Abstract
We used semi-quantitative grading of musculoskeletal ultrasound to evaluate wrist and hand lesions of subclinical synovitis, in order to make earlier diagnosis of rheumatoid arthritis. A total of 164 patients were included in this study. Physical examination and ultrasound examination were used to evaluate 30 joints of the wrist and hand. According to the clinical symptoms, the patients were divided into subclinical synovitis (SS) group and clinical synovitis (CS) group. The wrist and hand joints of patients with rheumatoid arthritis between the two groups were evaluated by semi-quantitative grading of musculoskeletal ultrasound, including synovitis, Power Doppler signal, joint effusion and bone erosion. We found that the total score of semi-quantitative ultrasound, synovitis score and Power Doppler signal score in the SS group were lower than those in the CS group (P<0.05). There was no significant difference in joint effusion score and bone erosion score (P>0.05). In the analysis of laboratory examination, the value of anti-RA33 antibody and ESR of SS group were decreased than that of CS group, with statistically significant difference (P=0.004), while that of RF, AKA and CCP had no significant difference between the two groups (P>0.05). In this study, the author also compared the tenosynovitis between the two groups. There was statistically significant difference (P=0.033). In conclusions, semi-quantitative grading of musculoskeletal ultrasound has certain diagnostic value for the diagnosis of subclinical synovitis in wrist and hand lesion.
Keywords: Musculoskeletal ultrasound, semi-quantitative grading, subclinical synovitis, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by synovitis, which could damage cartilage, bone, ligament and tendon [1]. Clinical synovitis (CS) refers to the joint swelling and tenderness which was found under the physical examination of patients, while subclinical synovitis (SS) refers to the synovitis found under ultrasound or magnetic resonance examination, with no signs of joint swelling [2].
Imaging examination has been used as effective methods to diagnose RA. Both magnetic resonance and ultrasound are useful to detect clinical synovitis. However, ultrasound is readily available at most hospitals and is more economic feasible for the majority of patients. Hence, the European League Against Rheumatism (EULAR) issued 10 recommendations on imaging examination in RA clinical practice, which clearly put forward that ultrasound could be used for the diagnosis, detection and follow-up of RA patients [3].
Subclinical synovitis has been widely confirmed, in the earliest phases of RA or in clinical remission after regular treatment. Early treatment is closely related to less joint deformity and functional loss for those patients suffered from RA. For patients discontinued treatment, it is still important to identify who need to receive therapy again. Musculoskeletal ultrasound is a useful method to detect subclinical synovitis. In a previous study, 43% of RA patients in remission have increased Power Doppler signal [4]. Importantly, scores of musculoskeletal ultrasound with reduced and feasible joint counts could be helpful for rheumatologists to make corrective treatment decisions.
In this study, semi-quantitative grading of musculoskeletal ultrasound was used to evaluate the subclinical synovitis of rheumatoid arthritis in wrist and hand lesions, in order to make earlier diagnosis of rheumatoid arthritis.
Patients and methods
Eligibility
We conducted this study concerning patients diagnosed with rheumatoid arthritis at Affiliated Dongguan Hospital, Southern Medical University from January, 2018 to January, 2021. A total of 164 patients were collected according to the diagnostic criteria of RA revised by American rheumatic Association in 1987 [5]. There were 122 females and 42 males, with an average age of 56.07 years (range, 18-87 years). Patients whose clinical information could not be obtained in full were excluded from this study. Physical examination and ultrasonography were used to evaluate 30 joints of wrist and hand for every patient. According to the clinical symptoms, the patients were divided into two groups: SS group and CS group. This study was approved by ethics committee of Affiliated Dongguan Hospital, Southern Medical University and was conducted according to the Declaration of Helsinki. Patients provided informed written consent.
Ultrasound assessment
Ultrasound machine Canon i900 and i18LX5 linear array transducer were used to scan 30 joints (bilateral wrists, MCP1-5, PIP1-5 and DIP 2-5 joints) for all patients, by both the gray scale (GS) and the power Doppler (PD) modes. US-determined joint inflammation was defined as synovitis and/or tenosynovitis and/or peritendinitis. The pathological changes and abnormal Power Doppler signals of synovium, tendon sheath and bone cortex were carefully observed. At the same time, semi-quantitative grading and scoring of synovitis, Power Doppler signal, joint effusion and bone erosion were performed according to the European guideline for musculoskeletal ultrasound in rheumatology medicine [6].
Joint effusion, synovitis, bone erosions and power Doppler signal in the synovial membrane of the preselected joints were evaluated and classified on 4-grade semi-quantitative scales. The selected parameters were defined as follows. Bone erosions were defined as changes in the bone surface of the area adjacent to the joint (Grade 0= regular bone surface, Grade 1= irregularity of the bone surface without formation of a defect seen in 2 planes, Grade 2= formation of a defect in the surface of the bone seen in 2 planes, Grade 3= bone defect creating extensive bone destruction) (Figure 1A-D). Joint effusion was defined as a compressible anechoic intracapsular area (Grade 0= no effusion, Grade 1= minimal amount of joint effusion, Grade 2= moderate amount of Joint effusion [without distension of the joint capsule], Grade 3= extensive amount of joint effusion [with distension of the joint capsule]) (Figure 1E-H).
Figure 1.
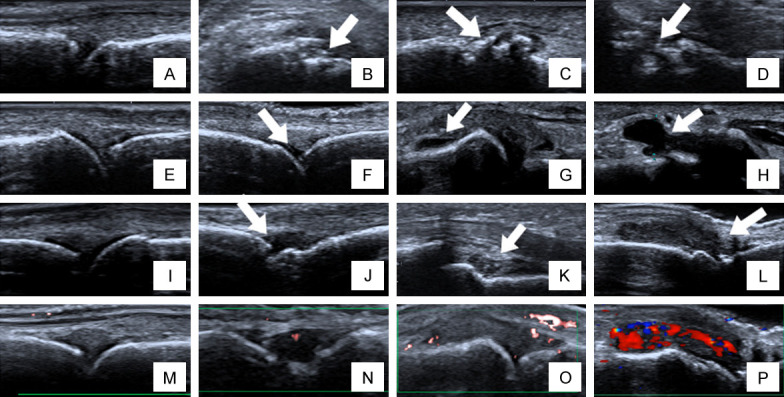
Ultrasonic scoring criteria for joint involvement. A-D. Bone changes scored with ultrasonography, with each joint visualized in 2 planes (longitudinal and transverse). A. Grade 0: regular bone surface; B. Grade 1: irregularity of the bone surface without formation of a defect seen in 2 planes (arrow); C. Grade 2: formation of a defect in the surface of the bone seen in 2 planes (arrow); D. Grade 3: bone defect creating extensive bone destruction (arrow). E-H. Joint effusion on ultrasonography. E. Grade 0: no fluid; F. Grade 1: minimal amount of fluid (arrow); G. Grade 2: moderate amount of fluid (without distension of the joint capsule) (arrow); H. Grade 3: extensive amount of fluid (with distension of the joint capsule) (arrow). I-L. Synovial changes on ultrasonography. I. Grade 0: no synovial thickening; J. Grade 1: minimal synovial thickening (filling the angle between the periarticular bones, without bulging over the line linking tops of the bones); K. Grade 2: synovial thickening bulging over the line linking tops of the periarticular bones but without extension along the bone diaphysis; L. Grade 3: synovial thickening bulging over the line linking tops of the periarticular bones and with extension to at least one of the bone diaphyses. M-P. Power Doppler signal. M. Grade 0: no flow in the synovium; N. Grade 1: up to three single Doppler spots or up to one confluent spot and two single spots or up to two confluent spots; O. Grade 2: greater than Grade 1 but <50% Doppler signals in the total GS background; P. Grade 3: greater than Grade 2 (>50% of the background GS).
Synovitis was defined as a noncompressible hypoechoic intracapsular area (synovial thickening) (Grade 0= no synovial thickening, Grade 1= minimal synovial thickening [filling the angle between the periarticular bones, without bulging over the line linking tops of the bones], Grade 2= synovial thickening bulging over the line linking tops of the periarticular bones but without extension along the bone diaphysis, Grade 3= synovial thickening bulging over the line linking tops of the periarticular bones and with extension to at least one of the bone diaphyses) (Figure 1I-L). Power Doppler signal was used in our study to display flow signal in the synovium (Grade 0= no flow in the synovium, 1= up to three single Doppler spots or up to one confluent spot and two single spots or up to two confluent spots, 2= greater than Grade 1 but <50% Doppler signals in the total GS background, 3= greater than Grade 2 (>50% of the background GS)) (Figure 1M-P).
For synovitis, Power Doppler signal, joint effusion and bone erosion, the maximum semi-quantitative grade recorded on the volar and dorsal side of the given joint area were taken as the recording grade. The sum of each grade score is the final score. Both tenosynovitis and peritendinitis were defined as the presence of GS or PD signal.
Manufacturer recommendations for musculoskeletal of the wrist and hand were used, including the color gain was set at the level at which noise artifacts appeared and then gradually reduced, until only a flow signal, if present, was left. All the ultrasound scanning was done by one ultransonographer with over 10 years of experience in maneuvering musculoskeletal ultrasound.
Joint and laboratory assessment
Joint assessment for tenderness or swelling was performed carefully and detailed clinical information of RA patients was obtained by rheumatologists at Affiliated Dongguan People’s Hospital, Southern Medical University. Laboratory parameters were measured and collected by laboratory technologist at our hospital. These parameters include serum concentrations of rheumatoid factor (RF), anti-keratin antibody (AKA), anti-cyclopanine antibody (CCP) and C-reactive protein (CRP).
Statistical analysis
Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) 22.0 software. For the descriptive analyses, continuous variables were presented as mean and standard deviation (SD) if normally distributed and median and interquartile range (IQR) if non-normally distributed. Rank sum test was used to compare the differences among the groups, a two-sided P<0.05 was considered statistically significant. Tenosynovitis cases were treated with routine treatment χ2 inspection.
Results
Demographics and clinical characteristics of patients
The characteristics of the enrolled patients are illustrated in Table 1. There were 23 cases in the SS group and 141 cases in the CS group. In the SS group, there were 18 females with an average age of 55.3 years. The median course of disease was 24 months. The laboratory test results showed that there were 19, 5, 8 and 1 positive cases of RF, AKA, CCP and anti-RA33 antibody, respectively. The median value of ESR was 11 mg/L. While in the CS group, 104 cases were female, with an average age of 56.2 years. The median course of disease was also 24 months. The positive cases of RF, AKA, CCP and anti-RA33 antibody were found in 101, 54, 109 and 18 patients, respectively. The median value of ESR in this group was 43 mg/L.
Table 1.
Demographics and clinical characteristics of 164 RA patients
| Parameters | Values | |
|---|---|---|
|
| ||
| SS group (23) | CS group (141) | |
| Age (years), mean ± SD | 55.3±12 | 56.2±15 |
| Female, n (%) | 18 (78%) | 104 (74%) |
| Disease duration (months), median (IQR) | 24 (5, 75) | 24 (3, 111) |
| Positive RF, n (%) | 19 (83%) | 101 (72%) |
| Positive AKA, n (%) | 5 (22%) | 54 (38%) |
| Positive CCP, n (%) | 8 (35%) | 109 (77%) |
| Positive Anti-RA33, n (%) | 1 (4%) | 18 (13%) |
| CRP (mg/L), median (IQR) | 11 (6, 41) | 43 (23, 73) |
Abbreviations: RF, rheumatoid factor; AKA, anti-keratin antibody; CCP, anti-cyclopanine antibody; CRP, C-reactive protein; SS, subclinical synovitis; CS, clinical synovitis.
The occurrence rate of synovial hyperplasia and synovitis in SS and CS groups
In the SS group, there were 21 cases of synovial hyperplasia (91.3%), 5 cases of increased synovial blood flow (21.7%), 1 case of joint effusion (4.3%), 1 case of bone erosion (4.3%) and 3 cases of tenosynovitis (13.0%), as compared with that of 137 cases (97.2%), 91 cases (64.5%), 21 cases (14.9%), 28 cases (19.9%) and 50 cases (35.5%) in the CS group, respectively (Figure 2).
Figure 2.
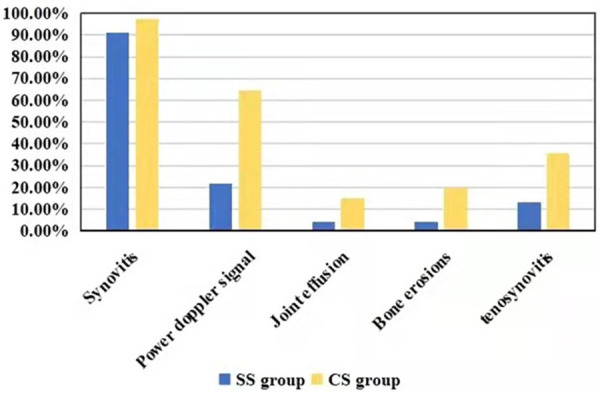
The occurrence rate of synovial hyperplasia and synovitis in SS and CS groups.
Analysis of laboratory examination
In our study, the median value of anti-RA33 antibody and ESR in the SS group were 2.6 and 11 respectively, while that in the CS group were 8.9 and 43 respectively. The difference between the two groups were statistically significant (P=0.004). The median value of RF, AKA, CCP in the SS group were 49, 1:5 and 13 respectively, while that in the CS group were 56, 1:5, 49.3 respectively (Table 2). Notably, the difference of these factors between the two groups was not statistically significant (P>0.05).
Table 2.
Comparison of related laboratory indexes between the two groups
| Group | RF (IU/mL) | AKA | CCP (U/mL) | RA33 (AU/mL) | ESR (mm/h) |
|---|---|---|---|---|---|
| SS group | 49 (21, 105.4) | 1:5 (1:5, 1:10) | 13 (4, 201) | 2.6 (1.3, 10.4) | 11 (6, 41) |
| CS group | 56 (16.6, 132.2) | 1:5 (1:5, 1:10) | 49.3 (1.7, 201) | 8.9 (5.2, 14) | 43 (23, 72.5) |
| Mann-Whitney U | 1583.000 | 836.000 | 1390.000 | 564.500 | 610.000 |
| P value | 0.898 | 0.235 | 0.360 | 0.004 | 0.000 |
Abbreviations: RF, rheumatoid factor; AKA, anti-keratin antibody; CCP, anti-cyclopanine antibody; CRP, C-reactive protein; SS, subclinical synovitis; CS, clinical synovitis.
Semi-quantitative grading score of musculoskeletal ultrasound
The total semi-quantitative ultrasound score, synovial hyperplasia score and synovial Doppler blood flow score of SS group were lower than that of clinical synovitis group, and the differences were statistically significant (P<0.05). However, there were no significant difference between the scores of joint effusion and joint bone erosion (P>0.05) (Table 3). In this study, the author also compared the occurrence rate of tenosynovitis between the two groups, the difference was statistically significant (P=0.033).
Table 3.
Ultrasound score between the two groups
| Synovitis | Power Doppler signal | Joint effusion | Bone erosions | Total score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| SS group | CS group | SS group | CS group | SS group | CS group | SS group | CS group | SS group | CS group | |
| Grade 0 | 2 | 5 | 18 | 50 | 18 | 121 | 22 | 113 | -- | -- |
| Grade 1 | 5 | 26 | 5 | 45 | 2 | 17 | 0 | 4 | -- | -- |
| Grade 2 | 14 | 58 | 0 | 34 | 1 | 1 | 0 | 9 | -- | -- |
| Grade 3 | 2 | 52 | 0 | 12 | 2 | 3 | 1 | 15 | -- | -- |
| median (IQR) | 2 (1, 2) | 2 (2, 3) | 0 (0, 0) | 1 (0, 2) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 2 (2, 3) | 4 (2, 5) |
| Mann-Whitney U | 1165.000 | 812.500 | 1476.500 | 1376.5 | 983.500 | |||||
| P value | 0.021 | 0.000 | 0.271 | 0.081 | 0.002 | |||||
Abbreviations: SS, subclinical synovitis; CS, clinical synovitis; IQR, interquartile range.
Discussion
Till date, RA cannot be completely cured. However, previous studies show that early intervention of this disease could slow down the damage of cartilage, bone, ligament and reduce the disability rate of RA patients [7]. Therefore, accurate judgment of joint inflammation is very important in the clinic. There were studies showed that ultrasound was more sensitive and superior to clinical examination in displaying synovial lesions in RA and that the incidence of subclinical synovitis by ultrasound in rheumatoid arthritis was 6.8% [8,9]. It was also proved that synovitis could be detected by ultrasound in the patients whose joint swelling was not found by physical examination.
However, the swelling and pain of joints are not objective indicators, the severity of which depends on the judgment of patients and clinicians [10]. In addition, if RA patients are also with obesity, edema and other factors, physical examination is difficult to determine joint swelling completely and accurately. Similarly, the perception of pain is highly subjective and may be affected by social and cultural factors [11-13]. In addition, neuroimaging evidence proved that RA is a mixed pain state with central sensitization characteristics [14]. Therefore, imaging detection of subclinical synovitis is very important for rheumatologists to make treatment decisions, especially disease improvement antirheumatic drugs (DMARD) therapy. Patients with subclinical synovitis under ultrasound should be given intensive treatment in order to achieve clinical remission [15].
The inflammatory processes of synovitis involve all synovial tissues, including intra-articular, tendon sheath and synovial sac, which could be easily observed by ultrasound [16]. Previous studies showed that ultrasound were more sensitive than clinical examination in detecting synovitis [17-25]. Ultrasound could evaluate the shape and quantity of synovitis and could also evaluate the vascular distribution of synovitis in RA patients. Semi-quantitative grading of musculoskeletal ultrasound could detect the inflammatory processes and severity of synovitis. In our study, we found that the total semi-quantitative ultrasound score, synovial hyperplasia score, and synovial Doppler blood flow score in the SS group were reduced to different degrees, as was compared with that in the CS group.
With the reduction of disease severity, the semi-quantitative grading score of patients shows a significant downward trend, which is highly consistent with results of previous studies [26,27]. Therefore, color Doppler ultrasound may be used to confirm the existence of synovitis, detect the activity and progress of RA and evaluate the inflammation severity [28]. Importantly, ultrasound could also show synovium, joint effusion and bone erosion. Doppler can sensitively detect the blood flow distribution in joint tissue, reflect synovitis [29]. However, the difference of joint bone erosion score and joint effusion score of SS and CS groups in our study was not statistically significant, which indicates that the grade of bone erosion and joint effusion could even happened at the subclinical stage of RA. Hence, early treatment of patients is critical in order to control the whole processes of this disease.
Other than imaging examination, laboratory results are also important for the diagnosis of rheumatoid arthritis. Anti-RA33 antibody is a kind of nuclear protein antibody with a molecular weight of 33 KD. It participates in the formation of nuclear protein splicing body and could react with the autoantibodies of RA patients. It is now recognized that the serological detection of anti-RA33 is very helpful for the early diagnosis of RA [30]. One previous study reported that the sensitivity, specificity, positive and negative predictive values of anti-RA33 antibody were 41.1%, 97.1%, 93.3% and 62.3% [31], respectively. In addition, ESR is often used to evaluate the condition of RA. If the ESR value is significantly accelerated, it indicates that the patient may suffered from RA [32]. In our study, we found that the median value of anti-RA33 antibody and ESR in the SS group were significantly lower than that in the CS group, which suggests that these two laboratory parameters are closed related with the disease processes of RA. This result is highly consistent with that of several previous studies.
Nonetheless, this study still had some limitations. Firstly, this was a retrospective analysis with a relatively small number of RA patients. Hence, comprehensive multivariable analyses were not allowed in the study. Secondly, it was inevitably affected by subjective and objective factors of both patients and rheumatologists, such as the enthusiasm and availability of ultrasound assessment. Finally, there may have been elevated risk of a patient selection bias in the study, as our study was only a single center investigation.
In conclusion, semi-quantitative grading of musculoskeletal ultrasound is valuable for the diagnosis of subclinical synovitis in wrist and hand lesions of RA patients. Compared with the clinical synovitis group, the semi-quantitative grading score of musculoskeletal ultrasound in the subclinical synovitis group decreased in varying degrees. Combined with results of relevant laboratory tests, the diagnostic efficiency of ultrasound could be further improved.
Acknowledgements
We thank colleagues at Affiliated Dongguan Hospital, Southern Medical University for their kind technical help, psychological support, theoretical guidance and writing assistance during the present study. The authors would also like to thank patients and their family members engaged in this clinical trial for their kindness to support our study. This study was funded by Dongguan Science and Technology of Social Development Program (20211800903592).
Disclosure of conflict of interest
None.
Abbreviations
- RA
Rheumatoid arthritis
- SS
subclinical synovitis
- CS
clinical synovitis
- RF
rheumatoid factor
- AKA
anti-keratin antibody
- CCP
anti-cyclopanine antibody
- CRP
C-reactive protein
- EULAR
the European League Against Rheumatism
- SPSS
Statistical Product and Service Solutions
- SD
standard deviation
- IQR
interquartile range
- DMARD
disease improvement antirheumatic drugs
References
- 1.Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84:1245–52. [PubMed] [Google Scholar]
- 2.Zhao J, Wang Y, Geng Y, Zhang X, Deng X, Ji L, Song Z, Zhang Z. Intensive therapy alleviates subclinical synovitis on ultrasound and disease activity and reduces flare in rheumatoid arthritis patients who have achieved clinical target-a randomized controlled trial. Semin Arthritis Rheum. 2020;50:673–679. doi: 10.1016/j.semarthrit.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Colebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, D’Agostino MA, Forslind K, Grassi W, Haavardsholm EA, Haugeberg G, Jurik AG, Landewé RB, Naredo E, O’Connor PJ, Ostendorf B, Potocki K, Schmidt WA, Smolen JS, Sokolovic S, Watt I, Conaghan PG. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–14. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 4.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, Wakefield RJ, O’Connor PJ, Emery P. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 6.Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–62. doi: 10.1002/art.10877. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Zhou D, Liu Q, Li D, Wang Q, Shi X, Wen C, Huang L. Network analysis indicating the pharmacological mechanism of Yunpi-Qufeng-Chushi-prescription in prophylactic treatment of rheumatoid arthritis. BMC Complement Med Ther. 2021;21:142. doi: 10.1186/s12906-021-03311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone M, Bergin D, Whelan B, Maher M, Murray J, McCarthy C. Power Doppler ultrasound assessment of rheumatoid hand synovitis. J Rheumatol. 2001;28:1979–82. [PubMed] [Google Scholar]
- 9.Ogishima H, Tsuboi H, Umeda N, Horikoshi M, Kondo Y, Sugihara M, Suzuki T, Matsumoto I, Sumida T. Analysis of subclinical synovitis detected by ultrasonography and low-field magnetic resonance imaging in patients with rheumatoid arthritis. Mod Rheumatol. 2014;24:60–8. doi: 10.3109/14397595.2013.854050. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Deng X, Xie W, Wang L, Wang Y, Zhang Z. The agreement between ultrasounddetermined joint inflammation and clinical signs in patients with rheumatoid arthritis. Arthritis Res Ther. 2019;21:100. doi: 10.1186/s13075-019-1892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courvoisier DS, Agoritsas T, Glauser J, Michaud K, Wolfe F, Cantoni E, Perneger TV, Finckh A Swiss Clinical Quality Management Program for Rheumatoid Arthritis; National Data Bank for Rheumatic Diseases. Pain as an important predictor of psychosocial health in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:190–6. doi: 10.1002/acr.20652. [DOI] [PubMed] [Google Scholar]
- 12.Bjork M, Trupin L, Thyberg I, Katz P, Yelin E. Differences in activity limitation, pain intensity, and global health in patients with rheumatoid arthritis in Sweden and the USA: a 5-year follow-up. Scand J Rheumatol. 2011;40:428–32. doi: 10.3109/03009742.2011.594963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(Suppl 69):S92–6. [PubMed] [Google Scholar]
- 14.Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, Waiter G, Clauw DJ. Neurobiologic features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol. 2018;70:1000–7. doi: 10.1002/art.40451. [DOI] [PubMed] [Google Scholar]
- 15.Shi YM, Wu LJ, Wang WJ, Luo CN, Chen YJ. Value of musculoskeletal ultrasound in assessing the disease activity in rheumatoid arthritis. Zhonghua Yi Xue Za Zhi. 2019;99:1008–11. doi: 10.3760/cma.j.issn.0376-2491.2019.13.010. [DOI] [PubMed] [Google Scholar]
- 16.Filippucci E, Cipolletta E, Mashadi Mirza R, Carotti M, Giovagnoni A, Salaffi F, Tardella M, Di Matteo A, Di Carlo M. Ultrasound imaging in rheumatoid arthritis. Radiol Med. 2019;124:1087–1100. doi: 10.1007/s11547-019-01002-2. [DOI] [PubMed] [Google Scholar]
- 17.Bruyn GA, Möller I, Garrido J, Bong D, d’Agostino MA, Iagnocco A, Karim Z, Terslev L, Swen N, Balint P, Baudoin P, van Reesema DS, Pineda C, Wakefield RJ, Naredo E. Reliability testing of tendon disease using two different scanning methods in patients with rheumatoid arthritis. Rheumatology (Oxford) 2012;51:1655–61. doi: 10.1093/rheumatology/kes103. [DOI] [PubMed] [Google Scholar]
- 18.Bruyn GA, Naredo E, Möller I, Moragues C, Garrido J, de Bock GH, d’Agostino MA, Filippucci E, Iagnocco A, Backhaus M, Swen WA, Balint P, Pineda C, Milutinovic S, Kane D, Kaeley G, Narvaez FJ, Wakefield RJ, Narvaez JA, de Augustin J, Schmidt WA. Reliability of ultrasonography in detecting shoulder disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:357–61. doi: 10.1136/ard.2008.089243. [DOI] [PubMed] [Google Scholar]
- 19.Bruyn GA, Pineda C, Hernandez-Diaz C, Ventura-Rios L, Moya C, Garrido J, Groen H, Pena A, Espinosa R, Möller I, Filippucci E, Iagnocco A, Balint PV, Kane D, D’Agostino MA, Angulo M, Ponte R, Fernandez-Gallardo JM, Naredo E. Validity of ultrasonography and measures of adult shoulder function and reliability of ultrasonography in detecting shoulder synovitis in patients with rheumatoid arthritis using magnetic resonance imaging as a gold standard. Arthritis Care Res (Hoboken) 2010;62:1079–86. doi: 10.1002/acr.20175. [DOI] [PubMed] [Google Scholar]
- 20.Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, Raber H, Hamm B, Burmester GR, Bollow M. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232–45. doi: 10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Østergaard M. Contrast-enhanced power Doppler ultrasonography of the metacarpophalangeal joints in rheumatoid arthritis. Eur Radiol. 2003;13:163–8. doi: 10.1007/s00330-002-1459-2. [DOI] [PubMed] [Google Scholar]
- 22.Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44:2018–23. doi: 10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Wakefield RJ, Freeston JE, O’Connor P, Reay N, Budgen A, Hensor EM, Helliwell PS, Emery P, Woodburn J. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis. 2008;67:1678–82. doi: 10.1136/ard.2007.079947. [DOI] [PubMed] [Google Scholar]
- 24.Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, Carmona L, Crespo M. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–24. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 25.Perricone C, Ceccarelli F, Modesti M, Vavala C, Di Franco M, Valesini G, Iagnocco A. The 6-joint ultrasonographic assessment: a valid, sensitive-to-change and feasible method for evaluating joint inflammation in RA. Rheumatology (Oxford) 2012;51:866–73. doi: 10.1093/rheumatology/ker405. [DOI] [PubMed] [Google Scholar]
- 26.Rezaei H, Af Klint E, Hammer HB, Terslev L, D’Agostino MA, Kisten Y, Arnaud L. Analysis of correlation and causes for discrepancy between quantitative and semi-quantitative Doppler scores in synovitis in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:255–62. doi: 10.1093/rheumatology/kew385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohrndorf S, Messerschmidt J, Reiche BE, Burmester GR, Backhaus M. Evaluation of a new erosion score by musculoskeletal ultrasound in patients with rheumatoid arthritis: is US ready for a new erosion score? Clin Rheumatol. 2014;33:1255–62. doi: 10.1007/s10067-014-2646-7. [DOI] [PubMed] [Google Scholar]
- 28.Gaujoux-Viala C, Gossec L, Cantagrel A, Dougados M, Fautrel B, Mariette X, Nataf H, Saraux A, Trope S, Combe B French Society for Rheumatology. Recommendations of the French society for rheumatology for managing rheumatoid arthritis. Joint Bone Spine. 2014;81:287–97. doi: 10.1016/j.jbspin.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi. 2018;57:242–251. doi: 10.3760/cma.j.issn.0578-1426.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Wang M, Zhang X, Li X, Cai G, Xia Q, Wang L, Xin L, Xu S, Pan F. Diagnostic accuracy of anti-RA33 antibody for rheumatoid arthritis: systematic review and meta-analysis. Clin Exp Rheumatol. 2016;34:539–47. [PubMed] [Google Scholar]
- 31.Syed Mohamed Suhail SM, Nur Atiqah I, Nur Salimi Zakirah ZA, Lailatul Syazwani Z, Batis WW, Md Monoto EM, Abdul Wahab A, Mohd Shahrir MS. Diagnostic performance of anti-RA33 antibody as a serological marker for rheumatoid arthritis. Malays J Pathol. 2019;41:259–265. [PubMed] [Google Scholar]
- 32.Harvey NC, Kanis JA, Odén A, Nakamura T, Shiraki M, Sugimoto T, Kuroda T, Johansson H, McCloskey EV. Efficacy of weekly teriparatide does not vary by baseline fracture probability calculated using FRAX. Osteoporos Int. 2015;26:2347–53. doi: 10.1007/s00198-015-3129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


