Abstract
Dysmenorrhea is the term for describing complex menstrual flow and painful spasmodic cramps during menstruation, and pain without any pathology is considered Primary Dysmenorrhea (PD). It is the most frequent ailment among women of all ages and races. The pain is dull and throbbing in character and occurs in the lower back and abdomen. Symptoms commonly appear 6 to 12 months after menarche, with the most significant incidence in the late teen and early twenties. Physical exercise is nearly a new non-medical intervention to relieve PD associated pain. Aerobics, stretching and Resistive exercises for 8-12 weeks, either supervised or unsupervised, relieves pain. Exercises are believed to cause hormonal changes in the uterine lining, which reduces PD symptoms. Researchers have presumed different pain-relieving methods, ranging from non-opioids to opioids to hormonal for variations in pain sensitivity. Exercise-induced analgesia provides the central pathway as the primary mechanism for pain reduction while, another way to reducing pain in PD may be a hormonal interaction. The hormonal changes causing exercise-induced pain modulation during the menstruation cycle is not clearly understood and the interaction and activation of all the central and endocrine components, which is a complex mechanism, is also not explained clearly. This study briefly reviews the physiological mechanism of Exercise-induced analgesia and its potent roles in controlling the pathogenesis of PD for pain relief.
Keywords: Exercise-induced analgesia, Analgesia, Exercise, Dysmenorrhea, Primary dysmenorrhea
INTRODUCTION
Dysmenorrhea, or unpleasant cramps associated with menstruation, is the most frequent ailment among women of all ages and races. Its prevalence ranges from 16.8% to 81%, with rates as high as 90% having been reported. It usually starts in adolescence and can result in school and work absences and restrictions on social, academic, and physical activities. In the absence of any pathology, it is classified as primary dysmenorrhea (PD); otherwise, it is classified as secondary. Symptoms commonly appear 6 to 12 months following menarche, with the most significant incidence in late teen and early twenties [1]. The pain is dull and throbbing in character and occurs in the lower back and abdomen. It is related to the commencement of flow and can remain for up to 72 hours. The majority of the girls report moderate-intensity pain and premenstrual symptoms (41.49%) such as headache, back and thigh pain, diarrhea, nausea, and vomiting, all of which have a significant negative influence on their quality of life [2]. Pain and discomfort during the menstrual cycle are due to inflammatory uterine changes which are regulated by hormones.
The hypothalamus-pituitary gonadal system regulates the menstrual cycle and secretes FSH (follicle-stimulating hormone) and LH (luteinizing hormone). These hormones promote the growth and maturation of follicles and release estrogen in the first phase of the cycle. In the late phase (luteal phase) progesterone concentration increases as follicle mature by corpus luteum [3]. If no fertilization occurs, progesterone concentration decreases, and Arachidonic Acid (AA) starts appearing onto the cell membrane and metabolites in Prostaglandin (PG) and Leukotrienes (LT) via Cyclooxygenase (COX) and 5-Lipoxygenase pathway (LOX). The PG is in the form of two active components PGE2 and PGF2α; PGE2 promotes vasodilatation of the endometrial blood vessels to enhance inflammation and swelling and attract the LT towards the active site, while PGF2α causes myometrial contraction and vasoconstriction to promote ischemia, and finally, pain sensitivity decreases for the free nerve endings and pain appears. LT simultaneously promote the vasoconstriction and contraction of the muscle and helps PGF2α for the same [4,5]. Proinflammatory cytokines also play an essential role in the pathogenesis of PD by increasing the synthesis of PG.
NSAIDs, OCPs, physical exercises, and other complementary therapies are among the recommended treatments for PD. Physical exercise is nearly a new non-medical intervention to relieve PD associated pain [6]. One crucial element in pain management is that intervention must be affordable, both in time and expense. Exercise meets these requirements by providing an inexpensive alternative or an adjunct to zother analgesic agents. The PD pain reduced when exercises were done for 30-45 minutes, 3 days/week with variable intensities. The VAS (visual analogue scale), McGill Pain Questionnaire (MPQ), Adverse Effects of exercises, overall menstrual symptoms (back pain, fatigue), Moos menstrual distress questionnaire (MMDQ), use of rescue analgesics medication, restriction of daily activity, quality of life, and absence of work were used as outcome measures of exercise’s effect on PD which improves significantly [7]. Aerobic exercise for 30-45 minutes at 70-85% HRmax, 3-5 days/week, for 8-12 weeks, either guided or unsupervised, relieves pain. Stretching or weight training for 10-20 minutes, 2-3 days/week, for 8 weeks shows substantial improvement, although the quality of research is weak, necessitating more investigation and assessment [8-13]. As most studies have shown, there is a considerable improvement in pain and posit various mechanisms involved in pain modulation in PD.
Researchers have presumed different pain-relieving methods, ranging from non-opioids to opioids to hormonal changes for variations in pain sensitivity. Evidence shows that concurrent exercise-induced increases in endorphins or endocannabinoids are mainly responsible for pain reduction in PD related pain. Similarly, a small number of studies show that the production of anti-inflammatory macrophages in physically exercising muscle suppresses pro-inflammatory cytokine activity and regulates inflammatory responses [8,14]. Scientific evidence shows that a rise in PG causes pain and the influence of exercise on PG can help to relieve pain. Mosler et al. 1914 suggest that reducing uterine contractions by shunting blood away from the uterus reduces pain. Exercises are believed to cause hormonal changes in the uterine lining, which assist in reducing symptoms of PD [15].
Exercise-induced analgesia, which has been extensively recognized in literature, relies on the central pathway as the primary mechanism for pain reduction, and hormonal interaction might be another approach to reduce pain in PD. However, the complicated process of pain relief by exercise training and the interaction of all central and hormonal components for pain modulation in PD does not adequately explain the changes during the cycle phases. Therefore, given the exercise type during different phases of the cycle, this review will cover all probable neural and hormonal mechanisms facts of pain relief involved in PD resulting from exercise training.
EFFECTS OF EXERCISES ON CENTRAL NERVOUS SYSTEM
Aerobically or anaerobically exercised either, most researchers study the effects of aerobic mode of exercise and very few on resistive training. A single aerobic exercise session improves pain, and its effect lasts for less than 30 minutes [16]. Exercise, regardless of the time of injury or insult, shows the same result either done before (preventive effect) or after the insult or injury (therapeutic Effect). Repetitive swimming for 50-90 minutes for 5 days/week or treadmill for 10-60 minutes for 3 to 7 days/week improves mechanical pain and thermal sensitivity [17,18]. The level of pain reduction is directly related to the intensity and volume, not the frequency of the exercises [19]. If the training continues at moderate intensity before and after the onset of injury, hypoalgesia will be more, and as the intensity reaches exhaustion point, the effect will start declining, or pain will be more severe than before [20]. It all suggests that the volume and intensity of exercise are the keys to pain relief. Most of the studies showing the hypoalgesia effect of exercise have been animal trials, and very few human trials are available. Therefore, we will have our discussion weighted towards the findings we got in the animal trials.
1. Endogenous opioids
One of the most familiar mechanisms studied for exercise-induced analgesia. It shows the effect via β-endorphin, met-enkephalin, 2-endogenous, which increases with aerobic training for 5 to 8 weeks in Periaqueductal Grey (PAG), Rostral Ventral Medial Medulla (RVM), and Hypothalamus and remains high for approximately 48 hours [21,22]. If the training continues for more than 9 weeks or 45 sessions, the effects start declining as mu-opioid receptors were absent or reduced [23], which suggests that regular stimulation of the system produces down regulation of the opioids and expression of the receptors. Several studies showed an elevated plasma β-endorphin level at 85% HRmax or up to 80% of VO2max, and its concentration increases with an increase in intensity [24,25].
2. Serotonergic system
The function of serotonin is for both pain onset and pain relief. It mediates through 5-HT receptors. The concentration of 5 HT receptors increases in the Brainstem, Lumber spinal cord, and Parieto-occipital cortex with 60 minutes of swimming or 15 minutes on the treadmill for 6 days/week, for 4 weeks and remains higher for a week after exercising than sedentary subjects [26,27]. The Serotonin analgesic effect at the level of RVM is regulated by serotonin transporter (SERT), extracellularly [28]. Only two weeks of treadmill training can decrease the concentration of SERT with corresponding increase in the levels of 5HT leading to hypo-analgesia [29]. Similarly, reductions in non-inflammatory muscle pain can be experienced with 8 weeks of training, done before the injury. It means SERT alters the pain behavior and exercise can affect it with regular training by activating the opioids receptors in PAG, which is required to control SERT activity [21].
3. Endocannabinoids system
Pain modulation occurs via endocannabinoid receptors CB1 and CB2 in the spinal cords PAG, RVM, and dorsal horn [30]. These receptors bind to anandamide (AEA) and 2-arachidonylglycerol (2-AG) and produce analgesia. CB receptors are on the pain-sensing C fibers, increasing the nociceptive threshold to stimuli and improving pain. The CB receptors expression increases in dorsolateral and ventrolateral PAG with aerobic and resistance training [31,32]. Similarly, to serotonin-opioids interaction, there are pieces of evidence that indicate a specific link between the opioid and the endocannabinoid system. The studies that show the relationship between them were not performed for exercise-induced analgesia, though it shows the positive result for fear stress [33]. While one researcher shows improvement in analgesia with 25% of Maximum Isometric contraction of handgrip dynamometer and found that here analgesia is produced primarily by endocannabinoids, its action depends on the opioid system [34].
4. NMDA receptor alteration and nor-adrenergic system
N-methyl D-aspartate (NMDA) receptor NR1 subunit (p-NR1) phosphorylation in the RVM causes chronic hyperalgesia, and its downregulation causes analgesia [35]. An hour per day of exercise done for a week can prevent an increase in NR1 expression in RVM [21] and suggests that NMDA receptor non-phosphorylation in the neurological system is related to exercise-induced analgesia. Exercise activates the nor-adrenergic system and leads to release of catecholamines [36]. These chemicals bind to their respective receptors (α1, α2, β2 adrenergic) which are located in PAG, dorsal raphe, and spinal cord dorsal root ganglion (DRG) and mediate pain. Thus, exercise increases catecholamines and upregulation of its receptors in the nervous system [37].
CENTRAL NERVOUS SYSTEM-MEDIATED ANALGESIA
Exercise increases opioid tone in the CNS by modifying Ca2+ activated K+ channels, resulting in a decrease in neural excitability, neural firing, and inhibition of neurotransmitters (Table 1). The excitatory interaction between PAG and RVM facilitates and inhibits nociceptive signals, resulting in analgesic action [38].
Table 1.
The mechanism of action of exercises on Central Nervous System and hormones for exercise induced analgesia in PD
| Systems | Exercise protocol | Exercise effects | Mechanism of action |
|---|---|---|---|
| CNS | |||
| Endogenous opioids | 85% HRmax or at 80% of VO2max 20-30 mins 5-8 wks (Max 45 Sessions) |
↑↑ concentration (max 48 hrs) | • Modify Ca2+ activated K+ channels • ↓↓ Neural excitability & Neural firing • ┤Neurotransmitters |
| Endocannabinoids system | 25% MIC or 75-85% HRmax 30 mins |
↑↑ concentration | • ↑ Nociceptive threshold • Hyperpolarization • ↓ in excitatory cell firing rate • SUPPR neurotransmitter release • ↓ nociceptive impulses |
| Serotonergic system | 75-85% HRmax 15 to 60 mins 2-8 wks |
↑↑ concentration (168 hrs) | • ↓↓ expression of SERT • ↑↑ 5HT • ↑↑ Serotonin levels in the RVM |
| NMDA receptor and nor-adrenergic system | 75-85% HRmax 1 hour 1 week |
↑ expression | • ↑ NR1 expression • NMDA receptors non-phosphorylation • ↓↓ Neural excitability & Neural firing • ┤Neurotransmitters |
| Hormones | |||
| Progesterone | 30 to 95% HRmax or 75% of VO2 max 20-30 mins, 3/d/wk 8 weeks |
↑↑ concentration (untrained) ↔ (trained) or ↑ (untrained) |
• regulates the synthesis of PG and LT • ┤MMP expression • ↓ IL-8 mRNA expression • Indirectly regulates Hypoxia |
| Prostaglandins | 100-150 Watts or 30 to 95% HRmax or 60% MVC Single session |
↑↑↑↑ PGE2 and ↑↑ PGF2α 60-90% metabolic clearance of concentration |
• ↓ PGF2α/PGE2 ratio • ↓ PGF receptor expression |
| Inflammatory markers | |||
| Arachidonic acid | 30% to 80% of HRmax 20-40 min Single session |
↑↑ Cytochrome P450 (EET, DHET & ratio) ↑ PG & LT |
• Hyperpolarize of the vascular smooth muscle • ↑ Pro-fibrinolysis • ↓ Inflammation |
↑: increase, ↑↑: highly increase, ↓: decrease, ↓↓: highly decrease, ┤: inhibition, HRmax: maximum heart rate, VO2max: maximum oxygen consumption, MIC: maximum isometric contraction, MVC: maximum voluntary contraction, EET: epoxyeicosatrienoic acid, DHET: dihydroxyeicosatricnoic acid, SERT: serotonin transporter, 5HT: 5-hydroxytryptamine receptors, NMDA: N-methyl D-aspartate, NR1: subunit of NMDA receptor, MMP: matrix metalloproteases, IL-8: interleukin 8, RVM: rostral ventral medial medulla, SUPPR: suppress.
Simultaneously, exercise also lowers the expression of SERT and raise the concentration of 5HT [29], which increases serotonin levels in the RVM and modulates pain. SERT activity is controlled by serotonin concentration in RVM and is mediated by opioid receptors in PAG [39]. Although in RVM, on the other hand, NMDA receptors also help with pain modulation with SERT. In RVM, non-phosphorylation of NMDA reduces channel conductance and its insertion into the synapse, resulting in analgesia [31].
As a result, exercise increases opioid receptor activation and neuronal pain activity through the non-phosphorylation of NMDA and restricts SERT expression, which either prevents hyperalgesia or induces hypoalgesia. All of this occurs in a single set of occasions for controlling pain, and opioids may be the prime cause of hypoalgesia as they control the other events for the same purpose. In contrast, overstimulation of the opioid system can cause downregulation, eliminating the hypoalgesia effects.
A variety of endogenous pathways, including non-opioid systems, affect pain perception. In the exercise analgesia as well the role of the nor-adrenergic (NA) system is studied. Catecholamines can modulate the nociceptors pathway by activating α2 adrenergic receptors (α2-AR) [40], which inhibits the development of cyclic adenosine monophosphate, induces hyperpolarization and in turn decreases the excitable cell firing rate [41]. It regulates pain via diverse locations in PAG and DRG. Anti-nociceptive effects of α2 receptors are associated to the interaction of opioid receptors, which results in the release of endogenous opioids such as β-endorphin and induces analgesia in inflamed conditions [42]. At the same time, NA activates α2, α1, and β adrenoceptors in non-inflamed models, causing anti-nociception against PGE2 [43].
Endocannabinoids (ECB) is another non-opioid system that helps with pain relief. The receptors are negatively coupled to the adenylate cyclase, positively coupled with the Mitogen-activated protein kinase [44], producing hyperpolarization and a decrease of the excitatory fire rate and inhibiting the release of neurotransmitters [45]. It has receptors in PAG, RVM, DRG, and immune cells, and it interacts with the opioid system, allowing one system to activate the other [46]. The AEA concentration increases when opioid receptors are stimulated during exercise training [33], but this alone is not enough to cause analgesia. While an increase in 2AG concentration induces substantial analgesia, the opioid system does not endorse its activation [25]. NA and partially ECB enable the opioid pathway to provide pain relief. As a result, it is reasonable to conclude that opioids are the leading cause of exercise-induced analgesia combined with endocannabinoids.
EFFECTS OF EXERCISE ON HORMONES AND INFLAMMATORY MARKERS
1. Progesterone
Progesterone has anti-inflammatory effects by regulating the synthesis of PG and LT. Its concentration increases with exercises, but more significant changes occur in the luteal phase of menstruation, and its concentration depends on the intensity of exercises, as it shows maximum concentration at exhaustion. All these changes are more evident in untrained individuals than trained [47]. The concentration of the circulation hormones can be increased or decrease from the source or by affectioning the metabolic clearance. Estimated hepatic blood flow decreases markedly up to 60% in 40 minutes during exercises [48]. As the metabolic clearance improves, the concentration will come back to normal as in trained subjects.
Researchers showed that if exercises performed for 20 to 30 mins for 8 weeks, 3 days in a week at 30 to 95 % of maximum output or 75% of VO2max would significantly improve progesterone’s plasma concentration [49]. Even in animal studies, the progesterone plasma concentration increases with 10 to 30 minutes of swimming [50].
Under the exercise condition, progesterone increases, but LH concentration did not significantly change in any phase of the cycle [47,50]. As LH, FSH, and progesterone are interrelated, it would therefore appear that any increase in progesterone is independent of the hypothalamus-pituitary gonadal system. However, lactate affects the cAMP of the ovary, which in turn increases progesterone concentration [50].
2. Prostaglandins
The contribution of Prostaglandins to exercise is controversial as PG has its potential role in proinflammatory action, and its concentration is higher in PD [5]. In symptomatic PD women, excess PG due to increased uterine production causes pain, but its actual plasma concentration differs significantly from symptom severity. The difference between pain and plasma amount could be because of the low metabolic clearance of PG. However, the metabolic clearance rate of PG increases with exercises [51].
The lung is a primary organ for the synthesis of PG and can take up and release catabolized PG into the circulation from other organs. PGs are moderately temporary molecules with a short life span, usually just a second to a minute. Nearly 60-90% circulating concentration of PGE2 and PGF2α is inactivated in a single passage through the pulmonary circulation [52]. More or less, approximately 90% of the PG is expelled out from the blood.
With exercises, PG synthesis increases, especially PGE2 and PGF2α in the skeletal muscle, which regulates the muscle protein synthesis and its adaptation for training [53]. PG also has a potential role in exercise-induced hyperaemia in men and women following rhythmic or isometric muscle contraction. When exercises are performed at 50-75% of maximum workload or 60% of Maximum Voluntary Contraction (MVC), hyperaemia appears in skeletal muscles as it expresses COX, PGE2, and PGF2α synthase [54,55].
3. Arachidonic acid
AA metabolized into PG and LT via COX and LOX. AA can be converted into other metabolites also via Cytochrome P450 (CYP). CYP converts AA into epoxyeicosatrienoic acid (EET), which further hydrolyzed in dihydroxyeicosatricnoic acid (DHET). EET is the potential substrate for endothelial-derived hyperpolarization factor (EDHF). The hyperpolarize of the vascular smooth muscle causes relaxation via Ca2+ dependent K+ channels [49].
The stable metabolite of EET, DHET, which causes vasodilatation in skeletal muscle and regional circulation like the heart shows a positive relationship with exercises. Dynamic exercises at different intensities (30% to 80% of HRmax) and duration (20-40 min) improve plasma concentration. The significant changes appear at a higher intensity and short duration (80% and 20 min) and moderate-intensity and more prolonged duration (60% and 40 min). The magnitude of changes in the plasma concentration depends on the intensity and duration of exercise [56]. The acute bouts of maximal Exercise (Bruce Protocol) at maximum HR and the point of exhaustion show the same result in isolated concentration and the EET/DHET ratio [57]. The changes in the plasma concentration convey that the AA metabolism shifted towards CYP pathways during exercises. The release of EET and DHET improves the vascular tone, BP, produces pro-fibrinolysis, and reduces inflammation [58].
4. Pro-inflammatory cytokines
The release of pro-inflammatory and anti-inflammatory cytokines is affected by exercises. Aerobic exercise lowers pro-inflammatory cytokine levels (TNF-α [tumor necrosis factor-α], IL-6, and IL-1β) [59] and activates macrophages to release anti-inflammatory cytokines (IL-10) and decrease proinflammatory cytokines (TNF-α, IL-6, and IL-1β) [23]. It has been demonstrated that physical exercise and C-Reactive Protein (CRP) have an inverse relationship and CRP and pro-inflammatory cytokines are directly related [60].
HORMONAL MEDICATED ANALGESIA
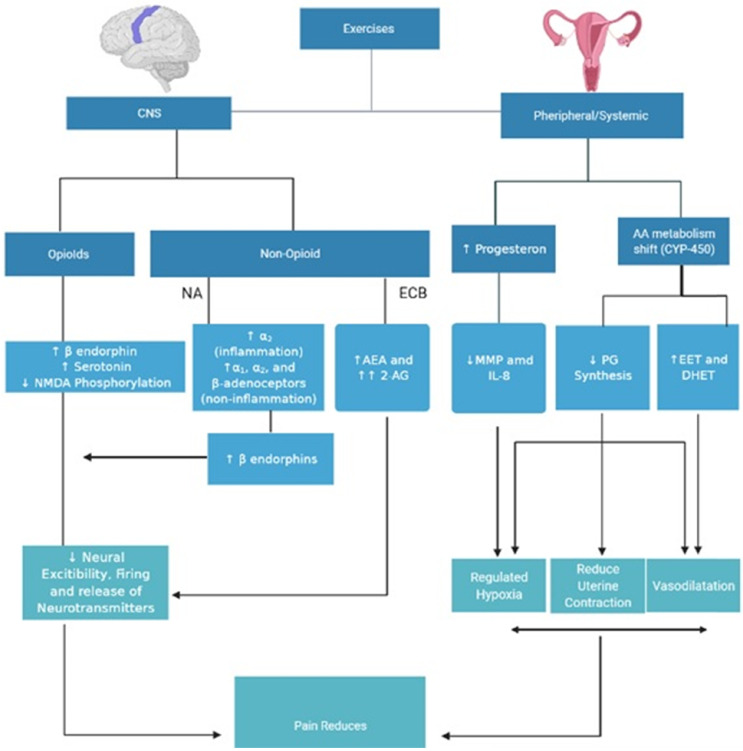
Hormonal action is the possible mechanism for exercise- induced analgesia in clinical trials (Fig. 1). Pain perception and the prevalence of many symptoms are similar in male and female in childhood, but they vary after puberty when gonadal hormones play a significant role in reproduction [61]. The central anti-inflammatory, neuroprotective, and analgesic properties of progesterone are particularly apparent [62], and pain relief may be provided by neurohormone, endorphins, hypnotic and depression effects on the brain during pregnancy and luteal menstrual phase [63]. Exercises demonstrate improvement of the plasma level of progesterone in untrained healthy females during the luteal stage [47].
Fig. 1.
Exercise induced analgesia in primary dysmenorrhea. AEA: anandamide, 2AG: 2-arachidonylglycerol, PG: prostaglandins, MMP: matrix metalloproteases, EET: epoxyeicosatrienoic acid, DHET: dihydroxyeicosatricnoic acid.
Researchers have reported that progesterone plays a protective role in fibromyalgia and Caesarian section pain sensitivity in females. It correlates negatively with the pain sensitivity, and the maximum response for pain modulation was in the luteal phase [64]. It also reduces pain and uterine contractions in cases of threatened abortion due to luteal phase insufficiency [65]. Still, the role of progesterone is controversial as few of the studies showed a positive correlation with mechanical pain, hyperalgesia, and ischemic pain in the luteal phase of menstruation [66]. In contrast, no association exists between progesterone and heat tolerance and threshold during end-term pregnancy and 4 to 8 weeks of the postpartum period [67]. Though progesterone spikes in the luteal phase during exercise training, it returns to its normal plasma levels after few weeks of training.
Progesterone has anti-inflammatory effects by regulating the synthesis of PG and LT [4]. In the absence of pregnancy, progesterone level declines during the late secretory phase, and the cycle starts with the vital enzyme Matrix Metalloproteases (MMP), as MMP expression inhibited by progesterone [68,69]. MMP action takes some time to initiate the cycle, and during that period if progesterone level improves, the menses will decrease. The critical duration to initiate the cycle and action of MMP is 36 hours, and a total of 36 hours is not a window for the irreversible action of progesterone. Initial first 24 hours progesterone will inhibit MMP 1, 2, and 3, and if the rise is in 48 hours after the decline, only MMP1 and 3 inhibited, and MMP2 will carry forward the inflammatory cycle [70]. It could be possible that as the progesterone rises with exercises, it controls all these activities and modulates pain but the raise exact time and phase in which it minimizes inflammatory action requires clinical trials to confirm the changes throughout the cycle.
Fall in progesterone causes local changes in the endometrium, induces vasoconstriction in arterioles, and reduces the MMP [71]. Lack of progesterone also upregulates the IL-8 mRNA expression after 48 hours of decline; IL-8 causes the production of MMP [72]. So MMP is activated by the decline in progesterone and IL-8 activation, but hypoxia regulates the production of IL-8 [73]. So, with progesterone rise, the MMP production decreases directly and indirectly by IL-8 and hypoxia. At the same time, hypoxia is essential to control the inflammation and repair of the endometrium and can affect the pain modulation, though it affects the uterine contraction and causes ischemia and pain [74]. Gannon et al. [75] failed to find episodes of menstrual ischemia, but his finding may be less impactful as of less resolution fluxmeter. This approach would not allow focused and prolonged ischemia-reperfusion events. In contrast, in animal studies transient hypoxia in the endometrium appears.
In PD, there is a hyper exaggerated inflammatory response, and the critical component of hyper contraction of the uterus is PGF2α only [76], which causes vasocontraction and hyperalgesia. In Heavy menstrual bleeding, the PGF2α/PGE2 ratio and PGF receptor expression reduce significantly, which results in vasodilatation of spiral arterioles by increasing PGE2 production at the expense of PGF2α [77]. PGF2α and endothelin-1 are two endometrial factors with known vasoconstriction properties [78]. As the CL ages from the mid to late luteal phase, PGF2α concentration increases and the ratio to PGE2 also improves. PGF2α production is an auto amplification loop as luteal cells with PGF2α induces production of further PGF2α by luteal cell. The intercellular mechanism via the action of cytosolic Phospholipase A2 and COX-2 and fall in progesterone induces COX-2 for PG synthesis [79].
PG increases during exercise; however, PGE2 rises faster than PGF2 possibly altering the ratio and reducing spiral arteriole constriction and pain in PD. As per our knowledge, none of the studies has analyzed PGE2/PGF2 ratio variations in any condition similar to PD despite the need for clarification. It is also crucial to know the benefits of higher progesterone levels and COX-2 expression on PG concentration during different phases of menstruation as even a small amount of PG is potent enough to show its effects. Exercise’s effect on hypoxia-induced ischemia in PD is essential to note as hypoxia causes hyperaemia in skeletal muscles [55]. Since hypoxia is an essential component of endometrial repair during the menstruation period, the alteration in blood flow in the endometrium with exercise during the early and late luteal phases must be determined. Exercise also affects AA metabolism, shifting the pathway towards CYP and controlling PG synthesis output. Exercise increases the EET/DHET ratio, which triggers arteriole vasodilation. Since it has a positive and negative effect on the EET/DHET and PGF2/PGE2 ratios, this may cause pain relief in PD.
CONCLUSION
Despite everything, pain improves with exercises in PD. The pain relief could be because of the synergistically working of both opioids and the non-opioids systems. Hormonal changes occur during exercises as raises progesterone concentration in the late luteal phase, high metabolic clearance rate of PGs, and shifting of AA metabolism towards the CYP. All of these changes influence blood flow by managing arteriole vasoconstriction and the sensory pathway. Though the parameters to assess the desired effect need clarification, whether the progesterone alone or any other changes in the inflammatory process cause pain reduction in PD. To evaluate the precise pain control, high-quality RCT’s are required to assess the effect before, during, and after the cycle by progesterone or any other mechanism. To assess pain regulation in PD, it is crucial to understand the gradual increment and long-term changes in plasma concentration and expression of all main hormones and inflammatory components involved in PD.
Footnotes
CONFLICTS OF INTERESTS
No conflict of interest.
References
- 1.Stevenson DR, Corrah T. Diagnosis and Initial Management of Dysmenorrhea. Br J Hosp Med. 2017;78(8):C114–17. doi: 10.12968/hmed.2017.78.8.C114. [DOI] [PubMed] [Google Scholar]
- 2.Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ. 2006 13;332(7550):1134–38. doi: 10.1136/bmj.332.7550.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan SA. The Treatment of Dysmenorrhea. Pediatr Clin North Am. 2017;6:1–7. doi: 10.1016/j.pcl.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Maybin JA, Critchley HOD. Progesterone: a pivotal hormone at menstruation. Ann N Y Acad Sci. 2011;1221(1):88–97. doi: 10.1111/j.1749-6632.2011.05953.x. [DOI] [PubMed] [Google Scholar]
- 5.Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13(4):277–88. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez E, Turk DC. Sensory and affective components of pain: Separation and synthesis. Psychol Bull. 1992;112(2):205–17. doi: 10.1037/0033-2909.112.2.205. [DOI] [PubMed] [Google Scholar]
- 7.Armour M, Ee CC, Naidoo D, Ayati Z, Chalmers KJ, Steel KA, de Manincor MJ, Delshad E. Exercise for dysmenorrhoea. Cochrane Database Syst Rev. 2019;9(9):CD004142. doi: 10.1002/14651858.CD004142.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora A, Yardi S, Gopal S. Effect of 12-Weeks of Aerobic Exercise on Primary Dysmennorrhea. Indian J Physiother Occup Ther - An Int J. 2014;8(3):130. doi: 10.5958/0973-5674.2014.00370.0. [DOI] [Google Scholar]
- 9.Israel RG, Suiton M, O'Brien KF. Effects of aerobic training on primary dysmenorrhea symptomatology in. College females. J Am Coll Health Assoc. 1985;33(6):241–4. doi: 10.1080/07448481.1985.9935033. [DOI] [PubMed] [Google Scholar]
- 10.Kannan P, Chapple CM, Miller D, Claydon-Mueller L, Baxter GD. Effectiveness of a treadmill-based aerobic exercise intervention on pain, daily functioning, and quality of life in women with primary dysmenorrhea: A randomized controlled trial. Contemp Clin Trials. 2019;81:80–6. doi: 10.1016/j.cct.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Fallah F, Mirfeizi M. How is the quality and quantity of primary dysmenorrhea affected by physical exercises? A study among Iranian students. Int J Women's Heal Reprod Sci. 2018;6(1):60–6. doi: 10.15296/ijwhr.2018.11. [DOI] [Google Scholar]
- 12.Jaibunnisha, Gomathi B, Goerge U. Effect of Selected Muscle Stretching Exercises on Primary Dysmenorrhoea among Student Nurses. Int J Nurs Educ. 2017;9(3):69. doi: 10.5958/0974-9357.2017.00073.3. [DOI] [Google Scholar]
- 13.Motahari-Tabari N, Shirvani MA, Alipour A. Comparison of the effect of stretching exercises and mefenamic acid on the reduction of pain and menstruation characteristics in primary dysmenorrhea: A randomized clinical trial. Oman Med J. 2017;32(1):47–53. doi: 10.5001/omj.2017.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelli F. Koltyn, Angelique G. Brellenthin, Dane B. Cook, Nalini Sehgal CH. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15(12):1294–304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannan P, Chapple CM, Miller D, Claydon-Mueller L, Baxter GD. Menstrual pain and quality of life in women with primary dysmenorrhea: Rationale, design, and interventions of a randomized controlled trial of effects of a treadmill-based exercise intervention. Contemp Clin Trials. 2015;42:81–9. doi: 10.1016/j.cct.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Rice D, Nijs J, Kosek E, Wideman T, Hasenbring MI, Koltyn K, Graven-Nielsen T, Polli A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J Pain. 2019;20(11):1249–66. doi: 10.1016/j.jpain.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Almeida C, Demaman A, Kusuda R, Cadetti F, Ida M, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156(3):504–13. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 18.Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg. 2012;114(6):1330–7. doi: 10.1213/ANE.0b013e31824c4ed4. [DOI] [PubMed] [Google Scholar]
- 19.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Malan TP. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain Model. Anesthesiology. 2011;114(4):940–8. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LGA, Santos ARS. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–48. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 21.Brito RG, Rasmussen LA, Sluka KA. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep. 2017;2(5):e618. doi: 10.1097/PR9.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Tzeng J, Lin M, Hung C, Wang J. Forced treadmill running suppresses postincisional pain and inhibits upregulation of substance P and cytokines in rat dorsal root ganglion. J Pain. 2014;15(8):827–34. doi: 10.1016/j.jpain.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira MSR, da Silva Fernandes MJ, Scorza FA, Persike SP, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull. 2010;83(5):278–83. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Colt EW, Wardlaw SL, Frantz AG. The effect of running on plasma beta-endorphin. Life Sci. 1981;28(14):1637–40. doi: 10.1016/0024-3205(81)90319-2. [DOI] [PubMed] [Google Scholar]
- 25.Sforzo GA. Opioids and Exercise. Sport Med. 1989;7(2):109–24. doi: 10.2165/00007256-198907020-00003. [DOI] [PubMed] [Google Scholar]
- 26.Dey S, Singh RH, Dey PK. Exercise Training : Significance of Regional Alterations in Serotonin Metabolism of Rat Brain in Relation to Antidepressant Effect of Exercise. Physiol Behav. 1992;52(6):1095–9. doi: 10.1016/0031-9384(92)90465-E. [DOI] [PubMed] [Google Scholar]
- 27.Gerin C, Teilhac J, Smith K, Privat A. Motor activity induces release of serotonin in the dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 2008;436(2):91–5. doi: 10.1016/j.neulet.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inase M, Nakahama H, Otsuki T, Fang J. Analgesic effects of serotonin microinjection into nucleus raphe magnus and nucleus raphe dorsalis evaluated by the monosodium urate (MSU) tonic pain model in the rat. Brain Res. 1987;426(2):205–11. doi: 10.1016/0006-8993(87)90874-2. [DOI] [PubMed] [Google Scholar]
- 29.Bobinski F, Ferreira TAA, Cordova MM, Dombrowski PA, da Cunha C, Caroline C, Poli A, Pires RGW, Martinssilva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohmann AG, Tsou K, Walker JM. Cannabinoid Suppression of Noxious Heat-Evoked Activity in Wide Dynamic Range Neurons in the Lumbar Dorsal Horn of the Rat. J Neurophysiol. 1999;81(2):575–83. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JD. Cannabinoids Modulate Pain by Multiple Mechanisms of Action. J Pain. 2000;1(1):2–14. doi: 10.1016/S1526-5900(00)90082-8. [DOI] [Google Scholar]
- 32.Galdino G, Romero T, Felippe J, da Silva FP, Aguiar D, de Paula AM, Cruz J, Parrella C, Piscitelli F, Duarte I, di Marzo V, Perez A. Acute Resistance Exercise Induces Antinociception by Activation of the Endocannabinoid System in Rats. Anesth Analg. 2014;119(3):702–15. doi: 10.1213/ANE.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler RK, Rea K, Lang Y, Gavin AM, Finn DP. Endocannabinoid-mediated enhancement of fear-conditioned analgesia in rats : Opioid receptor dependency and molecular correlates. Pain. 2008;140(3):491–500. doi: 10.1016/j.pain.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Endocannabinoid and Opioid System Interactions in Exercise-Induced Hypoalgesia. Pain Med. 2018;19(1):118–23. doi: 10.1093/pm/pnx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felipe L, da Silva S, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151(1):155–61. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc. 1996;28(2):204–09. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Nicholas PT, Hökfelt VAP. The distribution and significance of CNS adrenoceptors examined with in situ hybridization Anthony. Trends Pharmacol Sci. 1996;17(7):245–54. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- 38.Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: Further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187(3):513–31. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- 39.Rivot JP, Weil-Fugazza J, Godefroy F, Bineauthurotte M, Orylavollee L BJ. Involvement of serotonin in both morphine and stimulation-produced analgesia-electrochemical and biochemical approachesle. Adv Pain Res Ther. 1984;6:135–50. [Google Scholar]
- 40.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22(5):845–58. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald E, Kobilka BK, Scheinin M. Gene targeting- Homing in on α2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18(6):211–9. doi: 10.1016/S0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 42.Binder W, Mousa SA, Sitte N, Kaiser M, Stein C, Schäfer M. Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur J Neurosci. 2004;20(1):92–100. doi: 10.1111/j.1460-9568.2004.03459.x. [DOI] [PubMed] [Google Scholar]
- 43.Thiago A, Lima R, Rodrigues R, Petrocchi A, Guzzo LS, Klein A, Duarte IDG. Noradrenaline induces peripheral antinociception by endogenous opioid release. Pharmacol Rep. 2018;70(4):784–88. doi: 10.1016/j.pharep.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63(5):569–611. doi: 10.1016/S0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 45.Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Marzo VD, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans-Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Onodera K, Sakurada S, Furuta S, Yonezawa A, Hayashi T, Honma I, Miyazaki S. Age-related differences in forced walking stress-induced analgesia in mice. Drugs Exp Clin Res. 2001;27(5-6):193–8. [PubMed] [Google Scholar]
- 47.Bonen A, Ling WY, MacIntyre KP, Neil R, McGrail JC, Belcastro AN. Effects of exercise on the serum concentrations of FSH, LH, progesterone, and estradiol. Eur J Appl Physiol Occup Physio. 1979;42(1):15–23. doi: 10.1007/BF00421100. [DOI] [PubMed] [Google Scholar]
- 48.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971;50(12):2715–25. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balazy M, Schieber EB, McGiff JC. Identification of arachidonate epoxides in human platelets. Adv Prostaglandin Thromboxane Leukot Res. 1995;23(4):199–201. [PubMed] [Google Scholar]
- 50.Lu SS, Lau CP, Tung YF, Huang SW, Chen YH, Shih HC, Tsai SC, Lu CC, Wang SW, Chen JJ, Chien EJ, Chien CH, Wang PS. Lactate stimulates progesterone secretion via an increase in cAMP production in exercised female rats. Am J Physiol-Endocrinol Metab. 1996;271:E910–5. doi: 10.1152/ajpendo.1996.271.5.E910. [DOI] [PubMed] [Google Scholar]
- 51.Demers LM, Harrison TS, Halbert DR, Santen RJ. Effect of prolonged exrrcise on plasma prostaglandin levels Laurence. Prostaglandins Med. 1981;6(4):413–8. doi: 10.1016/0161-4630(81)90073-2. [DOI] [PubMed] [Google Scholar]
- 52.Ferreira SH, Vane JR. Prostaglandins: Their Disapperance from and Release in to the Circulation. Nature. 1967;216(2):868–73. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- 53.Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol. 2013;115(6):909–19. doi: 10.1152/japplphysiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol. 1976;257(1):109–21. doi: 10.1113/jphysiol.1976.sp011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowak J, Wennmalm Å. Effect of exercise on human arterial and regional venous plasma concentrations of prostaglandin E. Prostaglandines Med. 1978;1(6):489–97. doi: 10.1016/0161-4630(78)90119-2. [DOI] [PubMed] [Google Scholar]
- 56.Giordano RM, Newman JW, Pedersen TL, Ramos MI, Stebbins CL. Effects of dynamic exercise on plasma arachidonic acid epoxides and diols in human volunteers. Int J Sport Nutr Exerc Metab. 2011;21(6):471–9. doi: 10.1123/ijsnem.21.6.471. [DOI] [PubMed] [Google Scholar]
- 57.Gollasch B, Dogan I, Rothe M, Gollasch M, Luft FC. Maximal exercise and plasma cytochrome P450 and lipoxygenase mediators: a lipidomics study. Physiol Rep. 2019;7(13):1–11. doi: 10.14814/phy2.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang H, Anderson GD, McGiff JC. The red blood cell participates in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2012;98(3-4):91–3. doi: 10.1016/j.prostaglandins.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeanjean AP, Moussaoui SM, Maloteaux JM, Laduron PM. Interleukin-1/3 induces long-term increase of axonally transported opiate receptors and substance P. Neuroscience. 1995;68(1):151–7. doi: 10.1016/0306-4522(95)00106-S. [DOI] [PubMed] [Google Scholar]
- 60.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 61.Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain. 2010;149(1):76–88. doi: 10.1016/j.pain.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 62.González SL, Coronel MF. Beyond reproduction: The role of progesterone in neuropathic pain after spinal cord injury. Neural Regen Res. 2016;11(8):1238–40. doi: 10.4103/1673-5374.189177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Lee J, Ko S. The relationship between serum progesterone concentration and anesthetic and analgesic requirements: A prospective observational study of parturients undergoing cesarean delivery. Anesth Analg. 2014;119(4):901–5. doi: 10.1213/ANE.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 64.Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily Fluctuations of Progesterone and Testosterone Are Associated With Fibromyalgia Pain Severity. J Pain. 2018;19(4):410–7. doi: 10.1016/j.jpain.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palagiano A, Bulletti C, Pace MC, De Ziegler D, Cicinelli E, Izzo A. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Ann N Y Acad Sci. 2004;1034:200–10. doi: 10.1196/annals.1335.022. [DOI] [PubMed] [Google Scholar]
- 66.Pogatzki-Zahn EM, Drescher C, Englbrecht JS, Klein T, Magerl W, Zahn PK. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain. 2019;160(8):1781–93. doi: 10.1097/j.pain.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 67.Frölich MA, Banks C, Warren W, Robbins M, Ness T. The Association between Progesterone, Estradiol, and Oxytocin and Heat Pain Measures in Pregnancy: An Observational Cohort Study. Anesth Analg. 2016;123(2):396–401. doi: 10.1213/ANE.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 68.Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ. Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metallo-proteinases. Proc Natl Acad Sci (USA) 1996;93(17):9120–5. doi: 10.1073/pnas.93.17.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lockwood CJ, Krikun G, Hausknecht VA, Papp C, Schatz F. Matrix metalloproteinase and matrix metalloproteinase inhibitor expression in endometrial stromal cells during progestin-initiated decidualization and menstruation- related progestin withdrawal. Endocrinology. 1998;139(11):4607–13. doi: 10.1210/endo.139.11.6304. [DOI] [PubMed] [Google Scholar]
- 70.Slayden OD, Brenner RB. A critical period of progesterone withdrawal precedes menstruation in macaques. Reprod Biol Endocrinol. 2006;4(S1):1–10. doi: 10.1186/1477-7827-4-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markee JE. Menstruation in intraocular endometrial transplants in the Rhesus monkey. Am J Obstet Gynecol. 1978;131(5):558–9. doi: 10.1016/0002-9378(78)90119-9. [DOI] [PubMed] [Google Scholar]
- 72.Critchley HO, Jones RL, Lea RG, Drudy TA, Kelly RW, Williams AR, Baird DT. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrinol Metab. 1999;84(1):240–8. doi: 10.1210/jc.84.1.240. [DOI] [PubMed] [Google Scholar]
- 73.Hirani N, Antonicelli F, Strieter RM, Wiesener MS, Haslett C, Donnelly SC. The regulation of interleukin-8 by hypoxia in human macrophages - A potential role in the pathogenesis of the acute respiratory distress syndrome (ARDS) Mol Med. 2001;7(10):685–97. doi: 10.1007/BF03401959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawood MY. Dysmenorrhea. J Reprod Med. 1985;30(3):154–67. doi: 10.1055/s-2007-1022627. [DOI] [PubMed] [Google Scholar]
- 75.Gannon BJ, Carati CJ, Verco CJ. Endometrial perfusion across the normal human menstrual cycle assessed by laser Doppler fluxmetry. Hum Reprod. 1997;12(1):132–9. doi: 10.1093/humrep/12.1.132. [DOI] [PubMed] [Google Scholar]
- 76.Lundstrom V. The myometrial response to intra-uterine administration of PGF2alpha and PGE2 in dysmenorrheic women. Acta Obs Gynecol Scand. 1977;56(3):167–72. doi: 10.3109/00016347709162115. [DOI] [PubMed] [Google Scholar]
- 77.Smith OPM, Jabbour HN, Critchley HOD. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22(5):1450–6. doi: 10.1093/humrep/del503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baird DT, Cameron ST, Critchley HO, Drudy TA, Howe A, Jones RL, Lea RG, Kelly RW. Prostaglandins and menstruation. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):15–7. doi: 10.1016/S0301-2115(96)02568-7. [DOI] [PubMed] [Google Scholar]
- 79.Wiltbank MC, Ottobre JS. Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol. 2003;1:1–11. doi: 10.1186/1477-7827-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]