Abstract
Epilepsy, progressive myoclonic 3, with or without intracellular inclusions (MIM# 611726) is a rare autosomal recessive condition associated with pathogenic variants in KCTD7, which encodes the BTB/POZ domain-containing KCTD7 protein. We report four individuals from three Indian families presenting with an initial period of normal development, progressive myoclonic seizures followed by neuroregression and an abnormal electroencephalogram. We identified two novel missense variants, c.458G>C p.(Arg153Pro) and c.205C>G p.(Leu69Val) and one known disease-causing variant, c.280C>T p.(Arg94Trp) in KCTD7 by exome sequencing. We review the literature of 67 individuals with variants in KCTD7. Our study expands the molecular spectrum of KCTD7-related progressive myoclonic epilepsy.
Keywords: epilepsy, myoclonus, neuroregression, whole exome sequencing, KCTD7
Introduction
Progressive Myoclonic Epilepsies (PME) are a diverse group of rare disorders characterized by progressive myoclonus, tonic-clonic seizures, cerebellar ataxia, developmental regression and other neurological symptoms (Satishchandra and Sinha, 2010; Malek et al., 2015). Epilepsy, progressive myoclonic 3, with or without intracellular inclusions (MIM# 611726), caused by biallelic variants in KCTD7 was first described in three siblings with clinical features suggestive of PME (Van Bogaert et al., 2007). It is an autosomal recessive disorder, where patients show early onset myoclonic seizures or/and generalized tonic-clonic seizures, neurologic regression after the onset of seizures, dysarthria, ataxia, dementia, microcephaly and abnormal findings on electroencephalogram (EEG) with multifocal epileptiform discharges and slowed dysrhythmia (Van Bogaert et al., 2007; Kousi et al., 2012; Van Bogaert, 2016). Here, we report two novel and one known disease-causing missense variant in KCTD7 in four patients from three unrelated Indian families with myoclonic seizures, neuroregression and abnormal electroencephalogram.
Methods
Informed consent was obtained from all the three families. The Institutional Ethics Committee approved this study. The details of family 2 are included in another cohort of families with multilocus disease-causing variants, which is under consideration for publication. We reviewed the available phenotypic and genotypic information published in literature till date.
Clinical report
Family 1:
The proband (IV-1) was examined at the age of 20 months (Figure 1A). He was born to a consanguineously married couple following three spontaneous abortions. Development was normal till the age of one year followed by regression of milestones. He achieved neck holding at 3 months, rolling over at 4 months, sitting without support at 6 months, standing with support at 8 months and walking with support at 1 year. He started speaking monosyllables at 1 year of age. He had fever at 16 months of age, following which he developed head titubation and myoclonic seizures. Involuntary movement of limbs and tongue were also noted in him. At the time of examination, he was unable to speak, sit or stand. His occipito-frontal circumference (OFC) was 46 cm (−3 SD), length was 80 cm (−1.45 SD) and weight was 8.6 kg (−2.3 SD). He had a prominent forehead and low-set prominent ears. He was found to have choreoathetosis and chewing movements. His muscle tone was decreased, and deep tendon reflexes were normal. EEG at 16 months of age showed background activity of 3 to 4 Hertz of high amplitude, symmetrical and synchronous waves. Frequent bilateral central and para-sagittal epileptiform discharges were seen. These waves were present during photic stimulation and sleep. Magnetic resonance imaging (MRI) of the brain was unremarkable. Gas chromatography-mass spectrometry, tandem mass spectrometry, thyroid function tests, liver and renal function tests, complete blood counts, folate and vitamin B12, plasma lactate, ammonia and transferrin assay revealed normal results. Karyotype from blood leukocytes showed normal 46, XY constitution. No pathogenic copy number changes were detected by chromosomal microarray.
Figure 1:
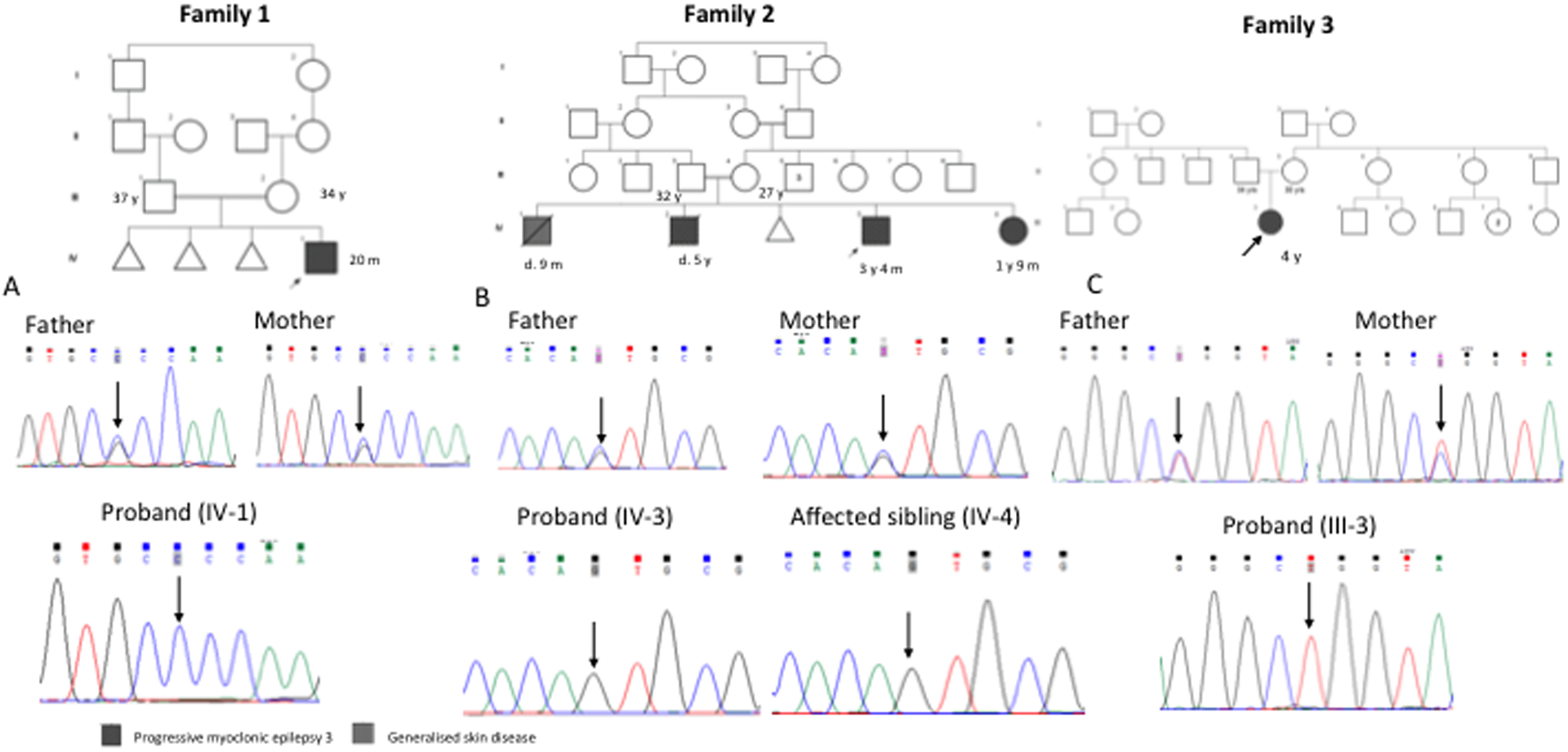
Family pedigree of family 1, family 2 and family 3. Sanger sequences: family 1 (A) shows the homozygous variant, c.458G>C in the proband and heterozygous carrier parents; family 2 (B) shows homozygous variant, c.205C>G in the proband, affected sibling and parents are heterozygous carriers. family 3 (C) shows homozygous variant, c.280C>T in the proband and parents are heterozygous carriers; family 3
Family 2:
A 3 years-4 months old boy (IV-3) was evaluated for generalized tonic clonic seizures and myoclonic seizures since the age of 7 months followed by gradual loss of achieved milestones (Figure 1B). He was born to a consanguineously married couple by normal vaginal delivery at term with a birth weight of 3.5 kg (0.2 SD). The first-born male (IV-1) of this couple deceased at 9 months of age. Details of illness and cause of death were not available with the family. The second child (IV-2), deceased at 5 years of age had a course of illness similar to the proband. IV-3 sat without support at 8 months, stood at 1 year 2 months and started to walk with support at 1 year 6 months. This was followed by neuroregression. On examination, his OFC was 48.5 cm (−2 SD) and length was 88 cm (−2.74 SD). He had myoclonic movements and increased tone in all limbs. Deep tendon reflexes were brisk. He had extensor plantar response. MRI of the brain in the proband at 3 years revealed bilateral parieto-occipital white matter T2 hyperintensities and EEG showed multiple epileptiform discharges. The fundus evaluation was unremarkable.
His younger sibling (IV-4), 1 year 9 months old girl, also presented with myoclonic movements, developmental delay and loss of achieved milestones. She was born with a birth weight of 2.25 kg (−2.37 SD) at full term and cried immediately after birth. She started holding her neck at 4 to 5 months, rolled over at 5 to 6 months, sat at 7 months and started walking without support at 14 months. This was followed by neuroregression. She developed myoclonic seizures since the age of 1 year 6 months. Her OFC was 47.5 cm (−0.8 SD), length was 82 cm (−0.68 SD) and weight was 10.5 kg (−0.27SD). Like her elder brother, she also showed increased tone in all the limbs, brisk deep tendon reflexes, myoclonic movements and extensor plantar response. Her fundus evaluation was normal. Complete blood count, liver function test and renal function tests were normal.
Family 3:
The proband (III-3) was examined at the age of four years (Figure 1C). She was the first-born of a non-consanguineously married couple and had a birth weight of 2.5 kg (−2 SD). Mother had an uneventful antenatal period. She attained neck holding at the age of 4 months, rolling over at 6 months, sitting without support at 8 months, standing without support at 10 months, walking without support at 1 year 5 months and running at 2 years. She started speaking bisyllables at the age of 1 year and 2–3 words short phrases at 2 years. She did not interact well with other children. She was able to draw and scribble. She developed myoclonic movements at 1 year, which increased in frequency subsequently. She had an episode of generalized tonic clonic type seizures associated with fever at the age of 2 years-6 months, controlled by sodium valproate and levetiracetam. She had frequent falls and dysarthria. On examination, her OFC was 48.5 cm (−0.5 SD), weight was 10 kg (−2.6 SD) and height was 92.5 cm (−2.23 SD). She had a triangular face, straight eyebrows, small mouth, and pes planus. She had decreased tone and sluggish deep tendon reflexes. EEG performed at the age of 3 years showed generalized epileptiform abnormalities with mild degree of generalized non-specific dysfunction of electric activity. Ophthalmologic and hearing evaluation was unremarkable. Complete blood count, serum lactate, CSF lactate, and creatinine phosphokinase levels were within normal limits. MRI of the brain showed thick corpus callosum. Chromosomal microarray did not reveal any clinically relevant pathogenic variants.
Whole Exome Sequencing (WES):
Trio exome sequencing was done for family 1 and singleton exome sequencing was done for family 2 (IV-3) and family 3 (III-3). In family 1 and family 2, genomic DNA was extracted from peripheral blood using phenol chloroform method. In family 3 genomic DNA was extracted from peripheral blood using the QIAamp DNA Blood Mini Kit (cat # 51106). For family 1 and 2, genomic DNA capture was performed using Illumina Nextera Rapid Capture Exome Kit (Illumina, San Diego, CA, USA). For family 3, genomic DNA capture was performed using SureSelect Clinical Research Exome V2 capture kit (Agilent Technologies, Inc. USA). Massive parallel sequencing was achieved by using NextSeq 500 High Output Kit and NextSeq 500 Sequencer (Illumina, Inc., San Diego, California, United States) as described previously (Shukla et al., 2017, Girisha et al., 2019). Sanger sequencing for validation and segregation of the variants was done.
Results
We observed an initial period of normal development followed by early onset progressive myoclonic seizures, neuroregression, and abnormal EEG patterns in the probands in all the three families. Analysis of WES data led to the identification of two homozygous rare novel missense variants, c.458G>C p.(Arg153Pro) in exon 3 and c.205C>G p.(Leu69Val) in exon 2 of KCTD7 (NM_153033.4) in the probands of family 1 and 2 respectively. WES revealed the presence of a known disease-causing variant, c.280C>T p.(Arg94Trp) in exon 2 of KCTD7 in the proband of family 3 (Kousi et al., 2012). These variants were not found in the homozygous state in population databases like gnomAD and our inhouse database of 1121 individuals. These variants, c.458G>C, c.205C>G, and c.280C>T have conservation scores (GERP) of 5.93, 2.96 and 4.91, respectively and CADD score of 32, 23.6 and 24.5 correspondingly (Davydov et al., 2010; Kircher et al., 2014). In family 1, the variant c.458G>C was observed in heterozygous state in the parents of the proband. In family 2, similarly affected younger siblings (IV-4) of proband also had c.205C>G in homozygous state and their parents were heterozygous for this variant. In addition to this variant, the proband in family 2, also had a de novo missense variant, c.726C>A p.Ser242Arg in MEFV (NM_000243.3) previously reported to cause autosomal dominant neutrophilic dermatosis, acute febrile (MIM# 608068) (Masters et al., 2016). In family 3, the variant c.280C>T was found in heterozygous state in the parents of the proband.
Biallelic variants in KCTD7 have been reported in 67 individuals till date including the individuals in this study. Complete clinical information was not available for 17 individuals. A summary of the clinical characteristics of 50 individuals, including the individuals of the present cohort is described provided in Table 1.
Table 1:
Summary of clinical characteristics of patients with biallelic variants in KCTD7
| Clinical features | Frequency (%) |
|---|---|
| Initial period of normal development | 26/38 (68.4) |
| Myoclonic epilepsy (Multifocal or status epilepticus) | 45/53 (84.9) |
| Neuroregression | 47/53 (88.6) |
| Speech abnormalities | 33/33 (100) |
| Ataxia | 31/49 (63.2) |
| Microcephaly | 7/15 (46) |
| Abnormalities in MRI brain | 27/38 (71) |
| Abnormalities in EEG | 38/39 (97.4) |
| Response to antiepileptic drugs | 19/43 (44) |
MRI: Magnetic Resonance Imaging
EEG: Electroencephalograph
Discussion
We report four affected individuals from three Indian families with clinical features suggestive of KCTD7-related progressive myoclonic epilepsy. Three typical clinical features characterize this form of progressive myoclonic epilepsy: an initial period of normal development, myoclonic seizures with onset at 1–2 years of age followed by neuroregression and a relatively stable course after a few years (Van Bogaert, 2016). Clinical findings observed in our patients are in concordance with the patients reported in the literature.
The patients described till date belonged to different ethnicities (Supplementary Table S1, S2). Patients from the Indian subcontinent were not reported previously. Consanguinity was seen in 40.6% (13/32) of the affected families. Family 1 and family 2 we described are consanguineous. However, consanguinity was denied by family 3, even though the proband had a homozygous variant in KCTD7.
An initial period of normal development and attainment of age appropriate milestones was observed in 26 out of 38 individuals (68.4%). The other 12 individuals had developmental delay noticed since early infancy. The age of onset of seizures varies from as early as 5 months to 2 years of age. The seizures in affected individuals were myoclonic, generalized tonic-clonic, absence seizures, atonic seizures or a combination of different types of seizures. Myoclonic seizures including myoclonic status epilepticus were observed in 45 out of 53 individuals (84.9%). Only one affected individual had frequent multifocal generalized spike waves in EEG without any clinical seizures (Dai et al., 2019). Almost 88% (47/53) of the affected individuals had neuroregression following the onset of seizures. Speech abnormalities were present in all the affected individuals whose clinical data is available for review. Severe dysarthria, no or limited speech, onomatopoeia and inability to form phrases and sentences were some of the speech abnormalities which were present in affected individuals. Ataxia was noted in 63.2% (31/49) of affected individuals. All individuals described in our study demonstrated the typical clinical profile with initial period of normal development, infantile onset myoclonic seizures, followed by neuroregression. However, none of them had ataxia at the time of examination.
Abnormalities in MRI of the brain were identified in 71% (27/38) affected individuals. Cerebral atrophy, cerebellar atrophy, periventricular white matter changes, corpus callosal hypoplasia and prominent ventricles were some of the abnormalities seen. Abnormalities in EEG were seen in 97.4% (38/39) individuals. Three of the four patients (IV-1 in family 1, IV-3 in family 2, III-3 in family 3) the present study in whom EEG was available, showed abnormal patterns. However, MRI brain was unremarkable except for bilateral parieto-occipital white matter T2 hyperintensities in IV-4 of family 2 and thick corpus callosum in family 3.
Response to antiepileptic drugs was seen only in 44% (19/43) of affected individuals in the literature. One patient underwent corpus callosotomy for seizure control (Metz et al., 2018). Only five affected individuals were reported to have deceased (Staropoli et al., 2012; Van Bogaert, 2016; Metz et al., 2018). Individuals IV-1 in family 1, IV-3 and IV-4 in family 2 did not show any response to antiepileptic drugs. Individual IV-1 in family 1 was treated with leviteracetam. Individual III-3 in family 3 responded to sodium valproate and leviteracetam.
One of the close differential diagnoses of this condition with overlapping clinical features is late infantile neuronal ceroid lipofuscinosis. Staropoli et al., 2012, demonstrated electron dense storage material in skin fibroblasts in an individual with biallelic KCTD7 variants. The authors concluded that biallelic variants in KCTD7 could be a cause for neuronal ceroid lipofuscinosis type 14. The alternate name for epilepsy, progressive myoclonic 3, with or without intracellular inclusions due to KCTD7 variants, is neuronal ceroid lipofuscinosis (MIM #611726). However screening for KCTD7 variants in patients suspected of having neuronal ceroid lipofuscinosis did not yield any positive result (Kousi et al., 2012). Storage material in skin fibroblasts could be demonstrated only in one additional patient (Mastrangelo et al., 2019). Clinical features, which help to differentiate the two phenotypes, are the presence of optic atrophy and retinitis in patients with late infantile neuronal ceroid lipofuscinosis. Individuals with KCTD7-related progressive myoclonic epilepsy have a stable course compared to individuals with late infantile neuronal ceroid lipofuscinosis.
We identified two novel variants, c.458G>C p.(Arg153Pro) and c.205C>G p.(Leu69Val) and one known disease-causing variant c.280C>T in KCTD7 (Kousi et al., 2012). Another variant c.458G>A p.(Arg153His) was described in a patient by Metz et al., 2018. KCTD7 encodes for BTB/POZ domain-containing protein KCTD7, belonging to KCTD family (UniProtKB - Q96MP8). It contains a highly conserved N- terminal BTB/POZ domain, which shares homology with T1 domain of voltage gated K+ channel (Stogios et al., 2005). In-silico tools such as SIFT, PaPI and Polyphen2 are concordant in predicting these novel variants to be damaging to the protein function (Kumar et al., 2009; Adzhubei et al., 2010; Limongelli et al., 2015). Till date, 38 missense variants, 2 splicing variants, 4 small deletions and 3 exonic deletions have been described as disease-causing or probable disease-causing variants in KCTD7 (Stenson et al., 2012). Among them, 21 variants occur in region coding for functional BTB domain and 23 variants falls outside region coding for the BTB domain. However, the variants occurring in the functional BTB domain did not produce a severe phenotype compared to the variants outside the domain suggesting a lack of genotype-phenotype correlation (Van Bogaert, 2016).
Experiments in mice demonstrated a relatively high amount of KCTD7 protein in the olfactory bulb, hippocampus, Purkinje cells and deep layers of cerebellar cortex (Azizieh et al., 2011). Recent studies on Xenopus oocytes revealed the role of KCTD7 protein in K+ dependent hyperpolarization of cells and also in regulating the SAT2 glutamine neuronal transporter. SAT2 transporters regulate concentration glutamine, precursors for neurotransmitters in the CNS. It is hypothesized, mutant KCTD7 protein may indirectly alter the glutamine concentration and synthesis of neurotransmitters (Moen et al., 2016). These experimental data suggest pathogenic variants in KCTD7 may alter the K+ homeostasis and neurotransmitter signaling in the brain leading to PME.
The chance of recurrence of a similar condition in families with biallelic variants in KCTD7 is 25% in every pregnancy. In family 2, in addition to the biallelic variants in KCTD7, the proband had a de novo heterozygous variant in MEFV, reported to cause autosomal dominant neutrophilic dermatosis, acute febrile (MIM# 608068) (Masters et al., 2016. In the previously reported individuals with this condition, the onset of symptoms was in late childhood. At the time of examination, IV-3 in family 2 did not have any suggestive symptoms. However, we were unable to re-examine and evaluate the child because the family was lost for follow up.
Our study adds two novel variants to the spectrum of KCTD7 pathogenic variants causing progressive myoclonic epilepsy 3. Initial period of normal development, early onset myoclonic seizures and neuroregression with a relatively stable course could be clinical pointers to consider KCTD7-related epileptic encephalopathy. Molecular testing is essential for providing genetic counseling and prenatal diagnosis to affected families.
Supplementary Material
Acknowledgements
We thank the patients and their families for participating in this study.
Funding
Funding for this work was provided by National Institutes of Health, United States of America under the project titled ‘Genetic Diagnosis of Neurodevelopmental Disorders in India’ Grant ID - 1R01HD093570-01A1 and ‘Genetic Diagnosis of Heritable Neurodevelopmental Disorders in India: Investigating the Use of Whole Exome Sequencing and Genetic Counseling to Address the High Burden of Neurodevelopmental Disorders’ (1R21NS094047-01).
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from corresponding author upon reasonable request.
References
- Adzhubei IA, Schmidt S, Peshkin L, et al. (2010). A method and server for predicting damaging missense mutations. Nat Methods 7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizieh R, Orduz D, Van Bogaert P, et al. (2011). Progressive myoclonic epilepsy-associated gene KCTD7 is a regulator of potassium conductance in neurons. Mol Neurobiol 44:111–121. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P (2016). KCTD7-related progressive myoclonus epilepsy. Epileptic Disord 18:115–119. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P, Azizieh R, Désir J, et al. (2007). Mutation of a potassium channel-related gene in progressive myoclonic epilepsy. Ann Neurol 61:579–586. [DOI] [PubMed] [Google Scholar]
- Dai L, Ding C, Fang F (2019). Two Chinese siblings with two novel KCTD7 mutations have dystonia or seizures and epileptic discharge on electroencephalograms. Seizure - Eur J Epilepsy 70:27–29. [DOI] [PubMed] [Google Scholar]
- Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S (2010). Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol 6:e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisha KM, von Elsner L, Neethukrishna K, et al. (2019). The homozygous variant c.797G>A/p.(Cys266Tyr) in PISD is associated with a Spondyloepimetaphyseal dysplasia with large epiphyses and disturbed mitochondrial function. Hum Mutat. 40:299–309. [DOI] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J (2014). A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi M, Anttila V, Schulz A, et al. (2012). Novel mutations consolidate KCTD7 as a progressive myoclonus epilepsy gene. J Med Genet 49:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC (2009). Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4:1073–1081. [DOI] [PubMed] [Google Scholar]
- Limongelli I, Marini S, Bellazzi R (2015). PaPI: pseudo amino acid composition to score human protein-coding variants. BMC Bioinformatics 16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek N, Stewart W, Greene J (2015). The progressive myoclonic epilepsies. Pract Neurol 15:164–171. [DOI] [PubMed] [Google Scholar]
- Masters SL, Lagou V, Jéru I, et al. (2016). Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med 8:332ra45. [DOI] [PubMed] [Google Scholar]
- Mastrangelo M, Sartori S, Simonati A, et al. (2019). Progressive myoclonus epilepsy and ceroidolipofuscinosis 14: The multifaceted phenotypic spectrum of KCTD7-related disorders. Eur J Med Genet 62:103591. [DOI] [PubMed] [Google Scholar]
- Metz KA, Teng X, Coppens I, et al. (2018). KCTD7 deficiency defines a distinct neurodegenerative disorder with a conserved autophagy-lysosome defect. Ann Neurol 84:766–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen MN, Fjær R, Hamdani EH, et al. (2016). Pathogenic variants in KCTD7 perturb neuronal K+ fluxes and glutamine transport. Brain 139:3109–3120. [DOI] [PubMed] [Google Scholar]
- Satishchandra P, Sinha S (2010). Progressive myoclonic epilepsy. Neurol India 58:514–522. [DOI] [PubMed] [Google Scholar]
- Shukla A, Upadhyai P, Shah J, Neethukrishna K, Bielas S, Girisha KM (2017). Autosomal recessive spinocerebellar ataxia 20: Report of a new patient and review of literature. Eur J Med Genet 60:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli JF, Karaa A, Lim ET, et al. (2012). A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitin-proteasome system. Am J Hum Genet 91:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson PD, Ball EV, Mort M, Phillips AD, Shaw K, Cooper DN (2012). The Human Gene Mutation Database (HGMD) and Its Exploitation in the Fields of Personalized Genomics and Molecular Evolution. Curr Protoc Bioinforma 39:1.13.1–1.13.20. [DOI] [PubMed] [Google Scholar]
- Stogios PJ, Downs GS, Jauhal JJS, Nandra SK, Privé GG (2005). Sequence and structural analysis of BTB domain proteins. Genome Biol 6:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from corresponding author upon reasonable request.


