Abstract
OBJECTIVE
Several studies support associations between relative leukocyte telomere length (rLTL), a biomarker of biological aging and type 2 diabetes. This study investigates the relationship between rLTL and the risk of glycemic progression in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In this cohort study, consecutive Chinese patients with type 2 diabetes (N = 5,506) from the Hong Kong Diabetes Register with stored baseline DNA and available follow-up data were studied. rLTL was measured using quantitative PCR. Glycemic progression was defined as the new need for exogenous insulin.
RESULTS
The mean (SD) age of the 5,349 subjects was 57.0 (13.3) years, and mean (SD) follow-up was 8.8 (5.4) years. Baseline rLTL was significantly shorter in the 1,803 subjects who progressed to insulin requirement compared with the remaining subjects (4.43 ± 1.16 vs. 4.69 ± 1.20). Shorter rLTL was associated with a higher risk of glycemic progression (hazard ratio [95% CI] for each unit decrease [to ∼0.2 kilobases]: 1.10 [1.06–1.14]), which remained significant after adjusting for confounders. Baseline rLTL was independently associated with glycemic exposure during follow-up (β = −0.05 [−0.06 to −0.04]). Each 1-kilobase decrease in absolute LTL was on average associated with a 1.69-fold higher risk of diabetes progression (95% CI 1.35–2.11). Two-sample Mendelian randomization analysis showed per 1-unit genetically decreased rLTL was associated with a 1.38-fold higher risk of diabetes progression (95% CI 1.12–1.70).
CONCLUSIONS
Shorter rLTL was significantly associated with an increased risk of glycemic progression in individuals with type 2 diabetes, independent of established risk factors. Telomere length may be a useful biomarker for glycemic progression in people with type 2 diabetes.
Introduction
Although type 2 diabetes is a progressive disease, the rate of progression is highly variable between individuals. The risk of diabetes complications, such as cardiovascular (1) and kidney dysfunction (2), is increased by hyperglycemia and is higher in those requiring pharmacologic therapy for glucose control. It is important to identify early those who may progress their diabetes rapidly for early intensive and individualized treatment to ameliorate glycemic deterioration and chronic complications. Understanding factors associated with and mediating diabetes progression may aid the development of specific therapies. Some previous studies have reported clinical or genetic factors associated with the rate of diabetes progression (3–5). Our recent study also noted during a median follow-up of 8 years, faster diabetes progression was significantly associated with low/high BMI, younger age at diagnosis, higher triglycerides (TG), and the presence of retinopathy (5).
Telomeres are protective “caps” of repetitive 5′-TTAGGG-3′ sequences at the ends of each DNA strand within every chromosome. As telomeres shorten with cell replication in proliferative somatic cells, telomere length is inversely related to the total number of cell divisions and therefore to biological age (6). Leukocyte telomere length (LTL) in patients with diabetes is shorter than their age-matched peers without diabetes. The exact mechanisms underlying this difference are not fully elucidated and may be contributed to by hyperglycemia per se or by oxidative stress and metabolic toxins (7–9). β-Cell telomeres have been shown to be shorter in people with diabetes than in subjects without diabetes (10), and LTL is positively correlated with tissue-specific telomere length, including pancreas (11). The Danish Twin Registry found that shorter baseline LTL was associated with increased progression of insulin resistance over an average period of 12 years (12). These findings suggest that LTL may also be a good candidate biomarker to predict diabetes progression in patients with type 2 diabetes.
To our knowledge, there is no previous study that has examined the relationship between telomere length and diabetes progression. Using a large cohort from Hong Kong with extensive longitudinal follow-up, we tested the hypothesis that shorter LTL is associated with more rapid diabetes progression. In addition, we performed a Mendelian randomization (MR) analysis using genetic variants associated with LTL to test the causal relationship between LTL and glycemic progression in Chinese patients with type 2 diabetes.
Research Design and Methods
Study Population
This study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee. A total of 5,506 adults with type 2 diabetes consecutively recruited between 1995 and 2007 with available DNA and clinical data were selected from the Hong Kong Diabetes Register (HKDR) (5). The details of enrollment and assessment have been published (5). Upon enrollment, all subjects provided written informed consent and agreed to additional blood collection for research purposes, including genetic studies.
LTL Measurements
Relative LTL (rLTL) was measured using an updated quantitative real-time PCR method (13–15) and calculated as ΔΔCt between telomere and single-copy gene (human β-globin [HBG]) relative to a normalization control. A no-template control (water) and a reference human sample (QC) were included for normalization of any plate-to-plate variability and calculation of ΔΔCt. There are no agreed procedures for normalizing rLTL measurement, with calculations based on no-template control or QC both being considered acceptable. Interplate coefficients of variation (CVs) of the telomere and HBG assays were 2.9% and 1.2%, respectively. The overall intraplate CV was 1.2% for rLTL and 0.4% for HBG. A total of 157 (2.9%) subjects were excluded due to failed QC or missing rLTL measures. Of the remaining 5,349 subjects, 905 patients were already on insulin treatment at baseline, 486 were considered to have failed noninsulin diabetes treatment at baseline based on two consecutive hemoglobin A1c (HbA1c) ≥8.5%, and 21 required insulin use within 1 year. Finally, a total of 3,937 patients were included for analysis.
We further estimated absolute LTL from whole-genome sequencing (WGS) data for whole blood samples in a subset of 251 subjects using Telseq software (16). Telseq defines the reads which contained seven or more TTAGGG repeats as telomeres, calculates the relative proportion of telomeric reads among all sequenced reads and transforms this value into absolute telomere length. The Pearson correlation between relative and absolute measurements was r = 0.148, P = 0.019, and 1 unit of rLTL was equivalent to 5.0 kilobases of absolute LTL estimated from WGS.
Outcome Definition
Clinical outcomes were defined using hospital discharge diagnoses based on the ICD-9 and mortality as censored on or before 30 June 2014. All hospitalization records were retrieved from the Hong Kong Hospital Authority system using a unique identifier number. As there is often delay in commencing insulin after the clinical indication has arisen, due to patient reluctance or clinical inertia, we used “clinical indication for insulin treatment” as the main end point. Glycemic progression was defined as: 1) progression to continuous insulin treatment (>6 months’ duration), or 2) failure of noninsulin diabetes treatment (two consecutive HbA1c values ≥8.5%, >3 months apart during treatment with two or more noninsulin diabetes therapies [metformin, sulfonylureas, or thiazolidinediones]), in line with the definition used in the DIRECT study (3,5). Follow-up time was defined as the period from baseline visit to the date of the first clinical end point or the censored dates, whichever occurred first. The alternative end point of continuing insulin treatment was defined as “actual use of insulin” and used for the sensitivity analysis. The delay of time to first insulin use was defined as the period from oral drug failure before insulin initiation.
In addition, we selected 3,757 subjects with at least three HbA1c measurements during follow-up. Glycemic exposure in these subjects was estimated by calculating the area under the individual HbA1c curve >7% (53 mmol/mol), from enrollment until the date of the first clinical end point or the censored date, whichever came first.
MR
We applied two MR methods based on individual-level data and published data. Ten LTL-associated genetic variants were selected (P < 5 × 10−8; linkage disequilibrium coefficient r2 < 0.5; minor allele frequency >0.01) from the Singapore Chinese Health Study (17). In the first one-sample method, we extracted these single nucleotide polymorphisms (SNPs) from imputed genome-wide genotyping data (Illumina Omni 2.5+ exome array; Illumina Inc., San Diego, CA), and developed a weighted genetic risk score (GRS). The β estimate for each SNP (SNP–LTL effect) was derived from the original genome-wide association study (GWAS) (17). A subset (N = 3,455) of the primary cohort had available genotyping data. A higher GRS value refers to shorter genetically predicted rLTL. We then explored the causal relationship between rLTL and glycemic progression through GRS and individual SNPs. One-sample MR was conducted using “ivtools” package (https://cran.r-project.org/web/packages/ivtools/index.html) in R software, adjusting for age, sex, and the top three principal components.
A second two-sample MR method using inverse variance weighting (IVW) with the Two-Sample MR package (https://github.com/MRCIEU/TwoSampleMR) was performed, complemented by the maximum likelihood, weighted median, and weighted mode approaches. We also performed sensitivity analyses to check heterogeneity using Cochran’s Q statistic and horizontal pleiotropy of the genetic instruments using the MR-Egger regression approach. To validate the robustness of results, we also performed leave-one-out analysis and single SNP analysis.
Statistical Analysis
All analyses were performed using R version 3.6.1 (www.r-project.org). Data are expressed as mean ± SD, median (Q1, Q3), or percentage as appropriate and Student t test, χ2, or Fisher exact tests were used for comparisons between groups, as appropriate. A general linear model was conducted to compare rLTL between groups after adjustment. We performed multivariate Cox regression to examine associations between baseline rLTL and diabetes progression, and linear regression was conducted to estimate the relationship between rLTL and glycemic exposure. Confounders included in the regression model were selected from an earlier study using the same cohort (5). Due to the nonlinear relationship between BMI and outcome and the violation of proportional hazard assumption for HbA1c, both BMI and HbA1c were included as strata variables in Cox models, whereas other covariates were assumed to have the same effects across strata. We excluded subjects with missing data from the regression models. As the association between rLTL and diabetes progression was linear, we investigated rLTL as a continuous variable in Cox regression. The hazard ratio (HR) represented the relative increase in the risk of diabetes progression associated with each ΔΔCt decrease of rLTL. Fine-Gray competing risk regression models were used to estimate the subdistribution HR of rLTL, with death caused by other diseases entered as the competing risk. We also conducted additional sensitivity analyses with rLTL normalized to QC, as well as absolute LTL from WGS. A two-tailed P value <0.05 was considered statistically significant.
Results
The Cohort
Table 1 shows baseline characteristics of 3,937 subjects, stratified according to the study outcome. Mean age was 57.0 ± 13.3 years, with 45.0% being male and mean time since diabetes diagnosis 5.8 ± 5.9 years. A total of 1,803 (45.8%) participants progressed to requiring insulin treatment during a mean follow-up period of 8.8 ± 5.4 years. The overall incidence rate of diabetes progression was 52.0 (95% CI 49.7–54.5)/1,000 person-years. The mean period from an established diagnosis of type 2 diabetes to requirement for insulin was 12.9 ± 6.8 years. Compared with patients who remained controlled on oral glucose-lowering drugs (OGLD), patients who progressed to requiring insulin had younger age and age of diagnosis, with longer diabetes duration, higher smoking rates, higher blood pressure glucose, lipids, and urine albumin-to-creatinine ratio (ACR). They were also more likely to have complications at baseline, including retinopathy, peripheral neuropathy, and albuminuria (Table 1). There was no significant difference in sex and BMI. Progressors, despite their younger age than nonprogressors at baseline, had significantly shorter rLTL (4.43 ± 1.16 vs. 4.69 ± 1.20; P < 0.001). After further adjustment for the age difference, age at diagnosis, sex, diabetes duration, smoking status, blood pressure, lipids, urine ACR, and HbA1c, the difference in rLTL remained significant (P < 0.001).
Table 1.
Baseline characteristics of progressors and nonprogressors for glycemic deterioration defined as need for insulin treatment
| Baseline variables | Nonprogressors | Progressors | P value |
|---|---|---|---|
| N | 2,134 | 1,803 | |
| Age (years) | 58.5 ± 13.3 | 55.3 ± 13.2 | <0.001 |
| Age at diagnosis (years) | 53.61 ± 12.6 | 48.5 ± 12.3 | <0.001 |
| Year of diagnosis | 1996 (1992, 2002) | 1993 (1988, 1997) | <0.001 # |
| Male (%) | 932 (43.7) | 839 (46.5) | 0.078 |
| Duration of diabetes (years) | 4.9 ± 5.4 | 6.8 ± 6.2 | <0.001 |
| Current smoker (%) | 242 (11.4) | 266 (14.8) | 0.002 |
| Ever smoked (%) | 579 (27.2) | 571 (31.7) | 0.002 |
| SBP (mmHg) | 134.3 ± 20.5 | 135.4 ± 21.0 | 0.104 |
| DBP (mmHg) | 75.7 ± 11.0 | 77.1 ± 10.8 | <0.001 |
| BMI (kg/m2) | 25.2 ± 4.0 | 25.4 ± 4.1 | 0.234 |
| HbA1c (mmol/mol) | 51.2 ± 15.1 | 65.0 ± 21.1 | <0.001 |
| HbA1c (%) | 6.8 ± 1.4 | 8.1 ± 2.0 | <0.001 |
| FPG (mmol/L) | 7.5 ± 2.5 | 9.4 ± 3.5 | <0.001 |
| TC (mmol/L) | 5.1 ± 1.1 | 5.3 ± 1.2 | <0.001 |
| HDL-C (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.4 | <0.001 |
| Non-HDL (mmol/L) | 3.8 ± 1.1 | 4.0 ± 1.2 | <0.001 |
| LDL-C (mmol/L) | 3.0 ± 1.0 | 3.2 ± 1.0 | <0.001 |
| TG (mmol/L) | 1.3 (0.9, 1.9) | 1.5 (1.0, 2.2) | <0.001 # |
| Urinary ACR (mg/mmol) | 1.4 (0.7, 5.3) | 2.7 (0.9, 12.8) | <0.001 # |
| eGFR (mL/min/1.73 m2) | 81.41 ± 23.03 | 83.22 ± 25.57 | 0.019 |
| Retinopathy (%) | 396 (18.6) | 482 (26.7) | <0.001 |
| Neuropathy (%) | 303 (14.2) | 397 (22.0) | <0.001 |
| Microalbuminuria (%) | 473 (23.1) | 512 (29.6) | <0.001 |
| Macroalbuminuria (%) | 215 (10.5) | 318 (18.4) | <0.001 |
| Lipid-lowering drugs (%) | 386 (18.1) | 248 (13.8) | <0.001 |
| Antihypertensive drugs (%) | 964 (45.2) | 702 (38.9) | <0.001 |
| Oral antihyperglycemic drugs (%) | 1,384 (64.9) | 1,264 (70.1) | 0.001 |
| RAS inhibitors (ACE inhibitors or ARBs) (%) | 374 (17.5) | 315 (17.5) | 0.998 |
| Absolute telomere length (kilobase pair) | 5.65 ± 0.21 | 5.61 ± 0.21 | <0.001 |
| Relative telomere length (ΔΔCt) | 4.69 ± 1.20 | 4.43 ± 1.16 | <0.001 |
Data are mean ± SD, number (%), or median (Q1, Q3). The t test or Mann-Whitney rank sum test was used for continuous variables, and χ2 test was used for categorical variables.
ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; FPG, fasting plasma glucose; RAS, renin-angiotensin system; SBP, systolic blood pressure; TC, total cholesterol.
Logarithmic transformation was used in TG and ACR. Boldface indicate P values < 0.05.
rLTL and Glycemic Progression
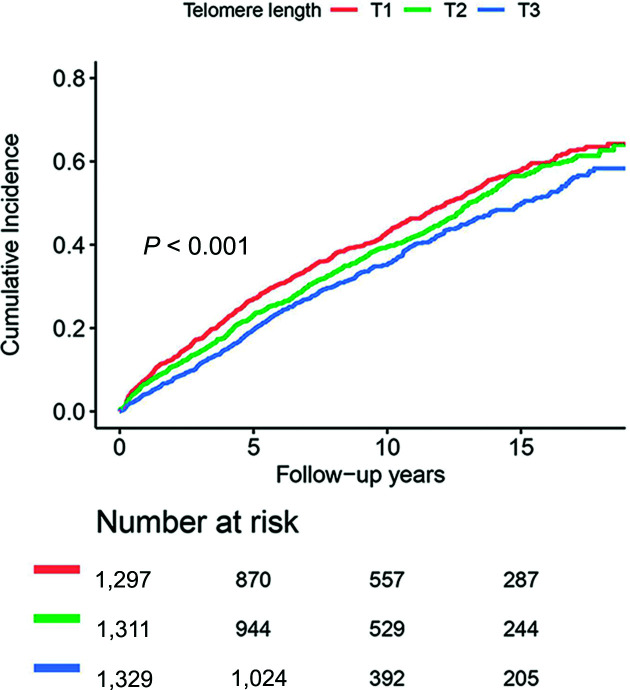
Subjects with shorter telomeres had increased risk of needing exogenous insulin, which persisted after adjusting for traditional risk factors (Fig. 1). In the Cox regression analysis (Table 2), each rLTL unit decrease was associated with a 1.098-times (95% CI 1.056–1.142) higher risk of glycemic progression. When further adjusted for age at diagnosis, diabetes duration, smoking, lipids [log(TG) and LDL-cholesterol (LDL-C)], lipid drug use, neuropathy, retinopathy, kidney function [log(ACR) and estimated glomerular filtration rate (eGFR)], and use of ACE inhibitors or angiotensin receptor blockers and of OGLD, the association remained significant (P = 0.023) (Table 2). Using competing risk regression models yielded similar results, which also indicated no significant competing risk from death before development of glycemic progression (Supplementary Table 1).
Figure 1.
Cumulative probability of patients with progression to insulin requirement according to tertiles of telomere length. Patients were divided by LTL < 4.153, 4.153 ≤ LTL < 5.092, and LTL ≥ 5.092. rLTL was calculated by negative control (water).
Table 2.
Cox regression analysis for association between baseline relative telomere length and glycemic progression defined as need for insulin treatment
| Variables | Unadjusted model | Fully adjusted model | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| rLTL | 1.098 (1.056–1.142) | <0.001 | 1.052 (1.007–1.100) | 0.023 |
| Age at diagnosis (per 1 year) | 0.971 (0.966–0.976) | <0.001 | ||
| Duration of diabetes (per 1 year) | 1.020 (1.011–1.029) | <0.001 | ||
| Smoking | ||||
| Non-smoker | Reference | |||
| Ex-smoker | 1.247 (1.088–1.430) | 0.002 | ||
| Current smoker | 1.158 (1.003–1.337) | 0.045 | ||
| Log (TG) | 1.323 (1.044–1.675) | 0.020 | ||
| LDL-C | 0.936 (0.886–0.987) | 0.015 | ||
| Log urinary ACR | 1.373 (1.262–1.495) | <0.001 | ||
| eGFR | 0.991 (0.988–0.994) | <0.001 | ||
| Sensory neuropathy | 1.268 (1.120–1.435) | <0.001 | ||
| Retinopathy | 1.235 (1.092–1.396) | 0.001 | ||
| Use of OGLD | 1.284 (1.143–1.443) | <0.001 | ||
| Use of lipid-lowering drugs | 1.049 (0.901–1.221) | 0.541 | ||
| Use of RAS inhibitors | 1.147 (0.999–1.318) | 0.052 | ||
BMI and baseline HbA1c categories were included as strata variables. BMI was categorized as four groups (<18.5, 18.5–23, 23–25, and ≥25 kg/m2), and baseline HbA1c was categorized as three groups (<7%, ≥7–9%, and ≥9%).
RAS, renin-angiotensin system. Boldface indicate P values < 0.05.
As an earlier age of type 2 diabetes diagnosis was strongly associated with glycemic progression, we divided participants into young-onset diabetes (diagnosis <40 years) and late-onset diabetes (≥40 years). Baseline rLTL was independently associated with progression to insulin requirement (HR [95% CI] 1.146 [1.038–1.267]; P = 0.007) in the late-onset group. The fully adjusted association differed between women and men, being positive for women (HR [95% CI] 1.119 [1.054–1.187]; P < 0.001), but not for men: P for interaction was 0.012 (Supplementary Table 2).
Sensitivity Analyses
To assess the robustness of our findings, sensitivity analyses were performed. First, we defined the subcomponent of glycemic deterioration using the end point of continuing insulin treatment, with those on insulin for >6 months being defined as progressors. Among 1,803 subjects who progressed to needing insulin, 300 (16.6%) experienced delay of insulin initiation, represented by the period of oral drug failure before insulin initiation. The median period of delay between OGLD failure and insulin initiation was 2.6 (1.4–4.7) years. During a mean (SD) 9.4 (5.4) years of follow-up, 38.2% (1,503) of participants progressed to prescription of insulin. Progressors had significantly shorter rLTL than those who remained on OGLD (4.39 ± 1.15 vs. 4.68 ± 1.20; P < 0.001). rLTL was associated with progression to actual insulin use (HR [95% CI] 1.121 [1.075–1.170] per each unit decreased of rLTL; P < 0.001), which persisted after adjusting for known risk factors (HR [95% CI] 1.065 [1.016–1.117] per each unit decreased; P = 0.009) (Supplementary Table 3).
Second, we explored the relationship between baseline rLTL and a subset of the subjects in whom total glycemic exposure during follow-up was available. Baseline rLTL negatively correlated with glycemic exposure during follow-up (β = −0.048; P < 0.001), which remained significant after adjusting for the same confounders from the Cox regression models (β = −0.018; P = 0.001) (Supplementary Table 4). Compared with subjects with the shortest telomeres (T1 < 4.152), those with longer rLTL (T3 and T2) had significantly lower glycemic exposure during follow-up (6.22 [3.90–10.84] and 7.76 [4.49–14.25] vs. 8.42 [5.01–15.70]; P = 0.025 and P < 0.001, respectively).
Thirdly, based on absolute LTL data extrapolated from WGS, 1-kilobase decrease in absolute LTL was associated with a 1.688-fold higher risk of diabetes progression (95% CI 1.353–2.105; P < 0.001), which remained significant after adjustment (HR [95% CI] 1.332 [1.040–1.705]; P = 0.023) (Supplementary Table 5).
Furthermore, we conducted analyses stratified by diabetes duration. We found that when stratified by duration of diabetes, the effect size of the association between baseline rLTL and diabetes progression was similar regardless of duration of diabetes, although results in some of the subgroups were no longer significant due to the smaller sample size (Supplementary Table 6).
Lastly, we compared LTL between subjects with GAD autoantibody positive (N = 69) and negative (N = 3,868). There was no significant difference in LTL between the two groups (4.74 ± 1.17 vs. 4.57 ± 1.19; P = 0.237). As an additional sensitivity analysis, we further removed those 69 individuals with known GAD+ and repeated the regression analysis (Supplementary Table 7) (N = 3,868). The results were consistent with the original analysis, with shorter rLTL being associated with increased risk of progression to need for insulin (HR 1.052 [1.006–1.100] in the fully adjusted model; P = 0.026) (Supplementary Table 7).
Genetically Determined LTL and MR
To explore whether rLTL may be directly related to glycemia progression, we examined the relationship between genetically determined telomere length and diabetes progression. Association of each SNP and the GRSTL with rLTL are shown in Supplementary Table 8, with the GRS inversely associated with rLTL (β = −0.525; P < 0.001). Replacing rLTL with genetically determined LTL, reflected by the GRSTL, highlights consistent association with the GRS being inversely associated with progression to insulin requirement (odds ratio [OR] 1.366 [95% CI 0.959–1.949]), though this relationship did not reach statistical significance (P = 0.085).
One-sample MR analysis provided evidence of an association between genetically predicted rLTL (per 1-unit decrease) and progression to insulin requirement (OR 1.820 [95% CI 0.911–3.638]); P = 0.090).
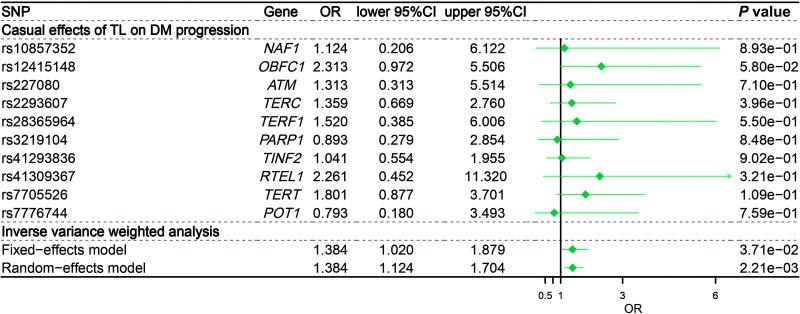
In two-sample MR analysis, data from the GWAS for rLTL from the Singapore Chinese Health Study (N = 23,096) were used to identify SNPs associated with rLTL in Chinese. We also included data from 3,455 HKDR subjects with available genotype data. This revealed a significant causal relationship between rLTL shortening and glycemic progression (IVW estimate of OR: 1.384 per 1-unit decrease in genetically determined rLTL [95% CI 1.124–1.704]; P = 0.002) (Fig. 2). The maximum likelihood, weighted mode, and weighted median yielded a similar pattern of effects, although results using some methods were no longer significant (Supplementary Table 9). To investigate the consistency and directional effect of the individual SNP association with rLTL and glycemic progression, we plotted the effect and SE of SNPs on rLTL with their corresponding effect on the risk of requiring insulin (Fig. 2). Furthermore, analyses leaving out each SNP one by one revealed that no single SNP drove these results, but the results rather reflected an overall combined pattern of opposite relationships between rLTL shortening and diabetes progression (Supplementary Fig. 2). Similarly, we observed no heterogeneity in the effect estimates for the 10 independent rLTL-associated SNPs (Cochran’s Q statistic from IVW: P = 0.899; MR-Egger: P = 0.853). Additionally, there was no evidence of directional pleiotropy in the MR-Egger analysis (P = 0.723). Furthermore, our MR results on actual use of insulin as the outcome were generally similar in direction and magnitude to estimates based on clinical indication for insulin requirement (IVW estimate of OR: 1.442 [95% CI 1.129–1.843]; P = 0.003) (Supplementary Table 10).
Figure 2.
OR for glycemic progression per 1-unit decreased in genetically determined rLTL. Glycemic progression was defined as need for insulin treatment. DM, diabetes mellitus.
Conclusions
In this large prospective study from HKDR, we analyzed relationships between baseline rLTL with progression to requirement for and for initiation of insulin treatment among 3,937 Chinese patients with type 2 diabetes during a mean follow-up of 8.8 years. Shorter baseline rLTL was independently associated with a higher risk of both requirement for and actual prescription of insulin. Using the largest available GWAS data for rLTL and glycemic progression, we performed both one-sample and two-sample MR analyses to assess the causal relationship between rLTL and glycemic progression.
In general, most studies have reported shorter rLTL among subjects with type 1 or type 2 diabetes compared with subjects without diabetes (7). In the largest study of LTL to date involving 472,174 participants from UK Biobank, Codd et al. (18) found LTL was significantly associated with glucose and type 2 diabetes in the observational data set, but the MR analysis did not support a causal relationship between LTL and diabetes. As far as we are aware, no study has so far, in Europeans or other populations, examined the relationship between LTL and diabetes progression. In our analysis, subjects who progressed to insulin requirement were younger at baseline compared with those who remained stable with OGLD. The mean LTL among progressors was significantly shorter than those of the nonprogressors independent of age. Biological age is impacted by many lifestyle factors, including diet, exercise, and sleeping habits. It is believed that biological age is more clinically relevant and tends to outperform chronological age in predicting healthy aging (19). Telomeres play a major role in how quickly cells age and die, and LTL is regarded as a good marker of biological age (20). Our results demonstrated that shorter LTL was associated with progression to insulin requirement, which was independent of chronological age. Those subjects who progressed to insulin use were younger but also had significantly shorter telomeres than those remaining on OGLD, which may be impacted by their longer diabetes duration and worse metabolic milieu or specific mechanisms underlying the link between telomere shortening and diabetes progression.
The phenotype at type 2 diabetes diagnosis provides information about future insulin requirement by which patients with pronounced hyperglycemia commonly continue to have hyperglycemia after medical nutritional therapy (21), and those who are nonobese most commonly require insulin therapy (22). Several studies have built clinical and genetic prediction models for glycemic progression in patients with type 2 diabetes (3–5). Younger onset of diabetes was strongly associated with earlier need for insulin therapy (3). In our study, LTL was associated with progression to insulin treatment among both young-onset (diagnosis <40 years) and late-onset diabetes (diagnosis ≥40 years), which was consistent with results from the whole cohort. In the sensitivity analysis, with consideration of delayed insulin use, LTL was still significantly associated with glycemic progression. Different normalization or changes in covariates did not affect this association, including adjusting for HDL-cholesterol (HDL-C). HDL modulates insulin secretion and β-cell survival, and lower HDL-C levels have been associated with faster progression to need for OGLD and/or insulin in the FIELD trial in type 2 diabetes (23). The sensitivity analyses, including exclusion of subjects with GAD+, or analysis stratified by diabetes duration, supported that the association between LTL and diabetic progression was not related to the impact of GAD+ autoimmunity or undiagnosed diabetes. Thus, our results support the independent association between LTL and glycemic progression.
The fully adjusted association differed between women and men. It is well established that men in general have shorter LTL compared with women (24). This sex difference was also observed in our previous reports on the risk association between rLTL and incident cardiovascular disease and kidney failure (2,15). We postulate that both insulin resistance and β-cell failure contribute to glycemic progression and the requirement of insulin therapy. Men generally have higher incidence of diabetes partly due to more severe metabolic syndrome. If insulin resistance has a greater contribution to glycemic progression than β-cell dysfunction in men, this may explain the lack of association between rLTL and glycemic progression in this group.
In this study, we showed that genetically decreased LTL was associated with increased risk of glycemic progression. The GRS used as the instrumental variable includes 11 SNPs, most of which are involved in telomere biology, including preserving telomere structure, regulating LTL, and functioning in DNA repair pathways (17,25). Our MR results further suggested the potential causal effect of rLTL on glycemic progression. Previous studies reported short telomeres can cause spontaneous insulin secretion defects in vivo and in vitro. Mouse β-cells with dysfunctional telomeres exhibit senescence and have an activated DNA damage response, altered gene expression, p16INK4A upregulation, and impaired proliferation. The dysregulation of gene expression was shown to alter pathways that are essential for insulin secretion and exocytosis signaling, include mitochondrial function and Ca2+ handling (26). Kuhlow et al. (27) generated mice that were homozygotically null for the telomerase RNA component gene and exhibited impaired insulin secretion and glucose intolerance due to reduced islet size and impaired β-cell replication capacity. Although the telomere and telomerase system differ between humans and animals, our data suggested a plausible link between genetically determined LTL and glycemic progression and establish that telomere attrition had an additive effect on glycemic progression pathogenesis.
Increased oxidative stress caused by hyperglycemia and the diabetes milieu accelerates telomere shortening (28), and, in fact, clinical studies have reported that LTL is negatively correlated with biomarkers of oxidative DNA damage in patients with type 2 diabetes (29). β-cells are extremely sensitive to oxidative stress, which may be due to reduced antioxidant enzymes and high oxygen consumption during insulin secretion (30). In pancreatic tissue from 47 patients with type 2 diabetes and 51 subjects without diabetes, telomere length in β-cells was reduced in patients with type 2 diabetes, and there was a negative correlation between HbA1c and β-cell telomere length (10). Human endothelial cells exposed to high glucose had decreased telomerase activity and shortened telomeres (31), supporting that oxidative stress induced by hyperglycemia can promote telomere dysfunction and further shorten β-cell life span. Of relevance, there is considerable interest in targeting β-cell regeneration, including via the effects of oxidative stress (32). Weight loss, the reversal of oxidative stress, and endoplasmic reticulum stress have been associated with restoration of β-cell function at the early stage of diabetes (33). Meanwhile, some longitudinal studies show protective effects of healthy lifestyle interventions, including exercise, nutrition, and drugs, on LTL (7). Hence, monitoring LTL in patients with diabetes may reflect oxidative stress levels and help predict β-cell function and glycemic progression. This is particularly relevant given recent data from the GTex Consortium highlighting the association between rLTL and tissue-specific telomere length, including TL in the pancreas (11). Prescription of more intensive treatment may restore β-cell function and prevent diabetes progression in high-risk patients.
We acknowledge several study limitations. First, LTL was measured only at one time point, which limits our ability to explore the impact of the LTL attrition rate on outcomes. Moreover, the WGS analysis sample size was relatively small, and the correlation between relative and absolute LTL was relatively weak. However, our results were similar to those obtained in the GTex study (11). Type 2 diabetes may exist prior to clinical diagnosis, and, as for most studies, we can only report known diabetes duration. Last, all analyses were based on a single cohort, and validation studies are merited. Nevertheless, strengths of our study include a large cohort size in a single prospective study in which telomere length was measured in >5,000 Chinese patients with type 2 diabetes. A robust laboratory assay with excellent CVs was used. The length of follow-up ensured adequate event rates and statistical power. Moreover, subjects in our study had comprehensive baseline data on demographics, metabolic profiles, genetic, and drug information which may influence rLTL.
In conclusion, our findings highlight the relationship between LTL and progression to insulin requirement in type 2 diabetes. In Chinese patients with type 2 diabetes, shorter LTL was associated with higher risk of progression to insulin treatment, which was independent of traditional risk factors. Further studies in other populations are merited to validate the use of LTL as a biomarker for prediction of glycemic progression and as a surrogate end point in intervention studies.
Article Information
Acknowledgments. The authors are thankful to all of the subjects who took part in and contributed to the study. The authors also thank the physicians and nurses of the Diabetes and Endocrine Centre and the endocrine team at the Prince of Wales Hospital and Dr Andrzej Januszewski for assistance with the individual subject DNA QC material.
Funding. This study was supported by the Research Grants Council Theme-based Research Scheme (T12-402/13N) and Research Impact Fund (R4012-18), the Chinese University of Hong Kong Vice-Chancellor One-off Discretionary Fund, a Direct Grant, and the Chinese University of Hong Kong-Shanghai Jiao Tong University Joint Research Fund. R.C.W.M. acknowledges support from a Croucher Foundation Senior Medical Research Fellowship. F.C., A.A.H., and R.C.W.M. acknowledge support from the Chinese University of Hong Kong Global Scholarship Programme for Research Excellence. R.C.W.M. also acknowledges support from the Internationalization Faculty Mobility Schemes (Outbound Research Mobility Scheme) from the Office of Academic Links, Chinese University of Hong Kong. F.C. acknowledges support from the Dragon Culture PhD Scholarships for Medical Studies and Faculty Postdoctoral Fellowship Scheme, Chinese University of Hong Kong. M.V.J. and A.A.H. acknowledge JDRF (U.S.) and JDRF (Australia) CRN for their fellowships, respectively.
Duality of Interest. A.O.L. has served as an advisory committee member for AstraZeneca, Boehringer Ingelheim, Sanofi, and Amgen; and has received research grants and travel grants from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, and Amgen. A.P.K. has received research grants and/or speaker honoraria from Abbott, AstraZeneca, Eli Lilly and Company, Merck Serono, Nestle, Sanofi, and Novo Nordisk. A.J.J. has served on advisory boards for Medtronic Australia, Abbott Diabetes Australia, and Sanofi; has received research grants from Abbott Europe, Mylan, and Sanofi; and has received speaker honorarium from Amgen. J.C.N.C. has received research grants and/or honoraria for consultancy or giving lectures from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly and Company, GlaxoSmithKline, Merck Serono, Merck Sharp & Dohme, Novo Nordisk, Pfizer, and Sanofi. R.C.W.M. has received research grants for clinical trials from AstraZeneca, Bayer, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Tricida Inc.; honoraria for consultancy or lectures from AstraZeneca and Boehringer Ingelheim, all used to support diabetes research at the Chinese University of Hong Kong; and is a cofounder of GemVCare, a technology startup initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities. The sequencing data was partially supported by an educational grant from AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.C. measured telomere length, performed statistical analysis, and wrote the manuscript. A.O.L., M.S., C.H., G.J., A.Y., H.W., C.H.T.T., B.F., E.S.H.L., E.C., S.K.W.T., and H.C.S. contributed to data analysis and interpretation of results. C.K.P.L., A.C.W.N., K.K.W., and H.M.L. contributed to study logistics and sample preparation. M.V.J. and A.A.H. developed the modified rLTL measurement method and trained F.C. L.C., A.C.K., M.V.J., A.A.H., and A.J.J. contributed to data analysis. J.C.N.C. is the principal investigator of HKDR and contributed to research design, study logistics, funding, and interpretation of results. A.O.L., A.P.K., W.Y.S., J.C.N.C., and R.C.W.M. contributed to subject recruitment and study logistics. R.C.W.M. designed the research, obtained funding to support the study, supervised the research work, performed statistical analysis, and wrote the manuscript. All authors contributed meaningfully to this manuscript and approved the final version. R.C.W.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.17699903.
References
- 1. Lawes CM, Parag V, Bennett DA, et al.; Asia Pacific Cohort Studies Collaboration . Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 2004;27:2836–2842 [DOI] [PubMed] [Google Scholar]
- 2. Cheng F, Luk AO, Wu H, et al. Relative leucocyte telomere length is associated with incident end-stage kidney disease and rapid decline of kidney function in type 2 diabetes: analysis from the Hong Kong Diabetes Register. Diabetologia 2022;65(2):375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou K, Donnelly LA, Morris AD, et al. Clinical and genetic determinants of progression of type 2 diabetes: a DIRECT study. Diabetes Care 2014;37:718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner R, Stratton I, Horton V, et al.; UK Prospective Diabetes Study Group . UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]
- 5. Jiang G, Luk AO, Tam CHT, et al.; Hong Kong Diabetes Register TRS Study Group; Hong Kong Diabetes Biobank Study Group . Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med 2020;17:e1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simm A, Nass N, Bartling B, Hofmann B, Silber R-E, Navarrete Santos A. Potential biomarkers of ageing. Biol Chem 2008;389:257–265 [DOI] [PubMed] [Google Scholar]
- 7. Cheng F, Carroll L, Joglekar MV, et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol 2021;9:117–126 [DOI] [PubMed] [Google Scholar]
- 8. Blazer S, Khankin E, Segev Y, et al. High glucose-induced replicative senescence: point of no return and effect of telomerase. Biochem Biophys Res Commun 2002;296:93–101 [DOI] [PubMed] [Google Scholar]
- 9. Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000;36:195–200 [DOI] [PubMed] [Google Scholar]
- 10. Tamura Y, Izumiyama-Shimomura N, Kimbara Y, et al. β-cell telomere attrition in diabetes: inverse correlation between HbA1c and telomere length. J Clin Endocrinol Metab 2014;99:2771–2777 [DOI] [PubMed] [Google Scholar]
- 11. Demanelis K, Jasmine F, Chen LS, et al.; GTEx Consortium . Determinants of telomere length across human tissues. Science 2020;369:eaaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhulst S, Dalgård C, Labat C, et al. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 2016;59:1258–1265 [DOI] [PubMed] [Google Scholar]
- 13. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joglekar MV, Satoor SN, Wong WKM, Cheng F, Ma RCW, Hardikar AA. An optimised step-by-step protocol for measuring relative telomere length. Methods Protoc 2020;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng F, Luk AO, Tam CHT, et al. Shortened relative leukocyte telomere length is associated with prevalent and incident cardiovascular complications in type 2 diabetes: analysis from the Hong Kong Diabetes Register. Diabetes Care 2020;43:2257–2265 [DOI] [PubMed] [Google Scholar]
- 16. Ding Z, Mangino M, Aviv A, Spector T, Durbin R; UK10K Consortium . Estimating telomere length from whole genome sequence data. Nucleic Acids Res 2014;42:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorajoo R, Chang X, Gurung RL, et al. Loci for human leukocyte telomere length in the Singaporean Chinese population and trans-ethnic genetic studies. Nat Commun 2019;10:2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Codd V, Wang Q, Allara E, et al. Polygenic basis and biomedical consequences of telomere length variation. Nat Genet 2021;53:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci 2013;68:667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine 2017;21:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. United Kingdom Prospective Diabetes Study Group . UK Prospective Diabetes Study 24: relative efficacy of sulfonylurea, insulin and metformin therapy in newly diagnosed non-insulin dependent diabetes with primary diet failure followed for six years. Ann Intern Med 1998;128:165–175 [DOI] [PubMed] [Google Scholar]
- 22. Nabarro JD. Diabetes in the United Kingdom: a personal series. Diabet Med 1991;8:59–68 [DOI] [PubMed] [Google Scholar]
- 23. Waldman B, Jenkins AJ, Davis TM, et al.; FIELD Study Investigators . HDL-C and HDL-C/ApoA-I predict long-term progression of glycemia in established type 2 diabetes. Diabetes Care 2014;37:2351–2358 [DOI] [PubMed] [Google Scholar]
- 24. Dalgård C, Benetos A, Verhulst S, et al. Leukocyte telomere length dynamics in women and men: menopause vs age effects. Int J Epidemiol 2015;44:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saxena R, Bjonnes A, Prescott J, et al. Genome-wide association study identifies variants in casein kinase II (CSNK2A2) to be associated with leukocyte telomere length in a Punjabi Sikh diabetic cohort. Circ Cardiovasc Genet 2014;7:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012;13:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuhlow D, Florian S, von Figura G, et al. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging (Albany NY) 2010;2:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jennings BJ, Ozanne SE, Hales CN. Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab 2000;71:32–42 [DOI] [PubMed] [Google Scholar]
- 29. Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006;29:283–289 [DOI] [PubMed] [Google Scholar]
- 30. Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 2017;26:501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsui-Hirai H, Hayashi T, Yamamoto S, et al. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: a relationship between telomeres and nitric oxide. J Pharmacol Exp Ther 2011;337:591–599 [DOI] [PubMed] [Google Scholar]
- 32. White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible β-cell dysfunction. Diabetes Care 2016;39:2080–2088 [DOI] [PubMed] [Google Scholar]
- 33. Pinnick K, Neville M, Clark A, Fielding B. Reversibility of metabolic and morpholo-gical changes associated with chronic exposure of pancreatic islet β-cells to fatty acids. J Cell Biochem 2010;109:683–692 [DOI] [PubMed] [Google Scholar]