Abstract
Circular RNAs (circRNAs) are a class of single‐stranded RNAs with closed loop structures formed by covalent bonds of head and tail. Exploration of circRNAs is continually increasing; however, their functional relevance largely remains to be elucidated. In general, they are stable, abundant, conserved and expressed in tissue‐specific manner. These distinct properties and their diverse cellular actions indicate that circRNAs modulate transcription and translation, and may even function as translation templates. Growing evidence reveals that circRNAs contribute to various physiological and pathological processes, including the initiation and progression of cancer. In this review, we present the current knowledge about circRNAs in cancer development, as well as their potential for use as biomarkers and even therapeutic targets. CircRNA’s role in immune regulation and antitumour immunotherapy is also discussed. In addition, possible challenges in antitumour therapy are raised, and current progress and future perspectives are provided.
Keywords: biomarker, cancer, circular RNAs, immunity, therapy
1. INTRODUCTION TO CIRCULAR RNA
Circular RNA (circRNA) has received increased attention in recent times, although it was first found in the 1970s and initially considered to be an abnormal and functionless RNA splicing product. 1 , 2 CircRNA belongs to the non‐coding RNA family and has a closed loop structure formed by covalent bonds of head and tail. Lacking 3’ termini makes it insusceptible to exonuclease digestion, and thus more stable than associated linear mRNA. 3 The development of technology and bioinformatics has facilitated the discovery of numerous circRNAs of different lengths and types, and research indicates that they are enriched in eukaryotic cells and highly conserved across species. 4
Both circular RNAs and linear RNAs are derived from precursor mRNAs and transcribed with the same efficiency. 5 Unlike linear RNAs’ classical splicing, circRNAs are generated by different modes, primarily by back‐splicing. According to the location of splice junction in genome, circRNAs are classified into four basic types, including exonic, intronic, exonic‐intronic and tRNA intronic. Exonic circRNAs (ecircRNAs) are formed by either single or several exons and account for the main body of cirRNAs; circular intronic RNAs (ciRNAs) are made up of introns alone; exonic‐intronic circRNAs (EIciRNAs) are composed of exons and introns; and tRNA intronic circRNAs (tricRNAs) are derived from splicing of pre‐tRNA introns. 5 , 6 Different types of circRNAs are distributed in different sites of cells. Generally, exonic circRNAs are located in cytoplasm, whereas some ciRNAs and EIciRNAs are in the nucleus, which is consistent with the location of their different biological functions, as shown below. Furthermore, circRNAs are reported to be packaged and released in certain vesicles (exosomes and microvesicles), and compared with in cells, circRNAs in exosomes are more enriched and widely expressed. 7 , 8
Although most of the biological functions of circRNAs have yet to be elucidated, the current knowledge about circRNAs has shown that they can regulate gene expression (transcription and translation), interact with proteins, act as miRNA sponges, translate proteins or peptides, be involved in rRNA processing and generate pseudogenes (Figure 1). In the nucleus, circRNAs function in the following: (A) competition in splicing. When the circRNA contains the same exon as the parental gene, circRNAs can compete with linear splicing of pre‐mRNA, thereby affecting the level of linear RNAs. 5 (B) Transcription regulation: ciRNAs and EIciRNAs can bind to U1 snRNP through RNA‐RNA interactions and further interact with the Pol II transcription complex to enhance parental gene expression. 9 (C) Regulation of parental gene by epigenetic mechanism: for example, circFECR1 from the FLI1 gene can bind to FLI1 promoter, recruit TET1 demethylase and bring extensive DNA demethylation in the CpG islands of promoter to activate FLI1. FECR1 can also directly inhibit the gene transcription of DNMT1 methyltransferase, an essential enzyme in DNA methylation maintenance, by binding to its promoter region, which is enriched in H3K27ac. 10 In cytoplasm, circRNAs function in (D) translation regulation: for example, CircYap can bind with Yap mRNA and the proteins associated with translation initiation, eIF4G and PABP, thereby suppressing Yap translation initiation. 11 (E) Binding to proteins: some circRNAs that have binding sites for RNA‐binding proteins may serve as protein sponges or scaffolds to affect their functions or translocations. For example, circ‐Foxo3 can bind both Foxo3, p53 and sponge for MDM2. This allows it to prevent Foxo3 ubiquitylation or to act as scaffold to facilitate MDM2‐dependent p53 ubiquitylation. 12 In other cases, circ‐Foxo3 can bind the transcription regulators ID1, E2F1, FAK and HIF1α, and prevent their nuclear translocation. 13 In this function, circ‐Foxo3 does not affect the expression level of these transcription factors, but affects their localization. (F) Acting as miRNA sponges or decoys: highly abundant circRNAs contain diverse miRNA‐binding sites, and whether the effect of sponging inhibits or protects the target miRNA may be dependent on the cellular context, 14 which is true for the majority of circRNA functions. (G) Templates for translation: circRNA was initially regarded as a class of endogenous non‐coding RNA that could not translate proteins, and it was considered to lack essential elements for cap‐dependent translation. Recently, several circRNAs have been shown to encode proteins or peptides in other cap‐independent manners, such as circMbl, circFBXW7 and circZNF609. 15 , 16 It may translate either from an artificial internal ribosomal entry site (IRES) within an open reading frame (ORF), or following the incorporation of m6A RNA modification in the 5′ untranslated region (UTR). 17 (H) Involvement in rRNA processing: for example, circANRIL can bind to pescadillo homologue 1 (PES1), an essential 60S‐preribosomal assembly factor, resulting in the impairment of exonuclease‐mediated pre‐rRNA processing and ribosome biogenesis. (J) CircRNAs can also generate pseudogenes like linear RNAs, such as circRFWD2‐derived pseudogenes. 18 In addition, circRNAs in exosomes can also be released for cell communication or can act as vectors for miRNAs or proteins, functions which are described below. 19
Figure 1.
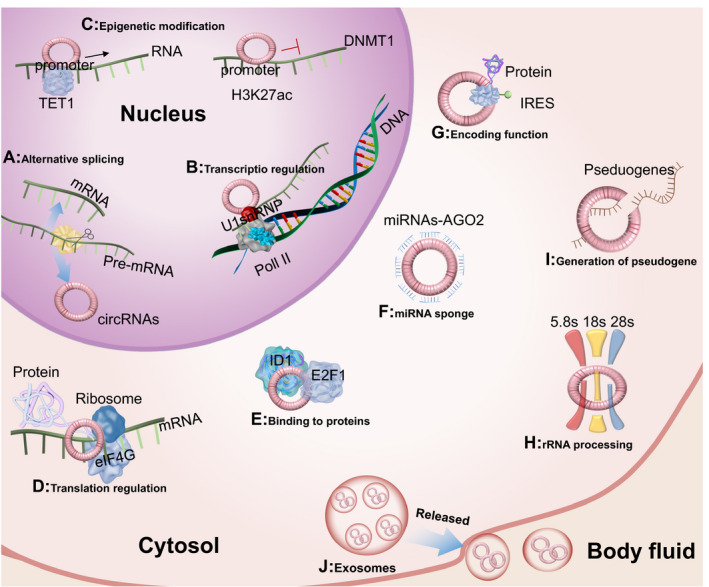
Illustration of Circular RNA function. Circular RNAs fulfil multiple functions. In nucleus, circRNAs can regulate transcription A, compete with the mRNA for the available splicing machinery and interfere in the alternative splicing process; B, regulate the transcription of their gene of origin through direct (circRNAs) or U1 snRNP‐mediated (EIcircRNA) interaction with the RNA polymerase II; C, regulate parental gene by epigenetic mechanism. In cytoplasm, circRNAs can D, regulate the translation of mRNAs by interacting with some translation initiation associated proteins; E, bind to some proteins as protein sponge or scaffold to affect their functions or translocations; F, act as miRNAs sponges, a majority of circRNAs function that interact with miRNA‐AGO2 complexes to affect miRNA functions; G, encode proteins or peptides, possibly with an internal ribosome entry site (IRES) or with m6A modification; H, interfere with the processing of pre‐rRNA subunits; I, generate pseudogenes. J, circRNAs may be released for cells communication or acting as vectors for miRNAs or proteins
Based on these biological functions, circRNAs contribute to diverse physiological and pathological processes, notably in the onset and progression of cancer. 20 , 21 In this review, we discuss the crucial roles of circRNAs pertaining to cancer, including their potential value as biomarkers and therapeutic targets. In view of the involvement of immune system in cancer, we also review the role of circRNAs in immune regulation and antitumour immunotherapy.
2. CIRCULAR RNA IN CANCER DEVELOPMENT
As a complex pathological process, cancer development contains many hallmarks, such as genome instability and mutation, reprogramming energy metabolism, sustaining proliferative signalling, enabling replicative immortality, evading growth suppressors, resisting cell death, activating invasion and metastasis, inducing angiogenesis, promoting tumour inflammation and evading immune destruction. Findings suggest that circRNAs are involved in these aspects of cancer development 12 , 22 , 23 , 24 , 25 , 26 (Figure 2).
Figure 2.
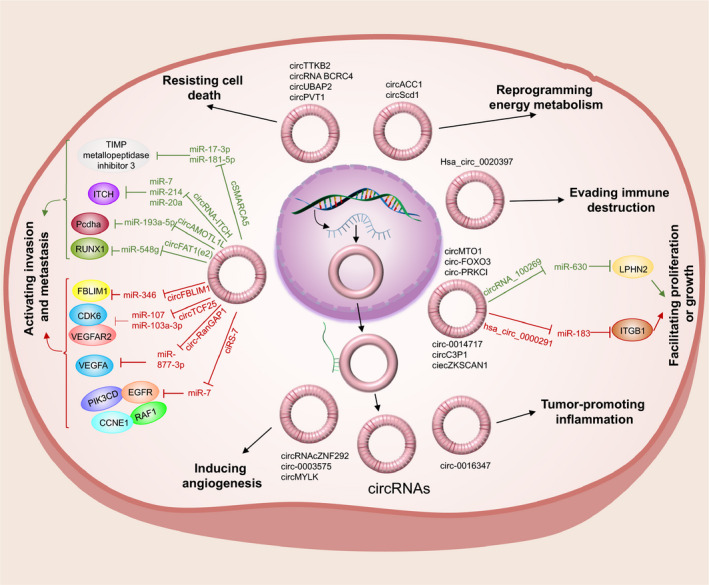
Illustration of Circular RNA in cancer. CircRNAs have been indicated (mainly in the cytoplasm) to contribute to various aspects of cancer progression. For example, some circRNAs may be involved in proliferation, growth, invasion, and metastasis. Tumour suppressor circRNAs are indicated by green lines and tumour‐ promotor circRNAs are shown by red lines
2.1. Tissue specificity
Research into the relationship between circRNAs and cancer found that circRNAs are often dysregulated in cancers which allows tumour tissue to be distinguished from adjacent normal tissue. 20 , 27 Studies have identified multiple functional cancer‐associated circRNAs, with differential expression, in different cancers. These circRNAs act as tumour suppressors or promotors and impact cancer phenotypes in diverse ways. For example, ciRS‐7, acting as an oncogene, was found to be up‐regulated in colorectal cancer (CRC) tissues, whereas overexpression of ciRS‐7 in vitro could induce a malignant phenotype. 14 Conversely, circ‐ITCH, as a sponge of oncogenic miR‐7 and miR‐214, was reported down‐regulated in lung cancer. Also, circ‐ITCH overexpression could suppress lung cancer cell proliferation. 28 In addition, circRNAs are ubiquitous across different species, tissues and cell types, and have a tissue‐ and developmental stage‐specific expression pattern, 21 , 29 which may depend on the specific function of circRNA itself in its context. Xia et al 21 first performed a global view of tissue‐specific (TS) circRNAs. They determined the TS circRNAs in human and mouse, identified their genomic location, conservation and binding of miRNAs or proteins to understand their function. Finally, they established an integrated database “TSCD” (Tissue‐Specific CircRNA Database: http://gb.whu.edu.cn/TSCD) as a better platform for characterizing features of TS circRNAs.
2.2. Invasion and metastasis
Invasion and metastasis play central roles in the progression of cancer. Indeed, a variety of circRNAs contribute to this process. 28 , 30 , 31 , 32 , 33 , 34 Zhong et al 27 investigated the up‐regulated circTCF25 in bladder cancer (BC), and they found that circTCF25 overexpression could promote proliferation and migration both in vitro and in vivo through sequestering miR‐103a‐3p and miR‐107, and thus increasing CDK6 expression. Lu et al 35 studied the up‐regulation of circ‐RanGAP1 in gastric cancer (GC). The loss‐ or gain‐of‐function experiments indicated that circ‐RanGAP1 was closely related to GC cell invasion and migration by sponging miR‐877‐3p to up‐regulate vascular endothelial growth factor A(VEGFA) expression. Furthermore, they found more circ‐RanGAP1 in plasma exosomes, and these exosomes with cargo could enhance GC cell migration and invasion capabilities, which suggested that exosomes can maintain the properties and original functions of circRNA, even transporting it as vectors. As mentioned above, the new circular RNA FECR1was identified when Chen et al 10 explored the mechanism of Friend leukaemia virus integration 1 (FLI1), which was up‐regulated and acted as a tumour metastasis driver in breast cancer. They found circFECR1 in the FLI1 promoter chromatin complex, and this circular RNA could enhance metastasis in breast cancer by epigenetic mechanisms rather than canonical oncoprotein pathway. As shown in Figure 2, a variety of circRNAs contribute to metastasis and invasion processing through diverse mechanisms via interacting with different molecules.
2.3. Angiogenesis
Tumour progression usually requires formation of new blood vessels to supply nutrients and dispose of waste. CircRNAs are implicated in diverse tumorigeneses including angiogenesis. Zhong et al 36 reported the up‐regulation of circRNA‐MYLK in BC and found that it could function as sponge of miR‐29a and activate target VEGFA. Ectopically overexpressing circRNA‐MYLK could facilitate cell proliferation, migration and tube formation of human umbilical vein endothelial cells (HUVEC) in vitro and promote tumour growth, metastasis and angiogenesis of BC xenografts in vivo. Li et al 37 discovered that the up‐regulation of Hsa_circ_0003575 in impaired vascular endothelial cells was caused by oxidized‐low density lipoprotein (oxLDL), whereas Hsa_circ_0003575 silencing could enhance proliferation and angiogenesis capacity of HUVES. Moreover, hypoxia is believed to be a notable stimulus for angiogenesis 38 ; thus some studies focused on the influence of hypoxia on endothelial cells and circRNAs in hypoxic cancer cells. Boeckel et al 23 identified several circRNAs in hypoxic human endothelial cells, and circZNF292 especially exhibited proangiogenic activities in vitro, whereas circZNF292 silencing led to reduced tube formation and spheroid sprouting of endothelial cells. These are also consistent with the finding that tumour cells and vascular endothelial cells can, respectively, secrete relevant factors to promote each other's growth. 39
2.4. Genome mutation
In general, accumulation of mutations accompanies the vast major of cancers. With an increase in studies focused on circRNAs in cancer, some circRNAs have been found to be associated with these mutations and post‐translational modifications. Mut‐p53 is known as the most common mutation in human cancers. Verduci et al 40 showed that, as an oncogene, circPVT1 was up‐regulated in head and neck squamous cell carcinoma (HNSCC) and regulated by YAP through forming the YAP/circPVT1 complex. However, this complex requires mut‐p53 to function as the stabilizer. In addition, genetic alterations can also directly impact non‐coding RNAs including producing aberrant circRNAs. When the back‐splicing of circRNAs is upon chromosomal translocation, it will result in generation of fusion circRNAs (f‐circRNA). Guarerio et al and Tan et al, 41 , 42 respectively, found F‐circM9, F‐circPR and F‐circEA/F‐circEA‐2 in leukaemia and solid cancer. Additionally, functional analysis showed that they also contributed to oncogenesis. Recently, Liu et al 43 systematically characterized circRNAs in the human dorsolateral prefrontal cortex and analysed circRNA expression variation by detecting partial quantitative trait loci (circQTLs) SNPs. Some circQTL SNPs exhibited an effect on circRNA formation by changing the canonical splicing site and further phenotypic changes. A similar approach is proposed to apply in cancer to identify whether these circQTL SNPs are involved in diverse tumorigenesis. In brief, some circRNAs may be involved in cancer genome mutations either through interacting with the mutant products, or via genetic alteration itself.
3. CIRCULAR RNA IN CANCER IMMUNE REGULATION
The immune system is responsible for defending the host from exogenous invasion and maintaining internal homeostasis. The emerging research on circRNAs has suggested that they are involved in the modulation of different immunocytes and diverse immune responses. For example, circular RNA100783 functions in CD8(+) T cell ageing and immunosenescence, and circRNA‐003780 and circRNA‐010056 function in macrophage differentiation and polarization. 44 , 45 A gene ontology analysis for 422 circRNAs identified from healthy human saliva categorized them into several groups, including establishment of T cell polarity, chemotaxis, inflammatory response and the integrin‐mediated signalling pathway, which indicated that these circRNAs may participate in intercellular signalling and immune responses. 22 However, inflammation can play vital roles during all stages of tumour development, especially in early tumour initiation as well as tumour promotion, tumour progression and metastasis. Immunosuppressive cells, inflammatory cytokines and signalling, chemokines and inhibitory receptors all contribute to tumour‐promoting inflammation. 46
3.1. Checkpoint
Immune checkpoints, such as PD‐1 and CTLA‐4 (inhibitory receptors), account for tumour immune escape. The rising hotspot of checkpoint blockade therapy and the emerging role of circRNAs has attracted studies seeking to elucidate the relationship between them. For instance, hsa_circ_0020397 was found to be up‐regulated in CRC and could mediate colorectal cancer cell viability and invasion by inhibiting miR‐138 and subsequently enhancing telomerase reverse transcriptase (TERT) and PD‐L1. 26 In another case, circ‐UBAP2 was found to be differentially expressed in pancreatic adenocarcinoma (PAAD) and could modulate the expression of CXCR4 and ZEB1, which are linked with M2 macrophages, exhausted T cells and T‐regulatory cells (Tregs), and are positively correlated with PD‐1 and CTLA‐4. 47 This relationship provides another perspective to apply PD‐1 in immunotherapy.
3.2. Chemokine
Chemokines are a type of cytokine that can recruit leucocytes and other types of cells. Evidence shows that some chemokines and chemokine receptors are frequently involved in cancer, even as tumour promoters. In one experiment, two human CRC cell lines were co‐stimulated by chemokines CCL20 and CXCL8, which resulted in CRC cell migration, invasion and the epithelial‐mesenchymal transition (EMT) process, accompanied by down‐regulation of circ_0026344. 48 In another case, Zhang et al 49 found that circFGFR1 could positively regulate CXCR4 expression in NSCLC cells. Knockout and enhancement experiments suggested that controlling the CXCR4‐related pathway in NSCLC cells might facilitate anti‐PD‐1 immunotherapy. Zhang et al 50 explored the relationship between circ_0067934 and CXCR1 in thyroid cancer (TC). The results indicated that circ_0067934 down‐regulation expedited cell apoptosis and repressed cell proliferation, migration and invasion by inhibiting miR‐1304 and modulating CXCR1 expression.
3.3. Inflammatory cytokines
Cytokines in tumour development are typically derived from cancer cells or immunosuppressive cells. In the process of tumour inflammatory microenvironment formation, caspase‐1 contributes to the activation of proinflammatory cytokines IL‐1β and IL‐18, which induce the key downstream inflammatory response. 51 Jin et al 52 reported that circ‐0016347 served as a tumour promoter in osteosarcoma cells by sponging miR‐214 and enhancing the downstream target caspase‐1, followed by enhancing the inflammation‐associated mechanism. In another case, Liu et al 53 found that overexpressed hsa_circRNA_002178 could sponge miR‐328‐3p and thus up‐regulate COL1A1, which resulted in progression of breast cancer, whereas hsa_circRNA_002178 silencing could inhibit tumour inflammation by decreasing the levels of IL‐6 and TNF‐α, preventing tumour growth.
3.4. Anti‐viral
A number of viruses have been proven to generate circRNAs after infecting the host, and some oncogenic viruses can encode circRNAs, which may contribute to oncogenesis, such as circEBNA_W1_C1 derived from Epstein‐Barr virus (EBV) and circE7 derived from human papillomavirus (HPV). 54 , 55 Under normal conditions, innate immunity provides the first defence against viral infection. Some dsRNA‐binding proteins, such as retinoic acid‐inducible gene‐I (RIG‐I), can recognize specific viral RNAs by their 5ʹ‐ppp and double‐stranded structure, and can also identify the exogenous circRNAs as nonself circRNAs. Additionally, the endogenous or host circRNAs are combined with RBPs, such as NF90/110, during biosynthesis in the nucleus, which enables self‐circRNAs to evade recognition and be distinguished from exogenous circRNAs. Takanobu et al identified differentially expressed host circRNAs in cells infected by Kaposi's sarcoma herpesvirus (KSHV), and they found that hsa_circ_0001400 activated by infection could inhibit expression of key viral genes LANA and RTA, and thus facilitate anti‐viral immune response. 56 Li et al revealed that the host circ‐RNP may resist viral infection, whereas the viral infection could suppress its biogenesis through export of NF90/NF110 from the nucleus. There may be a competitive relationship between host circRNAs and viral infection. 57 , 58 Furthermore, based on host‐virus interaction, Ghosal et al 59 constructed an integrated database, “HumanViCe” (http://gyanxet‐beta.com/humanvice), which could support ceRNA networks in virus‐infected cells, including those circRNAs enriched in pathways related to host immune responses and viral entry and replication.
4. CIRCULAR RNA AS A CANCER BIOMARKER
Considering the harmful effects of cancer, finding new and effective biomarkers to utilize in diagnostics, prognosis and/or to predict treatment response is vitally important. As described above, circRNAs are closely linked with cancer onset and progression, and there are differential expressions between tumour tissues and adjacent normal tissues. Furthermore, circRNAs have distinct properties of abundance, stability and tissue‐specific expression. More importantly, circRNAs can be secreted in human body fluids, such as saliva, urine, blood and cerebrospinal fluids, and are enriched in exosomes. Taken together, these characteristics indicate the advantages of circRNAs as biomarkers for detection and surveillance in cancer, which may be more accurate, convenient and noninvasive. 3 , 4 , 60
4.1. Diagnostics
A number of tumour‐associated circRNAs have been researched to examine their diagnostic value in cancer. For example, circPVT1 was found significantly up‐regulated in osteosarcoma (OS) tissues, serum and chemo‐resistant cell lines. Also, the receiver operating characteristic (ROC) curve illustrated that it had advanced diagnostic value with greater specificity and sensitivity than alkaline phosphatase (ALP) in OS. 61 Li et al 62 systematically performed a literature search, which demonstrated that plasma circRNAs have higher diagnostic accuracy than tissue and that combined circRNAs have better diagnostic efficacy than single circRNAs. The utility of circRNAs for diagnostic procedures is currently being evaluated in a clinical trial (NCT03334708), which compares the diagnostic value of a panel of circRNAs and other molecules(proteins and proteases, circulating tumour DNA, exosomes, functional DNA repair assays, stromal elements) for early‐stage pancreatic cancer. 63
4.2. Prognosis
Many groups have also investigated the prognostic value of circRNAs in cancer. Okholm et al 64 identified 113 differentially expressed circRNAs between low‐ and high‐risk groups of patients with non‐muscle‐invasive bladder cancer (NMIBC). They also discovered 13 circRNAs that independently correlated with BC progression, especially circCDYL and circHIPK3, which exhibited high prognostic value for early‐stage BC. Exosomes can be perfect carriers for circRNAs and exo‐circRNAs derived from tumours and can be secreted into human body fluids, ultimately impacting cancer development. 8 Li et al 65 found that exo‐circ‐PDE8A derived from the blood of patients with pancreatic cancer was significantly up‐regulated, and these blood exosomes extracted from pancreatic ductal adenocarcinoma (PDAC) patients exhibited high correlation with duodenal invasion, vascular invasion, TNM stage, and finally, survival expectancy. In this way, exo‐circPDE8A may be a superior early diagnostic and prognostic biomarker for PDAC.
In addition, circRNAs also exhibit certain potential in predicting treatment response. Recently, Liu et al 66 identified 1377 differentially expressed circRNAs between gefitinib‐effective and ineffective groups of non‐small‐cell lung carcinoma (NSCLC) patients. Among them, up‐regulated hsa_circ_0109320 was clearly correlated with longer progression‐free survival in gefitinib‐treated NSCLC patients. This provides a new approach to guide the clinical use of gefitinib. Moreover, studies into circRNAs and drug resistance also have indicated that circRNAs are implicated in the process of drug resistance in cancers by mediating diverse regulatory pathways and processes. For example, circPVT1 exhibits up‐regulation in patients with gastric cancer and cisplatin resistance during treatment, representing its potential as a biomarker related to cisplatin resistance. Circ_0081001 presents with gradual up‐regulation in patients with OS during treatment with cisplatin, indicating its potential in dynamic monitoring. 67 The sensitivity and specificity of circRNAs for monitoring drug resistance should be taken into account.
5. CIRCULAR RNA IN CANCER THERAPY
Exploring novel antitumour methods is urgent due to the harm and severity of cancer and the limitations of traditional treatment methods. CircRNAs are widely involved in tumorigenesis, making them an attractive target in the field of cancer therapy.
5.1. Gene therapy
There have been multiple circRNAs identified as tumour promotors or suppressors, which influence cancer phenotypes in different ways, especially angiogenesis, proliferation or growth, invasion and metastasis. Exogenous up‐regulation or down‐regulation of relevant circRNAs, by changing their gene expression, is common in studying their potential in ameliorating harmful phenotypes. In contrast, plasmid or lentiviral vectors are often used to increase circRNAs levels. For example, circ‐Foxo3 was reported to be down‐regulated in bladder cancer, whereas overexpression of circ‐Foxo3 by the above vectors could induce bladder cancer apoptosis through directly inhibiting miR‐191. 68 Similarly, as a translation template of FBXW7‐185aa, the circ‐FBXW7 level was found to be reduced in glioblastoma, whereas overexpression of FBXW7‐185aa could help inhibit cancer cell proliferation. 16 However, RNA‐mediated interference (RNAi) and antisense oligonucleotides (ASO) are used to decrease circRNA levels, and the CRISPR/Cas9 system can even directly knockout the specific circRNAs. Cao et al 69 reported the elevation of hsa_circ_0000291 in GC cell lines and found that silencing hsa_circ_0000291 could target the miR‐183/ITGB1 axis, resulting in inhibition of GC cell proliferation and metastasis. Yang et al 70 identified a novel function of circ‐Amotl1, which facilitates c‐myc nuclear translocation through binding protein and regulates its gene transcription. Silencing circ‐Amotl1 could decrease cancer cell proliferation and increase cell apoptosis. Additionally, based on the knowledge of m6A modification in the translation of circZNF609, Hung et al recently found that m6A‐generating enzyme METTL3 knockout or deleting one Alu element to completely abolish circRNA splicing did not influence translation or detectable protein products, suggesting the existence of a METTL3‐independent initiation mechanism. Alternatively, other m6A‐generating enzymes could exist, and circRNA overexpression constructs may undergo trans‐splicing. Therefore, such tools need to be evaluated carefully, and further research may help us better apply gene therapy with this strategy. 83
5.2. Therapeutics vectors
In addition to being targets in gene therapy, circRNAs can also be used as promising therapeutic vectors due to their unique stability and capacity for binding miRNA or proteins. They can be designed with miRNAs and/or proteins binding sites, which might be a simple, effective and convenient treatment strategy. Liu et al 71 were the first to successfully synthesize a circular RNA, scRNA21, which had the specific binding site of miR‐21. After transfection of scRNA21, three types of GC cells all increased apoptosis. In this way, synthetic circRNA targeting multiple miRNAs, proteins or the combination of the two may impair multiple oncogenic pathways. Thus, their group furtherly constructed a novel circRNA sponging both miR‐21 and miR‐93, which led to significant inhibition of cancer cell proliferation and migration in vitro and remarkable suppression of tumour growth in vivo. 72 Here, synthetic circRNA can be delivered by tumour cell exosomes as an agent, which can improve their targeting efficiency and increase their delivering amount. Furthermore, as transcription of circRNAs commences at Pol II promoters, another solution worth considering is designs with cell‐specific promoters or disease‐activated control elements, which may make the circRNAs optionally expressed in certain cells. Moreover, as some circRNAs can act as coding templates, it is expected that applying their expression cassettes in tumour suppressor proteins could be another approach to assist antitumour efforts.
5.3. Immuno‐oncology
Although both the innate and adaptive immune system function in antitumour response, tumours can escape from immune attack by lowing the exposure of their antigens and reducing infiltration and function of effector T cells and dendritic cells (DCs). Despite recent achievements of diverse antitumour immunotherapy, including checkpoint blockades, cellular therapies, cytokines and tumour vaccines, there are still a variety of issues to be resolved, partially due to tumour heterogeneity and side effects. 73 The emerging functions of circRNAs in tumour development and immune regulation give them great potential in antitumour immunotherapy.
To investigate influence of circRNAs on natural killer (NK) cells, Ma et al 74 used a specific plasmid for CircARSP91 overexpression and analysed the response of hepatocellular carcinoma (HCC) cells to NK cell cytotoxicity. They identified the up‐regulated UL16 binding protein 1 (ULBP1), which could impact NK cell activation. Ultimately, they concluded that CircARSP91 can strengthen NK cell antitumour cytotoxicity by up‐regulating ULBP1.
As described above, Zhang et al 26 reported that the overexpression of hsa_circ_0020397 in CRC cells could promote expression of TERT and PD‐L1, which contribute to immunocyte exhaustion and tumour escape from immune responses by sponging miR‐138. This provides us with new insights on “checkpoint therapy” in cancer patients, where alteration of specific circRNA expression may improve the effect of checkpoint therapy.
Due to the distinct properties of stability and specificity, circRNAs in different tumour tissues may act as tumour antigens for immune response. Moreover, circRNAs may carry tumour‐specific miRNAs or mRNAs as novel tumour antigens transported from the tumour cells to the recipient cells through exosomes and extracellular vesicles. 41 , 60 , 75 As coding template, proteins or peptides translated from abnormal circRNAs may also act as tumour antigens. 19 , 58 As is known, for cancer vaccine therapy or CAR T cell adoptive therapy, it is more attractive to explore novel and specific antigens to improve the treatment outcome. In this way, this new potential tumour antigen may be applied in cancer vaccine therapy or CAR T cell adoptive therapy.
Because research on miRNAs in antitumour immunity is clearer than that on circRNAs, the close relationship between circRNAs and miRNAs can help research into the role of circRNAs in antitumour immunity. Using circRNA databases (starBase v2.0 and circBasecan), we can predict a potential circRNA regulating tumour immunity‐associated miRNAs and identify potential new tumour antigens. 76 , 77 In addition to the circRNA‐miRNA network, some circRNAs indirectly contribute to antitumour immune responses by affecting some proteins. For example, Du et al 12 found that endogenous circ‐Foxo3 could elicit the degradation of p53, modulating immune response, by formation of a complex with MDM2. Silencing of circ‐Foxo3 could increase cell viability, whereas overexpression of circ‐Foxo3 could induce apoptosis and suppress tumour growth. These circRNAs identified in tumour immunity can be potential targets for antitumour therapy.
Taken together, this evidence exhibits the potential of circRNAs in regulation of antitumour immune responses and even as therapeutic targets. However, the underlying mechanisms of circRNAs in this field remain largely unknown and need to be further clarified.
Additionally, with increased recognition of circRNAs in antitumour‐drug resistance, targeting specific circRNA may ameliorate the drug resistance during antitumour treatment. As mentioned above, up‐regulated circPVT1 in gastric cancer is associated with multidrug resistance, such as resistance to cisplatin, paclitaxel, and oxaliplatin; thus, targeting circPVT1 may have the potential to treat drug resistance in gastric cancer.
6. CHALLENGES IN CANCER THERAPY
Using circRNAs as novel therapeutic targets will undoubtedly expand the field of potential “druggable” targets. However, despite the rapid growth of related research in recent years, knowledge of circRNAs is not sufficient compared to that of miRNAs and mRNA. Therefore, prior to clinical application, we should investigate the issues and challenges that currently exist in antitumour therapy.
6.1. Potential targets
Simply, discovery of a novel circRNA in one type of cancer cell or tissue and experimentally finding that it acts as an oncogene or tumour suppressor does not rule out the possibility that the circRNA may be involved in other vital physiological processes or has other targets that have yet to be identified. In addition, one circRNA may target multiple miRNAs in one cancer; for example, circ‐ITCH can sponge both miR‐7 and miR‐214 to inhibit the Wnt/β‐catenin pathway and repress proliferation of lung cancer cells. 28 Additionally, a specific circRNA may be down‐regulated in different tumours, which implies that its dysregulation cannot be uniquely related to a specific disease. 12 , 68 Furthermore, the same circRNA may exert opposite functions by targeting different miRNAs in different cancers. CircHIPK3 can target miR‐558 to down‐regulate HPSE and subsequently inhibit angiogenesis, migration and invasion of BC cells, but also can target miR‐7 to promote CRC growth and metastasis. 78 , 79 Thus, it is important to study the circRNA spectra as much as possible before focusing on single molecules related to a certain disease and to carry out enough trials to minimize the harm induced by other potential targets.
6.2. Specificity
Specificity is one of the most important matters to consider when drugs or therapeutic targets are applied clinically so as to avoid possible off‐target effects and assure safety and efficacy. First, targeting tumour‐associated circRNAs should preferably be exerted without disturbing expression of other RNAs, especially the corresponding linear mRNA. RNAi and ASOs are currently the two main approaches used to target RNAs, and minimizing the toxicity of off‐target is a major concern. 80 Considering the structure and biogenesis of circRNAs, RNAi or ASOs should be designed to be perfectly complementary to either the unique back‐splice junction site or the back‐splice signals in the pre‐mRNA, such as binding sites for trans‐acting splicing factors or flanking intronic Alu repeats. 4 Second, as mentioned above, circRNA has tissue‐, cell‐ and developmental stage‐specific expression patterns, which should be taken into account when targeting circRNAs in antitumour therapy. Recently, Lasse et al expanded the knowledge about spatially resolved cellular expression patterns of ciRS‐7 in colon cancer. 81 They were the first to identify not only the complete absence of ciRS‐7 in cancer cells, but also high expression in stromal cells within the tumour microenvironment, which provides guidance when selecting a target cell when targeting ciRS‐7 to treat colon cancer.
6.3. Gene therapy
In gene therapy, overexpression of circRNA is common, and plasmid or lentiviral vectors are frequently used. 82 During circRNA formation, inverted repeats, especially Alu repeats, are one of the most important factors to support successful circularization. 4 Thus, the cloned sequences contain the interesting exon(s) and splice sites flanked either by cognate Alu repeats or artificial long inverted repeats. The key point is to avoid the introduction of foreign circRNA, such as synthetic or exogenous circRNAs, which can stimulate RIG‐I signalling as nonself‐molecules and elicit immune responses. Unlike the endogenous circRNAs, exogenous circRNAs possess few RNA associated proteins. Moreover, the intracellular delivery of exogenous circRNA may induce a fast up‐regulation of various inflammation‐associated genes. In this sense, the mechanism for distinguishing between self and nonself circular RNAs should be explored by further research. 57 Alternatively, finding a proper delivery system may be taken into consideration as an optional strategy.
6.4. Delivery
How to safely deliver engineered circRNAs in vivo is an important question. As mentioned above, the abundance and stability of circRNAs in exosomes has been identified. Exosomes have a lipid bilayer structure, which can protect RNAs against degradation and ensure their effective concentration. 8 Furthermore, the small size and membrane structure of exosomes facilitate their absorption and fusion by cancer cells. Transporting circRNAs via exosomes can support intercellular communication in cancer. 19 For therapy purposes, modified exosomes may be an effective approach, because they contain engineered circRNAs or siRNAs targeting circRNA. 83 However, even if cancer‐derived exosomes have higher affinity with malignant cells, the possibility of targeting healthy ones still cannot be excluded. In recent times, nanoparticles, as a promising tool to deliver nucleic acids into cells, have emerged for application in therapy. Du et al 12 reported the application of gold nanoparticles to deliver circ‐Foxo3 in mice to inhibit tumour progression. However, this approach can only be applied to exonic circular RNAs, because nanoparticles have no ability to enter the nucleus. A recent study of combination of nanoparticles and microfluidics may provide an exciting strategy to increase nuclear entry for resolving this limitation. 84 Hence, it is expected that circular RNAs will become a new therapy target or tool to treat diverse cancers as the field continues to grow and delivery problems are resolved.
6.5. Current progress
Although various studies have indicated the promising potential of circRNAs in cancer therapy (Table 1), such as in gene therapy, immunotherapy and a combination of the two, there are still many drawbacks to overcome. Thus far, there have been no preclinical reports in which circRNAs are applied alone as therapeutic targets or vectors in cancer, but we believe the ongoing research will change this in the future. With the development of new technologies, more circRNAs will be discovered as diagnosis‐ and prognosis‐related markers, and these can be used to guide clinical medication selection. Therapeutic strategies that depend on circRNAs will be formulated, thereby providing new perspectives and directions for cancer therapy. Each experimental report will bring us closer to a patient‐tailored approach to achieve maximum anticancer effects with minimal side effects. Additional work with larger sample sets and long‐term follow‐up clinical information is needed for further validation.
Table 1.
Current Circular RNA in cancer therapy
| Tumour type | CircRNA | Function | Mechanism/Experiments | Ref. |
|---|---|---|---|---|
| Down‐regulation | ||||
| Hepatocellular carcinoma | circARSP91 | Interaction with protein | circARSP91/UL16 binding protein1. Overexpression of circARSP91 could enhance the cytotoxicity of natural killer cells against hepatocellular carcinoma | 74 |
| cSMARCA5 | MiRNA sponge | cSMARCA5/ miR‐17‐3p and miR‐181‐5p/ TIMP metallopeptidase inhibitor 3. Overexpression of cSMARCA5 suppresses the proliferation and migration of HCC cells | 33 | |
| hsa_circ_0079299 | Interaction with protein | Inhibiting cell proliferation through PI3K/AKT/mTOR signalling pathway. Over‐Overexpression of hsa_circ_0079299 suppressed tumour growth in vitro and in vivo, retarded cell cycle progression while had no effect on cell migration and apoptosis | 85 | |
| Bladder cancer | circ_ZKSCAN1 | MiRNA sponge | circ‐ZKSCAN1/miR‐1178‐3p/p21. Overexpressed circ‐ZKSCAN1 inhibited cell proliferation, migration, invasion and metastasis in vitro and in vivo | 86 |
| circHIPK3(BCRC‐2) | MiRNA sponge | circHIPK3/ miR‐558/heparinase. Overexpression of circHIPK3 effectively inhibited migration, invasion, and angiogenesis of bladder cancer cells in vitro and suppresses bladder cancer growth and metastasis in vivo. | 78 | |
| circ‐Foxo3 | MiRNA sponge | circ‐Foxo3/miR‐191‐5p. Overexpression of circ‐Foxo3 promoted bladder cancer cell apoptosis in BBN mice and in human bladder cancer cell lines | 68 | |
| Breast cancer | circ‐Foxo3 | Interaction with protein | the complex of circ‐Foxo3 and MDM2 induces the degradation of p53, modulating immune responses during tumorigenesis. Ectopic expression of circ‐Foxo3 triggered stress‐induced apoptosis and inhibited the growth of tumour xenograft. | 12 |
| Gastric cancer | circ_100269 | MiRNA sponge | CircRNA_100269/miR‐630/LPHN2. Overexpression suppresses tumour cell growth. | 87 |
| hsa_circ_0000096 | Interaction with protein | modulating cyclin D1, CDK6, matrix metalloproteinase 2 (MMP‐2), and MMP‐9. Knockdown of hsa_circ_0000096 significantly inhibited cell proliferation and migration in vitro and in vivo | 88 | |
| circFAT1(e2) | MiRNA sponge in cytoplasm and interaction with protein in nucleus | circFAT1(e2)/miR‐548g/RUNX1 in the cytoplasm and targeting YBX1 in the nucleus. Overexpression of circFAT1(e2) inhibiting proliferation, migration and invasion of gastric cancer cells | 31 | |
| circMRPS35 | Interaction with protein even directly mRNA regulator | circMRPS35/KAT7/FOXO1/3a. recruit KAT7 to and directly bind to FOXO1/3a promoter region. circMRPS35 overexpression suppressed the proliferation and invasion of gastric cancer cells in vitro and in vivo | 89 | |
| Kidney cancer cell | circC3P1 | MiRNA sponge | circC3P1/ miR‐21/PTEN and inactivating PI3K/AKT and NF‐κB signalling pathways. CircC3P1 overexpression declined cell viability, migration, and invasion and caused apoptosis | 90 |
| Lung cancer | circPTK2 | MiRNA sponge | circPTK2/miR‐429, miR‐200b‐3p/TIF1γ.CircPTK2 overexpression inhibited TGFβ‐induced EMT | 91 |
| circNOL10 | Interaction with protein | circNOL10 inhibits lung cancer development by promoting SCLM1‐mediated transcriptional regulation of the humanin polypeptide family in vitro and in vivo | 92 | |
| circRNA‐ITCH | MiRNA sponge | circRNA‐ITCH/ miR‐7, miR‐17 and miR‐214/ ITCH, and suppress the activation of Wnt/‐catenin signalling. Overexpressed circRNA‐ITCH suppress lung cancer cell proliferation | 28 | |
| Colorectal cancer | hsa_circ_0014717 | MiRNA sponge | hsa_circ_0014717/p16. Hsa_circ_0014717 overexpression could significantly suppress CRC cell proliferation and colony formation, as well as induce cell cycle G0/G1 phase arrest in vitro and inhibit xenograft tumour growth in vivo | 93 |
| Glioblastoma | circ‐FBXW7 | Translation | Translation template of FBXW7‐185aa. Up‐regulation of FBXW7‐185aa can help to inhibit proliferation and cell cycle acceleration | 16 |
| Prostate cancer | circAMOTL1L | MiRNA sponge | circAMOTL1L/miR‐193a‐5p/Pcdha.CircAMOTL1L overexpression inhibited progression and invasion of prostate cancer cells | 32 |
| Rectal cancer | circMTO1 | MiRNA sponge | circMTO1/miR‐19b‐3p/JAK1/STAT3 and AMPK signal pathways. CircMTO1 overexpression could suppress cell proliferation, migration, and incursion and induce apoptosis | 94 |
| CDR1as (or CiRS‐7) | MiRNA sponge | CDR1as/miR‐1270/SCAI signalling pathway. Overexpression of CDR1as sensitized ovarian cancer to cisplatin, inhibited cell proliferation and promoted the cisplatin‐induced cell apoptosis in ovarian cancer cells | 95 | |
| CDR1as (or CiRS‐7) | MiRNA sponge | CDR1as/miR‐135b‐5p/HIF1AN.CDR1as overexpression inhibited the proliferation, invasion and migration of ovarian cancer cells | 96 | |
| Up‐regulation | ||||
| Pancreatic cancer | hsa_circ_0020397 | MiRNA sponge | hsa_circ_0020397/miR138/TERT and PD‐L1, contributes to immunocytes exhaustion and tumour escape from immune responses, new insight for "checkpoint therapy" | 26 |
| Breast cancer | hsa_circRNA_002178 | MiRNA sponge | hsa_circRNA_002178/miR‐328‐3p/COL1A1.Hsa_circRNA_002178 silencing inhibited inflammation in vivo through reducing TNF‐α and IL‐6 levels and prevented tumour growth | 53 |
| circ‐AMOTL1 | Interaction with protein | Facilitate c‐myc nuclear translocation and regulate its gene transcription. Silencing circ‐Amotl1 decreased cancer cell proliferation but increased cell apoptosis | 70 | |
| Cervical cancer | circAMOTL1 | MiRNA sponge | circAMOTL1/miR‐485‐5p/AMOTL1. Gain‐ or loss‐of‐function assays and in vivo experiments demonstrated that AMOTL1 promoted cervical cancer cell growth both in vitro and in vivo | 97 |
| Colorectal cancer | circPTK2 | Interaction with protein | circPTK2 binding to protein vimentin. Silencing circPTK2 blunt tumour metastasis in a patient‐derived CRC xenograft model | 98 |
| circHIPK3 | MiRNA sponge | circHIPK3/miR‐7/c‐Myb. Knockdown of circHIPK3 markedly inhibited CRC cells proliferation, migration, invasion, and induced apoptosis in vitro and suppressed CRC growth and metastasis in vivo | 79 | |
| Colorectal cancer, osteosarcoma, hepatocellular carcinoma | CDR1as (or CiRS‐7) | MiRNA sponge | CDR1as (or CiRS‐7)/ miR‐7/EGFR, CCNE1, PI3KCD, and RAF1, promotion of tumorigenesis and invasion. CDR1as inhibition in vivo also induced tumour regression | 99 |
| Non‐small‐cell lung carcinoma | CDR1as (or CiRS‐7) | MiRNA sponge | CDR1as/miR‐219a‐5p/SOX5. Knockdown of circCDR1as inhibited the progression of NSCLC by decreasing cell viability, migration and invasion and increasing apoptosis | 100 |
| Gastric cancer | CDR1as (or CiRS‐7) | MiRNA sponge | CDR1as/miR‐7‐5p/REGγ. Knock‐down of CircRNA CDR1as specifically promoted the cytotoxic effects of low‐dose DB on GC cells instead of hepatocytes. | 101 |
| hsa_circ_0000291 | MiRNA sponge | hsa_circ_0000291/miR‐183/ITGB1. Silencing hsa_circ_0000291 suppressed GC cell metastasis and proliferation both in vivo and in vitro | 69 | |
| circ‐RanGAP1 | MiRNA sponge | circ‐RanGAP1/miR‐877‐3P/VEGFA. Inhibition of circ‐RanGAP1 decreased GC cell invasion and migration in vitro | 35 | |
| circ_PVT1 | MiRNA sponge | circ‐PVT1/miR‐124‐3p/ ZEB1. Circ‐PVT1 knockdown increased PTX sensitivity of GC in vivo | 102 | |
| circ_PVT1 | MiRNA sponge | circPVT1/miR‐199a‐5p/YAP1 and PI3K/AKT pathways. Silencing hsa_circ_PVT1 (circPVT1) suppressed the growth and metastasis of glioblastoma multiforme cells | 103 | |
| Osteosarcoma | circ_PVT1 | Knockdown of circPVT1 can weaken the resistance to doxorubicin and cisplatin of OS cells through decreasing the expression of ABCB1 | 61 | |
| Gliomas | circ‐TTBK2 | MiRNA sponge | circ‐TTBK2/miR‐217/ HNF1β/Derlin‐1. Knockdown of circ‐TTBK2 combined with miR‐217 overexpression can suppress tumorigenesis in vivo | 104 |
Reported circRNAs that have been identified to have tumour therapeutic potential in in vitro or in vivo experiments via different strategies, mainly gene therapy and immunotherapy.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Weizhen Li: Writing‐original draft (lead); Writing‐review & editing (lead). Jia‐Qiang Liu: Formal analysis (equal); Project administration (equal); Resources (supporting); Writing‐review & editing (equal). Ming Chen: Funding acquisition (equal); Project administration (equal); Resources (equal). Jiang Xu: Funding acquisition (equal); Project administration (equal); Resources (equal); Writing‐review & editing (equal). Di Zhu: Conceptualization (lead); Formal analysis (lead); Funding acquisition (equal); Project administration (lead); Resources (equal); Supervision (equal); Writing‐original draft (lead); Writing‐review & editing (lead).
Li W, Liu J‐Q, Chen M, Xu J, Zhu D. Circular RNA in cancer development and immune regulation. J Cell Mol Med.2022;26:1785–1798. 10.1111/jcmm.16102
Weizhen Li and Jia‐Qiang Liu contributed equally to this work.
Funding information
The current study was supported by projects on the Science and Technology Commission of Shanghai (18ZR1403900) (D. Zhu), the National Natural Science Foundation of China (81872895) (D.Z.), and Pudong Hospital ‐ Fudan University School of Pharmacy Joint Fund (RHJJ2018‐03) (D. Z. and T. Y.)
Contributor Information
Jiang Xu, Email: zhudi@fudan.edu.cn, Email: haeyxj@126.com.
Di Zhu, Email: zhudi@fudan.edu.cn.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available in the figshare repository.
REFERENCES
- 1. Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis‐splicing yields circular RNA molecules. FASEB J. 1993;7(1):155‐160. [DOI] [PubMed] [Google Scholar]
- 2. Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single‐stranded covalently closed circular RNA molecules existing as highly base‐paired rod‐like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852‐3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7(2):e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashwal‐Fluss R, Meyer M, Pamudurti N, et al. circRNA biogenesis competes with pre‐mRNA splicing. Mol Cell. 2014;56(1):55‐66. [DOI] [PubMed] [Google Scholar]
- 6. Conn S, Pillman K, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125‐1134. [DOI] [PubMed] [Google Scholar]
- 7. Preußer C, Hung L‐H, Schneider T, et al. Selective release of circRNAs in platelet‐derived extracellular vesicles. J Extracell Vesicles. 2018;7(1):1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569‐579. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Huang C, Bao C, et al. Exon‐intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256‐264. [DOI] [PubMed] [Google Scholar]
- 10. Chen N, Zhao G, Yan XU, et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu N, Yuan Z, Du KY, et al. Translation of yes‐associated protein (YAP) was antagonized by its circular RNA via suppressing the assembly of the translation initiation machinery. Cell Death Differ. 2019;26(12):2758‐2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du WW, Fang L, Yang W, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24(2):357‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38(18):1402‐1412. [DOI] [PubMed] [Google Scholar]
- 14. Weng W, Wei Q, Toden S, et al. Circular RNA ciRS‐7‐A promising prognostic biomarker and a potential therapeutic target in colorectal cancer. Clin Cancer Res. 2017;23(14):3918‐3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66(1):9‐21.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Gao X, Zhang M, et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Timoteo G, Dattilo D, Centrón‐Broco A, et al. Modulation of circRNA Metabolism by m(6)A Modification. Cell Rep. 2020;31(6):107641. [DOI] [PubMed] [Google Scholar]
- 18. Dong R, Zhang XO, Zhang Y, Ma XK, Chen LL, Yang L. CircRNA‐derived pseudogenes. Cell Res. 2016;26(6):747‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278‐294. [DOI] [PubMed] [Google Scholar]
- 20. Bachmayr‐Heyda A, Reiner AT, Auer K, et al. Correlation of circular RNA abundance with proliferation–exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia S, Feng J, Lei L, et al. Comprehensive characterization of tissue‐specific circular RNAs in the human and mouse genomes. Brief Bioinform. 2017;18(6):984‐992. [DOI] [PubMed] [Google Scholar]
- 22. Bahn JH, Zhang Q, Li F, et al. The landscape of microRNA, Piwi‐interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61(1):221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boeckel J‐N, Jaé N, Heumüller AW, et al. Identification and Characterization of Hypoxia‐Regulated Endothelial Circular RNA. Circ Res. 2015;117(10):884‐890. [DOI] [PubMed] [Google Scholar]
- 24. Li B, Xie F, Zheng FX, Jiang GS, Zeng FQ, Xiao XY. Overexpression of CircRNA BCRC4 regulates cell apoptosis and MicroRNA‐101/EZH2 signaling in bladder cancer. J Huazhong Univ Sci Technolog Med Sci. 2017;37(6):886‐890. [DOI] [PubMed] [Google Scholar]
- 25. Li Q, Wang Y, Wu S, et al. CircACC1 regulates assembly and activation of AMPK complex under metabolic stress. Cell Metab. 2019;30(1):157‐173.e7. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XL, Xu LL, Wang F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the miR‐138 targets TERT and PD‐L1. Cell Biol Int. 2017;41(9):1056‐1064. [DOI] [PubMed] [Google Scholar]
- 27. Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25‐miR‐103a‐3p/miR‐107‐CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Circular RNA‐ITCH suppresses lung cancer proliferation via inhibiting the Wnt/beta‐catenin pathway. Biomed Res Int. 2016;2016:1579490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westholm J, Miura P, Olson S, et al. Genome‐wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age‐dependent neural accumulation. Cell Rep. 2014;9(5):1966‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bai N, Peng E, Qiu X, et al. circFBLIM1 act as a ceRNA to promote hepatocellular cancer progression by sponging miR‐346. J Exp Clin Cancer Res. 2018;37(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang J, Hong H, Xue X, et al. A novel circular RNA, circFAT1(e2), inhibits gastric cancer progression by targeting miR‐548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett. 2019;442:222‐232. [DOI] [PubMed] [Google Scholar]
- 32. Yang Z, Qu C‐B, Zhang Y, et al. Dysregulation of p53‐RBM25‐mediated circAMOTL1L biogenesis contributes to prostate cancer progression through the circAMOTL1L‐miR‐193a‐5p‐Pcdha pathway. Oncogene. 2019;38(14):2516‐2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu J, Xu Q‐G, Wang Z‐G, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68(6):1214‐1227. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Hu H, Zhao Y, Zhao Y. CDR1as is overexpressed in laryngeal squamous cell carcinoma to promote the tumour's progression via miR‐7 signals. Cell Prolif. 2018;51(6):e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu J, Wang Y‐H, Yoon C, et al. Circular RNA circ‐RanGAP1 regulates VEGFA expression by targeting miR‐877‐3p to facilitate gastric cancer invasion and metastasis. Cancer Lett. 2019;471:38‐48. [DOI] [PubMed] [Google Scholar]
- 36. Zhong Z, Huang M, Lv M, et al. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305‐317. [DOI] [PubMed] [Google Scholar]
- 37. Li CY, Ma L, Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother. 2017;95:1514‐1519. [DOI] [PubMed] [Google Scholar]
- 38. Schito L, Semenza GL. Hypoxia‐inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758‐770. [DOI] [PubMed] [Google Scholar]
- 39. Zhu C, Kros JM, Cheng C, Mustafa D. The contribution of tumor‐associated macrophages in glioma neo‐angiogenesis and implications for anti‐angiogenic strategies. Neuro Oncol. 2017;19(11):1435‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verduci L, Ferraiuolo M, Sacconi A, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription‐competent complex. Genome Biol. 2017;18(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guarnerio J, Bezzi M, Jeong JC, et al. Oncogenic role of fusion‐circRNAs derived from cancer‐associated chromosomal translocations. Cell. 2016;166(4):1055‐1056. [DOI] [PubMed] [Google Scholar]
- 42. Tan S, Sun D, Pu W, et al. Circular RNA F‐circEA‐2a derived from EML4‐ALK fusion gene promotes cell migration and invasion in non‐small cell lung cancer. Mol Cancer. 2018;17(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z, Ran Y, Tao C, Li S, Chen J, Yang E. Detection of circular RNA expression and related quantitative trait loci in the human dorsolateral prefrontal cortex. Genome Biol. 2019;20(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang YH, Yu XH, Luo SS, Han H. Comprehensive circular RNA profiling reveals that circular RNA100783 is involved in chronic CD28‐associated CD8(+)T cell ageing. Immun Ageing. 2015;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Zhang Y, Li X, Zhang M, Lv K. Microarray analysis of circular RNA expression patterns in polarized macrophages. Int J Mol Med. 2017;39(2):373‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao R, Ni J, Lu SI, et al. CircUBAP2‐mediated competing endogenous RNA network modulates tumorigenesis in pancreatic adenocarcinoma. Aging (Albany NY). 2019;11(19):8484‐8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shen T, Cheng X, Liu X, et al. Circ_0026344 restrains metastasis of human colorectal cancer cells via miR‐183. Artif Cells Nanomed Biotechnol. 2019;47(1):4038‐4045. [DOI] [PubMed] [Google Scholar]
- 49. Zhang P‐F, Pei XU, Li K‐S, et al. Circular RNA circFGFR1 promotes progression and anti‐PD‐1 resistance by sponging miR‐381‐3p in non‐small cell lung cancer cells. Mol Cancer. 2019;18(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang H, Ma XP, Li X, Deng FS. Circular RNA circ_0067934 exhaustion expedites cell apoptosis and represses cell proliferation, migration and invasion in thyroid cancer via sponging miR‐1304 and regulating CXCR1 expression. Eur Rev Med Pharmacol Sci. 2019;23(24):10851‐10866. [DOI] [PubMed] [Google Scholar]
- 51. Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16(1):7‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin H, Jin X, Zhang H, Wang W. Circular RNA hsa‐circ‐0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget. 2017;8(15):25571‐25581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu T, Ye P, Ye Y, Lu S, Han B. Circular RNA hsa_circRNA_002178 silencing retards breast cancer progression via microRNA‐328‐3p‐mediated inhibition of COL1A1. J Cell Mol Med. 2020;24(3):2189‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ungerleider N, Concha M, Lin Z, et al. The Epstein Barr virus circRNAome. PLoS Pathog. 2018;14(8):e1007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao J, Lee EE, Kim J, et al. Transforming activity of an oncoprotein‐encoding circular RNA from human papillomavirus. Nat Commun. 2019;10(1):2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tagawa T, Gao S, Koparde VN, et al. Discovery of Kaposi's sarcoma herpesvirus‐encoded circular RNAs and a human antiviral circular RNA. Proc Natl Acad Sci U S A. 2018;115(50):12805‐12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen YG, Kim MV, Chen X, et al. Sensing self and foreign circular RNAs by intron identity. Mol Cell. 2017;67(2):228‐238.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li X, Liu C‐X, Xue W, et al. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67(2):214‐227.e7. [DOI] [PubMed] [Google Scholar]
- 59. Ghosal S, Das S, Sen R, Chakrabarti J. HumanViCe: host ceRNA network in virus infected cells in human. Front Genet. 2014;5:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dou Y, Cha DJ, Franklin JL, et al. Circular RNAs are down‐regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci Rep. 2016;6:37982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kun‐Peng Z, Xiao‐Long M, Chun‐Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. 2018;14(3):321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li J, Li H, Lv X, et al. Diagnostic performance of circular RNAs in human cancers: A systematic review and meta‐analysis. Mol Genet Genomic Med. 2019;7(7):e00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. A Study of Blood Based Biomarkers for Pancreas Adenocarcinoma. NCT03334708. https://clinicaltrials.gov/ct2/show/NCT03334708?cond=NCT03334708&rank=1
- 64. Okholm TLH, Nielsen MM, Hamilton MP, et al. Circular RNA expression is abundant and correlated to aggressiveness in early‐stage bladder cancer. NPJ Genom Med. 2017;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Z, Yanfang WU, Li J, et al. Tumor‐released exosomal circular RNA PDE8A promotes invasive growth via the miR‐338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237‐250. [DOI] [PubMed] [Google Scholar]
- 66. Liu Y‐T, Han X‐H, Xing P‐Y, et al. Circular RNA profiling identified as a biomarker for predicting the efficacy of Gefitinib therapy for non‐small cell lung cancer. J Thorac Dis. 2019;11(5):1779‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu T, Wang M, Jiang L, et al. CircRNAs in anticancer drug resistance: recent advances and future potential. Mol Cancer. 2020;19(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang C, Tao W, Ni S, Chen Q. Circular RNA circ‐Foxo3 induced cell apoptosis in urothelial carcinoma via interaction with miR‐191‐5p. Onco Targets Ther. 2019;12:8085‐8094. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69. Cao C, Han S, Yuan Y, et al. Downregulated circular RNA hsa_circ_0000291 suppresses migration and proliferation of gastric cancer via targeting the miR‐183/ITGB1 axis. Cancer Manag Res. 2019;11:9675‐9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang QI, Du WW, Wu N, et al. A circular RNA promotes tumorigenesis by inducing c‐myc nuclear translocation. Cell Death Differ. 2017;24(9):1609‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu XI, Abraham JM, Cheng Y, et al. Synthetic circular RNA functions as a miR‐21 sponge to suppress gastric carcinoma cell proliferation. Mol Ther Nucleic Acids. 2018;13:312‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Z, Ma KE, Cheng Y, et al. Synthetic circular multi‐miR sponge simultaneously inhibits miR‐21 and miR‐93 in esophageal carcinoma. Lab Invest. 2019;99(10):1442‐1453. [DOI] [PubMed] [Google Scholar]
- 73. Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12(5):631‐645. [DOI] [PubMed] [Google Scholar]
- 74. Ma Y, Zhang C, Zhang B, Yu H, Yu Q. circRNA of AR‐suppressed PABPC1 91 bp enhances the cytotoxicity of natural killer cells against hepatocellular carcinoma via upregulating UL16 binding protein 1. Oncol Lett. 2019;17(1):388‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lasda E, Parker R. Circular RNAs co‐precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PLoS One. 2016;11(2):e0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA‐ceRNA, miRNA‐ncRNA and protein–RNA interaction networks from large‐scale CLIP‐Seq data. Nucl Acids Res. 2014;42(D1):D92‐D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20(11):1666‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Zheng F, Xiao X, et al. CircHIPK3 sponges miR‐558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18(9):1646‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zeng K, Chen X, Xu MU, et al. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR‐7. Cell Death Dis. 2018;9(4):417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80. Chery J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 2016;4(7):35‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kristensen LS, Ebbesen KK, Sokol M, et al. Spatial expression analyses of the putative oncogene ciRS‐7 in cancer reshape the microRNA sponge theory. Nat Commun. 2020;11(1):4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Athanasopoulos T, Munye MM, Yanez‐Munoz RJ. Nonintegrating gene therapy vectors. Hematol Oncol Clin North Am. 2017;31(5):753‐770. [DOI] [PubMed] [Google Scholar]
- 83. Bai H, Lei K, Huang F, Jiang Z, Zhou X. Exo‐circRNAs: a new paradigm for anticancer therapy. Mol Cancer. 2019;18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maia FR, Reis RL, Oliveira JM. Finding the perfect match between nanoparticles and microfluidics to respond to the cancer challenges. Nanomedicine. 2019;24:102139. [DOI] [PubMed] [Google Scholar]
- 85. Zheng H, Chen T, Li C, et al. A circular RNA hsa_circ_0079929 inhibits tumor growth in hepatocellular carcinoma. Cancer Manag Res. 2019;11:443‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bi J, Liu H, Dong W, et al. Circular RNA circ‐ZKSCAN1 inhibits bladder cancer progression through miR‐1178‐3p/p21 axis and acts as a prognostic factor of recurrence. Mol Cancer. 2019;18(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Y, Liu H, Li W, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR‐630. Aging (Albany NY). 2017;9(6):1585‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li P, Chen H, Chen S, et al. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116(5):626‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jie M, Wu Y, Gao M, et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020;19(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen T, Yu Q, Xin L, Guo L. Circular RNA circC3P1 restrains kidney cancer cell activity by regulating miR‐21/PTEN axis and inactivating PI3K/AKT and NF‐ kB pathways. J Cell Physiol. 2020;235(4):4001‐4010. [DOI] [PubMed] [Google Scholar]
- 91. Wang L, Tong X, Zhou Z, et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF‐beta‐induced epithelial‐mesenchymal transition and metastasis by controlling TIF1gamma in non‐small cell lung cancer. Mol Cancer. 2018;17(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nan A, Chen L, Zhang N, et al. Circular RNA circNOL10 inhibits lung cancer development by promoting SCLM1‐mediated transcriptional regulation of the humanin polypeptide family. Adv Sci (Weinh). 2019;6(2):1800654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang F, Wang J, Cao X, Xu L, Chen L. Hsa_circ_0014717 is downregulated in colorectal cancer and inhibits tumor growth by promoting p16 expression. Biomed Pharmacother. 2018;98:775‐782. [DOI] [PubMed] [Google Scholar]
- 94. Fan M, Wang Y, Gao S. Circular RNA circMTO1 acts as an antitumor factor in rectal cancer cell lines by downregulation of miR‐19b‐3p. J Cell Biochem. 2019:1–13. [Google Scholar]
- 95. Zhao Z, Ji M, Wang Q, He N, Li Y. Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR‐1270 suppression. Mol Ther Nucleic Acids. 2019;18:24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Chen H, Mao M, Jiang J, Zhu D, Li P. Circular RNA CDR1as acts as a sponge of miR‐135b‐5p to suppress ovarian cancer progression. Onco Targets Ther. 2019;12:3869‐3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ou R, Lv J, Zhang Q, et al. circAMOTL1 motivates AMOTL1 expression to facilitate cervical cancer growth. Mol Ther Nucleic Acids. 2020;19:50‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang H, Li X, Meng Q, et al. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol Cancer. 2020;19(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR‐7 in non‐small‐cell lung cancer. Onco Targets Ther. 2018;11:3979‐3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Y, Zhang J, Pan S, Zhou J, Diao X, Liu S. CircRNA CDR1as knockdown inhibits progression of non‐small‐cell lung cancer by regulating miR‐219a‐5p/SOX5 axis. Thorac Cancer. 2020;11(3):537‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li C, Li M, Xue Y. Downregulation of CircRNA CDR1as specifically triggered low‐dose Diosbulbin‐B induced gastric cancer cell death by regulating miR‐7‐5p/REGgamma axis. Biomed Pharmacother. 2019;120:109462. [DOI] [PubMed] [Google Scholar]
- 102. Liu YY, Zhang LY, Du WZ. Circular RNA circ‐PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR‐124‐3p. Biosci Rep. 2019;39(12):BSR20193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chi G, Yang F, Xu D, Liu W. Silencing hsa_circ_PVT1 (circPVT1) suppresses the growth and metastasis of glioblastoma multiforme cells by up‐regulation of miR‐199a‐5p. Artif Cells Nanomed Biotechnol. 2020;48(1):188‐196. [DOI] [PubMed] [Google Scholar]
- 104. Zheng J, Liu X, Xue Y, et al. TTBK2 circular RNA promotes glioma malignancy by regulating miR‐217/HNF1beta/Derlin‐1 pathway. J Hematol Oncol. 2017;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the figshare repository.


