Abstract
Although technological advances and clinical studies on stem cells have been increasingly reported in stroke, research targeting hemorrhagic stroke is still lacking compared to that targeting ischemic stroke. Studies on hemorrhagic stroke are also being conducted, mainly in the USA and China. However, little research has been conducted in Korea. In reality, stem cell research or treatment is unfamiliar to many domestic neurosurgeons. Nevertheless, given the increased interest in regenerative medicine and the increase of life expectancy, attention should be paid to this topic. In this paper, we summarized pre-clinical rodent studies and clinical trials using stem cells for hemorrhagic stroke. In addition, we discussed results of domestic investigations and future perspectives on stem cell research for a better understanding.
Keywords: Stem cell, Hemorrhage stroke, Therapeutics
INTRODUCTION
Hemorrhagic stroke refers to spontaneous intracranial hemorrhage due to blood vessel rupture from hypertension, cerebral amyloid angiopathy, and other vascular abnormalities such as aneurysm, arteriovenous malformation, and Moyamoya disease [4]. Depending on the anatomical location, hemorrhagic stroke is divided into intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH), subarachnoid hemorrhage (SAH), and subdural hematoma. Hemorrhagic stroke accounts for 10–20% of all strokes. Its prevalence is higher in Asia than in the West [4]. Hong et al. [26] have reported that the relative proportion of hemorrhagic stroke at admission ranged from 23% to 35%. Once hemorrhagic stroke occurs, increased intracranial pressure (IICP) is the main problem. The mortality of patients with a hemorrhagic stroke is more than twice that of patients with an ischemic stroke [26]. Therefore, lowering IICP via medical treatment or surgical decompression is the main concerns for neurosurgeons. However, in addition to primary brain damage, secondary brain damage due to reactive oxygen species production, inflammation, and subsequent neuronal cell death can occur, leading to ultimate glial scars and cavity formation in the brain. Strategies of stem cellbased therapy for preventing secondary brain damage include blocking chain reactions of oxidative stress by red blood cell lysis or inflammatory reactions by thrombin, enhancing therapeutic effects by promoting vascular regeneration, or promoting nerve regeneration by inducing the secretion of growth factors. Considering that hemorrhagic stroke occurs at a relatively younger age than ischemic stroke and that 40% of patients remain functionally independent [4], neurosurgeons should consider additional treatment that can help prevent secondary brain damage and aid in rehabilitation as well as surgical treatment.
Stem cells are very attractive candidates in regenerative medicine due to their self-renewal and differentiation capacities as well as long-term ex vivo expansion. Stem cell can be considered for treating degenerative neurological diseases that cannot be transplanted with donated organs. Historical advances in stem cell research can be divided into three generations separated by their properties and use. Since the late 1950s, bone marrow-derived or hematopoietic stem cell (HSC) transplants have been used clinically for hematological cancers such as multiple myeloma and leukemia [57]. Thereafter, since the late 1990s, there have been significant developments in therapeutically useful stem cells in terms of their isolation, generation, and application of multiple cell types obtained from various tissues. The first-generation stem cell-based therapies using multipotent somatic stem cells such as HSCs, mesenchymal stem cells (MSCs), and fetal-derived neural stem cells (NSCs) are well known to have immunomodulatory, anti-inflammatory, angiogenic, anti-apoptotic, differentiation, and trophic properties to treat various diseases (e.g., autoimmune disease, bone and cartilage disorders, heart failure, neurodegenerative diseases, and gastrointestinal diseases) [52]. The second-generation stem cell-based therapies have been expanded to human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) due to their infinite self-renewal ability and pluripotent to differentiate into every cell type. Clinical trials using ESCs and iPSCs have been mainly conducted to investigate retinal degeneration, spinal cord injuries, type 1 diabetes mellitus, and ischemic heart failure [33]. Treatment with next-generation stem cells can be divided into two categories, as a delivery vehicle for therapeutic drugs and for enhancing treatment effectiveness. Currently, next-generation stem cell therapy aims to enhance its effectiveness through various engineering approaches using first and second-generation stem cells [34].
Despite advances in stem cell therapy for stroke, research targeting hemorrhagic stroke has been less than that targeting ischemic stroke [58]. Moreover, stem cell research is very unfamiliar to many domestic neurosurgeons who treat hemorrhagic stroke in their daily clinical practice. Thus, the aim of this review was to provide an update for various stem cell research studies on hemorrhagic stroke to improve neurosurgeon’s understanding of stem cells by focusing on research studies performed in the last 5 years. In addition, results of domestic investigations and future perspectives are discussed. This study was approved by the Institutional Review Board (No. 2021-03-010) of the participating hospital.
MSC
MSCs can be isolated from fetal, neonatal, and adult tissues such as bone marrow, adipose tissue, dental pulp, umbilical cord blood, Wharton’s jelly, placenta, peripheral blood, skin, and muscle. MSCs share similar characteristics such as self-renewal ability and multi-lineage differentiations into adipocytes, chondrocytes, and osteoblasts [7]. MSCs express surface markers of plastic-adherent cells such as CD29, CD44, CD73, CD90, and CD105. They are negative for CD11b, CD14, CD34, HLA-DR, and the hematopoietic marker CD45 [18,60]. Although similar characteristics and differentiation capacity are noted for MSCs derived from different origins, levels of several paracrine factors can differ according to their origins [62]. Preclinical rodent studies have been conducted using various MSCs isolated from bone marrow (BMSCs) [11], adipose tissue (ASCs) [71], umbilical cords (UC-MSCs) [45], and placenta (PSCs) [12]. However, clinical trials have been mainly performed using BMSCs or UC-MSCs due to their safety by avoiding immune rejection after autologous or allogeneic transplantation (Table 1).
Table 1.
Summary of recent major preclinical studies of stem cells using rodent hemorrhagic stroke models over the past 5 years
| Study | Type | Model | Species | Stem cell source | Dosage | Volume (μL) | Timing | Delivery route |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [11] (2020) | ICH | Coll | Rat | rBMSCs | 2×106 cells | 2 | 1 day | IC |
| Mello et al. [45] (2020) | ICH | Coll | Rat | hUC-MSCs | 3×106 cells | 500 | 1 day | IV |
| Huang et al. [28] (2019) | ICH | ABI | Rat | hBMSCs | 2×105, 5×105, 10×105/kg cells | N.C | 0.5 hours | IV |
| Deng et al. [17] (2019) | ICH | Coll | Rat | rBMSCs, GDNF overexpression | 5×105 cells | 20 | 3 day | IC |
| Han et al. [24] (2018) | ICH | ABI | Rat | rBMSC-derived exosomes | 100 μg of exosome | 500 | 1 day | IV |
| Zhang et al. [71] (2019) | ICH | Coll | Mouse | rASCs | 3–4×105 cells | 3 | 2 days | IC |
| Li et al. [41] (2019) | ICH | Coll | Mouse | rASCs, CX3CR1 overexpression | 2–4×05 cells | 2 | 3 days | IC |
| Kuramoto et al. [36] (2019) | ICH | Coll | Mouse | hASCs | 1×106 cells | 100 | 1 day | IV |
| Kim et al. [32] (2020) | IVH | ABI | Rat | hUC-MSCs | 1×105 cells | 10 | 2 days | IC |
| Zhang et al. [69] (2018) | ICH | Coll | Rat | rBMSC, miR21 overexpression | 1×106 cells | 10 | 0.5 hours | IC |
| Choi et al. [12] (2018) | ICH | Coll | Rat | hPSCs (placenta) | 1×106 cells | 500 | 1 hour | IV |
| Min et al. [46] (2018) | ICH | Coll | Rat | hPSCs (placenta) | 2.5×106 cells | N.C | 1 day | IV |
| Cui et al. [16] (2017) | ICH | ABI | Rat | rBMSCs | 5×103 cells | 100 | 1 day | IV |
| Cui et al. [15] (2017) | ICH | ABI | Rat | rBMSCs, CM | 10 μL of conditioned media | 10 | 0 hour | IV |
| Xie et al. [67] (2016) | ICH | Coll | Rat | hUC-MSCs | 2×105, 2×106 cells | 10, 1000 | N.C | IC, IV |
| Qin et al. [50] (2015) | ICH | Coll | Rat | iPSCs | 1×106 cells | 10 | 6 hours | IC |
| Han et al. [23] (2021) | SAH | EVP | Rat | rBMSC-derived exosome | 100 μg of exosome | 500 | 10 minutes | IV |
| Xiong et al. [68] (2020) | SAH | EVP | Rat | rBMSC-derived exosome | 200 μg of exosome | 200 | 5 minutes | IV |
| Chen et al. [9] (2020) | SAH | ABI | Rat | hUC-MSC, KO-TGF-b1 | 1×105 cells | N.C. | -1, 10 days | IC |
| Liu et al. [43] (2019) | SAH | EVP | Rat | rBMSC | 3×106 cells | 1000 | 1, 3 days | IV |
| Zhao et al. [72] (2019) | SAH | ABI | Rat | hUC-MSC-derived exosome | 400 μg of exosome | 200 | 1 hour | IV |
| Nijboer et al. [47] (2018) | SAH | EVP | Rat | rBMSC | 1.5×106 cells | 24 | 6 days | IN |
ICH : intracerebral hemorrhage, Coll : collagenase injection, r : rat, BMSCs : bone marrow mesenchymal stem cells, IC : intracerebral, h : human, UC-MSCs : umbilical cord-derived mesenchymal stem cells, IV : intravenous, ABI : autologous blood injection, N.C : no comment, GDNF : glial cell-derived neurotrophic factor, ASCs : adipose-derived mesenchymal stem cells, IVH : intraventricular hemorrhage, PSCs : placenta-derived mesenchymal stem cells, CM : conditioned medium, iPSCs : induced pluripotent stem cells, SAH : subarachnoid hemorrhage, EVP : endovascular perforation, IN : intranasal
The essential role of MSCs in the treatment of hemorrhagic stroke is due to their paracrine effects such as anti-inflammatory [70], angiogenesis [22], and chemoattractant effects [41] rather than their differentiation into neurogenic cells. The anti-inflammatory property of MSCs has been verified through coculture with UC-MSCs and microglia/astrocyte in vitro. MSCs can inhibit the activation of astrocytes by microglia, releasing pro-inflammatory cytokines in the presence of conditioned medium [32]. Results have indicated that intravenous administration of BMSCs or ASCs during the acute phase can alleviate microglia-mediated neuro-inflammation and improved neurobehavioral dysfunction after hemorrhage [36,43]. MSCs can also reduce cell apoptosis by modulating signal pathways with host cells after MSC transplantation [11,72]. Chen et al. [11] have reported that connexin 43 upregulation is attributed to nuclear factor erythroid 2-related factor 2 nuclear translocation after BMSC transplantation in a mouse ICH model. Vascular regeneration is important in supplying oxygen and nutrients to injured brain tissue during the recovery phase. BMSCs or UC-MSCs transplantation can promote angiogenesis and improved neurological function [24,67]. ASC transplantation in the injured site can stimulate the migration of circulating progenitor cells. Overexpression of CX3CR1, a chemokine fractalkine receptor, can stimulate cell migration in an experimental ICH model [41].
NSC
NSCs are known to exist in the subventricular zone and the sub-granular zone of the fetal or adult brain. NSCs have self-renewal ability and multipotent potential to differentiate into neuronal lineage cells including neurons, astrocytes, and oligodendrocytes. NSCs can also be directly differentiated from ESCs, iPSCs, or transdifferentiated from somatic cells such as fibroblasts and blood cells [56]. Intravenously (IV) transplanted NSCs can migrate to the perihematomal lesion and differentiated into astrocytes and neurons [29]. Compared to the control group, NSCs-transplanted rats exhibit better functional outcomes. Wang et al. [66] have reported that fetal NSCs can differentiate into three major neuronal cell types both in vitro and in vivo. Functional recovery can be obtained by secreted neurotrophic factors. Nevertheless, massive graft cell death after NSC transplantation is a limitation of its clinical effectiveness. Genetic modification to overexpress brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF), and superoxide dismutase 1 (SOD1) can improve the survival of grafted NSCs [63]. Wakai et al. [63] have reported that increasing the release of paracrine factor of SOD1 can result in reduced striatal atrophy and increased number of surviving neurons as well as better functional recovery in an ICH mouse model. Nevertheless, the feasibility of using NSCs for neurological diseases including hemorrhagic stroke has not been well-studied in clinical trials. In the clinical application of NSC transplantation, there are issues including ethics, difficulty in isolation and expansion, and the risk of tumorigenicity and immunological rejection [65]. Thus, further research is needed to overcome these issues.
iPSC
iPSCs are pluripotent cells that are reprogrammed from somatic cells similar to ESCs by inducing transcription factors such as Oct4, Sox2, Klf4 and c-Myc. iPSCs can differentiate into specific cell types such as those of ectoderm, mesoderm, and endoderm lineage. iPSCs generated from somatic cells of ICH patients could differentiate into neuroepithelial-like stem cells [51]. Compared to neurological diseases with rare monomorphic modeling (e.g., Parkinson’s and amyotrophic lateral sclerosis), the feasibility of using iPSCs has not been wellstudied in stroke due to limited genetic influence on stroke occurrence and multiple cell types affected by stroke [20]. Only a few studies have reported on the feasibility of using iPSCs in hemorrhagic stroke. Qin et al. [49] firstly injected iPSCs into the ipsilateral brain region of an ICH rat model. Grafted iPSCs derived from fibroblasts of an ICH patient could migrate into the injured brain tissue. Through follow-up research [50], the authors demonstrated attenuated cerebral inflammation, neuronal injuries, and glial scar formation as well as decreased cerebral edema and apoptosis after iPSC transplantation. Nevertheless, since the literature basis for hemorrhage stroke is relatively lacking, the specific effectiveness of iPSCs will be revealed through follow-up studies.
ESC
ESCs are classified as pluripotent stem cells, which are isolated from the inner cell mass of early embryogenesis. They also have characteristics of unlimited self-renewal, proliferation, and differentiation capacity. ESCs can differentiated into neurons and glial cells both in vitro and in vivo. Nonaka et al. [48] have reported that all-trans retinoic acid (ATRA) treated ESCs can induce neuronal cell-type differentiation. After intraventricular transplantation of ATRA-treated ESCs, surviving ESCs were found around injured sites, demonstrating that ESCs could replace neurons and glial cells and support neuroprotection and restoration in the brain [48]. Clinical research on ESCs transplantation to date has been performed for the treatment of central nervous system (CNS) or retinal tissues, which are less immunologically recognized [54]. To expand the use of ESCs in hemorrhagic stroke, ethical issues, technical difficulties of differentiating into specific cell types, complete exclusion of undifferentiated cells, and the possibility of immune reactions should be solved before their clinical application.
CLINICAL TRIALS OF STEM CELL TRANSPLANTATION
A total of 12 clinical trials on stem cells have been conducted on patients with hemorrhagic stroke [27]. Of these, six clinical trials have been completed with results reported, while results of the remaining six studies have not yet been officially reported (Table 2). Of the six completed clinical trials, four were conducted in China, one in India, and one in Korea. In terms of hemorrhagic stroke type, five trials were for ICH and the other one was for IVH [2]. Bhasin et al. [6] have reported results of BMSC transplantation in three patients with ICH in 2011 for the first time. BMSC-transplanted patients experienced an improvement in the motor function of their upper limbs and the modified Barthel index without adverse outcomes related to MSCs. However, there was no statistical significance in the degree of improvement compared to the control group. Chang et al. [8] have compared treatment outcomes after BMSC or UC-MSC transplantation in patients with moderate to severe neurologic deficits for more than 60 months. Hemorrhagic stroke patients treated with MSCs exhibited better functional outcomes than those who underwent surgical hematoma removal only. Phase I and II randomized controlled trials done by Tsang et al. [59] have also demonstrated that an autologous administration of BMSCs at a mean dosage of 4.57×107 cells can result in better functional improvement and neuro-restoration compared to placebo treatment. In Korea, Ahn et al. [2] have performed a phase I clinical trial to assess the safety of MSCs in nine preterm infants with severe IVH. Regardless of the dosage, neither treatment with a low dose of MSCs (5×106 cells/kg) or a high dose (1×107 cells/kg) showed serious adverse effects or dose-limiting toxicity.
Table 2.
Summary of major reported clinical trials of stem cell transplantation in patients with hemorrhagic stroke
| Study | Country | Trial design | Number/age | Source | Dosage | Volume (mL) | Timing | Delivery route | Follow-up | Result |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. [2] (2018) | Korea | Phase I, open-label | H(9)/24–30 weeks | hUC-MSCs | 5×106/kg, 1×107/kg | 1–2/kg | 7 days | ICV | 2, 4, 6, 8 weeks | Safe and feasible; no adverse events |
| Tsang et al. [59] (2017) | China | Phase I/II, blinded, randomization | H(5), C(4)/48–56 years | Autologous BMSCs | 2.65×105 to 1.45×106/kg | 10 | 12 months | IV | 12, 16, 24, 36, 60 weeks | Safe; improvements in motor and cognitive function; no adverse events |
| Chang et al. [8] (2016) | China | Phase I/II, blinded, randomization | H(16), C(8)/30–75 years | Autologous BMSCs, UC-MSCs | 1.8×108 cells | N.C | 2, 3 weeks | IP | 3, 6, 12, 36, 60 months | Safe; improvements in NIHSS, mRS, and modified BI; no adverse events |
| Chen et al. [10] (2013) | China | Phase I/II, blinded, randomization | H(10)/42–87 years | OECs, NPCs, SCs, UC-MSCs | Various* | Various† | 1 week | IP, IT, IV‡ | 6–24 months | Safe and feasible; increased BI and CNIS; no adverse events |
| Li et al. [42] (2013) | China | Phase I/II, non-randomization | H(60), C(40)/35–74 years | Autologous BMSCs | 7.25×105 to 1.35×106/L | 3.5 | 5–7 days | IT | 6 months | Improvement in NIHSS and BI; one patient had lung cancer |
| Bhasin et al. [6] (2011) | India | Phase I/II, open-label | H(6), C(6)/20–60 years | BMSCs | 5–6×107 cells | 250 | 3–12 months | IV | 8, 24 weeks | Safe and feasible; no improvement; no adverse events |
Indicates OEC : 1×106; OEC+NPC : 1–2×106, 2–4×106; NPC : 2–5×106; NPC+SC : 2–5×106, 2×106; and UCMSCs : 1–2.3×107.
OEC, NPC/50 μL and NPC/500 μL + 5 mL CSF, UC-MSCs/100 mL.
IP (OECs, NPCs), IT (NPCs, SCs), and IV (UC-MSCs).
H : the number of hemorrhage patients, UC-MSCs : umbilical cord mesenchymal stromal cells, ICV : intracerebralventricle, C : the number of control patients, BMSCs : bone marrow mesenchymal stem cells, IV : intravenous, N.C : no comment, IP : intracranial parenchymal, NIHSS : National Institute of Health Stroke Scale, mRS : modified Rankin Scale, BI : Barthel Index, OEC : olfactory ensheathing cells, NPCs : neural progenitor cells, SCs : Schwann cells, IT : intrathecal, CNIS : clinic neurologic impairment scale
Despite positive research findings on treatment effects, clinical studies have not been performed on a large scale for patients with hemorrhagic stroke. Although stem cell-related adverse effects including de novo tumor development were not observed, sufficient research on safety is required prior to clinical application. In addition, the next phase of research should be carried out in consideration of the effective delivery method to enhance therapeutic effects, proper dosage, the timing of injections, and whether large-scale production of stem cells is possible.
STEM CELL RESEARCH IN KOREA
A review of the literature published since 2000 identified ten animal studies and one clinical trial carried out by domestic researchers. Stem cell research using NSCs was the main subject in 2000. However, MSCs have become the main research topic recently. IV-transplanted human NSCs differentiated into neurons and astrocytes and resulted in a better functional performance [29]. After treatment with human NSCs along with a retroviral vector encoding V-myc, functional performance was markedly improved [37]. F3 human NSCs overexpressing VEGF induced more behavioral improvement and cell survival [38]. Transplanted F3 human NSCs overexpressing GDNF [40] or BDNF [39] also significantly increased levels of anti-apoptotic protein and showed better functional recovery than the control group. In severe IVH models, intraventricular transplantation of human UC-MSCs attenuated hydrocephaly and impairment based on behavioral tests, reduced corpus callosum thickness, and increased astrogliosis [1]. Regarding the mechanism of MSC treatment for severe IVH, MSCs could impede reactive microglia more than astrocytes [32]. MSC-conditioned medium can inhibit thrombin-induced microglial activation and proinflammatory cytokines through phosphorylation of signal transducer and activator of transcription 1 and p38 mitogen-activated protein kinase [32]. Recently, MSC-derived EVs have shown therapeutic effects as parental MSCs in IVH models [3]. MSCs and MSC-derived EVs can significantly attenuated neuronal cell death and the inflammatory response compared to MSC-derived EVs without BNDF knockdown. Accordingly, it can be assumed that BNDF transfer via EVs has a neuroprotection effect. Unlike studies on ICH or IVH, domestic stem cell studies on SAH have been reported yet. Therefore, further studies on stem cell transplantation in SAH are needed.
CONTROVERSIAL ISSUES AND FUTURE PERSPECTIVES
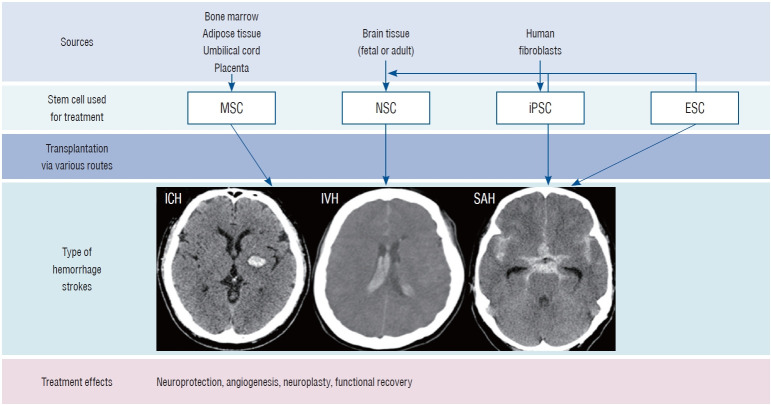
Pluripotent stem cells such as ESCs and iPSCs are capable of infinite proliferation through self-replication and differentiation into all cells, drawing attention as stem cell therapy for intractable diseases. ESCs have the biggest advantage, in that they can directly replace cells required for degenerative diseases because they can induce differentiation into specific cells. However, ethical and immunogenicity issues arising from the separation of ESCs from fertilized eggs remain a challenge [33]. iPSCs have been developed as an alternative to address these shortcomings of ESCs. iPSCs reprogrammed using autologous or allogenic somatic cells, have almost the same capabilities as ESCs. However, iPSCs need to be further investigated to determine whether they are safe to use as a cell therapy due to genetic modification by gene carriers such as lentivirus and adeno-associated viruses. In addition, they have higher tumorigenicity than ESCs [52]. NSCs are most suitable as stem cell therapy for degenerative neurological diseases because they can differentiate into specific cell types that compose the brain and nerves. Moreover, they can release neurotrophic factors. NSCs are located deep in the brain. Thus, additional risk is associated with the process of obtaining these cells [55]. MSCs are the most studied in stem cell research since they can be easily separated and cultured from a variety of adult tissues. In particular, MSCs can support the surrounding cells by secreting various factors related to anti-inflammatory, angiogenesis, anti-apoptosis, and growth function [31]. The ability of MSCs to differentiate into a neurogenic lineage remains controversial. Several studies have demonstrated that MSCs could be induced into neuronal or glial-like cells [21]. However, other studies have suggested that the neurogenic differentiation of MSCs may not be due to an intrinsic differentiation ability, but a temporary neurogenic feature of cell fusion or stressful culturing conditions. In addition, neuronal differentiation can stop or revert to MSC features without constant stimulation [19]. Therefore, the therapeutic use of MSCs in hemorrhagic stroke should be considered in anticipation of the mechanism by which the release of trophic factors can enhance endogenous regeneration. Moreover, therapeutic strategies that combine biomaterials, bioengineering, genetic overexpression, and preconditioning before transplantation also need to be considered (Fig. 1).
Fig. 1.
Sources of stem cells used for treating hemorrhagic stroke. MSC : mesenchymal stem cell, NSC : neural stem cell, iPSC : induced pluripotent stem cell, ESC : embryonic stem cell, ICH : intracerebral hemorrhage, IVH : intraventricular hemorrhage, SAH : subarachnoid hemorrhage.
There is still controversy regarding the optimal delivery route of stem cells transplanted into a damaged brain. IV injection of stem cells has the advantages of being noninvasive and easy to handle. However, it has the disadvantage of affecting the entire body, not just the specifically targeted organ. Although some preclinical studies have proven that IV-injected stem cells can migrate into injured brain tissues, organs where the injected stem cells that are most often found are the lungs and liver [61]. Also, the major action of IV-injected stem cells is thought to through suppression of systematic immune reactions, not brain-related immune reactions due to its limited ability to cross the blood-brain barrier (BBB). Intra-arterial delivery of stem cells using catheterization has the advantage of being able to reach the damaged brain without filteration by the lung or the liver [64]. However, there is a risk of further damage to the injured brain by blocking cerebral blood flow due to aggregation of grafted cells. ICV transplantation via stereotactic surgery can stimulate the proliferation and differentiation of quiescent NSCs and affect the entire CNS through cerebral spinal fluid circulatoin [14]. Compared to IV delivery, the transplantation of relatively few cells and the possibility of procedure-related complications can be concerns for their use. Intracerebral (IC) delivery is likely to have a high therapeutic effect due to direct replacement of neuronal cells and differentiation, supporting functional recovery in targeted brain lesions [5]. Nevertheless, the possibility of procedure-related complications is also a concern. Stem cell transplantation via the intranasal (IN) approach is a relatively noninvasive method compared to ICV and IC. Stem cells that migrated into the lesion have been observed from an IN route administration with functional recovery demonstrated in a preclinical research [30]. Considering anatomical differences between human and rodents, follow-up studies on their usefulness in actual clinical practice are needed.
Preconditioning effects and exosomes should be topics of future stem cell research. When a stem cell is exposed to certain conditions before transplantation, they can reflexively increase their potential for survival and angiogenesis. For example, hypoxia-conditioned BMSCs can improve regeneration and functional recovery in a stroke model. The responsible mechanism might be anti-apoptotic effects of the NF-κb pathway and angiogenesis via promoting VEGF by hypoxia-inducible factor-1-alpha [53]. Additionally, preconditioning methods for stem cells can be used for overexpressing a specific gene or chemical treatment. Currently, extracellular vesicles (EVs) have been used as pathological markers and genetic anti-cancer drug vehicles. EVs are often broadly categorized into microvesicles (<1000 nm) and exosomes (30–150 nm) after excluding apoptotic bodies. EVs released by membranebound packaging possess various information that can transfer message-containing specific origin-derived cytosolic proteins, membrane proteins, DNA, and mRNAs to across other organs [13]. In particular, the small size of exosomes can make them an alternative candidate for treating ICH due to their superior biocompatibility such as low immunogenicity and low toxicity as well as their ability to cross the BBB. In the CNS, neuronal EVs released from neurons and glia contribute to the maintenance of cellular homeostasis and support neuronal functions [25]. Recent MSC studies have investigated specific functions of exosomes. Han et al. [23] have reported that MSCs-derived EVs can inhibit NF-κB and the activation of AMP-activated protein kinase to reduce inflammation after SAH. Xiong et al. [68] have also shown that exosomes from BMSCs can alleviate SAH-induced early brain injury via anti-inflammatory and anti-apoptotic effects by miRNA129-5ps. Considering these results, effective exosome delivery and the identification of certain exosomes with therapeutic effects are required through further studies on hemorrhagic stroke.
ICH modeling in rodents can be achieved in two main ways : by intraparenchymal injection of bacterial collagenase and autologous blood [35,44]. Bacterial collagenase can disrupt the basal mania layer of cerebral arteries and causes hemorrhage over several hours [35]. Compared to autologous blood injection, collagenase-induced ICH model exhibits more injury to the brain parenchyma with extensive BBB damage [44]. However, human ICH can occur from a variety of causes with diverse hemorrhage locations. In addition, the timing of stem cell administration also varies. In most animal experiments, stem cells were injected within 24 hours after ICH induction. In contrast, the timing of stem cells injections in clinical studies varied from 2 weeks to several years following ICH. Accordingly, differences in stem cell effects might occur in pre-clinical animal models and clinical trials. To confirm the reproducibility of the clinical efficacy of stem cell treatment, future studies should focus on ICH modeling that can more accurately reflect clinical patients and the timing of stem cell injections. Also, since the generation of tissue-specific differentiated cells from various stem cells is a key technology that must first be addressed for the development of stem cell therapy, research on differentiation into NSCs, which are precursors of cells that make up the CNS, should be studied intensively using ESCs.
CONCLUSION
There has been a gradual increase in reports of preclinical and clincial studies regarding effects of stem cells on hemorrahgic stroke. Nevertheless, there are few studies on adult hemorrhagic stroke in Korea. Considering the fact that life expectancy continues to increase and hemorrhagic stroke occurs at a relatively young age, domestic neurosurgeons should also be interested in stem cell therapy and conduct more reseach on stem cells.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HR21C0198) and Hallym University Research Fund.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : JPJ
Data curation : JTK, JPJ
Funding acquisition : JPJ
Project administration : JPJ
Visualization : BJK, DHY, JKR
Writing - original draft : JTK, JPJ
Writing - review & editing : JPJ
References
- 1.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504. doi: 10.1161/STROKEAHA.112.679092. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I doseescalation clinical trial. Stem Cells Transl Med. 2018;7:847–856. doi: 10.1002/sctm.17-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cellderived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med. 2021;10:374–384. doi: 10.1002/sctm.20-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker EW, Platt SR, Lau VW, Grace HE, Holmes SP, Wang L, et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci Rep. 2017;7:10075. doi: 10.1038/s41598-017-10406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Chang Z, Mao G, Sun L, Ao Q, Gu Y, Liu Y. Cell therapy for cerebral hemorrhage: five year follow-up report. Exp Ther Med. 2016;12:3535–3540. doi: 10.3892/etm.2016.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Chen L, Xie D, Niu J. Protective effects of transforming growth factor-β1 knockdown in human umbilical cord mesenchymal stem cells against subarachnoid hemorrhage in a rat model. Cerebrovasc Dis. 2020;49:79–87. doi: 10.1159/000505311. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Xi H, Huang H, Zhang F, Liu Y, Chen D, et al. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant 22 Suppl. 2013;1:S83–S91. doi: 10.3727/096368913X672154. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Liang H, Xi Z, Yang Y, Shan H, Wang B, et al. BM-MSC transplantation alleviates intracerebral hemorrhage-induced brain injury, promotes astrocytes vimentin expression, and enhances astrocytes antioxidation via the Cx43/Nrf2/HO-1 axis. Front Cell Dev Biol. 2020;8:302. doi: 10.3389/fcell.2020.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BY, Kim OJ, Min SH, Jeong JH, Suh SW, Chung TN. Human placenta-derived mesenchymal stem cells reduce mortality and hematoma size in a rat intracerebral hemorrhage model in an acute phase. Stem Cells Int. 2018;2018:1658195. doi: 10.1155/2018/1658195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Martinez P, González-Granero S, Molina-Navarro MM, Pacheco-Torres J, García-Verdugo JM, Geijo-Barrientos E, et al. Intraventricular injections of mesenchymal stem cells activate endogenous functional remyelination in a chronic demyelinating murine model. Cell Death Dis. 2016;7:e2223. doi: 10.1038/cddis.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui C, Cui Y, Gao J, Li R, Jiang X, Tian Y, et al. Intraparenchymal treatment with bone marrow mesenchymal stem cell-conditioned medium exerts neuroprotection following intracerebral hemorrhage. Mol Med Rep. 2017;15:2374–2382. doi: 10.3892/mmr.2017.6223. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Cui C, Cui Y, Li R, Sheng H, Jiang X, et al. Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem. 2017;42:137–144. doi: 10.1159/000477122. [DOI] [PubMed] [Google Scholar]
- 17.Deng L, Gao X, Fan G, Yang C. Effects of GDNF-transfected marrow stromal cells on rats with intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2019;28:2555–2562. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Faroni A, Smith RJ, Lu L, Reid AJ. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur J Neurosci. 2016;43:417–430. doi: 10.1111/ejn.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Susavila H, Bugallo-Casal A, Castillo J, Campos F. Adult stem cells and induced pluripotent stem cells for stroke treatment. Front Neurol. 2019;10:908. doi: 10.3389/fneur.2019.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S, Hamblin MR, Abrahamse H. Differentiation of mesenchymal stem cells to neuroglia: in the context of cell signalling. Stem Cell Rev Rep. 2019;15:814–826. doi: 10.1007/s12015-019-09917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Zhen Y, Wang A. Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatr Dis Treat. 2017;13:2757–2765. doi: 10.2147/NDT.S141534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han M, Cao Y, Guo X, Chu X, Li T, Xue H, et al. Mesenchymal stem cell-derived extracellular vesicles promote microglial M2 polarization after subarachnoid hemorrhage in rats and involve the AMPK/NF-κB signaling pathway. Biomed Pharmacother. 2021;133:111048. doi: 10.1016/j.biopha.2020.111048. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Seyfried D, Meng Y, Yang D, Schultz L, Chopp M, et al. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2018;131:290–300. doi: 10.3171/2018.2.JNS171475. [DOI] [PubMed] [Google Scholar]
- 25.Holm MM, Kaiser J, Schwab ME. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 2018;41:360–372. doi: 10.1016/j.tins.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J Stroke. 2013;15:2–20. doi: 10.5853/jos.2013.15.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang AP, Hsu YH, Wu MS, Tsai HH, Su CY, Ling TY, et al. Potential of stem cell therapy in intracerebral hemorrhage. Mol Biol Rep. 2020;47:4671–4680. doi: 10.1007/s11033-020-05457-9. [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Freeman WD, Edenfield BH, Brott TG, Meschia JF, Zubair AC. Safety and efficacy of intraventricular delivery of bone marrow-derived mesenchymal stem cells in hemorrhagic stroke model. Sci Rep. 2019;9:5674. doi: 10.1038/s41598-019-42182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 30.Ji G, Liu M, Zhao XF, Liu XY, Guo QL, Guan ZF, et al. NF-κB signaling is involved in the effects of intranasally engrafted human neural stem cells on neurofunctional improvements in neonatal rat hypoxic-ischemic encephalopathy. CNS Neurosci Ther. 2015;21:926–935. doi: 10.1111/cns.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji XL, Ma L, Zhou WH, Xiong M. Narrative review of stem cell therapy for ischemic brain injury. Transl Pediatr. 2021;10:435–445. doi: 10.21037/tp-20-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Kim YE, Hong S, Kim KT, Sung DK, Lee Y, et al. Reactive microglia and astrocytes in neonatal intraventricular hemorrhage model are blocked by mesenchymal stem cells. Glia. 2020;68:178–192. doi: 10.1002/glia.23712. [DOI] [PubMed] [Google Scholar]
- 33.Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14:681–692. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
- 34.Kimbrel EA, Lanza R. Next-generation stem cells - ushering in a new era of cell-based therapies. Nat Rev Drug Discov. 2020;19:463–479. doi: 10.1038/s41573-020-0064-x. [DOI] [PubMed] [Google Scholar]
- 35.Krafft PR, Rolland WB, Duris K, Lekic T, Campbell A, Tang J, et al. Modeling intracerebral hemorrhage in mice: injection of autologous blood or bacterial collagenase. J Vis Exp. 2012;(67):e4289. doi: 10.3791/4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuramoto Y, Takagi T, Tatebayashi K, Beppu M, Doe N, Fujita M, et al. Intravenous administration of human adipose-derived stem cells ameliorates motor and cognitive function for intracerebral hemorrhage mouse model. Brain Res. 2019;1711:58–67. doi: 10.1016/j.brainres.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010;88:3282–3294. doi: 10.1002/jnr.22474. [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Park IH, Kim HJ, Kim SU. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16:1066–1076. doi: 10.1038/gt.2009.51. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Yu H, Liu N, Zhang P, Tang Y, Hu Y, et al. Overexpression of CX3CR1 in adipose-derived stem cells promotes cell migration and functional recovery after experimental intracerebral hemorrhage. Front Neurosci. 2019;13:462. doi: 10.3389/fnins.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZM, Zhang ZT, Guo CJ, Geng FY, Qiang F, Wang LX. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-a prospective clinical observation. Clin Neurol Neurosurg. 2013;115:72–76. doi: 10.1016/j.clineuro.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Li R, Yin J, Guo S, Chen Y, Fan H, et al. Mesenchymal stem cells alleviate the early brain injury of subarachnoid hemorrhage partly by suppression of Notch1-dependent neuroinflammation: involvement of Botch. J Neuroinflammation. 2019;16:8. doi: 10.1186/s12974-019-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 45.Mello TG, Rosado-de-Castro PH, Campos RMP, Vasques JF, Rangel-Junior WS, Mattos RSAR, et al. Intravenous human umbilical cordderived mesenchymal stromal cell administration in models of moderate and severe intracerebral hemorrhage. Stem Cells Dev. 2020;29:586–598. doi: 10.1089/scd.2019.0176. [DOI] [PubMed] [Google Scholar]
- 46.Min S, Kim OJ, Bae J, Chung TN. Effect of pretreatment with the NADPH oxidase inhibitor apocynin on the therapeutic efficacy of human placenta-derived mesenchymal stem cells in intracerebral hemorrhage. Int J Mol Sci. 2018;19:3679. doi: 10.3390/ijms19113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nijboer CH, Kooijman E, van Velthoven CT, van Tilborg E, Tiebosch IA, Eijkelkamp N, et al. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. 2018;27:313–325. doi: 10.1089/scd.2017.0148. [DOI] [PubMed] [Google Scholar]
- 48.Nonaka M, Yoshikawa M, Nishimura F, Yokota H, Kimura H, Hirabayashi H, et al. Intraventricular transplantation of embryonic stem cell-derived neural stem cells in intracerebral hemorrhage rats. Neurol Res. 2004;26:265–272. doi: 10.1179/016164104225014049. [DOI] [PubMed] [Google Scholar]
- 49.Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y, et al. Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett. 2013;554:70–75. doi: 10.1016/j.neulet.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, et al. Transplantation of induced pluripotent stem cells alleviates cerebral inflammation and neural damage in hemorrhagic stroke. PLoS One. 2015;10:e0129881. doi: 10.1371/journal.pone.0129881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin J, Song B, Zhang H, Wang Y, Wang N, Ji Y, et al. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci Lett. 2013;548:95–100. doi: 10.1016/j.neulet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Ratcliffe E, Glen KE, Naing MW, Williams DJ. Current status and perspectives on stem cell-based therapies undergoing clinical trials for regenerative medicine: case studies. Br Med Bull. 2013;108:73–94. doi: 10.1093/bmb/ldt034. [DOI] [PubMed] [Google Scholar]
- 53.Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. Biores Open Access. 2014;3:137–149. doi: 10.1089/biores.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 55.Shoemaker LD, Kornblum HI. Neural stem cells (NSCs) and proteomics. Mol Cell Proteomics. 2016;15:344–354. doi: 10.1074/mcp.O115.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang Y, Yu P, Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8:e3108. doi: 10.1038/cddis.2017.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 58.Toyoshima A, Yasuhara T, Date I. Mesenchymal stem cell therapy for ischemic stroke. Acta Med Okayama. 2017;71:263–268. doi: 10.18926/AMO/55302. [DOI] [PubMed] [Google Scholar]
- 59.Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, et al. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9:133–143. doi: 10.4252/wjsc.v9.i8.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vahidy FS, Rahbar MH, Zhu H, Rowan PJ, Bambhroliya AB, Savitz SI. Systematic review and meta-analysis of bone marrow-derived mononuclear cells in animal models of ischemic stroke. Stroke. 2016;47:1632–1639. doi: 10.1161/STROKEAHA.116.012701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2:154–162. [PMC free article] [PubMed] [Google Scholar]
- 63.Wakai T, Sakata H, Narasimhan P, Yoshioka H, Kinouchi H, Chan PH. Transplantation of neural stem cells that overexpress SOD1 enhances amelioration of intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2014;34:441–449. doi: 10.1038/jcbfm.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Ji X, Leak RK, Chen F, Cao G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res Rev. 2017;34:39–50. doi: 10.1016/j.arr.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, et al. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15:2624–2633. doi: 10.1111/j.1582-4934.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie J, Wang B, Wang L, Dong F, Bai G, Liu Y. Intracerebral and intravenous transplantation represents a favorable approach for application of human umbilical cord mesenchymal stromal cells in intracerebral hemorrhage rats. Med Sci Monit. 2016;22:3552–3561. doi: 10.12659/MSM.900512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong L, Sun L, Zhang Y, Peng J, Yan J, Liu X. Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury after subarachnoid hemorrhage through miRNA129-5p-HMGB1 pathway. Stem Cells Dev. 2020;29:212–221. doi: 10.1089/scd.2019.0206. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z. MicroRNA-21 overexpression promotes the neuroprotective efficacy of mesenchymal stem cells for treatment of intracerebral hemorrhage. Front Neurol. 2018;9:931. doi: 10.3389/fneur.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Deng H, Hu Y, Pan C, Wu G, Li Q, et al. Adipose-derived mesenchymal stem cells stereotactic transplantation alleviate brain edema from intracerebral hemorrhage. J Cell Biochem. 2019;120:14372–14382. doi: 10.1002/jcb.28693. [DOI] [PubMed] [Google Scholar]
- 72.Zhao H, Li Y, Chen L, Shen C, Xiao Z, Xu R, et al. HucMSCs-derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience. 2019;417:11–23. doi: 10.1016/j.neuroscience.2019.07.051. [DOI] [PubMed] [Google Scholar]