |
| Respiratory failure requiring ventilatory support | 2 | 2.5 |
| Pneumothorax | 1 | 1.3 |
| Septic shock | 1 | 1.3 |
| Superimposed lymphadenitis | 1 | 1.3 |
Figure 1. Frequency of monthly admitted positive bocavirus-infected children with respiratory complaints.
Regression analyses identified comorbidities and the need for oxygenation during management as the significant predictors of extended LOS (> 5.3 days) (Table 4). After adjusting for age, gender, and body mass index (BMI), having at least one comorbidity was associated with a fourfold increased odd of extended stay (OR=4.5; 95% CI=2.1-9.5). Irrespective of age, gender, or BMI, patients who received oxygen therapy required as twice as the duration of hospital stay of those without oxygen requirement (OR=2.2; 95% CI=1.0-9.5).
Table 4. Logistic regression model of the association between patients’ characteristics and extended hospital stay (defined as > 5.3 days).
*Significant at 0.05 level
BMI, body mass index; CI, confidence interval; LOS, length of hospital stay; OR, odd ratio
| Variable | Extended LOS unadjusted, OR (CI) | Extended LOS adjusted, OR (CI) |
| Age at admission time (months) | 1.0 (0.99-1.0) | 1.0 (0.98-1.0) |
| Gender | 1.0 (0.53-2.0) | 0.91 (0.44-1.8) |
| BMI | 0.84 (0.73-.97) | 0.88 (0.76-1.0) |
| O2 treatment (yes vs. no as reference) | 2.4 (1.1-5.0) | 2.2* (1.0-4.9) |
| Comorbidity (yes vs. no as reference) | 5.5 (2.6-11.3) | 4.5* (2.1-9.5) |
Discussion
Bocavirus infection was less common in our study group compared to worldwide reports and previous prevalence studies in Saudi Arabia [11-16]. Affected children were of younger age group and were more prone to get infected during winter months. Respiratory symptoms were non-specific, but there were also gastrointestinal symptoms in most of our patients. In our tertiary institute, the majority of patients received oxygen, antibiotics, corticosteroids, and bronchodilators, but the LOS was relatively short in duration compared to other viruses.
HBoV has variable prevalence in different parts of the world [12-16]. Similar to the findings in previous international studies, our study showed low prevalence of bocavirus infection among inpatient children [9,17,18]. Nevertheless, in a previous study in Al Taif in Saudi Arabia, which lies in a high altitude (1.879 meters, 6.16 feet in the slopes of the Hejaz mountains), a notably higher prevalence of bocavirus infection of 22.5% was reported [11]. This could be related to more cool weather during winter season that enhances viral replication, which is the same cause of higher reports of the disease during winter months in our cohort [11]. On average, temperature in Riyadh ranges from 49°F to 110°F (rarely below 41°F or above 114°F). The age of children who were infected with bocavirus was lower in our study compared to a previous study in Al Qatif, Saudi Arabia [19]. However, our results were in keeping with the age distribution reported in most of the other studies with predominance in children less than 24 months old possibly related to suboptimal immune response against the virus in this age group [4,9,20]. Unlike most of previous pediatric studies on bocavirus infection that reported male predominance, our study showed almost equal gender distribution [11,19,21]. Understandably, younger age group in our cohort, likewise in other studies, had more respiratory complaints compared to older age group. However, systemic symptoms and immunological manifestations such as fever, lymph node inflammation, body aches, and convulsions accompanying fever. Similar to many other viruses, bocavirus had the highest incidence in our study during winter and fall quarters of the year [4,17,18]. On the other hand, some studies found bocavirus to be detected more in the summer season [11,22]. Another study in China found that the incidence of bocavirus increased more during the spring and summer seasons (May-June 2010) [23].
Characterizing viral respiratory tract infections is difficult because various symptoms can be caused by a wide range of viruses. In all studies of bocavirus, including ours, researchers have agreed that bocavirus usually presents predominantly as a lower respiratory tract infection [21,23,24]. In consistence with other bocavirus studies, our study showed a significant rate of co-infection with other viruses [25-28]. The most common coexisting viruses in our study were human rhinovirus, human adenovirus, and RSV. This phenomenon could be explained by the fact that bocavirus weakened children’s immunity, and, therefore, children were more likely to get other infections, or it could be that the pathogenesis of bocavirus led to introduction of other viruses to the systems. Another possibility is that bocavirus is acting as a bridge for other viruses or it needed other virus(es) to replicate. Moreover, our study indicated that bocavirus could present with gastrointestinal symptoms such as vomiting and diarrhea, which have also been reported in previous studies [9,24,29]. However, the most common reason for hospital admission with bocavirus infection in children is respiratory distress. We found that 75/193 children we studied experienced respiratory distress of various degrees of severity. This was also observed in a study in Thailand, which showed that the bocavirus could cause severe respiratory distress that led to pneumonia [25]. An explanation of this could be due to the pathogenesis of bocavirus or because of the higher rate of co-infections. It could also be related to immaturity of the immune system in children younger than two years of age contributing to the severe forms of the infection [25,26]. In our study, bocavirus-infected children with predominantly gastrointestinal complaints and/or those with coexisting positive viral infection tended to have more of an immune response resulting in apparent constitutional and systemic manifestations such as fever, tiredness, and lymphadenopathy.
Most patients had shorter LOS in our study. Another study in China also found that bocavirus-positive admitted patients had a short period of hospitalization compared to other common viruses [22]. The main predictors of extended LOS in our study were comorbidity and the requirement of oxygen treatment. A similar finding with regard to oxygenation being an influencing factor of LOS of children with bocavirus infection was reported in a previous study in California [30]. Interestingly, as also observed in a study in Brazil, children who received oxygen in our study were younger (infants and toddlers) and stay longer in the hospital with the bocavirus infection. When adjusted in our logistic regression analyses, the prolonged LOS was mainly related to the oxygen requirement but not to the age factor. Also, as expected, comorbid patients had a greater need for oxygen therapy in our study than those who are otherwise healthy, and that certainly adds to the prolonged LOS. Last but not least, at all times and especially in this era of post-COVID-19 pandemic, education about hand washing and other measures of infection control for parents and caregivers of hospitalized children should not be forgotten as this would certainly improve LOS.
Most patients received bronchodilators, antipyretics, antibiotics, and steroids in our study, irrespective of complexity level of their medical condition. The top administered medical therapy in our study was bronchodilators followed by oxygen therapy. The mean oxygenation duration was 10.74 + 6.4 hours on average. Children infected with bocavirus and co-existing viruses were less likely to require oxygen supply. Although RSV is usually associated with oxygen requirement, it only coexisted with bocavirus infection in 7% of our cohort. Given this interesting observation the study lent on the oxygen requirement, the authors postulate that coexistence with other viruses probably resulted in a better immune response observed as more apparent constitutional symptoms in these patients. This could also hypothetically reduce the severity of bocavirus infection and related oxygen requirement in these patients. Steroids, specifically prednisolone, were not found effective in treating children with bocavirus [10]. Around 47% of our patients, however, were treated with steroids, and around 60% of those who received oxygen were also treated with steroids. Most of the children in our study were placed on at least one kind of antibiotic therapy during hospital admission despite the viral nature of the disease. An additional diagnosis of a possible bacterial infection such as otitis media or pneumonia in these patients has warranted the use of the antibiotic. However, the use of antibiotics was empirical in most of these patients based on the severity of presentation and suspicion of co-existing bacterial infection. Therefore, a stewardship antimicrobial program was implemented in our institute to minimize the overuse of antibiotics in such patients. Understandably, the majority of bocavirus admitted cases received anti-pyretic medications to relieve their fever.
A limitation of our study was that it was a single-center study conducted over a limited period of time. Probably, a larger multicenter study is required to evaluate the global impact of this virus over a longer period of time.
Conclusions
Bocavirus is a recently recognized virus that predominantly affects respiratory and gastrointestinal systems. Similar to other viruses, it tends to affect children more during winter months with a complicated course in vulnerable patients who have other comorbidities; hence, patients had a prolonged LOS in hospital. Oxygen and bronchodilators were commonly used modalities of management, and steroids were not found to be effective in our cohort.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. King Abdullah International Medical Research Centre issued approval RC/18/065/R. The study was reviewed and approved by the Institutional Review Board of King Abdullah International Medical Research Center (KAIMRC).
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Human bocavirus: lessons learned to date. Schildgen O. Pathogens. 2013;2:1–12. doi: 10.3390/pathogens2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human bocavirus: current knowledge and future challenges. Guido M, Tumolo MR, Verri T, et al. World J Gastroenterol. 2016;22:8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Human bocavirus-the first 5 years. Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. Rev Med Virol. 2012;22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 4.Cloning of a human parvovirus by molecular screening of respiratory tract samples. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Proc Natl Acad Sci U S A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Human bocavirus commonly involved in multiple viral airway infections. Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. J Clin Virol. 2008;41:34–37. doi: 10.1016/j.jcv.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Debiaggi M, Canducci F, Ceresola ER, Clementi M. Virol J. 2012;9:247. doi: 10.1186/1743-422X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.[Very rare and life-threatening complications of bocavirus bronchiolitis: pneumomediastinum and bilateral pneumothorax] Yeşilbaş O, Kıhtır HS, Talip Petmezci M, Balkaya S, Hatipoğlu N, Meşe S, Şevketoğlu E. Mikrobiyol Bul. 2016;50:159–164. doi: 10.5578/mb.10312. [DOI] [PubMed] [Google Scholar]
- 8.Serodiagnosis of human bocavirus infection. Kantola K, Hedman L, Allander T, et al. Clin Infect Dis. 2008;46:540–546. doi: 10.1086/526532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Human bocavirus: passenger or pathogen in acute respiratory tract infections? Schildgen O, Müller A, Allander T, Mackay IM, Völz S, Kupfer B, Simon A. Clin Microbiol Rev. 2008;21:291-304, table of contents. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.No efficacy of prednisolone in acute wheezing associated with human bocavirus infection. Jartti T, Söderlund-Venermo M, Allander T, Vuorinen T, Hedman K, Ruuskanen O. Pediatr Infect Dis J. 2011;30:521–523. doi: 10.1097/INF.0b013e318216dd81. [DOI] [PubMed] [Google Scholar]
- 11.Detection of bocavirus in children suffering from acute respiratory tract infections in Saudi Arabia. Abdel-Moneim AS, Kamel MM, Al-Ghamdi AS, Al-Malky MI. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0055500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viral load of human bocavirus-1 in stools from children with viral diarrhoea in Paraguay. Proenca-Modena JL, Martinez M, Amarilla AA, et al. Epidemiol Infect. 2013;141:2576–2580. doi: 10.1017/S095026881300023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Human metapneumovirus and human bocavirus associated with respiratory infection in Apulian population. Guido M, Quattrocchi M, Campa A, Zizza A, Grima P, Romano A, De Donno A. Virology. 2011;417:64–70. doi: 10.1016/j.virol.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.'Human bocavirus in Pakistani children with gastroenteritis'. Alam MM, Khurshid A, Shaukat S, et al. J Med Virol. 2015;87:656–663. doi: 10.1002/jmv.24090. [DOI] [PubMed] [Google Scholar]
- 15.Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. Kapoor A, Simmonds P, Slikas E, et al. J Infect Dis. 2010;201:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A novel bocavirus associated with acute gastroenteritis in Australian children. Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. PLoS Pathog. 2009;5:0. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. J Infect Dis. 2006;194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detection of human bocavirus in Japanese children with lower respiratory tract infections. Ma X, Endo R, Ishiguro N, Ebihara T, Ishiko H, Ariga T, Kikuta H. J Clin Microbiol. 2006;44:1132–1134. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical description of human bocavirus viremia in children with LRTI, Eastern Province, Saudi Arabia. Bubshait DK, Albuali WH, Yousef AA, et al. Ann Thorac Med. 2015;10:146–149. doi: 10.4103/1817-1737.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.High rate of human bocavirus and adenovirus coinfection in hospitalized Israeli children. Hindiyeh MY, Keller N, Mandelboim M, et al. J Clin Microbiol. 2008;46:334–337. doi: 10.1128/JCM.01618-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Human bocavirus infections among children less than two years old in Iran during fall and winter 2012-2013. Tabasi M, Mokhtari-Azad T, Eshraghian MR, Shadab A, Shatizadeh S, Shafiei-Jandaghi NZ, Yavarian J. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4833746. Iran J Microbiol. 2016;8:80–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Human bocavirus and human metapneumovirus in hospitalized children with lower respiratory tract illness in Changsha, China. Zhou JY, Peng Y, Peng XY, et al. Influenza Other Respir Viruses. 2018;12:279–286. doi: 10.1111/irv.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. Liu WK, Chen DH, Liu Q, Liang HX, Yang ZF, Qin S, Zhou R. BMC Infect Dis. 2011;11:345. doi: 10.1186/1471-2334-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Human bocavirus infection, Canada. Bastien N, Brandt K, Dust K, Ward D, Li Y. Emerg Infect Dis. 2006;12:848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A newly identified bocavirus species in human stool. Kapoor A, Slikas E, Simmonds P, et al. J Infect Dis. 2009;199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. Fry AM, Lu X, Chittaganpitch M, et al. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Severe pneumonia and human bocavirus in adult. Kupfer B, Vehreschild J, Cornely O, et al. Emerg Infect Dis. 2006;12:1614–1616. doi: 10.3201/eid1210.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Choi EH, Lee HJ, Kim SJ, et al. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. Lau SK, Yip CC, Que TL, et al. J Infect Dis. 2007;196:986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Human bocavirus: prevalence and clinical spectrum at a children's hospital. Arnold JC, Singh KK, Spector SA, Sawyer MH. Clin Infect Dis. 2006;43:283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]