Abstract
Introduction: Prostate cancer (PCa) is the second most prevalent neoplasm among men in the world. Its treatment has a wide spectrum of alternatives and variables, ranging from active surveillance through radio and/or brachytherapy, to surgery. Objective: The present work aimed to identify the predictive factors for biochemical recurrence and to evaluate the toxicity of the treatment using the association of external beam radiation therapy (EBRT) with high dose rate brachytherapy (HDR-BT) applied in the treatment of patients with prostate cancer. Methods: Longitudinal retrospective study, using a prospectively collected database between 2005 and 2014 of 186 consecutive patients records with a diagnosis of low, intermediate, or high-risk prostate cancer treated with EBRT combined with HDR-BT, in a single medical institution located in the city of Campinas, SP, Brazil (Radium Institute). PSA increase over 2 ng/ml above the nadir PSA was considered as biochemical recurrence, following the definition of the Phoenix Consensus. Continuous and clinically relevant categorical variables (age, initial PSA, delivered dose in EBRT, number of implants, number of positive cores in transrectal biopsy, use of hormone blockade, Gleason score, TNM staging, post treatment PSA and PSA Nadir) were evaluated with absolute (n) and percentage (%) values using multiple logistic regression and validated our previously described optimal PSA nadir as predictor of biochemical recurrence. Results: Post treatment PSA was the only independent predictor of biochemical recurrence, P<0.0001. The lower the PSA nadir the lower the biochemical recurrence risk (P=0.0009). PSA nadir >1 was the best cutoff (P=0.018) determinant of biochemical recurrence. The incidence of grade 3 late toxicity to the genitourinary tract was 0.6%, and there were no cases of severe complications to the gastrointestinal tract. Conclusion: External Beam Radiation Therapy conjugated to Brachytherapy in the treatment of Prostate Cancer has demonstrated low biochemical recurrence rates, mainly when PSA nadir <1, with low toxicity into both GU and GI tracts.
Keywords: External Radiotherapy, high dose rate brachytherapy, prostate cancer, biochemical relapse, toxicity, efficacy, PSA nadir
Introduction
Prostate cancer is the second most common malignant neoplasm among men in Brazil and worldwide [1,2]. Several factors are associated with the risk of prostate cancer such as age [3], ethnic group [4], heredity [5], environmental and lifestyle factors [6,7].
The choice of localized cancer treatment may include active surveillance [8,9], radio and/or brachytherapy [10-14] and surgery [15,16], considering not only tumor characteristics and staging but also individual patient features, expectations, and agreement with the treatment risks and benefits. D’Amico et al. proposed a risk stratification of biochemical relapse after the many treatment options, based on PSA, Gleason score and clinical staging (TNM) [17]. This classification system has simplified the way in which doctors predict the response to treatment modalities.
Brachytherapy as monotherapy for a low-risk prostate cancer is regarded as a good choice according to the D’Amico’s classification [18]; however, in the intermediate and high-risk cases, isolated brachytherapy presents poor results in terms of biochemical control [19], in which the association of brachytherapy and external beam radiation has been considered a viable alternative [20-23].
Objective
To identify predictive factors for biochemical recurrence and evaluate treatment toxicity using the association of external beam radiation therapy (EBRT) with high dose rate brachytherapy (HDR-BT) applied in the treatment of patients with PCa.
Methods
This is a retrospective longitudinal study, with a prospectively collected database of prostate cancer patients from January 2005 to January 2014, ethics committee approval number 374.513. Inclusion criteria consisted of patients diagnosed with prostate cancer, confirmed by transrectal ultrasound-guided biopsy, treated with an association of EBRT and HDBT at the Instituto do Radium, Campinas, SP, Brazil.
A total of 186 consecutive patients diagnosed with low, intermediate, and high-risk prostate cancer treated with EBRT, associated with HDR-BT in a single medical institution located in the city of Campinas, SP, Brazil (Instituto do Radium) was selected. Continuous and clinically relevant categorical variables were evaluated with absolute (n) and percentage (%) values using multiple logistic regression.
Twenty-four patients were later submitted to other treatment modalities or lost segment at the institution and were excluded. All patients were informed of all possible treatment methods, their risks and benefits, adverse side effects and complications. The study design is shown in Figure 1.
Figure 1.
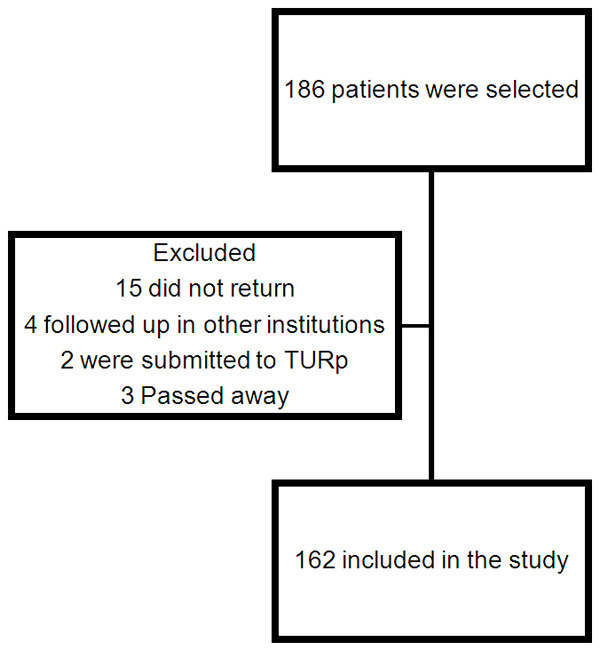
Study design.
The patients were submitted to physical and digital rectal exams [24] and blood samples for laboratory testing. Distant metastases were excluded by total abdominal computed tomography, simple chest x-ray, and bone scintigraphy (if PSA >20 ng/ml). The Gleason score was used to determine tumor differentiation [25,26]. The American Joint Committee on Cancer (AJCC) TNM system (2009) was used for clinical staging [27], and the patients were divided into risk groups according to D’Amico’s classification [17].
Brachytherapy
Patients were treated in a lithotomy position (dorsal decubitus on leg holders), under spinal anesthesia or general anesthesia. A Foley catheter was used to fill the bladder with distilled water. The transrectal ultrasound probe was inserted, and the prostate, seminal vesicles, urethra, bladder, and rectum were visualized, thus preparing for the introduction of the radioactive implants. The entire prostate volume was targeted for HDR-BT. The maximum dose for the urethra and rectum was defined as being lower than 120% of the surrounding tissues and 7 Gy for the rectum, respectively. The implants (on average 20) were placed under ultrasound guidance. The first 45 patients received 2 fractions of 8 Gy, through HDR 192-Ir, Varian Gammamed brand and Vitesse Varian planning system. The other 117 patients received a single fraction of 10 Gy (Figures 2 and 3). High dose rate brachytherapy was performed after external beam radiation therapy, following a gap of 2 to 3 weeks.
Figure 2.
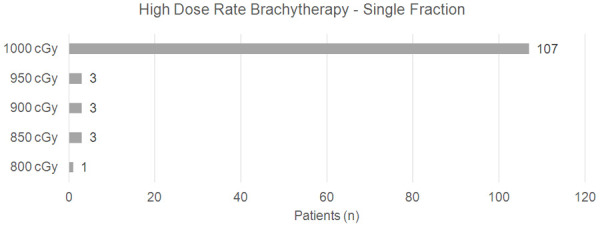
Patients who underwent HDRB (Gy) in a single fraction.
Figure 3.
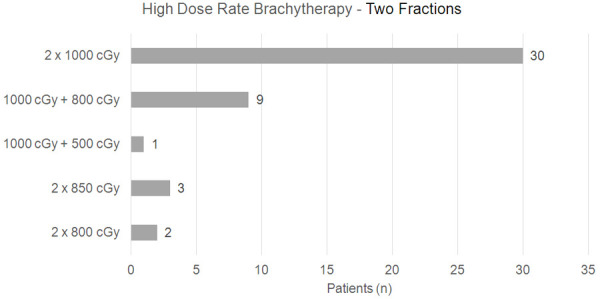
Patients who underwent HDRB (Gy) in two fractions.
External beam radiation therapy (EBRT)
Three-dimensional (3D) or conformational EBRT, with photon energy generated by a linear particle accelerator (Varian®, model 6EX, 120 slides) was used, and CT scan was performed in all patients using the software EclipseTM-Varian, version 11.0. The target area included the prostate, seminal vesicles and, in high-risk cases, the pelvic lymph nodes. The patients were placed in dorsal decubitus position and with pelvic fixation system. The duration of EBRT treatment was 5 to 6 weeks, according to the brachytherapy planning. The applied dose was 50 Gy in fractions of 2 Gy (five times a week). When the brachytherapy planning was of only one insertion, the dose of external radiotherapy was 60 Gy in the prostate, 50 Gy in the seminal vesicles and in the cases of radiotherapy in pelvic lymph nodes, the dose was 50 Gy.
Androgen deprivation treatment (ADT)
Neoadjuvant treatment was performed in 46 (28.4%) subjects and was reserved for patients with prostate volumes greater than 40 g, confirmed by US. Adjuvant treatment was performed in 19 (11.7%) high risk cases. The mean duration of treatment was 6 to 18 months.
Follow-up
Follow-up averaged 57 months (4.2-163). The patients were evaluated every three months in the first 2 years, every six months in the third year, and then annually. Post-treatment PSA levels were analyzed using the validated Immulite® PSA kit.
Statistical analysis
The sample was evaluated according to the frequency of categorical variables, with absolute (n) and percentage (%) values. Descriptive statistics of continuous variables were also analyzed, with mean values, standard deviation, minimum and maximum values, median and quartiles. Chi-Square or Fisher’s Exact Test were used, when necessary, for the comparison of categorical variables: (i) age range, (ii) Gleason score (<7 vs. ≥7), (iii) pre-treatment PSA (<10 vs. ≥10 ng/ml), and (vi) TNM staging, among the groups.
For comparison of continuous variables: (i) age, (ii) PSA pre-treatment, (iii) PSA nadir at 12 months, (iv) number of needles used, (v) radiation dose, (vi) total number of fragments and positive fragments in the biopsy, (vii) percentage of positive fragments in the biopsy, and (viii) follow-up time. The Mann-Whitney test was applied due to the absence of normal distribution of the variables.
Biochemical recurrence after primary RT, with or without short-term hormonal manipulation is considered any PSA increase greater than 2 ng/ml above nadir, following the definition of the Phoenix Consensus [28]. The PSA as a parameter to define “biochemical recurrence” in the absence of clinical or histopathological evidence of recurrence which aims to define the best time for a new intervention to prevent disease progression. We validated our optimal PSA nadir as predictor of biochemical recurrence, previous calculated by receiver operating characteristic [ROC] curve [29].
Statistical Analysis in System (SAS) software, version 9.4 (SAS Institute Inc, 2002-2012, Cary, NC, USA) was used for statistical analysis and the significance level adopted for the statistical tests was 5% (P<0.05).
Results
Measured variables
The mean age found was 66 years (39-86), with an initial PSA of 11.6 ng/ml (1.06-28). Nadir PSA mean was 0.20 ng/ml (0.0-3.51). About 2/3 of patients had stage T2, followed by 23% of T3 cases. About half of the men had an intermediate degree of risk. Only twenty-six patients (16%) were low risk and 84% were intermediate/high risk. One hundred and four patients (64.2%) had a Gleason score ≥7 on prostate biopsy (Table 1). The mean follow-up time was 57 months (4-163). Table 2 shows continuous and Table 3 categorical measured variables.
Table 1.
Clinical features of patients
| Variables | N (%) |
|---|---|
| Age (years) | |
| <65 | 63 (38.9) |
| ≥65 | 99 (61.1) |
| Gleason Score | |
| 6 | 58 (35.8) |
| 7 | 69 (42.6) |
| 8 | 19 (11.7) |
| 9 | 13 (8.0) |
| 10 | 3 (1.9) |
| Initial PSA (ng/ml) | |
| <10 | 111 (68.5) |
| 10-20 | 30 (18.5) |
| >20 | 21 (13) |
| T Stage (AJCC) | |
| T1-T2a | 62 (38.3) |
| T2b-T2c | 77 (47.5) |
| T3a-T3b | 23 (14.2) |
Table 2.
Descriptive analysis of the categorical measured variables
| Frequency | Percentage (%) | Cumulative frequency | |
|---|---|---|---|
| Preliminary PSA | |||
| <10 ng/mL | 110 | 68.75 | 110 |
| 10-20 ng/mL | 29 | 18.13 | 139 |
| >20 ng/mL | 21 | 13.13 | 160 |
| Gleason | |||
| 6 | 58 | 35.80 | 58 |
| 7 | 69 | 42.59 | 127 |
| 8 | 19 | 11.73 | 146 |
| 9 | 13 | 8.02 | 159 |
| 10 | 3 | 1.85 | 162 |
| Stage | |||
| Low risk | 26 | 16.05 | 26 |
| Medium risk | 79 | 48.77 | 105 |
| High risk | 57 | 35.19 | 162 |
| T stage | |||
| T1 | 41 | 25.31 | 41 |
| T2 | 99 | 61.11 | 140 |
| T3 | 22 | 13.58 | 162 |
| Neoadjuvant hormone blockade | |||
| No | 116 | 71.60 | 116 |
| Yes | 46 | 28.40 | 162 |
| Post treatment hormone blockade | |||
| No | 143 | 88.27 | 143 |
| Yes | 19 | 111.73 | 162 |
| Perineural invasion (PNI) | |||
| No | 142 | 87.65 | 142 |
| Yes | 20 | 12.35 | 162 |
| PSA nadir | |||
| <1 | 121 | 96.03 | 121 |
| ≥1 | 5 | 3.97 | 126 |
| Biochemical Recurrence | |||
| No | 156 | 96.30 | 156 |
| Yes | 6 | 3.70 | 162 |
Table 3.
Descriptive analysis of continuous variables
| n | Mean | SD | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| Age | 162 | 66.21 | 8.51 | 39.00 | 60.00 | 67.50 | 72.00 | 86.00 |
| Preliminary PSA | 160 | 11.63 | 17.19 | 1.06 | 4.98 | 7.40 | 11.40 | 28.00 |
| Post treatment PSA | 135 | 0.60 | 1.15 | 0.00 | 0.03 | 0.10 | 0.35 | 8.60 |
| EBRT dose (Gy) | 162 | 56.20 | 71.47 | 30.00 | 48.00 | 60.00 | 61.20 | 67.00 |
| Number of implants | 147 | 20.62 | 6.41 | 10.00 | 15.00 | 19.00 | 24.00 | 48.00 |
| IPSS | 121 | 14.04 | 8.61 | 0.00 | 7.00 | 13.00 | 22.00 | 35.00 |
| Number of cores in biopsy | 125 | 15.67 | 7.19 | 6.00 | 12.00 | 14.00 | 16.00 | 29.00 |
| PSA nadir | 126 | 0.20 | 0.44 | 0.00 | 0.01 | 0.04 | 0.21 | 3.50 |
| Follow-up (months) | 147 | 56.92 | 31.58 | 4.17 | 32.43 | 53.49 | 79.70 | 163.65 |
SD: Standard Deviation; EBRT: External Beam Radiation Therapy.
Biochemical recurrence
We assess that biochemical recurrence occurred in 6 patients (3.7%), mean ± SD of PSA nadir 1.1±1.3, compared to 0.2±0.3 in those with oncological control (P=0.0009). Pre-treatment characteristics were comparable between those with oncological control and those presenting biochemical recurrence. The only independent predictor of oncological control was PSA nadir.
Toxicity
Grade 3 gastrointestinal toxicity was not found in any case, and only one patient (0.6%) had genitourinary Grade 3 toxicity (urethral stenosis). Table 4 shows the comparison between the main clinical and pathological variables and biochemical recurrence. PSA nadir >1 was validated as the best cutoff (P=0.018) determinant of biochemical recurrence.
Table 4.
Comparison between the main clinical and pathological variables and biochemical recurrence
| Variables | Without biochemical recurrence (n=156) | With biochemical recurrence (n=6) | P-value |
|---|---|---|---|
| Age | |||
| (mean ± SD) (n) | 66.4±8.5 (N=156) | 61.5±9.3 (N=6) | 0.20 |
| (median) (min-max) | 68.0 (39.0-86.0) | 63.0 (49.0-72.0) | |
| Initial PSA | |||
| (mean ± SD) (n) | 11.5±17.3 (N=154) | 14.5±13.6 (N=6) | 0.41 |
| (median) (min-max) | 7.4 (1.1-189.0) | 9.1 (4.9-40.5) | |
| RT dose (Gy) | |||
| (mean ± SD) (n) | 5606.7±722.9 (N=156) | 5970±309.3 (N=6) | 0.43 |
| (median) (min-max) | 6000.0 (3000.0-6700.0) | 6040 (5580.0-6400.0) | |
| Number of implants | |||
| (mean ± SD) (n) | 20.8±6.4 (N=141) | 16.2±4.3 (N=6) | 0.62 |
| (median) (min-max) | 20.0 (10.0-48.0) | 14.5 (13.0-24.0) | |
| IPSS | |||
| (mean ± SD) (n) | 14.0±8.6 (N=117) | 14.0±9.9 (N=4) | 0.98 |
| (median) (min-max) | 13.0 (0.0-35.0) | 13 (3.0-27.0) | |
| Number of cores | |||
| (mean ± SD) (n) | 15.8±7.3 (N=120) | 13.6±1.7 (N=5) | 0.67 |
| (median) (min-max) | 14.0 (6.0-69.0) | 14.0 (12.0-16.0) | |
| PSA nadir | |||
| (mean ± SD) (n) | 0.2±0.3 (N=120) | 1.1±1.3 (N=6) | 0.0009 |
| (median) (min-max) | 0.0 (0.0-2.2) | 0.6 (0.1-3.5) | |
| Post PSA | |||
| (mean ± SD) (n) | 0.4±0.6 (N=129) | 4.4±2.3 (N=6) | <0.0001 |
| (median) (min-max) | 0.1 (0.0-2.2) | 4.3 (2.3-8.6) | |
| Follow-up (months) | |||
| (mean ± SD) (n) | 56.6±31.9 (N=141) | 63.4±22.3 (N=6) | 0.49 |
| (median) (min-max) | 53.6 (4.2-163.6) | 52.4 (44.8-96.8) | |
| Gleason | |||
| 7 | 67 (42.9%) | 2 (33.3%) | 0.76 |
| <7 | 56 (35.9%) | 2 (33.3%) | |
| >7 | 33 (21.2%) | 2 (33.3%) | |
| Perineural invasion | |||
| No | 137 (87.8%) | 5 (83.3%) | 0.55 |
| Yes | 19 (12.2%) | 1 (16.7%) | |
| Neoadjuvant hormone blockade | |||
| No | 111 (71.2%) | 5 (83.3%) | 0.68 |
| Yes | 45 (28.8%) | 1 (16.7%) | |
| Post treatment hormone blockade | |||
| No | 137 (87.8%) | 6 (100%) | 1.00 |
| Yes | 19 (12.2%) | 0 (0.0%) |
SD: Standard deviation; RT: Radiation Therapy; IPSS: International Prostate Symptom Score; PSA: Prostatic Specific Antigen.
Discussion
This retrospective study demonstrated that the association of EBRT with HDR-BT is an effective and safe therapeutic option for localized prostate cancer, with a biochemical recurrence rate of 3.7%. PSA nadir <1.0 ng/dL was the only categorical variable predictive of biochemical recurrence. In relation to grade 3 late toxicities, only one case (0.6%) was observed in the GU (urethral stenosis) and no case in the GI. PSA nadir has been revealed as an important predictor of oncological control in the context of radiotherapy [29].
The association of EBRT with BT (high/HDR or low/LDR dose rate) was initially proposed to combine the many advantages of each technique. On the one hand, EBRT allows a large range radiation to treat possible tumor invasions in the seminal vesicles and prostatic capsule, while BT offers a dose of intraprostatic radiation, superior to that offered by EBRT and several studies have demonstrated the feasibility of this combination [30-32].
This study is one of the first carried out in Brazil referring specifically to the treatment of localized PCa, using the association of EBRT and HDR-BT as a therapeutic modality. The first one was published in 2006 by Esteves et al. [29], at the Hospital Beneficência Portuguesa-São Paulo (SP), with 46 patients, followed by two other articles published by Pellizzon et al. in 2008 [30] and 2011 [31] at the Hospital AC Camargo-São Paulo (SP).
Several other studies [32-37] have demonstrated the potential benefit of the synergism between EBRT and HDR-BT, in view of tumor control, biochemical recurrence and toxicity (Table 5).
Table 5.
Comparison between literature studies: External beam radiatilon therapy associated to high dose rate brachytherapy in the treatment of localized Prostate Cancer-low (L), intermediate (I) and high (H) risk
| Authors | Study Design | Patients (n) | Total HDR BT dose (Gy) | Gy/fractionation | Total EBRT dose (Gy) | Follow-up (years) | Recurrence free (%) | 3rd or 4th degree late toxicity (%) | Erectile preservation (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Low | Intermediate | High | GU | GI | ||||||||
| Borghede (1997) | Prospective | 50 | 10 | 5 | 50 | 1.5 | 97 | 97 | 92 | 2 | 0 | 74 |
| Demanes (2005) | Prospective | 209 | 23 | 6 | 36 | 7.3 | 90 | 87 | 69 | 7.7 | 0 | 67 |
| Kalkner (2007) | Phase I | 154 | 20 | 10 | 50 | 6.1 | 97 | 83 | 83 | 5 | 1 | NA |
| Pellizzon (2008) | Phase II | 209 | 20 | 10 | 45 | 5.3 | 92 | 90 | 89 | NA | NA | NA |
| Demanes (2009) | Prospective | 211 | 23 | 6 | 36 | 6.4 | 92 | 87 | 63 | 0 | 0 | NA |
| Liu (2016) | Prospective | 156 | 18 | 9 | 39 | 3.1 | 100 | 100 | 96.9 | 2.6 | 0 | NA |
| This study | Retrospective | 162 | 8-20 | 8-10 | 45-66 | 4.75 | 96.5 | 96.2 | 96.2 | 0.6 | 0 | NA |
| Mean | 170 | 19.6 | 7.4 | 41.7 | 5.1 | 94.3 | 90.2 | 79.4 | 2.9 | 0.2 | 69.5 | |
EBRT: External beam radiation therapy; high dose rate brachytherapy (HDR-BT); BT: Brachytherapy; Gy: Radiation dose Unit (Gray); GU: genitourinary system; GI: gastrointestinal system; NA: not available.
In 2012, Hoskin Póse et al. proposed that the combined treatment (EBRT + HDR-BT) resulted in significant improvement of biochemical recurrence rates, when compared to isolated EBRT. In their study, a 31% reduction in the risk of recurrence (P=0.01) was obtained, alongside a reduction of acute morbidity and similar incidence of late severe toxicity in the genitourinary and gastrointestinal tracts [38]. In 2013, Kotecha et al. published results on recurrance-free survival and morbidity in 229 patients with localized PCa treated with EBRT + HDR-BT. They concluded that this combination provided a high rate of radiation to the prostate and was associated with better tumor control and grade 3 toxicity in the genitourinary tract (GU) remained inferior to 4% [39].
Regarding the prognostic value of nadir PSA, Tsumura et al. (2016) analyzed data from 216 high-risk or locally advanced PCa patients who underwent EBRT associated HDR-BT with long-term androgen deprivation therapy (ADT) for a period of 6 years. A post-radiotherapy nadir PSA value of ≤0.02 ng/mL was associated with better long-term biochemical control [40].
Data from 3,424 patients treated with EBRT + HDR-BT between 1997 and 2014 were collected from 16 Asian hospitals (Japan and Singapore), using a standardized database. The risk category was defined as low, intermediate, high and very high risk, according to NCCN criteria (www.nccn.org). The mean dose of HDR-BT was 18 Gy and the EBRT was 39 Gy. Neoadjuvance was given to 27.7% and 49.5% received both. The mean follow-up was 66 months (1-250). Biochemical control at 5 and 10 years was 90.6% and 81.4%, respectively. High risk was detected as a predictor of biochemical recurrence. They concluded that, in cases of very high risk, the time of ADT should be prolonged, even with HDR-BT; while also being useful to suppress late toxicity [41].
An Australian study published in July 2017 retrospectively evaluated the results (biochemical relapse and incidence of urethral stenosis) in 507 patients with intermediate and high-risk PCa treated with EBRT + HDR-BT in the period of August 2000 to December 2009. All patients received neoadjuvant hormonal blockade (6 months), and only 11 (2.1%) required adjuvant treatment (all high risk). Three doses of HDR-BT were prescribed (the first dose on the day of implant and the other two on the following day, with a minimum interval of 6 hours with a dose of 6.6 Gy each). The EBRT dose was 46 Gy (divided in 23 sessions). With a mean follow-up of 124 months (10.3 years), the authors concluded that, with the association of EBRT + HDR-BT, the results were better in terms of biochemical recurrence when compared to previous results from EBRT only treatments in the same institution. The biochemical recurrence free rates for intermediate and high-risk cases were 93.3% and 74.2% at 5 years and 86.9% and 56.1% at 10 years, respectively. The rate of urethral stenosis was 28.9% before 2005 and 4.2% after 2005 [42].
In our study, from 2005 to mid-2007, almost all patients received 2 HDR-BT fractions, the majority being two 10 Gy doses. From 2007 until the end of the first half of 2008 there was a diversification of the treatment, sometimes 1 dose of 10 Gy, or 2 doses, ranging from 16 to 20 Gy. From August 2008, all patients underwent a single dose of HDR-BT of 10 Gy. This trend was demonstrated by Falk et al. [43] and Hoskin et al. [44] and concluded that the single fraction for PCa produces similar results in terms of biochemical control and late toxicity compared to two or three fractions’ schemes and is acceptable to “boost” the final EBRT with similar rates.
In the present study the only categorical variable that presented a statistically significant difference was the nadir PSA value <1 ng/ml (P=0.018) and patients who presented nadir PSA 0.2±0.3 (n=120) did not present biochemical recurrence (P=0.009). Post treatment PSA (P<0.0001) showed statistical value in terms of biochemical recurrence: 1) PSA nadir as continuous variable (P=0.0009) and PSA nadir <1 (P=0.018). No other variable (age, initial PSA, dose employed in EBRT, number of implants, number of positive cores in the biopsy, use of hormone blockade, Gleason score or TNM staging) was determinant for biochemical recurrence.
The incidence of grade 3 late toxicity in the GU was 0.6%, and there were no cases of severe complications in the gastrointestinal tract (GI).
We acknowledge the limitations of a retrospective study, carried out in a single reference center with a restricted number of patients and heterogeneous characteristics. In addition, different HDR-BT schemes (1 or 2 fractions) have been used over the years. However, this is also found in the literature, as seen in a systematic review published by Zaorsky et al. [45] in which the authors concluded that the limitations of current EBRT + HDR-BT studies include reports from single institution experiments and unrefined results in terms of patient toxicity or quality of life. Regarding disease control, biochemical relapse, and side effects (toxicity), our study presents results compatible with other series published before.
High-dose brachytherapy associated with image-guided EBRT is an effective and safe method for dose delivery with a similar and safe tumoricidal effect added to the advantage of treatment optimization with fewer sessions and a greater number of patients treated with same resources. In addition, recent radiobiological data on the treatment of prostate cancer suggest that HDBT should produce tumor control and late side effects that are at least as good as those achieved with conventional fractionation, with the additional possibility that acute side effects may be reduced [46].
While we have identified the PSA nadir >1 as the best marker of biochemical recurrence both for monotherapy (29) and for combined radiotherapy in the current study, future studies should look for new and more specific markers with potential for higher precision.
Conclusion
External Beam Radiation Therapy conjugated to Brachytherapy in the treatment of Prostate Cancer has demonstrated low biochemical recurrence rates (3.7%), mainly when PSA nadir <1, with low toxicity (0.6%) into both GU and GI tracts.
Acknowledgements
To the involved institution(s), the patients, and those that provided and cared for study patients.
Disclosure of conflict of interest
None.
References
- 1.Instituto Nacional de Câncer José Alencar Gomes da Silva. Rio de Janeiro: INCA; 2015. Estimativa 2016: Incidência de câncer no Brasil. [Google Scholar]
- 2.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours. Lyon: IARC Press; 2004. Pathology and genetics of tumours of the urinary system and male genital organs. [Google Scholar]
- 3.Yancik R, Ries LA. Cancer in older persons: an international issue in an aging world. Semin Oncol. 2004;31:128–36. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Romero FR, Romero AW, Almeida RM, Tambara Filho R. The prevalence of prostate cancer in Brazil is higher in black men than in white men: systematic review and meta-analysis. Int Braz J Urol. 2012;38:440–7. doi: 10.1590/s1677-55382012000400002. [DOI] [PubMed] [Google Scholar]
- 5.Alberti C. Hereditary/familial versus sporadic prostate cancer: few indisputable genetic differences and many similar clinicopathological features. Eur Rev Med Pharmacol Sci. 2010;14:31–41. [PubMed] [Google Scholar]
- 6.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–8. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Richman EL, Kenfield SA, Chavarro JE, Stampfer MJ, Giovannucci EL, Willett WC, Chan JM. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern Med. 2013;173:1318–26. doi: 10.1001/jamainternmed.2013.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez P, Manoharan M, Sved P, Kim SS, Soloway MS. Radical retropubic prostatectomy in Hispanic patients. Cancer. 2004;100:1628–32. doi: 10.1002/cncr.20127. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L. Active surveillance for prostate cancer: a review. Curr Urol Rep. 2010;11:165–71. doi: 10.1007/s11934-010-0110-z. [DOI] [PubMed] [Google Scholar]
- 10.Eade TN, Hanlon AL, Horwitz EM, Buyyounouski MK, Hanks GE, Pollack A. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–9. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojan L, Harrer K, Schäfer J, Voss M, Welzel G, Bolenz C, Wenz F, Alken P, Michel MS. Complications and side effects of low dose rate brachytherapy for the treatment of prostate cancer: data on a 13 year follow-up study from Mannheim. Urologe A. 2007;46:1542–7. doi: 10.1007/s00120-007-1369-7. [DOI] [PubMed] [Google Scholar]
- 12.Pellizzon AC, Salvajoli J, Novaes P, Maia M, Fogaroli R. Updated results of high-dose rate brachytherapy and external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO phoenix definition. Int Braz J Urol. 2008;34:293–301. doi: 10.1590/s1677-55382008000300006. [DOI] [PubMed] [Google Scholar]
- 13.Zamboglou N, Tselis N, Baltas D, Buhleier T, Martin T, Milickovic N, Papaioannou S, Ackermann H, Tunn UW. High-dose-rate interstitial brachytherapy as monotherapy for clinically localized prostate cancer: treatment evolution and mature results. Int J Radiat Oncol Biol Phys. 2013;85:672–8. doi: 10.1016/j.ijrobp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Morton GC. High-dose-rate brachytherapy boost for prostate cancer: rationale and technique. J Contemp Brachytherapy. 2014;6:323–30. doi: 10.5114/jcb.2014.45759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bill-Axelson A, Holmberg L, Filén F, Ruutu M, Garmo H, Busch C, Nordling S, Häggman M, Andersson SO, Bratell S, Spångberg A, Palmgren J, Adami HO, Johansson JE Scandinavian Prostate Cancer Group Study Number 4. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–54. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briganti A, Chun FK, Salonia A, Gallina A, Farina E, Da Pozzo LF, Rigatti P, Montorsi F, Karakiewicz PI. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. 2006;98:788–93. doi: 10.1111/j.1464-410X.2006.06318.x. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 18.Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Kane CJ, Kawachi MH, Kuettel M, Lee RJ, Meeks JJ, Penson DF, Plimack ER, Pow-Sang JM, Raben D, Richey S, Roach M 3rd, Rosenfeld S, Schaeffer E, Skolarus TA, Small EJ, Sonpavde G, Srinivas S, Strope SA, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 19.Cosset JM, Flam T, Belin L, Thiounn N, Pierrat N, Pontvert D, Wakil G, Savignoni A, Chauveinc L. Long-term results of permanent implant prostate cancer brachytherapy: a single-institution study of 675 patients treated between 1999 and 2003. Cancer Radiother. 2016;20:261–7. doi: 10.1016/j.canrad.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Dattoli M, Wallner K, True L, Bostwick D, Cash J, Sorace R. Long-term outcomes for patients with prostate cancer having intermediate and high-risk disease, treated with combination external beam irradiation and brachytherapy. J Oncol. 2010;2010:471375. doi: 10.1155/2010/471375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz MD, Halabi S, Archer L, McGinnis LS, Kuettel MR, DiBiase SJ, Small EJ. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer. 2011;117:5579–88. doi: 10.1002/cncr.26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez AA, Gonzalez J, Ye H, Ghilezan M, Shetty S, Kernen K, Gustafson G, Krauss D, Vicini F, Kestin L. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363–70. doi: 10.1016/j.ijrobp.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Chapet O, Bossi A, Horn S, Créhange G. Combination external beam radiation and brachytherapy boost for prostate cancer. Cancer Radiother. 2017;21:473–477. doi: 10.1016/j.canrad.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Reis LO, Simão AF, Baracat J, Denardi F, Gugliotta A. Digital rectal examination standardization for inexperienced hands: teaching medical students. Adv Urol. 2013;2013:797096. doi: 10.1155/2013/797096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 26.Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, Montironi R, Wheeler TM, Delahunt B, Egevad L, Epstein JI ISUP Prostate Cancer Group. International Society of Urological Pathology (ISUP) Consensus Conference on handling and staging of radical prostatectomy specimens. Working group 5: surgical margins. Mod Pathol. 2011;24:48–57. doi: 10.1038/modpathol.2010.155. [DOI] [PubMed] [Google Scholar]
- 27.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 28.Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Reis LO, Sanches BC, Zani EL, Castilho LN, Monti CR. PSA-nadir at 1 year as a sound contemporary prognostic factor for low-dose-rate iodine-125 seeds brachytherapy. World J Urol. 2014;32:753–9. doi: 10.1007/s00345-013-1148-6. [DOI] [PubMed] [Google Scholar]
- 30.Pellizzon AC, Salvajoli J, Novaes P, Maia M, Fogaroli R. Updated results of high-dose rate brachytherapy and external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int Braz J Urol. 2008;34:293–301. doi: 10.1590/s1677-55382008000300006. [DOI] [PubMed] [Google Scholar]
- 31.Pellizzon AC, Fogaroli RC, Silva ML, Castro DG, Maia MC, Lopes A. High-dose-rate brachytherapy combined with hypofractionated external beam radiotherapy for men with intermediate or high risk prostate cancer: analysis of short- and medium-term urinary toxicity and biochemical control. Int J Clin Exp Med. 2011;4:43–52. [PMC free article] [PubMed] [Google Scholar]
- 32.Borghede G, Hedelin H, Holmäng S, Johansson KA, Aldenborg F, Pettersson S, Sernbo G, Wallgren A, Mercke C. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother Oncol. 1997;44:237–44. doi: 10.1016/s0167-8140(97)00121-7. [DOI] [PubMed] [Google Scholar]
- 33.Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys. 2005;61:1306–16. doi: 10.1016/j.ijrobp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Kälkner KM, Wahlgren T, Ryberg M, Cohn-Cedermark G, Castellanos E, Zimmerman R, Nilsson J, Lundell M, Fowler J, Levitt S, Hellström M, Nilsson S. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46:909–17. doi: 10.1080/02841860601156140. [DOI] [PubMed] [Google Scholar]
- 35.Pellizzon AC, Salvajoli J, Novaes P, Maia M, Fogaroli R, Gides D, Horriot R. The relationship between the biochemical control outcomes and the quality of planning of high-dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO phoenix definition. Int J Med Sci. 2008;5:113–20. doi: 10.7150/ijms.5.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demanes DJ, Brandt D, Schour L, Hill DR. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am J Clin Oncol. 2009;32:342–7. doi: 10.1097/COC.0b013e31818cd277. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Kaidu M, Sasamoto R, Ayukawa F, Yamana N, Sato H, Tanaka K, Kawaguchi G, Ohta A, Maruyama K, Abe E, Kasahara T, Nishiyama T, Tomita Y, Aoyama H. Two-fraction high-dose-rate brachytherapy within a single day combined with external beam radiotherapy for prostate cancer: single institution experience and outcomes. J Radiat Res. 2016;57:280–7. doi: 10.1093/jrr/rrw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoskin PJ, Rojas AM, Ostler PJ, Bryant L, Lowe GJ. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2021;154:214–219. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Kotecha R, Yamada Y, Pei X, Kollmeier MA, Cox B, Cohen GN, Zaider M, Zelefsky MJ. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy. 2013;12:44–9. doi: 10.1016/j.brachy.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Tsumura H, Satoh T, Ishiyama H, Tabata K, Komori S, Sekiguchi A, Ikeda M, Kurosaka S, Fujita T, Kitano M, Hayakawa K, Iwamura M. Prostate-specific antigen nadir after high-dose-rate brachytherapy predicts long-term survival outcomes in high-risk prostate cancer. J Contemp Brachytherapy. 2016;8:95–103. doi: 10.5114/jcb.2016.59686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishiyama H, Kamitani N, Kawamura H, Kato S, Aoki M, Kariya S, Matsumura T, Kaidu M, Yoshida K, Hashimoto Y, Noda Y, Lim KHC, Kawase T, Takahashi T, Inaba K, Kumano M, Yoshikawa N, Yoshioka Y, Nakamura K, Hiratsuka J, Itami J, Hayakawa K. Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: an Asian Prostate HDR-BT Consortium. Brachytherapy. 2017;16:503–510. doi: 10.1016/j.brachy.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Yaxley JW, Lah K, Yaxley JP, Gardiner RA, Samaratunga H, MacKean J. Long-term outcomes of high-dose-rate brachytherapy for intermediate- and high-risk prostate cancer with a median follow-up of 10 years. BJU Int. 2017;120:56–60. doi: 10.1111/bju.13659. [DOI] [PubMed] [Google Scholar]
- 43.Falk AT, Demontoy S, Chamorey E, Chand ME, Gautier M, Azria D, Zaki S, Chevallier D, Cham Kee DL, Hannoun-Lévi JM. High-dose-rate brachytherapy boost for prostate cancer: comparison of three different fractionation schemes. Brachytherapy. 2017;16:993–999. doi: 10.1016/j.brachy.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Hoskin P, Rojas A, Ostler P, Hughes R, Alonzi R, Lowe G. Single-dose high-dose-rate brachytherapy compared to two and three fractions for locally advanced prostate cancer. Radiother Oncol. 2017;124:56–60. doi: 10.1016/j.radonc.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Zaorsky NG, Ohri N, Showalter TN, Dicker AP, Den RB. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treat Rev. 2013;39:728–736. doi: 10.1016/j.ctrv.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Cerqueira MA, Laranja WW, Sanches BC, Monti CR, Reis LO. Burden of focal cryoablation versus brachytherapy versus active surveillance in the treatment of very low-risk prostate cancer: a preliminary head-to-head comprehensive assessment. Eur J Cancer Care (Engl) 2015;24:929–37. doi: 10.1111/ecc.12307. [DOI] [PubMed] [Google Scholar]


