Abstract
Background
People with HIV are at increased risk of human papillomavirus (HPV) disease progression, given the persistence of immune activation and residual inflammation despite effective combination antiretroviral therapy (cART). Whether a low CD4:CD8 T-cell ratio, known to mirror peripheral immune dysfunction, is associated with squamous intraepithelial lesions (SILs) is unknown.
Methods
This was a retrospective cohort study on cART-treated HIV-positive subjects undergoing screening for HPV-related dysplasia (anal/cervical cytology and HPV genotyping). SIL was defined as the presence of either atypical squamous cells of undetermined significance (ASCUS), low-grade SILs, or high-grade SILs. Demographic and viro-immunological parameters (T-cell count, CD4:CD8 T-cell ratio, CD8+ CD38+ T-cell percentage) at the time of screening were analyzed by the chi-square test, Mann-Whitney test, and multivariate logistic regression analysis.
Results
A total of 419 cART-treated subjects were included. Half of the patients had cervical/anal SIL. Individuals with SIL were more commonly males, were men who have sex with men, were coinfected with Treponema pallidum, had been treated with integrase inhibitor (INSTI)–based cART regimens, and had a shorter time since HIV diagnosis and cART initiation than subjects with normal cytology. CD38+ CD8+ T-cell percentage, but not the CD4:CD8 T-cell ratio, correlated with SILs. HPV infection, especially with multiple and high-risk genotypes, was confirmed to be associated with SIL. In multivariate analysis, the only factors independently associated with cervical/anal dysplasia were HPV infection and harboring higher percentages of peripheral activated CD38+ CD8+ T cells.
Conclusions
HPV infection is the major driver of dysplasia in the setting of HIV infection. In this study, CD8+ CD38+ T cells were an independent predictor of dysplasia in cART-treated subjects, while CD4:CD8 T-cell ratio was not. In the setting of HIV–HPV coinfection, CD4:CD8 T-cell ratio may not fully capture the alterations of HPV-specific immunity.
Keywords: activated CD8+ CD38+ T cells, CD4:CD8 ratio, cervical–anal dysplasia, HIV infection, HPV infection
With the advent of combination antiretroviral therapy (cART), cancers are the leading cause of death in people with HIV (PWH) [1]. Worldwide, human papillomavirus (HPV) is the causative agent of all cervical cancers and almost all anal cancers, as well as the majority of other genital male and female neoplasms [2]; when compared with their uninfected counterparts, women and men who have sex with men (MSM) with HIV experience a 2–40 times greater risk of cervical cancer [3] and a 4.5–9.2 times higher anal cancer incidence [4, 5], respectively. This phenomenon is the consequence of the negative impact of HIV-associated disruption of T-cell homeostasis on the natural history of HPV infection with a higher prevalence [4, 6, 7] and lower clearance [6, 7] of multiple and high-risk (HR) HPV genotypes [7, 8] in PWH than in general population.
All these findings translate into a greater risk of precancerous lesions as squamous intraepithelial lesions (SILs) [9, 10] and faster progression to cancer [9, 11] in the setting of HIV–HPV coinfection compared with HPV alone. The reasons for this scenario are several: Next to a higher behavioral exposure of PWH to HPV [12] and a greater prevalence of cancer co-factors (eg, smoking) [13, 14], a central role is attributable to HIV-related mucosal and systemic immune alterations, which are both key factors in hindering HPV infection and its progression. At a local level, HIV-induced mucosal damage is coupled with a significant density reduction of immune cells—CD4+ T lymphocytes [15], especially naïve CD4+ and memory CD8+ CD45R0+ T-cell pools [16], macrophages, neutrophils, and natural killer (NK) cells—an important downrepresentation of both pro- and anti-inflammatory cytokines [15], as well as increased but dysfunctional CD8+ T infiltrates [16–18]. As CD4+ T-cell count is a marker of HIV-induced immunosuppression and viral replication, it inversely correlates with HPV infection risk (especially by HR-HPV genotypes), SIL development, and progression to cancer [6]. Even if cART effectively reduces the rates of acquisition and persistence of HPV and the incidence of dysplastic lesions [19, 20], literature has shown conflicting data on the potential impact of antiretroviral therapy on lowering the risk of HPV-related cervical and anal cancer development in PWH [4, 21, 22].
In the setting of HIV infection, CD4+ T-cell count and HIV viremia are generally used to monitor the viro-immunological response to cART. More recently, CD4:CD8 ratio emerged as an interesting parameter of immune system competence. Indeed, a low CD4:CD8 ratio is linked to a T-cell phenotype skewed to terminally differentiated CD8+ T cells, increased CD8+ activation, and senescence [23]. These features are also well described in the course of physiological non-HIV aging [24], so, in the context of HIV infection, the CD4:CD8 ratio is also a hallmark of non-AIDS-defining events [25] and predicts cardiovascular, muscle, and renal age-associated diseases [26], and therefore non-AIDS morbidity and mortality [25]. All these characteristics make the CD4:CD8 ratio an ideal tool for monitoring PWH, aiming to identify those at higher risk for the development non-AIDS comorbidities and for whom a stricter follow-up may be advocated [25].
The role of CD4:CD8 ratio as a marker of immune system dysregulation and a predictor of HPV disease has yet to be assessed; thus, the aim of our work was to investigate whether the CD4:CD8 ratio value correlates with HPV-dependent SILs.
METHODS
Study Population
We conducted a cross-sectional retrospective study enrolling all HIV-positive patients of the San Paolo Infectious Diseases (SPID) cohort, San Paolo Hospital, University of Milan, Italy, who underwent anal or cervical HPV screening from January 2016 to February 2019. Inclusion criteria were being on combination antiretroviral therapy with at least 2 consecutive determinations of HIV-RNA <50 copies/mL.
HPV-Related Dysplasia Screening
All patients were screened for HPV-related dysplasia and underwent anal (males) or cervical (females) cytology and HPV genotyping during a surgical/gynecological examination. Anal and cervical specimens were collected using a disposable plastic cytological brush (Ningbo HLS Medical Products Co.) to perform a cervical/anal cytology. SIL was defined according to the 2001 Bethesda System and included atypical squamous cells of undetermined significance (ASCUS), low-grade SILs (LSILs), high-grade SILs (HSILs), or carcinoma. Cytological samples were considered invalid, and thus excluded from the analyses, if the result was insufficient cellularity.
Specimen Collection
Anal/cervical samples were collected in a liquid-based cytology medium (PreservCyt-Hologic). Total DNA was extracted using a commercial kit (QIAamp DNA Blood Mini KIT, Qiagen).
Flow Cytometry
T-cell surface phenotypes were evaluated using fresh peripheral blood (FACSCanto II; Becton Dickinson Italia Spa, Milan, Italy), CD4-PE-cy7, CD8-PE-cy5, and CD38-FITC (Becton Dickinson).
HPV Genotyping
HPV-DNA was detected with polymerase chain reaction using both the L1 consensus primers MY09/MY11 and the E6/E7 consensus primers PU-1M/PU-2R [27, 28]. Viral genotyping was performed using a direct sequencing kit (BD Terminator Kit, version 1.1; Applied Biosystems, Life Technologies) on an automated capillary electrophoresis sequencer (ABI3130; Applied Biosystems, Life Technologies). The sequences obtained were blasted against the NCBI nucleotide DNA database using the Basic Local Alignment Search Tool (BLAST: http://blast.ncbi.nlm.nih.gov/) to identify the viral genotype. The different viral genotypes were classified according to the oncogenic risk in low- and high-risk genotypes [29].
Demographic and Viro-immunological Parameters
Demographic, HIV-related, and viro-immunological parameters (CD4+ and CD8+ T-cell count, CD4:CD8 ratio, activated CD8+ CD38+ T-cell percentage in peripheral blood) at the time of the cytological evaluation were collected in an electronic data set.
Statistical Analysis
Chi-square and Mann-Whitney tests were used to compare patients with and without anal/cervical HPV-related dysplasia. Spearman’s correlation coefficient was used to investigate the correlation between immune activation (CD8+ CD38+ T cells) and CD4:CD8 ratio. We investigated the association between immunosuppression (measured by CD4:CD8 ratio) and HPV-related dysplasia by fitting a multivariable logistic regression analysis, adjusting for possible confounders (HPV infection, time since HIV diagnosis, cART duration, age, CD4+ nadir, and smoking). A second model of logistic regression analysis was also performed to explore the association between peripheral immune activation (measured by percentages of CD8+ CD38+ T cells) and HPV-related dysplasia. Statistical analyses were performed by SPSS software (version 19.0).
RESULTS
A total of 419 patients with HIV on effective cART (HIV-RNA <50 copies/mL) undergoing anal or cervical HPV screening were analyzed. The median age of the study population (interquartile range [IQR]) was 42 (36–48) years, 72.3% of subjects were males, and most of the subjects were MSM (49.8%). Demographic, HIV-related, and HPV-related characteristics of the study population, according to anal/cervical dysplasia, are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of Study Population According to Cervical/Anal Dysplasia
| Characteristics | Population (n = 419) |
SIL+ (n = 214, 51%) |
SIL- (n = 205, 51%) |
P Values |
|---|---|---|---|---|
| Males, No. (%) | 303 (72.3) | 171 (79.9) | 132 (64.4) | <.0001 |
| Age, median (IQR), y | 42 (36–48) | 42 (36–48) | 43 (36–48) | .373 |
| Mode of HIV transmission, No. (%) | .001 | |||
| Homosexual contact | 209 (49.8) | 126 (60.3) | 83 (40.5) | |
| Heterosexual contact | 124 (29.6) | 55 (25.9) | 69 (33.7) | |
| IDU | 48 (11.5) | 15 (7.1) | 33 (16.1) | |
| Other/unknown | 38 (9.1) | 18 (7.5) | 20 (9.8) | |
| Syphilis coinfection, No. (%) | .001 | |||
| TPPA negative | 251 (59.9) | 111 (51.9) | 140 (68.6) | |
| TPPA positive | 131 (31.3) | 85 (39.7) | 46 (22.5) | |
| Unknown | 37 (8.8) | 18 (8.4) | 19 (8.8) | |
| HCV coinfection, No. (%) | .198 | |||
| Negative | 340 (81.1) | 175 (81.8) | 165 (80.5) | |
| HCV-Ab positive, RNA negative | 48 (11.5) | 21 (9.8) | 27 (13.2) | |
| HCV-Ab positive, RNA positive | 22 (5.3) | 15 (7) | 7 (3.4) | |
| Unknown | 9 (2.1) | 3 (1.4) | 6 (2.9) | |
| HBV coinfection, No. (%) | .914 | |||
| Negative | 291 (69.4) | 150 (70.1) | 141 (68.8) | |
| HBsAg positive | 11 (2.6) | 6 (2.8) | 5 (2.4) | |
| HBcAb positive | 105 (25.1) | 52 (24.3) | 53 (25.9) | |
| Unknown | 12 (2.9) | 6 (2.8) | 6 (2.9) | |
| AIDS-defining conditions, No. (%) | 86 (20) | 40 (21.4) | 46 (25.1) | .394 |
| Time since HIV diagnosis, median (IQR), mo | 88 (30–165) | 71 (20–151) | 95 (37–189) | .019 |
| Nadir CD4 + T-cell count, median (IQR), cells/mmc | 250 (144–358) | 260 (148–358) | 234 (128–355) | .583 |
| Pre-cART CD4/CD8 ratio | n = 250 0.35 (0.21–0.54) |
n = 138 0.36 (0.21–0.54) |
n = 112 0.34 (0.21–0.54) |
.913 |
| cART duration, median (IQR), mo | 43 (12–99.7) | 33 (9–92) | 49 (17–108) | .055 |
| cART regimen, No. (%) | .021 | |||
| PI-based | 133 (31.8) | 68 (31.8) | 65 (31.7) | |
| NNRTI-based | 167 (39.8) | 76 (35.5) | 91 (44.4) | |
| INSTI-based | 74 (17.8) | 49 (22.9) | 25 (12.2) | |
| Other | 41 (9.7) | 18 (8.4) | 23 (11.2) | |
| Unknown | 4 (0.9) | 3 (1.4) | 1 (0.5) | |
| Smoking history, No. (%) | .099 | |||
| Never smokers | 163 (38.9) | 81 (37.9) | 82 (40) | |
| Active smokers | 209 (49.9) | 114 (53.2) | 95 (46.3) | |
| Ex-smokers, quit >1 y ago | 39 (9.3) | 18 (8.4) | 21 (10.3) | |
| Unknown | 8 (1.9) | 1 (0.5) | 7 (3.4) |
Quantitative variables are presented as median (interquartile range), categorical variables as absolute number (percentage); P values were calculated by chi-square test or nonparametric Mann-Whitney test, as appropriate.
Abbreviations: Ab, antibody; cART, combination antiretroviral therapy; HBcAb, anti-HBV core antigen antibodies; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; IDU, intravenous drug users; INSTI, integrase strand transfer inhibitor; IQR, interquartile ranges; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SIL, squamous intraepithelial lesion; TPPA, Treponema pallidum particle agglutination test.
Demographic Characteristics Associated With SIL
Half of the patients (214/419, 51%) were SIL positive (Table 1). The presence of SILs was more commonly associated with male gender (171/214, 79.9%, vs 132/205, 64.4%; P < .0001), men who have sex with men (MSM) as risk category for HIV transmission (126/214, 60.3%, vs 83/205, 40.5%; P = .001), and syphilis coinfection (85/214, 39.7%, TPPA positive vs 46/205, 22.5%; P = .001), compared with patients presenting a normal cytology. No correlation between SIL status and HCV and/or HBV coinfection was found.
SIL-positive patients also showed a more recent median (IQR) HIV diagnosis (71 [20–151] months vs 95 [37–189] months; P = .019), a shorter time from cART introduction (33 [9–92] months vs 49 [17–108] months; P = .055), and were more frequently treated with integrase strand transfer inhibitor (INSTI)–based cART (49/211, 22.9%, vs 25/204, 12.2%; P = .021) compared with patients without SIL on anal/cervical cytology.
We also assessed demographic characteristics associated with SIL separately in males and females (Supplementary Table 1). In males, SIL was associated with syphilis coinfection, shorter cART duration, and active or former smoking; in females, no association was seen between SIL and the demographic or HIV-related parameters described above.
HPV-Related Characteristics Associated With SIL
As expected, SIL-positive patients were more commonly infected with HPV than SIL-negative individuals (95/201, 47.3%, vs 30/207, 14.5%; P < .0001).
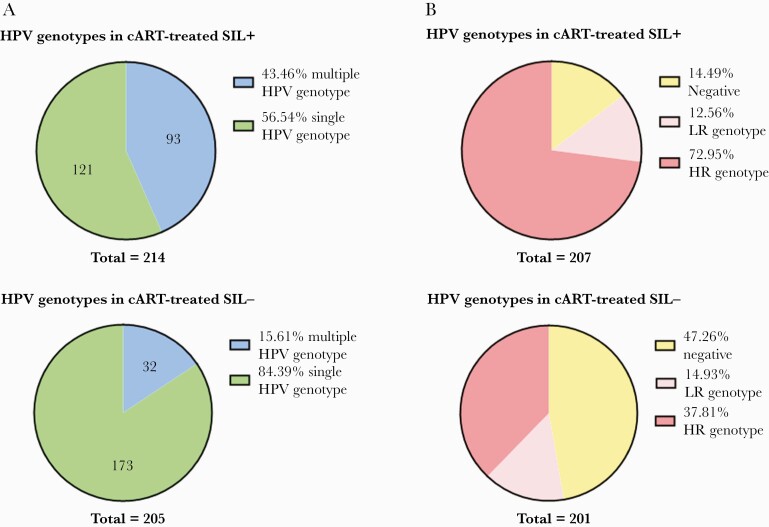
Furthermore, SIL-positive patients more frequently harbored multiple (93/214, 43.5%, vs 32/205, 15.6%; P = .002) (Figure 1A) and high-risk (151/207, 72.9%, vs 76/201, 37.8%; P < .0001) (Figure 1B) HPV genotypes, compared with subjects with negative cytology. The HR genotypes most frequently detected were 16, 18, 33, 58, 53, 31.
Figure 1.
Multiple–HPV genotype infection and low/high-risk HPV genotypes according to anal/cervical dysplasia. Abbreviations: cART, combination antiretroviral therapy; HPV, human papillomavirus; HR, high-risk; LR, low-risk; SIL, squamous intraepithelial lesion.
HIV-Related Characteristics Associated With SIL
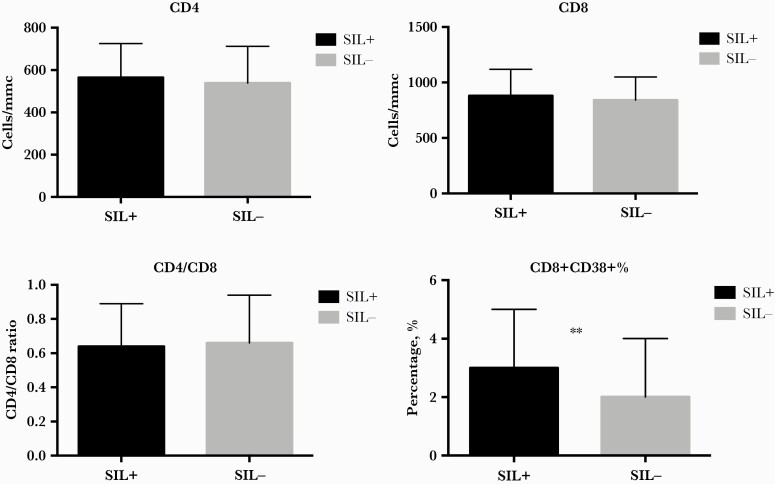
In terms of immunological parameters associated with cervical/anal SIL, no difference in median current CD4:CD8 ratio (IQR) between SIL-positive and SIL-negative patients (0.64 [0.44–0.89] vs 0.66 [0.47–0.9]; P = .404) was reported. Likewise, median (IQR) nadir CD4+ T-cell count (260 [148–358] cells/mmc vs 234 [128–355] cells/mmc; P = .583), current CD4+ T-cell count (565 [409–725] cells/mmc vs 539 [414–712] cells/mmc; P = .76), and CD8+ T-cell count (881 [692–1119] cells/mmc vs 840 [640–1049] cells/mmc; P = .107) were similar between the 2 groups of patients (Table 1, Figure 2).
Figure 2.
CD4+ T-cell count, CD8+ T-cell count, CD4/CD8 ratio and activated CD8+ CD38+ T-cell percentage according to anal/cervical dysplasia. CD8+ CD38+ T-cell percentage correlates with SILs. P values were calculated by Mann-Whitney test. ∗P < .05. Abbreviation: SIL, squamous intraepithelial lesion.
Interestingly, we found a higher proportion of peripheral activated CD8+ CD38+ T-cell percentages (IQR) in patients diagnosed with SILs (3% [1%–5%] vs 2% [1%–4%]; P = .018) (Figure 2).
CD8+ CD38+ T-cell percentages negatively correlated with CD4:CD8 ratio in the entire population (r = –0.202; P = .005) and in males (r = –0.152; P = .004), yet not reaching statistical significance in females (r = –0.19; P = .161).
Multivariate Logistic Regression Analysis
The association between percentage of CD38+ CD8+ T-cell and HPV-related anal/cervical dysplasia was explored by a multivariable logistic regression model, adjusting for HPV infection, age, time since HIV diagnosis, cART duration, CD4+ T-cell nadir, and smoking (model 1, Table 2A). We also investigated the possible association between CD4:CD8 ratio and SILs by fitting a second model of logistic regression (model 2, Table 2B).
Table 2.
Parameters Independently Associated With Cytologic HPV-Related Dysplasia by Fitting 2 Models of Multivariable Logistic Regression Analyses
| A, Model 1 of logistic regression analysis: association between CD8+ CD38+ T-cell percentages and HPV-related anal/cervical dysplasia | |||
|---|---|---|---|
| Parameter | aOR | 95% CI | P Value |
| Log10 CD8+ CD38+ T cells, percentages, each additional unit | 3.253 | 1.602–6.605 | .001 |
| B, Model 2 of logistic regression analysis: association between CD4/CD8 ratio and HPV-related anal/cervical dysplasia | |||
| Parameter | aOR | 95% CI | P Value |
| CD4/CD8 ratio, each unit more | 0.998 | 0.242–4.109 | .997 |
Adjusted for HPV infection, age, time since HIV diagnosis, cART duration, CD4+ nadir T cells, and smoking.
Time since HIV diagnosis is the time from HIV diagnosis to surgical/gynecological evaluation for detection of HPV-related dysplasia; cART duration is time from cART introduction to surgical/gynecological evaluation for detection of HPV-related dysplasia.
Abbreviations: aOR, adjusted odds ratio; cART, combination antiretroviral therapy; HPV, human papillomavirus.
A higher proportion of activated CD8+ CD38+ T cells (adjusted odds ratio [aOR], 3.253; 95% CI, 1.602–6.605; P = .001), but not CD4:CD8 ratio (aOR, 0.998; 95% CI, 0.242–4.109; P = .997), was independently associated with anal/cervical dysplasia (Table 2). The association between immune activation/immune senescence and cytologic HPV-related dysplasia was also investigated, stratifying for anal and cervical dysplasia (Supplementary Table 2). Immune activation was confirmed to be independently associated with anal dysplasia (aOR, 2.625; 95% CI, 1.142–6.033; P = .023); conversely, no association was reported between CD8+ CD38+ T cells or CD4:CD8 ratio and cervical dysplasia, probably due also to the small sample size of females.
DISCUSSION
With the present study, we aimed to evaluate if, in the setting of HIV infection controlled by effective cART, HPV-related SILs are associated with peripheral T-cell immune activation and immune senescence. For this purpose, we used a well-recognized marker of poor immunological rescue, CD4:CD8 ratio, and an index of peripheral immune T-cell activation, CD8+ CD38+ T-cell percentage [23].
In our study population, anal/cervical SILs were associated with HPV detection in both univariate and multivariate analyses, but this result was expected and not surprising, considering that HPV is the causal agent of virtually all cervical dysplastic lesions and cancers and is responsible for 88% of anal cancer cases [2]. Since 2007, HPV vaccination programs have been introduced in many countries worldwide, and very recent evidence has proven the efficacy of vaccines in reducing HR-HPV genotype infections and SIL incidence in women, as well as the occurrence of genital warts in men [30]. This highlights the importance of HPV vaccination for both males and females as a unique instrument of primary prevention, which is potentially able to eradicate all cervical cancers and most anal cancer cases.
In our study cohort, SIL detection was higher in men, especially MSM. This is in line with previous data from the literature [31, 32] and is probably linked to a high number of sexual partners. The same epidemiological interpretation could justify our findings of the association between dysplasia and a history of syphilis infection or multiple–HPV genotype detection. As already described, MSM are more likely to harbor multiple HPV genotypes or to be syphilis coinfected than men who have sex with women [33].
As a relevant result of our analysis, we found that SIL-positive patients experienced higher levels of peripheral activated CD8+ CD38+ T cells than their negative counterparts and, interestingly, CD8+ CD38+ T-cell percentage was an independent predictor of SILs even in multivariate analysis. This finding is in line with the well-documented phenomenon of residual immune activation under successful cART, which specifically affects the compartment of CD8+ T cells, which present altered cytotoxic functions and a more activated, exhausted, and terminally differentiated phenotype [34, 35]. This skewed and dysfunctional arm of cellular immunity sustains and is sustained by high levels of systemic inflammation and is associated with an increased risk of non-AIDS-related events [35].
This scenario calls attention to the problem of the inability of cART to restore broad immune competence despite stable HIV replication inhibition [24], thus creating a permissive environment for HPV persistence, replication, and SIL onset.
In the present work, we were not able to test for HPV antigen–specific CD8+ T-cell responses. Given the possible link between T-cell activation and impaired HPV-specific immunity in HIV infection, future studies are needed to assess this correlation and provide insights into the pathogenesis of HPV-related dysplasia in this context.
The more activated immune profile of patients with SILs than that of those without dysplasia may be well explained by our finding that the former had a more recent HIV diagnosis as well as shorter time from cART introduction, given that the introduction of antiretroviral therapy is the primum movens to immunological recovery [36]. This concept is sustained by several authors who have demonstrated that starting cART affects a reduction in prevalence of high-risk HPV genotypes and SILs, as well as dysplasia progression [21, 22]. Moreover, as the beneficial effect of antiretroviral therapy on the risk of HR-HPV acquisition and dysplasia development seems to compound with time [21, 37], we can speculate that, in our cohort study, the association between SILs and high-risk HPV genotypes might mirror a shorter time from cART introduction. Analogously, the finding of a correlation between SIL detection and INSTI-based cART could be justified by the fact that, in our cohort of patients, people with a more recent diagnosis of HIV were more likely to be treated with relatively new drugs—such as INSTIs—than patients with an older diagnosis. Both of these findings, that is, time from cART introduction and INSTI-based cART, relate to younger age, which, in turn, is associated more frequently with risky sexual behavior.
As regards the possible capability of CD4:CD8 ratio to predict SILs, our analysis failed to demonstrate such an association. As previously described, several non-AIDS-defining conditions correlated with an inverted CD4:CD8 ratio [26], so, given the noninvasive nature of this marker, we postulated its possible predictive role in detecting HPV-related dysplasia. Even though previous reports have shown the validity of our hypothesis in the setting of HIV infection and in both female and male patients [38, 39], our data concluded for a lack of correlation between CD4:CD8 ratio and dysplastic lesions. There are several reasons for this result: First, our study is retrospective in nature, and our cohort is relatively small and composed of subjects with heterogeneous courses of HIV infection (different times since HIV diagnosis and cART initiation, different types and numbers of antiretroviral regimens experienced). This could translate into deep immunological modifications, only in small part attributable to HPV infection, so CD4:CD8 ratio may not fully capture those relative to HPV disease. In this respect, an elegant work by Tong et al. demonstrated that HIV infection is an independent variable associated with systemic HPV-specific CD8+ T-cell responses and that HIV-infected subjects with AIDS had decreased HPV-specific CD4+ T-cell immunity, which the authors found to be linked to HSIL clearance [40]. These literature findings, together with our results on the lack of association between CD4:CD8 ratio and dysplasia, indeed demonstrate that inferring HPV antigen-specific T-cell responses from crude measures of T-cell function is problematic and highlight the need for subtle assessments of HPV-specific immunity in large-cohort studies.
Otherwise, we may have investigated the wrong “side of the coin”: in fact, the CD4:CD8 ratio is calculated using peripheral T cells and may therefore not be able to completely mirror the features of a process—as the anti-HPV immune defense—that is mostly local and mucosal [41]. However, as the assessment of the possible correlation between CD4:CD8 ratio and SILs derives mainly from small, monocentric, retrospective studies [38, 39], future investigation with prospective studies and larger cohorts of patients are needed to ascertain the accuracy of this association. Finally, a further limitation of the study is that we analyzed only cytologic HPV-related dysplasia without a histological validation.
In conclusion, in our cohort of men and women with HIV on suppressive cART, CD4:CD8 ratio did not associate with SILs, despite its already recognized capacity to predict non-AIDS-defining conditions. This was probably due to the limits of our work and advocates for further studies to better define its role in this setting, especially in the cART era, during which age-related and non-AIDS comorbidities represent the most important cause of mortality in this population. Otherwise, our analysis revealed a trend toward a correlation between the activated CD8+ T-cell immunophenotype and HPV-related anal/cervical dysplasia. This result underlines the importance of residual immune dysfunction, which is not fully captured by conventional parameters of immunological evaluation—such as CD4+ T-cell count and CD4:CD8 ratio—even under effective antiretroviral therapy. Stemming from these observations is the need for additional methods to identify patients with a higher risk of SIL development as well as adjuvant strategies to correct residual immune dysfunction.
Supplementary Material
Acknowledgments
We are thankful to all patients of the Clinic of Infectious Diseases, San Paolo Hospital, ASST Santi Paolo e Carlo, University of Milan, Milan, Italy, for their participation in the study.
Financial support. This work was supported by (i) ERANET-Transcan 2, Third Joint Transnational Call (JTC, 2016), funding agency: Fondazione Regionale della Ricerca Biomedica (FRRB; grant ID 073) awarded to C.T.; and (ii) University of Milan, Italy, Piano di Sostegno alla Ricerca 2015/17 awarded to C.T.
Potential conflicts of interest. The authors have no conflicts of interest related to this work to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. G.M. and C.T. conceived the study. D.M., C.T., F.B., M.A., M.G., and A.P.C. were involved in the clinical care of the patients. D.M. and V.B. collected data for the study. E.O., A.d.A.M., G.M., and C.T. supervised the study. F.B. performed the statistical analyses. D.M., F.B., and C.T. wrote the manuscript. D.M., F.B., M.A., M.G., A.P.C., V.B., E.O., A.d.A.M., G.M., and C.T. revised the manuscript.
Patient consent. All patients provided written informed consent, and the local ethical committees approved the study.
References
- 1. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13:607–15. [DOI] [PubMed] [Google Scholar]
- 3. De Vuyst H, Lillo F, Broutet N, Smith JS.. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev 2008; 17:545–54. [DOI] [PubMed] [Google Scholar]
- 4. Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 5. Clifford GM, Georges D, Shiels MS, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2021; 148:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu G, Sharma M, Tan N, Barnabas RV.. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018; 32:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poljak M, Šterbenc A, Lunar MM.. Prevention of human papillomavirus (HPV)-related tumors in people living with human immunodeficiency virus (HIV). Expert Rev Anti Infect Ther 2017; 15:987–99. [DOI] [PubMed] [Google Scholar]
- 8. Clifford GM, Gonçalves MA, Franceschi S; Group HaHS. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20:2337–44. [DOI] [PubMed] [Google Scholar]
- 9. Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS 2014; 25:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Critchlow CW, Surawicz CM, Holmes KK, et al. Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. AIDS 1995; 9:1255–62. [DOI] [PubMed] [Google Scholar]
- 11. Frisch M, Biggar RJ, Goedert JJ.. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–10. [DOI] [PubMed] [Google Scholar]
- 12. Torres-Ibarra L, Conde-Glez CJ, Salmerón J, et al. Risk factors for anal HPV-16/18 infection in Mexican HIV-infected men who have sex with men. Prev Med 2014; 69:157–64. [DOI] [PubMed] [Google Scholar]
- 13. Minkoff H, Feldman JG, Strickler HD, et al. Relationship between smoking and human papillomavirus infections in HIV-infected and -uninfected women. J Infect Dis 2004; 189:1821–8. [DOI] [PubMed] [Google Scholar]
- 14. Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst 1999; 91:226–36. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi A, Greenblatt RM, Anastos K, et al. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res 2004; 64:6766–74. [DOI] [PubMed] [Google Scholar]
- 16. Tincati C, Rainone V, Comi L, et al. Cell-mediated immunity in HIV-infected males with human papillomavirus-related anal dysplastic lesions. Clin Infect Dis 2016; 63:1396–8. [DOI] [PubMed] [Google Scholar]
- 17. Fernandes AT, da Rocha NP, Avvad E, et al. Balance of apoptotic and anti-apoptotic marker and perforin granule release in squamous intraepithelial lesions. HIV infection leads to a decrease in perforin degranulation. Exp Mol Pathol 2013; 95:166–73. [DOI] [PubMed] [Google Scholar]
- 18. Lucena AA, Guimarães MV, Michelin MA, et al. Evaluation of T, B and natural killer lymphocyte in the cervical stroma of HIV-positive and negative patients with cervical intraepithelial neoplasia. Immunol Lett 2016; 169:98–103. [DOI] [PubMed] [Google Scholar]
- 19. Blitz S, Baxter J, Raboud J, et al. Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis 2013; 208:454–62. [DOI] [PubMed] [Google Scholar]
- 20. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis 2010; 201:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly H, Chikandiwa A, Alemany Vilches L, et al. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2020; 7:e262–78. [DOI] [PubMed] [Google Scholar]
- 22. Kelly H, Weiss HA, Benavente Y, et al. ; ART and HPV Review Group. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5:e45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med 2012; 20:101–5. [PMC free article] [PubMed] [Google Scholar]
- 25. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 26. Serrano-Villar S, Moreno S, Fuentes-Ferrer M, et al. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med 2014; 15:40–9. [DOI] [PubMed] [Google Scholar]
- 27. Laconi S, Greco M, Milia G, et al. Simultaneous detection and typing of human papillomavirus in cervical biopsies using PCR-reverse hybridization. Pathologica 2000; 92:524–9. [PubMed] [Google Scholar]
- 28. Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst 1990; 82:1477–84. [DOI] [PubMed] [Google Scholar]
- 29. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 30. Drolet M, Bénard E, Pérez N, Brisson M; Group HVIS. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Revollo B, Videla S, Sirera G, et al. Natural history of anal squamous intraepithelial lesions in HIV-positive men with normal baseline cytology. AIDS Patient Care STDS 2019; 33:459–65. [DOI] [PubMed] [Google Scholar]
- 32. Darwich L, Videla S, Cañadas MP, et al. Distribution of human papillomavirus genotypes in anal cytological and histological specimens from HIV-infected men who have sex with men and men who have sex with women. Dis Colon Rectum 2013; 56:1043–52. [DOI] [PubMed] [Google Scholar]
- 33. Müller EE, Rebe K, Chirwa TF, et al. The prevalence of human papillomavirus infections and associated risk factors in men-who-have-sex-with-men in Cape Town, South Africa. BMC Infect Dis 2016; 16:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perdomo-Celis F, Velilla PA, Taborda NA, Rugeles MT.. An altered cytotoxic program of CD8+ T-cells in HIV-infected patients despite HAART-induced viral suppression. PLoS One 2019; 14:e0210540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao W, Mehraj V, Kaufmann DE, et al. Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era. J Int AIDS Soc 2016; 19:20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yao Y, Luo Y, He Y, et al. The effect of a year of highly active antiretroviral therapy on immune reconstruction and cytokines in HIV/AIDS patients. AIDS Res Hum Retroviruses 2013; 29:691–7. [DOI] [PubMed] [Google Scholar]
- 37. de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis 2011; 52:1174–81. [DOI] [PubMed] [Google Scholar]
- 38. Geltzeiler CB, Xu Y, Carchman E, et al. CD4/CD8 ratio as a novel marker for increased risk of high-grade anal dysplasia and anal cancer in HIV+ patients: a retrospective cohort study. Dis Colon Rectum 2020; 63:1585–92. [DOI] [PubMed] [Google Scholar]
- 39. Zarcone R, Bellini P, Carfora E, et al. Incidence of CIN in HIV-positive women. Minerva Ginecol 1998; 50:181–3. [PubMed] [Google Scholar]
- 40. Tong WW, Shepherd K, Garland S, et al. Human papillomavirus 16-specific T-cell responses and spontaneous regression of anal high-grade squamous intraepithelial lesions. J Infect Dis 2015; 211:405–15. [DOI] [PubMed] [Google Scholar]
- 41. Castle PE. Cervical concentrations of interleukin-10 and interleukin-12 do not correlate with plasma levels. J Clin Immunol 2002; 22:23–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.