Abstract
Zebrafish are highly social teleost fish and an excellent model to study social behavior. The neuropeptide Oxytocin is associated different social behaviors as well as disorders resulting in social impairment like autism spectrum disorder. However, how Oxytocin receptor signaling affects the development and expression kinetics of social behavior is not known. In this study we investigated the role of the two oxytocin receptors, Oxtr and Oxtrl, in the development and maintenance of social preference and shoaling behavior in 2- to 8-week-old zebrafish. Using CRISPR/Cas9 mediated oxtr and oxtrl knock-out fish, we found that the development of social preference is accelerated if one of the Oxytocin receptors is knocked-out and that the knock-out fish reach significantly higher levels of social preference. Moreover, oxtr−/− fish showed impairments in the maintenance of social preference. Social isolation prior to testing led to impaired maintenance of social preference in both wild-type and oxtr and oxtrl knock-out fish. Knocking-out either of the Oxytocin receptors also led to increased group spacing and reduced polarization in a 20-fish shoal at 8 weeks post fertilization, but not at 4. These results show that the development and maintenance of social behavior is influenced by the Oxytocin receptors and that the effects are not just pro- or antisocial, but dependent on both the age and social context of the fish.
Subject terms: Neuroscience, Psychology
Introduction
Many species, including humans, live in groups to enhance their fitness—their lifetime reproductive success. Living in a social context offers many benefits like improved predator and food detection1, availability of mating partners, reduction of energy consumption2 as well as the opportunity to learn vital behaviors from conspecifics3. In order to optimize cohabitation within a group, different forms of social behavior evolved.
The zebrafish (Danio rerio), a small teleost fish, is a powerful animal model used in biomedical research, including drug discovery4, developmental biology5,6 and neurobiology7,8. Furthermore, zebrafish exhibit a variety of behaviors including avoidance9, foraging and hunting10, responses to stress11 and different forms of sociality12–16. Examples of sociality include mating behavior, aggressive behavior and other simpler behaviors that occur in groups. Zebrafish prefer to swim in cohesive shoals, a tendency that develops within the first weeks of age17. Swimming in close proximity to conspecifics, also called social preference, starts to develop as early as 1–2 weeks-post-fertilization (wpf)18,19. Although the development of shoaling behavior has been correlated with age-dependent changes in the dopaminergic and serotonergic system20, the mechanisms underlying the development and maintenance of social preference and shoaling behavior are not well understood.
The nonapeptide Oxytocin is a highly conserved neuropeptide, present in humans and with only minor alterations in most other animals21. The zebrafish orthologue Isotocin (abbreviated Oxt) differs by only two amino acids from human Oxytocin21. In addition to its role in parturition22 and lactation23, Oxytocin has been described in the context of memory consolidation24 and nocifensive behavior25–28. Intracerebral Oxytocin levels also influence anxiety in mice29–31, rats32,33 or humans34,35. Moreover, the association between Oxytocin and social behavior has been demonstrated in multiple studies36–38 and the human Oxytocin receptor may influence social traits affected in neurodevelopmental disorders such as autism spectrum disorder39,40.
Social isolation for multiple weeks has an impact on different rodent behaviors including anxiety41, aggression42 and depressive-like behavior43. Gilles and Polston44 reported that a two-week isolation of rats starting after weaning (postnatal day 21) led to increased pro- and antisocial behavior. They further described an inverse correlation between the number of oxytocinergic neurons in the anterior parvicellular division of the hypothalamic paraventricular nucleus (PVN) and the level of antisocial behavior44. After long-term social isolation, the application of Oxytocin was shown to reduce depression-like behavior of prairie voles43 or aggressive behavior in male mice42. Furthermore, different studies using rats described the number of oxytocinergic neurons in PVN to be reduced45 (postnatal day 38–48), increased46 (postnatal day 74) or unchanged44 (postnatal day 103–107) following social isolation. Isolation has also been reported to reduce the activity of Oxytocin receptors (OTR) in the nucleus accumbens of rats46 and to affect the expression of OTR in specific brain regions to different degrees: a four-week long social isolation of prairie voles led to reduced hypothalamic expression of OTR47, whereas six- and eight-week social isolation did not change the expression of OTR in murine hippocampus48.
In mice, the Oxytocin receptor is expressed in different brain regions including the hippocampus, amygdala, suprachiasmatic nucleus and prelimbic cortex49. As teleost fish like zebrafish experienced a round of whole genome duplication approximately 320–350 million years ago50, they possess two orthologous Oxytocin receptors, the Oxytocin receptor (Oxtr) and the Oxytocin receptor like (Oxtrl). Several studies51–53 investigated how Oxytocin receptors influence social recognition or social preference in adult zebrafish by blocking both Oxtr and Oxtrl with antagonists51,52 or knocking out the oxtr53. Often however, these studies came to contradictory conclusions, underscoring the complexity of Oxytocin’s effects. The discrete impact of Oxtr or Oxtrl on the development and maintenance of social behavior in socially or isolation reared zebrafish remains to be elucidated.
Making use of CRISPR/Cas9 generated oxtr and oxtrl knock-out lines, the primary objective of this study was to investigate the role of the Oxytocin receptors in the development and maintenance of social behavior, specifically social preference and shoaling behavior in zebrafish (Danio rerio).
Results
To investigate the role of the Oxytocin receptors in development and maintenance of social behavior we compared the oxtr−/− and oxtrl−/− fish and their wild-type controls in two behaviors: social preference (assesses the fish’s preference for a social versus a non-social area) and shoaling.
Social preference
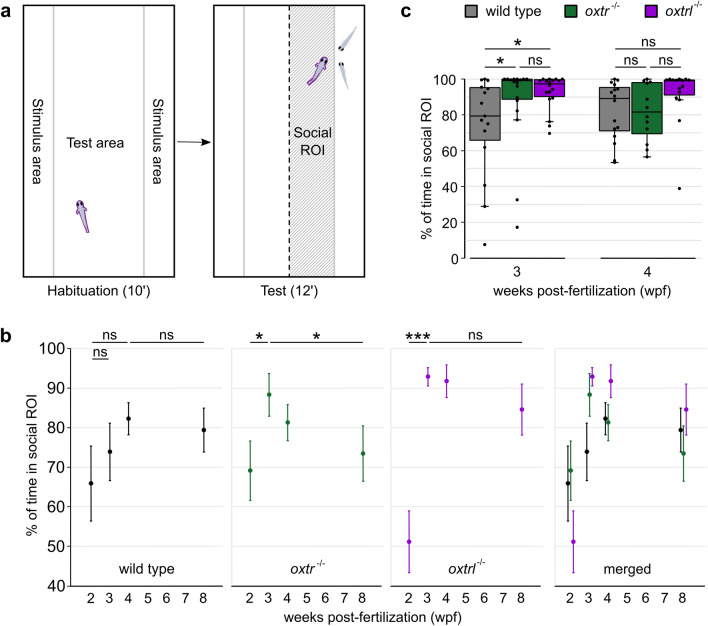
To measure social preference, individual zebrafish larvae at different developmental stages were placed in a rectangular tank with one area where conspecifics could be seen through a transparent glass wall (Fig. 1a). Following a period of habituation, two conspecifics were added to the stimulus area of the tank and the behavior of the experimental fish was observed. We measured the time spent in proximity to conspecifics (social region-of-interest) as an indicator for social preference. In wild-type zebrafish, this preference developed incrementally over the first 4 wpf and persisted up to 8 wpf (Fig. 1b,c). The increase in social preference observed between 2 and 4 wpf was comparable to the results of previous studies19,54, although not significant (p = 8.03 × 10−1). The oxtr−/− fish, by contrast, exhibited an early peak of maximal social behavior at 3 wpf. In contrast to the wild-type fish, this high sociability was not maintained but rather significantly (p = 4.95 × 10−2) decremented over 4–8 wpf (Fig. 1b,c). The development of social preference was also significantly (p = 2.09 × 10−4) altered in the oxtrl−/− fish, which also exhibited an early onset of social preference, reaching a peak precociously at 3 wpf (Fig. 1b,c). Furthermore, the maximal level of social preference exhibited by both Oxytocin receptor knock-out fish lines was substantially greater than the maximal level exhibited by the wild-type fish.
Figure 1.
Oxytocin receptors affect the development, intensity and maintenance of social preference. (a) The behavioral chamber for social preference tests was composed of one test area and two stimulus areas, divided by transparent walls. After a habituation period, two stimulus fish were added to one stimulus area and the half next to the stimulus fish was defined as “social ROI” during analysis. (b) Social preference developed faster in both the oxtr−/− and oxtrl−/− fish and in oxtr−/− fish the maintenance of social preference was impaired. Values are reported as mean ± standard error of the mean (s.e.m.). Asterisks represent the statistical comparison of age in the different genotypes. n(social wildtype, 2 wpf) = 15, n(social wildtype, 3 wpf) = 15, n(social wildtype, 4 wpf) = 16, n(social wildtype, 8 wpf) = 13, n(social oxtr−/−, 2 wpf) = 15, n(social oxtr−/−, 3 wpf) = 18, n(social oxtr−/−, 4 wpf) = 12, n(social oxtr−/−, 8 wpf) = 16, n(social oxtrl−/−, 2 wpf) = 18, n(social oxtrl−/−, 3 wpf) = 18, n(social oxtrl−/−, 4 wpf) = 15, n(social oxtrl−/−, 8 wpf) = 11. Kruskal–Wallis-Test: p(social wildtype) = 9.31 × 10−1, p(social oxtr−/−) = 1.65 × 10−2, p(social oxtrl−/−) = 4.09 × 10−5. Post-hoc Wilcoxon rank-sum test for socially reared oxtr−/−: p(2wpf⇔3wpf) = 3.66 × 10−2, p(2wpf⇔4wpf) = 6.11 × 10−1, p(2wpf⇔8wpf) = 6.42 × 10−1, p(3wpf⇔4wpf) = 7.40 × 10−2, p(3wpf⇔8wpf) = 4.95 × 10−2, p(4wpf⇔8wpf) = 6.42 × 10−1. Post-hoc Wilcoxon rank-sum test for socially reared oxtrl−/−: p(2wpf⇔3wpf) = 2.09 × 10−4, p(2wpf⇔4wpf) = 3.54 × 10−4, p(2wpf⇔8wpf) = 7.20 × 10−3, p(3wpf⇔4wpf) = 9.86 × 10−1, p(3wpf⇔8wpf) = 9.86 × 10−1, p(4wpf⇔8wpf) = 9.86 × 10−1. (c) At 3 and 4 wpf, the level of social preference was increased in the oxtrl−/− fish. Fish of the oxtr−/− line also showed enhanced social preference at 3 wpf, but did not maintain this high level at 4 wpf. Each dot represents one experimental fish. Asterisks represent the statistical comparison of genotype in two age groups. n(social wildtype, 3 wpf) = 15, n(social oxtr−/−, 3 wpf) = 18, n(social oxtrl−/−, 3 wpf) = 18, n(social wildtype, 4 wpf) = 16, n(social oxtr−/−, 4 wpf) = 12, n(social oxtrl−/−, 4 wpf) = 15. Kruskal–Wallis-Test: p(social 2 wpf) = 2.25 × 10−1, p(social 3 wpf) = 2.35 × 10−2, p(social 4 wpf) = 3.61 × 10−2, p(social 8 wpf) = 2.87 × 10−1. Post-hoc Wilcoxon rank-sum test for socially reared 3 wpf: p(wt⇔oxtr−/−) = 2.93 × 10−2, p(wt⇔oxtrl−/−) = 2.93 × 10−2, p(oxtr−/−⇔oxtrl−/−) = 7.00 × 10−1. Post-hoc Wilcoxon rank-sum test for socially reared 4 wpf: p(wt⇔oxtr−/−) = 9.26 × 10−1, p(wt⇔oxtrl−/−) = 5.34 × 10−2, p(oxtr−/−⇔oxtrl−/−) = 5.34 × 10−2. Number of replicates (n) and excluded n can be found in Supplementary Table S1, significance values in Supplementary Table S2. Significance is reported as *p < 0.05, **p < 0.01, ***p < 0.001.
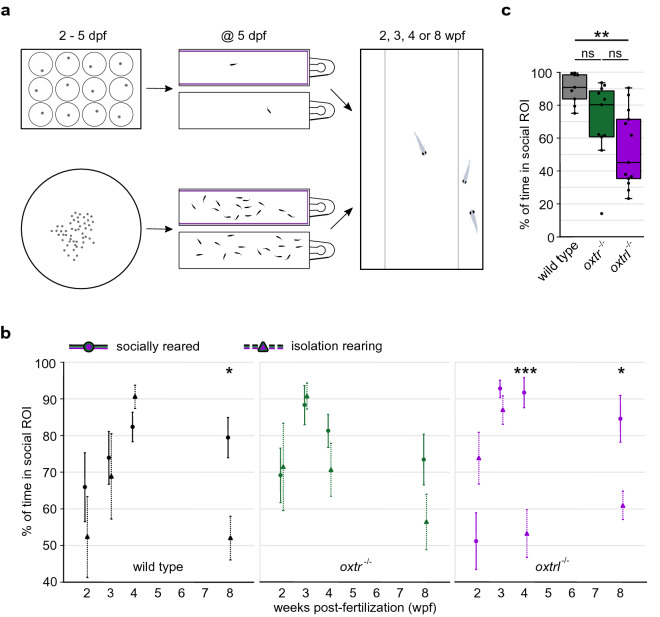
Isolation has been reported to alter social behavior in different species55–58. Therefore we next examined the effects of rearing in isolation on the development and expression of social preference in both wild-type and oxtr−/− and oxtrl−/− fish. We individually raised (in the absence of conspecifics) wild-type, oxtr−/− and oxtrl−/− fish from 2 days post-fertilization (dpf) to the day of experiment (Fig. 2a). In wild-type fish isolation rearing did not significantly (p = 3.39 × 10−1) affect the onset kinetics of social preference, but it dramatically diminished the maintenance of social preference typically observed at 8 wpf (Fig. 2b). Indeed, at 8 wpf, isolation-reared wild-type fish failed to exhibit any significant (p = 3.26 × 10−1) social preference. In contrast to socially reared wild-type fish, the increase in social preference from 2 to 4 wpf was significant after isolation rearing (p = 1.22 × 10−2). However, a direct comparison of same age socially and isolation-reared wild-type fish revealed no significantly different levels of social preference at 2 to 4 wpf. Isolation rearing had a similar effect on the oxtr−/− fish: isolation did not alter the onset kinetics of social preference but led to an accelerated decline in social preference measured at 8 wpf (Fig. 2b). Interestingly, the oxtrl−/− fish showed the same pattern, but the accelerated decline in social preference following isolation rearing was precociously evident at 4 wpf (Fig. 2b and c). In addition, RT-PCR of oxt expression in socially- and isolation-reared fish revealed that isolation led to significantly reduced oxt levels at 8 wpf, but not at 4 wpf (see Supplementary Fig. S1c). The expression of oxtr and oxtrl, however, was unaffected by isolation rearing (Supplementary Fig. S1d and S1e). These data show that the social preference develops following isolation rearing in wild-type, oxtr−/− and oxtrl−/− fish, but this social preference is not maintained. The isolation rearing induced decline in social preference is evident earlier in oxtr−/− and oxtrl−/− fish (Fig. 2c).
Figure 2.
Isolation rearing impairs the maintenance, but not the development of social preference. (a) Rearing conditions: Experimental fish were raised in isolation (2–5 days post-fertilization (dpf) in 12-well plates, from 5 dpf until experiment in 1.1L tanks with visual barriers (here shown in purple) in every second tank) or with conspecifics (2–5 dpf in a dish with 145 mm diameter, from 5 dpf until experiment in 1.1L tanks with visual barriers in every second tank at densities of 10–15 fish/L). The social preference test was performed at 2, 3, 4 or 8 wpf. (b) The development of social preference was not influenced by isolation rearing. Similar to the phenotype observed for socially reared fish, knocking-out one of the Oxytocin receptors led to an accelerated development of social preference in isolated fish. Isolation rearing impaired the maintenance of social preference, but 4 wpf old oxtr−/− fish were less susceptible to isolation. Values are reported as mean ± standard error of the mean (s.e.m.). Asterisks represent the statistical comparison of rearing conditions (social vs. isolation) in different age groups of each genotype. Data for socially reared fish are also presented in Fig. 1b. n(isolated wildtype, 2 wpf) = 11, n(isolated wildtype, 3 wpf) = 11, n(isolated wildtype, 4 wpf) = 9, n(isolated wildtype, 8 wpf) = 16, n(isolated oxtr−/−, 2 wpf) = 9, n(isolated oxtr−/−, 3 wpf) = 18, n(isolated oxtr−/−, 4 wpf) = 11, n(isolated oxtr−/−, 8 wpf) = 10, n(isolated oxtrl−/−, 2 wpf) = 13, n(isolated oxtrl−/−, 3 wpf) = 12, n(isolated oxtrl−/−, 4 wpf) = 13, n(isolated oxtrl−/−, 8 wpf) = 17. Kruskal–Wallis-Test: p(wildtype) = 6.70 × 10−3, p(oxtr−/−) = 2.00 × 10−4, p(oxtrl−/−) = 7.79 × 10−9, Post-hoc Wilcoxon rank-sum test for social vs. isolation reared wildtype: p(wildtype, 2wpf) = 3.39 × 10−1, p(wildtype, 3wpf) = 9.59 × 10−1, p(wildtype, 4wpf) = 3.39 × 10−1, p(wildtype, 8wpf) = 1.64 × 10−2, Post-hoc Wilcoxon rank-sum test for social vs. isolation reared oxtr−/−: p(oxtr−/−, 2wpf) = 4.09 × 10−1, p(oxtr−/−, 3wpf) = 4.09 × 10−1, p(oxtr−/−, 4wpf) = 4.09 × 10−1, p(oxtr−/−, 8wpf) = 2.76 × 10−1, Post-hoc Wilcoxon rank-sum test for social vs. isolation reared oxtrl−/−: p(oxtrl−/−, 2wpf) = 9.09 × 10−2, p(oxtrl−/−, 3wpf) = 3.50 × 10−1, p(oxtrl−/−, 4wpf) = 3.58 × 10−4, p(oxtrl−/−, 8wpf) = 1.46 × 10−2. (c) oxtr−/− and oxtrl−/− fish showed decreased social preference at 4 wpf after isolation rearing. Each dot represents one experimental fish. Asterisks represent the statistical comparison of genotype after isolation rearing at 4 wpf. n(isolated wildtype, 4 wpf) = 9, n(isolated oxtr−/−, 4 wpf) = 11, n(isolated oxtrl−/−, 4 wpf) = 13. Kruskal–Wallis-Test: p(isolated 2 wpf) = 1.85 × 10−1, p(isolated 3 wpf) = 3.51 × 10−1, p(isolated 4 wpf) = 1.80 × 10−3, p(isolated 8 wpf) = 4.82 × 10−1. Post-hoc Wilcoxon rank-sum test for isolation-reared 4 wpf: p(wt⇔oxtr−/−) = 5.01 × 10−2, p(wt⇔oxtrl−/−) = 2.52 × 10−3, p(oxtr−/−⇔oxtrl−/−) = 9.29 × 10−2. Number of replicates (n) and excluded n can be found in Supplementary Table S1, significance values in Supplementary Table S2. Significance is reported as *p < 0.05, **p < 0.01, ***p < 0.001.
An analysis of swim speed revealed significant differences between wildtype and mutant fish at 2 and 3 wpf (see Supplementary Figure S3a and S3b) these differences were not correlated with the alterations in social preference development described. For example, both 2- and 3-week old oxtrl−/− fish swam significantly faster than wildtype fish, but showed significantly enhanced (3 wpf) or decreased (2 wpf) social preference. Another example is the significantly reduced social preference of 4-week old isolation reared oxtrl−/− or oxtr−/− fish with an average swim speed comparable to wildtype zebrafish. Taken together, these data indicated that changes in swim speed induced by the loss of either Oxytocin receptor do not provide a straightforward explanation for the changes in social behavior observed.
Shoaling
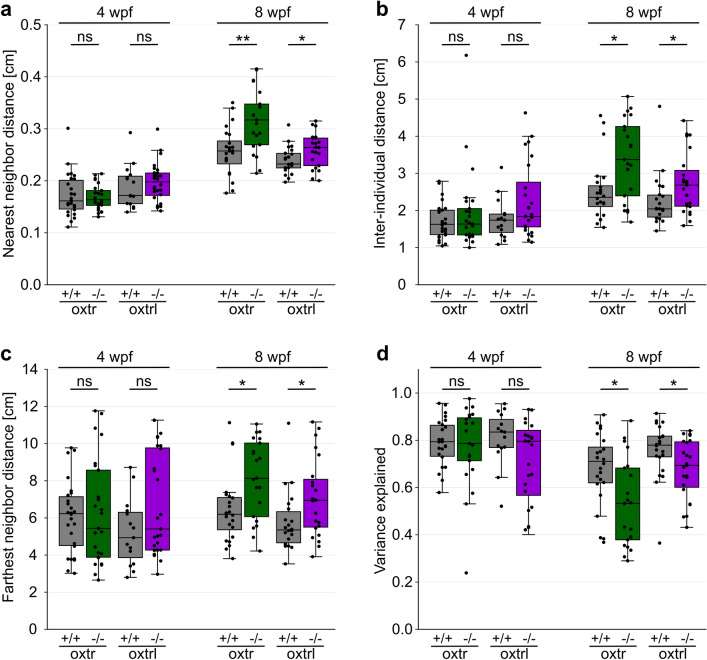
We also investigated another form of social behavior: shoaling. To measure shoaling we used a setup which allowed 20 zebrafish to move freely in a round tank (see Supplementary Fig. S2a) for 30 min. We tested socially reared wild-type, oxtr−/− and oxtrl−/− fish at ages of 4 and 8 wpf (younger fish could not be reliably tracked owing to their small size). To quantify shoaling, we analyzed the nearest-neighbor distance, inter-individual distance and farthest-neighbor distance of each fish as well as the cumulative shoal distance and the polarization parameter obtained from principal component analyses of individual videos (see “Materials and methods”). At 4 wpf, neither of the Oxytocin receptor knock-outs exhibited shoaling characteristics that were significantly different from their associated wild-type controls (p(oxtr+/+ vs. oxtr−/−) = 4.74 × 10−1 (a), 4.09 × 10−1 (b), 4.74 × 10−1 (c) and 8.88 × 10−1 (d), p(oxtrl+/+ vs. oxtrl−/−) = 3.17 × 10−1 (a), 2.59 × 10−1 (b), 1.29 × 10−1 (c) and 4.80 × 10−1 (d)) (Fig. 3a-d). At 8 wpf, however, both knock-out lines exhibited significant differences in their shoaling features, all consistent with the general phenotype of a less cohesive shoal (p(oxtr+/+ vs. oxtr−/−) = 1.64 × 10−3 (a), 1.38 × 10−2 (b), 1.91 × 10−2 (c) and 4.95 × 10−2 (d), p(oxtrl+/+ vs. oxtrl−/−) = 4.31 × 10−2 (a), 2.01 × 10−2 (b), 1.91 × 10−2 (c) and 4.95 × 10−2 (d)) (Fig. 3a-d). The cumulative shoal distance was not altered in both oxtr−/− and oxtrl−/− (see Supplementary Figure S2b). Moreover, knocking out one of the Oxytocin receptors did not lead to increased anxiety—represented by boldness (see Supplementary Figure S2c) and freezing (see Supplementary Figure S3c and S3d). Taken together, these two experiments revealed that both Oxytocin receptors play a crucial role in the development of social preference and shoal cohesion.
Figure 3.
Shoal cohesion and polarization is positively influenced by the Oxytocin receptors at 8 wpf. (a) The absence of either Oxytocin receptor led to increased nearest neighbor distance at 8 wpf, but not at 4 wpf. n for shoals: see below. Kruskal–Wallis-Test for nearest-neighbor distance: p(4wpf) = 1.69 × 10−2, p(8wpf) = 4.72 × 10−5, Wilcoxon rank-sum test for the nearest-neighbor distance of wild-type vs. mutant genotype: p(oxtr+/+⇔oxtr−/−, 4wpf) = 4.74 × 10−1, p(oxtrl+/+⇔oxtrl−/−, 4wpf) = 3.17 × 10−1, p(oxtr+/+⇔oxtr−/−, 8wpf) = 1.64 × 10−3, p(oxtrl+/+⇔oxtrl−/−, 8wpf) = 4.13 × 10−2. (b) The inter-individual distance was enlarged in both Oxytocin receptor KO lines at 8 wpf, but not at 4 wpf. n for shoals: see below. Kruskal–Wallis-Test for inter-individual distance: p(4wpf) = 2.07 × 10−1, p(8wpf) = 2.40 × 10−3, Wilcoxon rank-sum test for the inter-individual distance of wild-type vs. mutant genotype: p(oxtr+/+⇔oxtr−/−, 4wpf) = 4.09 × 10−1, p(oxtrl+/+⇔oxtrl−/−, 4wpf) = 2.59 × 10−1, p(oxtr+/+⇔oxtr−/−, 8wpf) = 1.38 × 10−2, p(oxtrl+/+⇔oxtrl−/−, 8wpf) = 2.01 × 10−2. (c) oxtr−/− and oxtrl−/− showed increased farthest neighbor distance at 8 wpf, but not at 4 wpf. n for shoals: see below. Kruskal–Wallis-Test for farthest-neighbor distance: p(4wpf) = 4.79 × 10−1, p(8wpf) = 2.65 × 10−3, Wilcoxon rank-sum test for the farthest-neighbor distance of wild-type vs. mutant genotype: p(oxtr+/+⇔oxtr−/−, 4wpf) = 4.74 × 10−1, p(oxtrl+/+⇔oxtrl−/−, 4wpf) = 1.29 × 10−1, p(oxtr+/+⇔oxtr−/−, 8wpf) = 1.91 × 10−2, p(oxtrl+/+⇔oxtrl−/−, 8wpf) = 1.91 × 10−2. (d) Coordinated swimming, represented by the variance explained, was reduced in both knockout groups at 8 wpf but remained unaffected at 4 wpf. n for shoals: see below. Kruskal–Wallis-Test for variance explained: p(4wpf) = 5.64 × 10−1, p(8wpf) = 7.00 × 10−4, Wilcoxon rank-sum test for the variance explained of wild-type vs. mutant genotype: p(oxtr+/+⇔oxtr−/−, 4wpf) = 8.88 × 10−1, p(oxtrl+/+⇔oxtrl−/−, 4wpf) = 4.80 × 10−1, p(oxtr+/+⇔oxtr−/−, 8wpf) = 4.95 × 10−2, p(oxtrl+/+⇔oxtrl−/−, 8wpf) = 4.95 × 10−2. Number of shoals (containing 20 fish each) per group: n(oxtr+/+, 4wpf) = 26, n(oxtr−/−, 4wpf) = 25, n(oxtrl+/+, 4wpf) = 15, n(oxtrl−/−, 4wpf) = 28, n(oxtr+/+, 8wpf) = 24, n(oxtr−/−, 8wpf) = 21, n(oxtrl+/+, 8wpf) = 22, n(oxtrl−/−, 8wpf) = 23. Each dot represents one shoal. Asterisks represent the statistical comparison between mutant and wild-type genotype at different ages. Number of replicates (n) and excluded n can be found in Supplementary Table S1, significance values in Supplementary Table S2. Significance is reported as *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Many studies59–61 have demonstrated a connection between Oxytocin and social behavior, but the exact role of the two Oxytocin receptors in development and maintenance of social behavior remained to be clarified. Our data indicate that preference for companions develops gradually in wild-type zebrafish in the first few weeks, reaching a maximum social preference at 4 wpf, which is then maintained at a high level until at least 8 wpf. If one of the Oxytocin receptors (Oxtr or Oxtrl) was deleted, the development of social preference was accelerated, reaching its maximal level precociously at 3 wpf. Although Autism spectrum disorder is caused by multiple factors like mutations in the Fragile × mental retardation 1 (FMR1) gene62, intranasal application of Oxytocin can ameliorate some of the social impairments of autistic patients40 and additionally, specific single-nucleotide polymorphisms (SNPs) of the human OXTR39 gene are frequent across the ASD population and have been described as potential genetic risk factor for ASD63. We have shown here that the development of social preference is affected by Oxytocin receptors in a distantly-related vertebrate, the zebrafish. It is interesting to note, that a recent study reported that the development of social preference is also sped up in fmr1−/− zebrafish64.
Isolation rearing of zebrafish did not affect the time course of the development of social preference, but significantly affected its maintenance. A recent study by Tunbak and colleagues65 described decreased social preference in about 40% of fish after isolation rearing. They used wild-type fish with the genetic background AB and a behavioral chamber described previously19, which, in contrast to the chamber used here, does not allow simultaneous visual access to both stimulus areas. Unfortunately, the age of fish was not specifically defined, but they classified the experimental fish as “juvenile”, suggesting an age between 30 and 89 dpf66. In our experiments, isolation-reared wild-type fish exhibited social preference that was comparable to socially-reared fish at age 14 to 28 dpf, but they were less social after 8 weeks of isolation. These data suggest that reduced social preference is a response to isolation periods longer than 28 dpf but differences in experimental design (different isolation rearing environments, behavioral chambers, light conditions, number of stimulus fish) can influence the results of social preference tests.
The isolation-induced decline in social preference we observed for wild-type zebrafish in our study was accelerated in both of the Oxytocin receptor knock-outs. RT-PCR of oxt expression in socially- and isolation-reared fish revealed that isolation led to significantly reduced oxt levels at 8 wpf, but not at 4 wpf (see Supplementary Fig. S1c). Furthermore, and in contrast to socially-reared fish, isolation-reared fish did not show significantly higher oxt expression with increasing age. These data are consistent with the observed behavioral changes after isolation rearing. The expression of oxtr and oxtrl, however, was unaffected by isolation rearing (see Supplementary Figure S1d and S1e), which is contrary to a study reporting reduced expression of the Oxytocin receptor (OTR) in prairie voles following four weeks of social isolation47. In addition to the reduced oxt expression in isolated fish, another possible explanation for the dramatically reduced social preference level at 8 wpf might be the downregulation of pth2, as isolation leads to reduced expression of this neuropeptide67 as well.
Our results differ from the work of Landin and co-workers, who tested the social preference of adult and 3-week-old zebrafish after application of the Oxytocin receptor antagonist L-368,899, a specific inhibitor of both Oxtr and Oxtrl52. At both developmental stages they detected a decreased social preference. Unlike the Landin et al. study, a previous study by Zimmermann and colleagues did not find a significant decrease in social preference after intraperitoneal injection of L-368,899. The conflicting results in the two studies were attributed to the use of different concentrations of the antagonist, 0.01 ng/g51 and 100 µg/g52. In contrast to these two studies, we used CRISPR/Cas9 generated genetic mutants lacking only one of the two Oxytocin receptors allowing us to dissect their distinct individual roles throughout development. Both receptors influenced the development of social preference, but in contrast to oxtrl−/−, oxtr−/− fish did not maintain a high level of social preference as they became older. Wircer and colleagues68 described a subpopulation of oxytocinergic neurons, which are located in the posterior tuberculum and express oxtr. Ablation of these neurons led to reduced social preference in adult zebrafish68. Interestingly, a study by Ribeiro and colleagues53 showed impaired social recognition but unchanged social preference in a single Oxytocin receptor (oxtrwz16/wz16) knock-out zebrafish line. Difference in the results obtained between Ribeiro et al. and our study may be due to the difference in experimental condition or age as they used a different behavioral chamber for the social preference test and three- to six-month old fish. oxtrl knock-out fish were not tested in their study. In another study, Ribeiro and co-workers described a non-significant tendency for less social preference in adult oxtr wild-type and knock-out fish after rearing in presence of oxtr knock-out conspecifics69. In our experiments, we reared wild-type fish with wild-type and knock-out fish with knock-out conspecifics. Different studies70–73 with Oxtr knock-out mice have shown decreased social behavior and impaired social recognition with enhanced aggression. Remarkably, the deficits in social behavior were also present in heterozygous Oxtr+/− mice73. In these studies, adult mice were tested. In a very recent study74, Nunes, Gliksberg and colleagues observed impaired social affiliation in adult zebrafish after chemical ablation of oxytocinergic neurons in the dorsorostral part of the neurosecretory preoptic area at early developmental stages (4–6 dpf and 12–14 dpf), but later ablation (20–22 dpf and 90 dpf) did not affect social preference of adult fish. As the ablated oxytocinergic neurons fully recovered within 42 days74, the described impaired social preference is likely due to the requirement of these oxytocinergic neurons at early developmental stages. The receptor knock-out fish we used in this study lacked Oxtr and Oxtrl, respectively, during all developmental stages. For oxtr−/−, we observed a significant reduction in social preference between the peak of maximal social preference at 3 wpf and 8 wpf. It is possible that this significant decrease persists until adulthood, resulting in impaired social preference of adult oxtr−/−, but this remains to be tested with knock-out fish. In comparison to oxtrl−/− fish, the maintenance of social preference was impaired at higher degrees in oxtr−/− fish, suggesting that this receptor might be more important for the Oxytocin influenced development of adult affiliative behavior in larval stages.
Our data suggest no alteration in anxiety or aggression levels in oxtr−/− and oxtrl−/− fish at the ages tested, as two indicators of anxiety—boldness (see Supplementary Figure S2c) and freezing (see Supplementary Figure S3c and S3d)—were not different between wildtype and mutant fish. In addition, the frequency of aggressive behavior, like biting, was not changed. In line with the Ribeiro et al. study53, a study with Oxytocin receptor knock-out prairie voles75 showed impaired social recognition in Oxtr−/−, but did not find significant changes in social behavior. Our results further show that Oxtr and Oxtrl do not have same functions since oxtr−/− and oxtrl−/− knockouts exhibit different degrees of social preference throughout development. Importantly, when considering the role of oxytocin receptors, one may need to evaluate the potential role of Vasotocin, the zebrafish orthologue to mammalian Vasopressin, which can also bind to Oxtr and Oxtrl, though with a much lower affinity than Oxt52.
As oxtrl−/− fish show enhanced social preference level at 3 and 4 wpf, we expected them to shoal more tightly, with decreased group spacing compared to wild-type. The increase in social preference was not, however, accompanied by changes in the shoaling parameters of nearest-neighbor, inter-individual, farthest-neighbor distances and the polarization parameter “variance explained” in either the oxtr−/− and oxtrl−/− at 4 wpf. And yet, at 8 wpf the nearest-neighbor, inter-individual, and farthest-neighbor distances were significantly increased while the variance explained significantly decreased in both oxtr−/− and oxtrl−/−. These data indicate less polarized shoaling behavior with increased group spacing in oxtr−/− and oxtrl−/−. In line with our results, intraperitoneal injection of L-368,899 also led to enhanced nearest-neighbor, inter-individual, and farthest-neighbor distances in shoals of four adult fish52, supporting a pro-cohesive role of the two Oxytocin receptors in the organization of shoals with fish older than 8 wpf. The major differences between the shoaling experiments of Landin and colleagues and ours are the age (adult vs. 8 or 4 wpf), shoal size (4 fish vs. 20 fish per shoal) and the behavioral chamber (trapezoid, 1.8 L vs. round, 2.4 or 3.0 L). Furthermore, in Landin et al., both Oxytocin receptors were blocked by antagonist injection 1 h prior to experiment whereas in our experiments only one of the two Oxytocin receptors was knocked-out since the time of fertilization. Although not significant, the first signs of reduced oxtrl−/− shoaling capability were visible as early as 4-weeks of age. As shoaling behavior develops continuously until adulthood17, the most parsimonious explanation for both sets of data is that Oxytocin receptors are likely to be important for shoal organization at later stages of development, whereas other signaling pathways may be able to compensate at 4 wpf. Similar to the social preference, both Oxytocin receptors modulate shoaling behavior, but to different degrees. Since shoaling parameters are also influenced by predator avoidance76,77 and experience78, shoaling should not be considered as an exclusive expression of social behavior. Moreover, shoaling and schooling are regulated by both visual and mechanosensory79 (lateral line) inputs.
In a study by Tang and colleagues80 the shoaling behavior of 90 CRISPR/Cas9 generated knock-out lines (for different genes) was compared (6 adult fish per shoal). This study revealed the influence of multiple genes on collective behavior of zebrafish—in particular on swim speed, group spacing and polarization. Tang et al. further showed that a single gene does not necessarily affect swimming speed, group spacing and polarization, but can influence only one or two of these shoaling parameters. Our data are consistent with this finding as we did not detect significant changes in the cumulative shoal distance at 8 wpf (see Supplementary Fig. S2b), whereas the group spacing and polarization parameters were significantly altered at this age. At first glance, the observations that the Oxytocin receptor mutant fish develop a preference for social companions precociously, yet exhibit poor coordination and shoaling behavior at 8 wpf may seem inconsistent. However, this may be explained by the observations of a decline in preference for social companions over ontogeny. oxtr−/− mutants, for example, show a larger effect in diminished shoaling parameters at 8 wpf compared to oxtrl−/− which agrees with the larger reduction in the preference for social companions at this age. The modest or no change in shoaling parameters at 4 wpf could reflect either a technical limitation of this assay at an early age, or, that there may be a “ceiling effect” to detect improved shoaling characteristics.
Taken together, our results show that the Oxytocin receptors play an important role in the development and maintenance of zebrafish social behavior and the impact of Oxytocin signaling depends on the age and environment of the fish.
Materials and methods
The materials and methods section follows the recommendations in the ARRIVE2.0 guidelines81.
Study design, sample size and exclusion criteria
The number of biological replicates for each experiment can be found in Supplementary Table S1.
For the investigation of social preference, we planned to test 18 fish per group. The sample size was determined prior to experiments using the E-equation method82. Unfortunately, some experimental fish died during rearing and therefore, 15 to 18 fish were tested per group. Data was excluded from further analysis if the test fish’s average swim speed was below a threshold (73/411). This threshold was calculated for each age group by determining the average swimming speed of each genotype group (wild type, oxtr−/− or oxtrl−/−) and multiplying the smallest with 0.6. The fish of three genotype groups (oxtr+/+;oxtrl+/+ = “wild type”, oxtr−/−;oxtrl+/+ = “oxtr−/−“ and oxtr+/+;oxtrl−/− = “oxtrl−/−”) were reared either in isolation or with conspecifics at 10–15 fish/L density and were tested for their social preference at age of 2, 3, 4 or 8 wpf. To keep the testing conditions as similar as possible, the different genotypes were tested without unnecessary delay (performing all experiments of one age group within one month excect for 8 wpf (groups were tested within four months) and the isolation and socially reared fish of one genotype were tested on the same day, whenever possible.
In the 30-min long shoaling experiment, shoaling parameters were analyzed in a shoal of 20 fish at age of 4 or 8 wpf in three different genotypes (oxtr+/+;oxtrl+/+ = “oxtr+/+” or “oxtrl+/+”, oxtr−/−;oxtrl+/+ = “oxtr−/−“and oxtr+/+;oxtrl−/− = “oxtrl−/−”). In contrast to social preference, we detected a significant difference in some shoaling parameters between the homozygous wild-type cousins of oxtr−/− and the homozygous wild-type cousins of oxtrl−/−. Therefore, we analyzed the wild-type zebrafish as two genotype groups, “oxtr+/+” and “oxtrl+/+”, respectively. We tested 23–28 socially reared (10–15 fish/L) shoals per group.
Test fish were used only once, sacrificed and genotyped after the experiment. If the fish/shoal was identified as heterozygous (22/611) it was excluded from further analysis.
Experimental animals and generation of knock-out
The oxtr (ZDB-GENE-110805-2/NCBI mRNA Reference Sequence: NM_001199370.1) and oxtrl (ZDB-GENE-110805-1/NCBI mRNA Reference Sequence: NM_001199369.1) genes were mutated by Ajay Mathuru and Caroline Kibat using the sgRNA:Cas9 system described in 83. Two CRIPSR targets (GGAAGTTACCGTGTTGGCCT and GGCTGATAAGCTTTAAAATA for oxtr; GTGCGTCCTTGTGGCCATCC and GGGGGGATTTTGTTCAGCCC for oxtrl) for each gene were identified using ZiFit (http://zifit.partners.org/zifit/). Customized sgRNAs with 20 nucleotide sequence complementary to a target site were synthesized by first cloning the target sequences into the expression construct pDR274 (Addgene #42,250) and then in vitro transcribed using T7 promoter according to the manufacturers protocol (Thermo Fisher # AMB13345).The Cas9 mRNA was transcribed from linearized plasmid MLM3616 (Addgene #42,251) following the manufacturers protocol (Thermo Fisher # AMB1344). The sgRNA:Cas9 RNAs cocktail (containing 12.5 ng/μL sgRNA and 300 ng/μL Cas9) were injected into single-cell embryos of AB wild-type background. The efficiency of the CRISPR targets and quality of the Cas9 endonuclease were determine 24 h post injection of sgRNA:Cas9 by PCR on 10% of the injected embryos. The remaining embryos were raised to adulthood and genotyped at three months post injection. A total of 64 individuals were genotyped. The sequence of the genotyping primer set is in detailed in the genotyping methods section. The PCR products were cloned into pGEMT (Promega #1360) and sequenced to verify the mutations. The F0 mutants were then then outcrossed to Danio Reds (https://doi.org/10.1016/S0006-291X(03)01282-8). F1 fish were in-crossed to establish germline transmitting homozygous mutant lines.
The lines are named oxtrync02 and oxtrlync03, in this paper abbreviated as oxtr−/− and oxtrl−/−, respectively. The homozygous wild-type cousins of these fish were used as wild-type control. Moreover, these wild-type cousins were used for regular out-crossing of the homozygous knockouts (KO) to keep the genetic background of the experimental fish as similar as possible. We could not test oxtr−/− oxtrl−/− double KO fish as their generation by breeding failed, possibly because the presence of at least one oxytocin receptor is vital at specific developmental stages. When evaluating the expression of the knocked-out receptor using specific primers by real time PCR, amplification signals were detected in the wild-type but not in the mutant fish, indicating the successful knock-out. Furthermore, a compensation of the knocked-out receptor by its orthologue Oxytocin receptor was not detectable (see Supplementary Fig. S1b).
Experimental fish were bred by incrossing homozygous mutant or wild-type fish in a 1:1 sex ratio per batch. Each group consisted experimental fish of multiple clutches (up to 17) and the clutch size was 300–400 eggs (no difference between wild-type and mutant fish). On the day of experiment the test fish were 2, 3, 4 or 8 wpf old, depending on the age group. We used experimental fish with a total body length comparable to the total body lengths described in the Zebrafish Book66: 6 mm (2 wpf), 8 mm (3 wpf), 10 mm (4 wpf) and 14 mm (8 wpf). No differences in development or growth were detected comparing mutant and wild-type fish. In the social preference test the stimulus fish (two per test fish) had the same age and similar size than the test fish. As it is difficult (juveniles) to impossible (larvae) to distinguish males and females at the developmental stages used in this study, fish became experimental fish independent of their sex.
Zebrafish housing and husbandry
Up to 5 dpf, the larvae were kept in a 28.5 °C incubator with a 14/10 light–dark cycle. At 2 dpf, eggs were either individually isolated in a 12-well plate (3 mL E3 medium [5 mM NaCl, 17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4] per 22 mm diameter well) or kept in pertri dishes (145 mm diameter, filled with 150 mL E3 medium) in groups of 50.
From 5 dpf on, they were raised isolated (including visual barriers placed in every second tank, see Fig. 2a, center) in 1.1L ZebTEC tanks or in groups of mixed sexes at densities of 10–15 fish/L in 1.1L ZebTEC tanks (social preference test) or 3.5L ZebTEC tanks (shoaling test).
From 5 dpf on, zebrafish were housed in a ZebTEC Active Blue Stand Alone System, with the water temperature of 28.5 °C ± 1 °C, the pH = 7.4 ± 0.3 and the conductance = 650 µS ± 100. The light–dark cycle in the fish facility is 14 h-light/10 h-dark with a 30 min twilight phase. Fish were fed three times per day: Up to 10 dpf with vinegar eelworms (Turbatrix aceti, bred in house), CAVIAR 50–100 (SAFE) and crushed Gemma Micro 75 (Skretting), from 10 to 20 dpf with vinegar eelworms, brine shrimp (Artemia salina, Ocean nutrition V154019) and CAVIAR 50–100, from 21 to 56 dpf with brine shrimp and CAVIAR 100–200 (SAFE).
Experimental procedures
Social preference test
Behavioral chamber (height 10 mm) for testing social preference consisted of the test area (25 × 75 mm (for 2 to 4 wpf) and 50 × 75 mm (for 8 wpf)) and the stimulus areas (8 × 75 mm (for 2 to 4 wpf) and 16 × 75 mm (for 8 wpf)). The behavioral chamber was placed on a screen, providing white backlight illumination and a camera positioned above recorded the fish’s movements with 20 (3–8 wpf) or 30 (2 wpf) frames per second using the program “Pylon recorder”. To reduce visual and acoustic disturbances, the setup was surrounded by a black, sound-absorbing box (LBH: 450 × 670 × 850 mm). Prior to the experiment the test and stimulus fish were moved from their home tank to a 1L breeding tank (TECNIPLAST, Part Number: ZB10BTE) with a nursery insert (TECNIPLAST, Part Number: ZB300BTI) and kept in a 28.5 °C incubator (test and stimulus fish in different tanks). After each test, the behavioral chamber was cleansed with hot water (~60 °C) and refilled with fresh ZebTEC Stand Alone system water (28.5 °C). The fish were moved using a disposable 3 ml pipette (the tip was cut off to generate a sufficient big diameter) by elevating the nursery insert and then transferred to the center of test area (test fish) or edge of stimulus area (stimulus fish) of the behavioral chamber. At 8 wpf the chamber was covered with the lid of a 145 mm dish to prevent fish from jumping out. The test fish habituated to the chamber without any other fish for ten minutes followed by a 12 min test phase in which two stimulus fish of same age and size were placed to one of the stimulus areas. A transparent wall between test and stimulus area allowed visual access to the stimulus fish whereas a white opaque wall determined the rear side of the stimulus area.
Shoaling experiment
The shoaling setup (see Supplementary Fig. S2a) consisted of a white round behavioral chamber with a diameter of 70 cm which was surrounded by 29.5 °C water (heated by a pump) to prevent cooling of the water inside the behavioral chamber during the 30 min of experiment. A high-resolution camera (Basler acA4112-30um), positioned 73 cm over the chamber, recorded the fish with 30 frames per second using the program “Pylon recorder” and 1200 white LEDs in the ceiling provided the necessary illumination (approximately 650 Lux). A white box surrounding the setup reduced visual and acoustic disturbances of the fish. For each replicate the chamber was completely emptied, dried off and refilled with 2.4 L (4 wpf)/ 3.0 L (8 wpf) fresh 28.5 °C warm Stand Alone system water. In the morning of an experimental day, the home tanks were moved from the ZebTEC stand alone to a 28.5 °C incubator. Approximately 5 min before the experiment started, 20 fish of same age and similar size were transferred from their home tank and to a 1L breeding cage (TECNIPLAST) with a nursery insert (TECNIPLAST, Part Number: ZB300BTI). With the nursery insert all 20 fish of one shoal were moved simultaneously to the center of the behavioral chamber. The recording of shoaling behavior started immediately and continued for 30 min.
Genotyping
In order to identify homozygous knockouts (KO) and homozygous wild types (wt) for generation of experimental fish, fin clip PCR was performed according to the ZIRC genotyping protocols (https://zebrafish.org/wiki/protocols/genotyping). Additionally, each experimental fish was genotyped after the experiment. The following primer sequences were used.
| Forward | Reverse | Band size | |
|---|---|---|---|
| oxtr | TGGAGGACATCTTCAAGGA | CTTCCCTCGGTGCTTCAGGA | 916 bp (wt), 413 bp (KO) |
| oxtrl | TGATCCTCTGGCCCATTAAC | TGGTCCATAAAAGCGAAAGG | 314 bp (wt), 304 bp (KO) |
The PCR products were loaded on a 1% (oxtr) or 2% (oxtrl) agarose gel and run at 100 V for 30 min (oxtr) or > 2 h (oxtrl). But as the difference between oxtrl+/+ and oxtrl−/− is only 10 base pairs, PCR products were also sequenced using the forward primer.
Real-time PCR
RNA was extracted from isolated brains as described before67 and using the QuantiTect Reverse Transcription Kit (QIAGEN Cat. No. 205311) 200 ng of RNA was transcribed into cDNA. 5μL of 1:10 diluted cDNA template were mixed with 1.3μL primers (10 μM) and 6.25 μL SYBR Green PCR master mix (Applied Biosystems/Thermo Fisher Art. No. 4309155). The cycling parameters were 10 min at 95 °C, followed by 40 cycles of denaturation (15 s at 95 °C) and amplification (60 s at 60 °C). A Real Time PCR System (Applied Biosystems/Thermo Fisher Art. No. 4376600) was used for qRT-PCR. The fluorescence threshold was set to 0.9 for all experiments and genes and we used TATA box binding protein (tbp) as reference gene. We used the ΔCT method and the following primer sequences.
| Forward | Reverse | |
|---|---|---|
| oxt | AAGCTCTCGGTGTCAGCCTT | GGCATACACTGTCGAATGGG |
| oxtr | CCAACCTCTTCGTTCTTTACAC | ACAAAATCTCCCCAGCAATC |
| oxtrl | AAACCTGTGCGTCCTTGTG | ATAAAAGCGAAAGGTGATGTCC |
| tbp | GTACTCACAGGTGCCAAGGT | GATTGCGTAGGTCACCCCAG |
Outcome measures
In the social preference test, the location of the test fish was measured in each frame of habituation and during the last 10 min of test, as the stimulus fish needed up to 2 min after being transferred to the stimulus area to exhibit normal social behavior. During analysis the test area was divided into two regions of interest (ROI). The “social ROI” was an area next to the stimulus fish whereas the “antisocial ROI” was on the opposite site of the test area. The percentage of total time was determined, in which the fish was located in the “social ROI”. The average swimming speed in absence (habituation) or presence (test) of stimulus fish was calculated and used as exclusion criteria for fish with reduced motion (e.g. because of freezing).
In the shoaling experiments all twenty fish of each shoal were tracked using TRex84 (tracking threshold 15 (4 wpf) and 50 (8 wpf)) and the nearest-neighbor distance, the inter-individual distance and the farthest-neighbor distance of each fish were calculated and cumulative shoal distance and variance explained as parameter for coordinated swimming were determined. For determination of the variance explained, x- and y-components of all individual trajectories (20 per shoal) were first assembled in a 40 × 54,000 (30 min with 30 frames per second) matrix, whose covariance matrix was then eigen decomposed to obtain principal components (PCs). We found the first two PCs corresponded to the x- and y-component of the shoal centroid trajectory over time. Principal component analysis (PCA) is a dimensionality reduction approach that can be used to quantify the degree of linear correlation in a dataset. If individual features are highly correlated, they can be compressed in one PC while virtually no data are lost. This can be measured with the variance of the original dataset that is explained by the derived PCs. In our case, the higher the variance explained by the first two PCs, the higher the correlation between the individual fish’s movement and the movement of the shoal overall (see Supplementary Fig. S2d). Additionally, we analyzed the variance explained from 20 fish of randomly chosen movies, expecting no coordinated swimming. In line with this expectation the variance explained was very low and increased continuously with increasing number of fish from the same movie (see Supplementary Fig. S2e). Thus, the variance explained can be used to quantify coordinated swimming of the fish.
Statistical methods
Significance is reported as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
All significance values can be found in Supplementary Table S2.
Normal distribution of data was checked using the Kolmogorov-Smirnoff-Test which revealed a non-normal distribution. Unpaired data was tested with a Kruskal–Wallis Test followed by a post-hoc Mann–Whitney-U Test (= Wilcoxon rank-sum test). The statistical analyses were carried out using python (from scipy.stats: kstest, kruskal and mannwhitneyu) or MATLAB (kstest, signrank, kruskalwallis and ranksum). In the social preference test n = 332 fish were included in the analysis (79 excluded), in the shoaling experiment n = 184 shoals were included in the analysis (18 excluded).
Blinding and randomization
The investigators were not blinded to the genotype of the experimental fish, but both experiments were recorded with an overhead camera and analyzed using a tracking software (custom written tracking and analysis script for social preference and TRex84 plus a custom written python script for shoaling) to provide an unbiased data analysis. The test fish were chosen randomly from their home tank group and in the social preference test the location of stimulus fish was changed between replicates in a random order. The experiments were performed from 8:00 AM to 5:00 PM and the order of genotype groups per day was changed between experimental days to minimize a potential circadian confounder. Furthermore, the position of tank during rearing was chosen in a way that all replicates of one experimental group were reared in all possible heights/light conditions.
Ethical statement
All procedures were conducted in accordance with the institutional guidelines of the Max Planck Society and were approved by the Regierungspräsidium Darmstadt, Germany (governmental ID: V 54-19 c20/15-F126/1013 and V54-19 c20/15-F126/1016).
Supplementary Information
Acknowledgements
We thank Anett-Yvonn Loos, Annette Hüttling, Dmitrji Burgard and Hans Siegert for the zebrafish husbandry and Fabian Bayer, Thomas Maurer, Guido Schmalbach, Andreas Umminger and Erik Papuschin for the manufacturing of our behavioral setups. We thank IMCB Zebrafish Facility staff for husbandry and Suresh Jesuthasan for sharing the oxtr and oxtrl knock-out lines with us. Furthermore we are grateful to Friedrich Kretschmer for the development of the program “Pylon Recorder” and Florian Vollrath for the tracking software and MATLAB script used for analysis of the social preference test. Finally we thank Helene Will for post-hoc genotyping of the experimental fish and Anett-Yvonn Loos for fruitful discussions. A.S.M. was supported by Yale-NUS College and Ministry of Education, Singapore through a Start-Up Grant.
Author contributions
A.G. and E.M.S. conceived the project. A.G., S.R. and E.M.S. designed the experiments, C.K. and A.S.M. generated the oxtr and oxtrl knock-out fish. A.G. and K.M. conducted the experiments, bred and genotyped the experimental fish, L.A. and T.E. wrote the python script for analysis of the shoaling experiments, A.G., L.A. and T.E. analyzed the data, A.G. and E.M.S. wrote the manuscript and prepared the figures. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated and analyzed during this study and the custom written scripts are available under the public repositories GitHub (https://github.com/GemmerA/OxytocinReceptorSocial) and Edmond (https://edmond.mpdl.mpg.de/imeji/collection/_pyBtaEsY6X9liD).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07990-y.
References
- 1.Harpaz R, Schneidman E. Social interactions drive efficient foraging and income equality in groups of fish. Elife. 2020;9:56196. doi: 10.7554/eLife.56196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marras S, et al. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 2015;69:219–226. doi: 10.1007/s00265-014-1834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucore EC, Connaughton VP. Observational learning and irreversible starvation in first-feeding zebrafish larvae: is it okay to copy from your friends? Zoology (Jena) 2021;145:125896. doi: 10.1016/j.zool.2021.125896. [DOI] [PubMed] [Google Scholar]
- 4.Bowman TV, Zon LI. Swimming into the future of drug discovery: In vivo chemical screens in zebrafish. ACS Chem. Biol. 2010;5:159–161. doi: 10.1021/cb100029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg ES, et al. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- 6.Pietsch J, et al. lessen encodes a zebrafish trap100 required for enteric nervous system development. Development. 2006;133:395–406. doi: 10.1242/dev.02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 8.Meshalkina DA, et al. The effects of chronic amitriptyline on Zebrafish behavior and monoamine neurochemistry. Neurochem. Res. 2018;43:1191–1199. doi: 10.1007/s11064-018-2536-5. [DOI] [PubMed] [Google Scholar]
- 9.Groneberg AH, et al. Early-life social experience shapes social avoidance reactions in larval Zebrafish. Curr. Biol. 2020;30:4009–4021 e4021. doi: 10.1016/j.cub.2020.07.088. [DOI] [PubMed] [Google Scholar]
- 10.Bianco IH, Engert F. Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol. 2015;25:831–846. doi: 10.1016/j.cub.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steenbergen PJ, Richardson MK, Champagne DL. The use of the zebrafish model in stress research. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1432–1451. doi: 10.1016/j.pnpbp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Sessa AK, et al. The effect of a depth gradient on the mating behavior, oviposition site preference, and embryo production in the zebrafish, Danio rerio. Zebrafish. 2008;5:335–339. doi: 10.1089/zeb.2008.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira RF, Silva JF, Simoes JM. Fighting zebrafish: characterization of aggressive behavior and winner-loser effects. Zebrafish. 2011;8:73–81. doi: 10.1089/zeb.2011.0690. [DOI] [PubMed] [Google Scholar]
- 15.Buske C, Gerlai R. Diving deeper into Zebrafish development of social behavior: analyzing high resolution data. J. Neurosci. Methods. 2014;234:66–72. doi: 10.1016/j.jneumeth.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R. Social behavior of zebrafish: From synthetic images to biological mechanisms of shoaling. J. Neurosci. Methods. 2014;234:59–65. doi: 10.1016/j.jneumeth.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Buske C, Gerlai R. Shoaling develops with age in Zebrafish (Danio rerio) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1409–1415. doi: 10.1016/j.pnpbp.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz FI, Aizenberg M, Tushev G, Schuman EM. Protein synthesis-dependent associative long-term memory in larval zebrafish. J. Neurosci. 2013;33:15382–15387. doi: 10.1523/JNEUROSCI.0560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreosti E, Lopes G, Kampff AR, Wilson SW. Development of social behavior in young zebrafish. Front. Neural Circuits. 2015;9:39. doi: 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buske C, Gerlai R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Dev. Psychobiol. 2012;54:28–35. doi: 10.1002/dev.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unger JL, Glasgow E. Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr. Patterns. 2003;3:105–108. doi: 10.1016/s1567-133x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 22.Smith CW, Chan S, Walter R. Dose-response behavior on the isolated rat uterus of oxytocin analogs with modifications at binding sites. J. Pharmacol. Exp. Ther. 1977;203:120–124. [PubMed] [Google Scholar]
- 23.Nickerson K, Bonsness RW, Douglas RG, Condliffe P, Du Vigneaud V. Oxytocin and milk ejection. Am. J. Obstet. Gynecol. 1954;67:1028–1034. doi: 10.1016/0002-9378(54)90261-6. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs GL, Bohus B, Versteeg DH, de Kloet ER, de Wied D. Effect of oxytocin and vasopressin on memory consolidation: sites of action and catecholaminergic correlates after local microinjection into limbic-midbrain structures. Brain Res. 1979;175:303–314. doi: 10.1016/0006-8993(79)91009-6. [DOI] [PubMed] [Google Scholar]
- 25.Nersesyan Y, et al. Oxytocin modulates nociception as an agonist of pain-sensing TRPV1. Cell Rep. 2017;21:1681–1691. doi: 10.1016/j.celrep.2017.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UryvaevIu V, Petrov GA. Decreased pain sensitivity in man after treatment with superlow doses of oxytocin. Biull Eksp. Biol. Med. 1996;122:487–489. [PubMed] [Google Scholar]
- 27.Uvnas-Moberg K, Bruzelius G, Alster P, Bileviciute I, Lundeberg T. Oxytocin increases and a specific oxytocin antagonist decreases pain threshold in male rats. Acta Physiol. Scand. 1992;144:487–488. doi: 10.1111/j.1748-1716.1992.tb09327.x. [DOI] [PubMed] [Google Scholar]
- 28.Wee CL, et al. Zebrafish oxytocin neurons drive nocifensive behavior via brainstem premotor targets. Nat. Neurosci. 2019;22:1477–1492. doi: 10.1038/s41593-019-0452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- 30.Ring RH, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 32.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 33.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 34.Ellenbogen MA, Linnen AM, Cardoso C, Joober R. Intranasal oxytocin attenuates the human acoustic startle response independent of emotional modulation. Psychophysiology. 2014;51:1169–1177. doi: 10.1111/psyp.12263. [DOI] [PubMed] [Google Scholar]
- 35.Nissen E, Gustavsson P, Widstrom AM, Uvnas-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or Cesarean section. J. Psychosom. Obstet. Gynaecol. 1998;19:49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerer E, et al. Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Mol. Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 40.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Grippo AJ, et al. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress. 2012;15:149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karpova IV, Mikheev VV, Marysheva VV, Bychkov ER, Proshin SN. Oxytocin-induced changes in monoamine level in symmetric brain structures of isolated aggressive C57Bl/6 mice. Bull. Exp. Biol. Med. 2016;160:605–609. doi: 10.1007/s10517-016-3228-2. [DOI] [PubMed] [Google Scholar]
- 43.Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilles YD, Polston EK. Effects of social deprivation on social and depressive-like behaviors and the numbers of oxytocin expressing neurons in rats. Behav. Brain. Res. 2017;328:28–38. doi: 10.1016/j.bbr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka K, Osako Y, Yuri K. Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience. 2010;166:1036–1042. doi: 10.1016/j.neuroscience.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira VEM, Neumann ID, de Jong TR. Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology. 2019;156:107504. doi: 10.1016/j.neuropharm.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Pournajafi-Nazarloo H, et al. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Lesse A, Rether K, Groger N, Braun K, Bock J. Chronic postnatal stress induces depressive-like behavior in male mice and programs second-hit stress-induced gene expression patterns of OxtR and AvpR1a in adulthood. Mol Neurobiol. 2017;54:4813–4819. doi: 10.1007/s12035-016-0043-8. [DOI] [PubMed] [Google Scholar]
- 49.Mitre M, et al. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci. 2016;36:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J. Mol. Evol. 2004;59:190–203. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann FF, Gaspary KV, Siebel AM, Bonan CD. Oxytocin reversed MK-801-induced social interaction and aggression deficits in zebrafish. Behav. Brain Res. 2016;311:368–374. doi: 10.1016/j.bbr.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 52.Landin J, et al. Oxytocin receptors regulate social preference in Zebrafish. Sci. Rep. 2020;10:5435. doi: 10.1038/s41598-020-61073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro D, et al. Oxytocin receptor signalling modulates novelty recognition but not social preference in zebrafish. J. Neuroendocrinol. 2020;32:e12834. doi: 10.1111/jne.12834. [DOI] [PubMed] [Google Scholar]
- 54.Engeszer RE, Barbiano LA, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 2007;74:1269–1275. doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav. Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- 56.Kercmar J, Budefeld T, Grgurevic N, Tobet SA, Majdic G. Adolescent social isolation changes social recognition in adult mice. Behav. Brain Res. 2011;216:647–651. doi: 10.1016/j.bbr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keesom SM, Finton CJ, Sell GL, Hurley LM. Early-life social isolation influences mouse ultrasonic vocalizations during male-male social encounters. PLoS ONE. 2017;12:e0169705. doi: 10.1371/journal.pone.0169705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shams S, Amlani S, Buske C, Chatterjee D, Gerlai R. Developmental social isolation affects adult behavior, social interaction, and dopamine metabolite levels in zebrafish. Dev. Psychobiol. 2018;60:43–56. doi: 10.1002/dev.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm. Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 60.Oettl LL, et al. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Festante F, et al. Oxytocin promotes prosocial behavior and related neural responses in infant macaques at-risk for compromised social development. Dev. Cogn. Neurosci. 2021;48:100950. doi: 10.1016/j.dcn.2021.100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown WT, et al. Autism is associated with the fragile-X syndrome. J. Autism Dev. Disord. 1982;12:303–308. doi: 10.1007/BF01531375. [DOI] [PubMed] [Google Scholar]
- 63.Campbell DB, et al. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J. Neurodev. Disord. 2011;3:101–112. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu YJ, et al. Fragile × mental retardation-1 knockout Zebrafish shows precocious development in social behavior. Zebrafish. 2017;14:438–443. doi: 10.1089/zeb.2017.1446. [DOI] [PubMed] [Google Scholar]
- 65.Tunbak H, Vazquez-Prada M, Ryan TM, Kampff AR, Dreosti E. Whole-brain mapping of socially isolated Zebrafish reveals that lonely fish are not loners. Elife. 2020;9:55863. doi: 10.7554/eLife.55863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westerfield M, ZFIN . The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio (Brachydanio) rerio. 4. Eugene: ZFIN; 2000. [Google Scholar]
- 67.Anneser L, et al. The neuropeptide Pth2 dynamically senses others via mechanosensation. Nature. 2020;588:653–657. doi: 10.1038/s41586-020-2988-z. [DOI] [PubMed] [Google Scholar]
- 68.Wircer E, et al. Homeodomain protein Otp affects developmental neuropeptide switching in oxytocin neurons associated with a long-term effect on social behavior. Elife. 2017;6:22170. doi: 10.7554/eLife.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro D, et al. Genetic variation in the social environment affects behavioral phenotypes of oxytocin receptor mutants in zebrafish. Elife. 2020;9:56973. doi: 10.7554/eLife.56973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimori K, et al. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Prog. Brain Res. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- 72.Sala M, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Sala M, et al. Mice heterozygous for the oxytocin receptor gene (Oxtr(+/-)) show impaired social behaviour but not increased aggression or cognitive inflexibility: Evidence of a selective haploinsufficiency gene effect. J. Neuroendocrinol. 2013;25:107–118. doi: 10.1111/j.1365-2826.2012.02385.x. [DOI] [PubMed] [Google Scholar]
- 74.Nunes AR, et al. Developmental effects of oxytocin neurons on social affiliation and processing of social information. J. Neurosci. 2021;41:8742–8760. doi: 10.1523/JNEUROSCI.2939-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horie K, et al. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm. Behav. 2019;111:60–69. doi: 10.1016/j.yhbeh.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav. Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav. Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sykes DJ, Suriyampola PS, Martins EP. Recent experience impacts social behavior in a novel context by adult zebrafish (Danio rerio) PLoS ONE. 2018;13:e0204994. doi: 10.1371/journal.pone.0204994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitcher TJ, Partridge BL, Wardle CS. A blind fish can school. Science. 1976;194:963–965. doi: 10.1126/science.982056. [DOI] [PubMed] [Google Scholar]
- 80.Tang W, et al. Genetic Control of Collective Behavior in Zebrafish. iScience. 2020;23:100942. doi: 10.1016/j.isci.2020.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Percie du Sert N, et al. The ARRIVE guidelines 2.0. J. Physiol. 2020;598:3793–3801. doi: 10.1113/JP280389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charan J, Kantharia ND. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang WY, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walter T, Couzin ID. TRex, a fast multi-animal tracking system with markerless identification, and 2D estimation of posture and visual fields. Elife. 2021;10:64000. doi: 10.7554/eLife.64000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during this study and the custom written scripts are available under the public repositories GitHub (https://github.com/GemmerA/OxytocinReceptorSocial) and Edmond (https://edmond.mpdl.mpg.de/imeji/collection/_pyBtaEsY6X9liD).