Abstract
Human primary in vitro cell cultures are among the most challenging procedures in cellular biology laboratory practice. Myoblasts-progenitor of skeletal muscle origin represent a promising therapeutic cell source since the procedure of their isolation is not technically demanding, and the in vitro culture is relatively straightforward. Myoblasts could be considered as the candidates for clinical applications due to their regenerative potential, and as the carriers of therapeutic proteins introduced through genetic modifications. The main goal of this prospective study was to evaluate different myoblasts isolation strategies based on the pre-plating technique and cells density characteristics. Moreover, testing of different myoblast media formulations-both commercially available and in-house made was performed. Our goal was to establish the in vitro protocol of myoblasts culture allowing for preservation of the proliferative potential and desired phenotype. Our results revealed that in culture of myoblasts of human muscle origin, the pre-plate technique and cell density differences did not correlate with changes in the proliferative potential, however it was observed that low density cells maintained expression of the CD56 marker up to the higher passages. Assessment of different types of culture media confirmed the best performance for DMEM based media without Chicken Embryo Extract (CEE) addition. Cells cultured in DMEM+FBS medium revealed high expression of CD56 and CD90 antigens, absence of the hematopoietic markers and presented stable proliferation profile. This finding is in line with guidelines of regulatory agencies recommending removal of the xeno-derived reagents from the manufacturing process of Advanced Therapy Medicinal Products (ATMP). In this study, human myoblasts culture was optimized in vitro under different media conditions. The next approach in assessment of myoblasts propagation for potential clinical applications will be testing of the clinical grade human platelet lysate (hPL) instead of the FBS.
Keywords: Myoblasts, muscle progenitor, in vitro culture, cell therapy, CD markers, growth media
Introduction
Human primary myoblasts (MB) derived directly from patient’s skeletal muscle tissue biopsy and cultured in vitro could serve as an important tool to study myocytes biology, metabolism and regulation of myogenic cell differentiation [1]. Despite of their unipotent characteristics, myoblasts could serve as a valuable source for stem cell-based therapies not only for patients with neuromuscular disorders such as muscular dystrophies or other types of myopathies, but also for treatment of other types of muscle tissue damage such as myocardial infraction or sphincters injury [2-4].
Skeletal muscle is a rich source of stem/progenitor cells. Among them two main types could be distinguished: satellite cells and non-satellite cells. Satellite cells (SC), well-known myogenic precursor cells, discovered over 50 years ago are located between muscle fibers and remain quiescent in their niche until activation signal is delivered. After activation, their gene expression profile and physiological properties change and begin interaction with other components of muscle tissue including extracellular matrix and other cell types to initiate muscle regeneration. The activation, proliferation, myotube and myocytes formation is accompanied by secretion of different growth factors, orchestrated by expression of MRF family transcription factors [5].
The non-satellite cells found in skeletal muscle could serve as the tissue homeostasis safeguards. Several authors showed that non-SC population could include mesenchymal progenitor, PW1+PAX7-interstitial cells (PICs), fibrocyte/adipocyte progenitors (FAPs), side population cells (SP) and pericytes [6]. Mesenchymal progenitors’ role is to coordinate the environment required for legitimate regeneration process via immunomodulation supported by trophic and mechanical factors [7]. PICs are thought to be the bi-potent cells and are involved in both, the smooth muscle and skeletal muscle regeneration. They are distinguished from the SC based on their location in the interstitium instead of the basal lamina and by the molecular markers, since the PICs are negative for Pax7-one of the key regulators of myogenesis. PICs derived from the mice muscle showed expression of some pluripotency markers including Sox2, Oct3/4 and Nanog [8]. FAPs according to several studies are critical for the efficient muscle repair. They share mesenchymal cell markers and have ability to differentiate into osteo-, chondro- and adipogenic lineages. Quiescent FAPs reside close to the blood vessels and during regeneration support satellite cells in both proliferation and maintenance of the SC pool. After acute phase of regeneration, the activated FAPs are cleared from the tissues by the apoptosis process. It was noticed, that in pathological conditions such as the dystrophic or atrophic alterations, the population of FAPs increases and augments tissue deterioration [9]. Pericytes are associated with capillaries of the skeletal muscle and are in close contact with the endothelial cells [10]. The clear link was presented between pericytes and tissue specific MSC found in the skeletal muscle. The definition of pericytes was based on the CD146 and other pericyte markers as reported in details by Crisan et al. [10] and was associated with hematopoietic antigens absence. These investigators have shown that the same cell population expresses the MSC markers such as the CD73, CD90 and CD44 [10]. The complexity, diversity and myogenic potential of non-satellite cells represents an unrecognized field, and the interspecies differences and distinctive cell populations in relation to the muscle type makes this topic extremely difficult to study.
NCAM1 (Neural Cell Adhesion Molecule 1) is a gene coding the transmembrane protein which is a member of superimmunoglobulin family. This protein is widely known as CD56 and is expressed in the nervous system, immune cells (mostly on NK cells surface) as well as in the muscle tissue. As the protein involved in cell-to-cell interactions and cell-to-ECM (extracellular matrix) interactions, its role is crucial for neurogenesis and neurite growth. CD56 was defined as the myogenic cell markers almost 40 years ago and still is one of the most recognized antigen for the myoblast isolation and characterization [11,12]. The function of this protein is not fully defined but expression of CD56 as the marker of myoblasts is widely approved by many researchers [13]. The CD56 was used as a reference in the study published by Thurner et al. and was considered as the critical parameter for development of potency assay based on acetylcholinesterase activity in myoblasts [13]. Establishment of potency assay as the quantitative measure of crucial biological function of cell-based ATMP is essential for presentation of cell-based therapy candidates to the regulatory agencies such as European Medicines Agency (EMA) or Food and Drug Administration (FDA). It is worth to mention that lack of CD56 on the muscle-derived cell’s surface correlates with diminished ability of myotube formation [13].
The limitations of the previous studies reported in the literature included: separate evaluation of the pre-plate method; optimization of media formulation, or conduction of studies on the rodent models rather than on the human tissues [12,14,15]. Thus, our goal was to evaluate the effect of myoblast isolation procedure on the stablity of myoblasts phenotype and proliferation potential as well as to assess the effect of different culture media on the reproducibility and stability of the CD56 marker during myoblast propagation up to 6 passages. These two features are crucial for propagation of myoblasts with top quality and quantity and would serve as the predictive values for optimization of the myoblast culture for future development of the myoblast-based cellular therapies.
Materials and methods
Cells isolation and in vitro culture
Tissue samples were obtained from patients (n=27) who underwent ACL procedure after signing informed consent in accordance with the ethical committee guidance and approval. All procedures employed in this study were approved by the Bioethical Committee of Poznan University of Medical Sciences (permission no. 672/18) and were conducted in accordance with the principles of Good Laboratory Practice. Human myoblasts were isolated according to our established procedure described in detail in previous publication [1,16] and the literature reports [14,17,18].
Briefly, remnants of muscle tissue were collected after ACL surgery and were placed into 50 ml vial, suspended in HBSS solution, and transported to the R&D laboratory. Next, the concomitant connective and adipose tissue was discarded, and only skeletal muscle part of tissue was minced and subjected to collagenase treatment for 45 min. After digestion, cells and tissue debris were filtered through 70 µm mesh and centrifuged. Cells were suspended in culture medium (four different formulations) and plated on gelatin covered T25 flask. The modified pre-plate method of in vitro culture system was applied to isolate fast and slow adherent cell sub-populations [17,18]. Additional centrifugation step performed during cells isolation procedure was applied for the distinction of low- and high-density sub-populations. The scheme of preplate-density isolation approach is shown on Figure 1. The isolation medium was transferred to new gelatin covered T25 flask every other day (p0 flask 1-4) whereas fresh culture medium was added to the original T25 flask.
Figure 1.
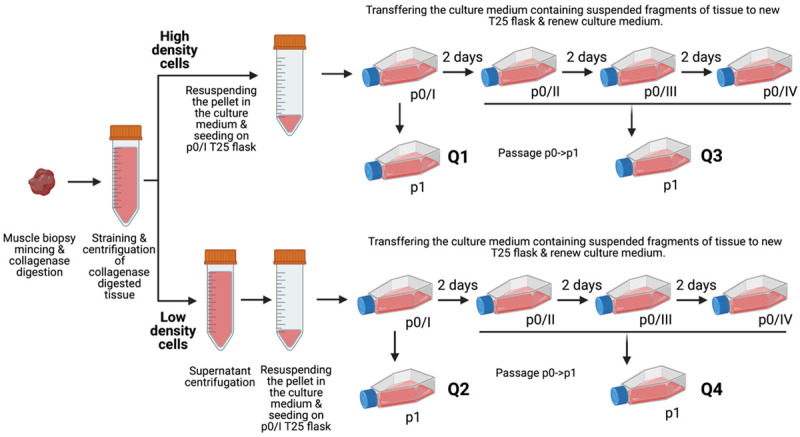
Schematic summary of the timeline of myoblasts isolation and pre-plating procedure from muscle biopsy to the creation of myoblasts subpopulations via subsequent passages. Four fractions were extracted based on two features: cell density and rate of cell adhesion: Q1-fast adherence; high density; Q2-fast adherence; low density; Q3-slow adherence, high density; Q4-slow adherence; low density. Created with BioRender.
The following culture media were tested: (1) Skeletal medium (Sigma); (2) SkGM-2 Bullet Kit (LONZA); (3) DMEM (4500 g/l glucose) supplemented with 20% of FBS (Fetal Bovine Serum; LONZA), antibiotics (LONZA), ultraglutamine (LONZA) and 1% CEE (Chick Embryo Extract) and (4) DMEM (4500 g/l glucose) supplemented with 20% of FBS (Fetal Bovine Serum; LONZA), antibiotics (LONZA), ultraglutamine (LONZA). The bFGF (Sigma) growth factor was added to both DMEM-based culture media.
Cell viability was evaluated by ADAM cell counter (NanoEntek) or Countess cell counter (Invitrogen).
Population doubling time
Population doubling time was counted according to the following formula:
Population doubling time = duration * log(2)/(log(final concentration)-log(initial concentration))
Where: duration = number of days; final concentration = final cell count; initial concentration = number of cells seeded.
Flow cytometry evaluation cell phenotype
Each cell line before passage was checked for the presence of the following surface markers: CD56 (APC mouse anti-human CD56, clone B159, BD Pharmingen), CD90 (PE/Cy7 anti-human CD90, clone 5E10, BioLegend), CD34 (APC anti-human CD34, clone 581, BioLegend), CD45 (PE/Cy7 anti-human CD45, clone HI30, BioLegend). Although this panel was chosen based on the literature, the criteria are not theoretical-since human myoblasts from muscle tissues used and assessed in this study are obtained from the human tissue remnants from surgical procedures. 105 of cells were suspended in 50 µl 1% FBS (Fetal Bovine Serum, Biological Industries) in PBS (Dulbecco’s Phosphate Buffered Saline w/o Ca, Mg 1×, Biological Industries) and labeled with antibodies for 20 min RT. Double washed samples were fixed in 1% PFA and measured by flow cytometery (BriCyte E6, Mindray or FACSCallibur, BD).
Immunofluorescence analysis
The 104 of cells per well were seeded on non-coated (DES) or Matrigel-coated (DMD, MyHC) coverslips. After 24 h or 7-days differentiation cells were fixed in 4% PFA (Sigma-Aldrich) for 15 min at 4°C. The cell membranes were permeabilized with 0,1% Triton X-100 (15 min, RT) (Sigma-Aldrich), and blocked in 5% albumin (Alburex20, CSL Behring) with 0.5% Triton X-100 for an hour in RT. Primary antibodies were diluted according to the protocols provided by Vendors: anti-desmin 1:200 (Sigma-Aldrich); anti-myosin heavy chain 1:400 (Sigma-Aldrich); anti-DMD 1:100 (Sigma-Aldrich), and incubated overnight with the specimens at 4°C. Then cells were washed three times in PBS (Biological Industries) and were incubated on the coverslips with a secondary anti-mouse IgG antibody conjugated with AlexaFluor 488 1:500 (Invitrogen). A DAPI anti-fade solution was used as a counterstain to visualize the nucleus (Sigma-Aldrich).
Fusion index
Cells were seeded in concentration of 5×104 per well on Matrigel-coated 12-well plates. The cells after differentiation in 2% HS (horse serum) based medium were fixed in cold methanol for 3 minutes, stained by May-Grunwald dye for 3 min and then stained by 10% Giemsa solution for 20 min. Fusion index has been defined as a ratio of nuclei number present in differentiated myotubes and nuclei in undifferentiated cells.
Statistical analysis
Results were reported as a mean value ± Standard Error of the Mean (SEM). The Kruskal-Wallis test was performed to compare the population doubling time (PDT) and cells phenotype depending on the cell passage. Difference in the phenotype of cells derived from 4 subpopulations, as well as cell count, PDT and phenotype of myoblast cultured in four different media was evaluated using the Mixed-effects model (REML) with Tukey post-hoc multiple comparisons test. P values were considered significant below 0.05. All analyses were performed using GraphPad Prism 9.10 software.
Results
Expression of the CD56, CD90, CD34 and CD45 markers during myoblasts culture
Standard myoblast isolation and in vitro culture was performed for n=27 muscle tissue biopsies according to the previously published protocol [1]. The isolated myoblasts presented expression of the myogenic markers such as desmin and after differentiation formed multinucleated myotubes which were positive for myosin heavy chain and dystrophin proteins. The typical panel of myoblasts characteristics is shown on Figure 2. The average fusion index calculated for the cultured in vitro myoblasts reached 53% as shown in Figure 3.
Figure 2.
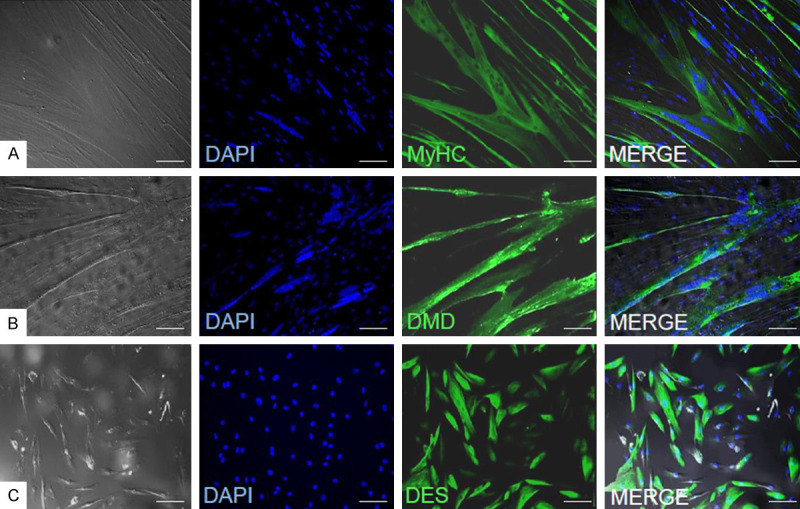
Immunofluorescence assessment of myogenic markers expression in myoblasts cultured in DMEM+FBS+FGF medium (A) and myocytes (B, C). Myosin heavy chain (A), dystrophin (B) and desmin (C). Cells were seeded on non-coated (DES) or Matrigel-coated (DMD, MyCH) coverslips, differentiated and incubated with the use of relevant antibodies. A DAPI anti-fade solution was used as a counterstain to visualize the nucleus. Magnification: ×200, scale bar 100 µm.
Figure 3.
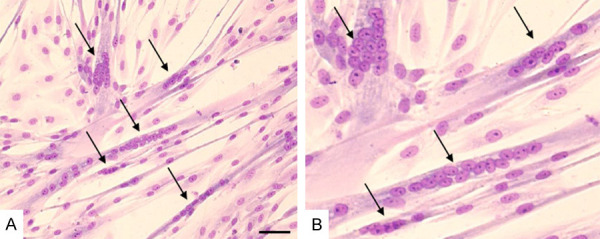
Example of myotubes formation in differentiated myoblasts cultured in DMEM+FBS+FGF medium and stained with Pappenheim staining method (May-Grunwald/Giemsa staining). Myotubes formation is shown under two magnifications: A. ×200, scale bar 100 µm; B. ×954. Arrows indicate examples of differentiated multinuclear myotubes.
The myoblasts culture in medium was carried on until the cells reached passage 6. The cell phenotype was evaluated by flow cytometry and data are presented in Figure 4. All obtained myoblasts populations show high expression of CD56 (82.03±6.51%) and CD90 (66.32%±11.34%) antigens and very low presence of hematopoietic markers (<5%) on the p1. We next assessed if the cell’s phenotype underwent a change during in vitro culture from p1 to p6.
Figure 4.
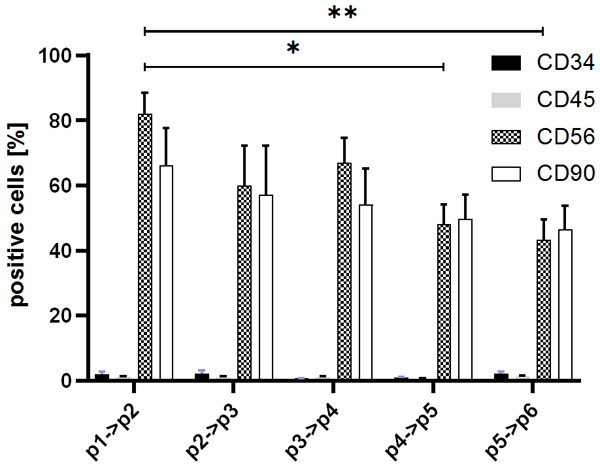
Comparison of the percentage of myoblast cells positive for the following markers: CD34, CD45, CD56 and CD90 based on the cell passages. Cells were labeled with relevant antibodies and visualized with FACS cytometer. The Kruskal-Wallis test showed a diminish of CD56 and CD90 positive cells during cell in vitro culture: Data is presented as mean ± SEM, *P<0.05; **P<0.01.
The CD56 antigen showed subsequent reduction of expression on the myoblasts surface and after p4 this difference reached statistical significance in relation to the expression recorded on the starting population. The CD90 antigen presence showed similar expression pattern from 60% positive cell on p1 (66.32%±11.34%) to 50% (46.46%±7.39%) on p5 but this observation did not reach statistical significance.
The pre-plate procedure verification
The pre-plate procedure is often applied during isolation of adherent cells including myoblasts as a step improving target cells purity. We used modified protocol assuming collection of four different myoblasts populations based on their adherence and density properties (Figure 1) classified as: Q1-fast adherence, high density; Q2-fast adherence, low density; Q3-slow adherence, high density; Q4-slow adherence, low density. We monitored the phenotype (CD56, CD90, CD34 and CD45) during 6 subsequent passages. As shown on Figure 5 there was no difference in evaluated antigens presentation between myoblasts sub-population during first passages, but it seems that low density cells are capable to maintain the expression of CD56 antigen on higher level on the later passages. The deterioration of expression of the positive myogenic marker-CD56 was observed during in vitro culture in all evaluated sub-populations as presented in Figure 5.
Figure 5.
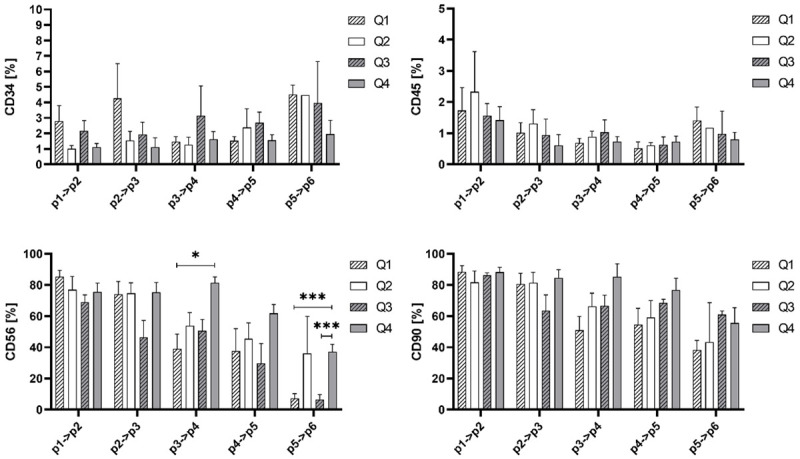
The percentage of CD34, CD45, CD56 and CD90 positive cells in myoblasts derived from the following subpopulations: Q1 (Fast adherence, high density), Q2 (Fast adherence, low density), Q3 (Slow adherence, high density), Q4 (Slow adherence, low density) fractions from isolation. Statistical analysis was performed using the Mixed-effects model (REML) with Tukey multiple comparisons test. Asterisks: Data is presented as mean ± SD; *P<0.05; **P<0.01; ***P<0.001.
Evaluation of PDT for myoblast subpopulations (Q1-Q4) was only feasible on p2 and p3 passages due to the technical reasons. Although, the shortening of PDT was observed comparing p2 to p3, however the data analysis has not confirmed statistical significance (Figure 6A). Taking this into consideration we conclude that density and adherence features do not influence the rate of myoblast proliferation. The combined analysis of PDT obtained for all analyzed myoblasts subpopulations showed that the proliferation rate was increasing (shorter PDT) when cells reached the p3 (Figure 6B).
Figure 6.
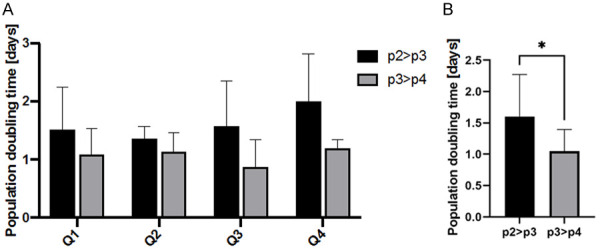
Population doubling time (PDT) calculated for subsequent passages of myoblasts subpopulation. Evaluation of PDT for myoblast subpopulations (Q1-Q4) was feasible on p2 and p3 passages. (A) Comparison of PDT for cell subpopulations: Q1, Q2, Q3, Q4. (B) Comparison of PDT between p2>p3 and p3>p4 for all cell subpopulations. PDT = duration * log(2)/(log(Final concentration)-log(Initial concentration)). Statistical analysis was performed using the Kruskal-Wallis test. Data is presented as mean ± SEM: *P<0.05.
The influence of composition of the myoblasts culture medium on the cell properties
We evaluated four different media formulations dedicated for myogenic cells. Two of them were commercially available i.e., Skeletal Cell Medium from Sigma and SkGM-2 Bullet Kit (LONZA) the following two were the DMEM based with 20% FBS and bFGF supplementation. The only difference between latter two was addition of another animal derived factor-CEE (Chick Embryo Extract). Similarly, to previous experiments the two main features of myoblasts were evaluated: the proliferation dynamics and presence of the specific surface antigens. As shown on Figure 7 the cells cultured in tested media did not differ in the passage pattern although commercially available media seems to stimulate cell proliferation more intensively. This phenomenon was not confirmed by statistical analysis, but evaluation of PDT clearly showed that cells cultured in commercially available medium need significantly less time to double the population compared to cells cultured in DMEM based in-house made medium (Figure 8).
Figure 7.
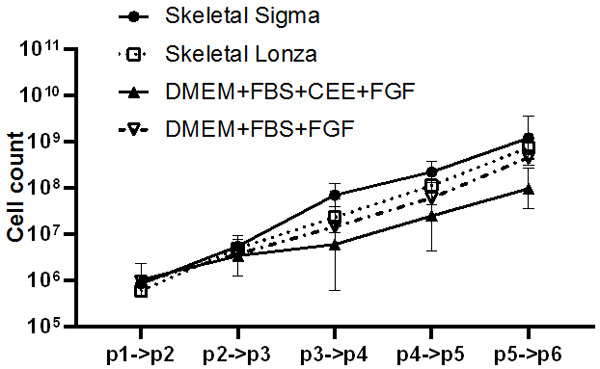
The cell count of myoblasts cultured in vitro on the subsequent passages in four different media formulations dedicated for myogenic cells. Statistical analysis was performed using the Mixed-effects model (REML) with Tukey multiple comparisons test. Data is presented as mean ± SEM, no significant difference was observed.
Figure 8.
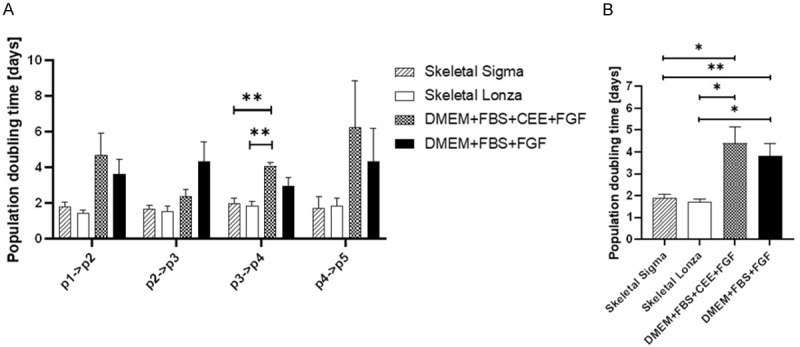
Population doubling time (PDT) between subsequent passages calculated for myoblasts cultured in four different media formulations dedicated for myogenic cells. (A) Calculated on individuals passaged (B) Calculated for long-term cell culture Population doubling time = duration * log(2)/(log(Final concentration)-log(Initial concentration)). Statistical analysis was performed using the Mixed-effects model (REML) with Tukey multiple comparisons test. Data is presented as mean ± SEM; asterisks: *P<0.05; **P<0.01.
Assessment of hematopoietic stem cell markers
CD34 and CD45 showed weak expression on the vast majority of evaluated cells. The expression of CD45 and CD34 was found to be below 5% in all examined cell populations except for the myoblasts cultured in Skeletal medium from Sigma which revealed approximately 10% on p2>p3 stage. This was the highest CD34 expression across all studied cell populations. The evaluation of CD56 and CD90 expression in tested cells revealed significant diversity between cells cultured in commercially available and in-house made media. As show in Figure 9, skeletal medium purchased from LONZA did not support high level of the CD56 and CD90 marker between passage p1 to p5. The percentage of cells positive for CD56 and CD90 antigens was relatively low from the p1 and did not show significant changes. The myoblast dedicated medium from Sigma showed preservation of CD90 markers at 53.75%±9.23% level, however did not maintain higher levels of CD56 markers (15.43%±3.56%).
Figure 9.
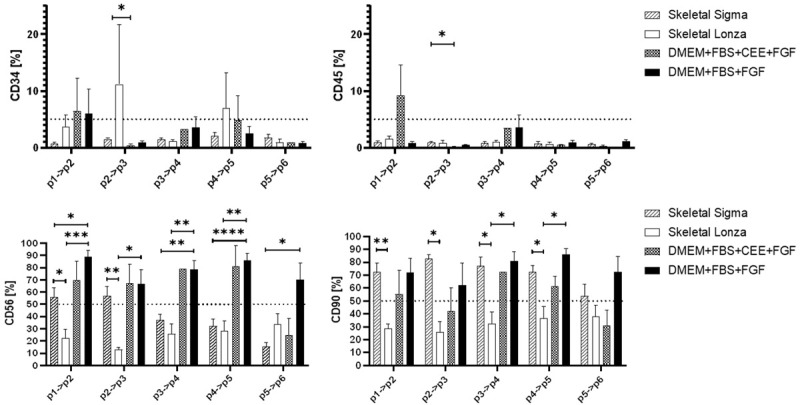
The percentage of CD34, CD45, CD56 and CD90 positive cells in myoblasts cultured in four different media formulations dedicated for myogenic cells. Cells were labeled with relevant antibodies and visualized with FACS cytometer. Statistical analysis was performed using the Mixed-effects model (REML) with Tukey multiple comparisons test. Data is presented as mean ± SEM; asterisks: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Discussion
Skeletal muscle is a complex heterogeneous tissue constituted of multinucleated myocytes and plethora of mononuclear cells including, muscle stem cells (satellite cells), non-myogenic mesenchymal progenitors (e.g., fibro-adipogenic progenitors, or FAPs), endothelial cells and other mononuclear cells such as immune cells. The aim of this study was to evaluate the impact of myoblasts isolation procedure based on the diverse cell density and adherence properties, on the proliferation rate and the phenotype of the propagated cells assessed by CD56, CD90, CD34 and CD45 surface markers. We were interested to evaluate these markers and characteristics of myoblasts due to their significance during delivery of the myoblasts-based therapies such as the Advance Therapy Medicinal Products (ATMP). The CD56 marker is described as the specific and efficient marker for assessment of myoblasts isolated from a dissociated human tissue biopsies by fluorescence-activated cell sorting (FACS). The other markers confirm mesodermal origin of isolated cells (CD90) and exclude the possibility of contamination of the isolated myogenic precursors with the cells of hematopoietic origin (CD45 and CD34). We have established basic in vitro culture conditions based on both-the literature reports [14,19] and our experience [1,16]. The primary in vitro culture condition represented the first step, leading to more detailed and comprehensive optimization of cell culture for future application in the clinically relevant ATMP-tailored conditions.
The pre-plate technique in myoblasts isolation was introduced by Huard et al. [14,19] over twenty years ago. The main goal of the pre-plate technique was to obtain the most suitable cell population to improve the survival rate and proper homing and integration of the injected myoblasts into dystrophic muscle. Since then, regardless of the reports on the successful outcomes of the in vitro and preclinical studies, no major improvements were proposed for the cell-based therapies for DMD (Duchenne Muscular Dystrophy) and other muscular genetic disorders. One of the reasons could be the differences between the rodent models for musculoskeletal disorders and the human muscle physiology and homeostasis. It is worth mentioning that not only the molecular pattern of mouse and human skeletal muscle transcriptome profiles varies [20] but also differences in muscle function between the two species could be noticed [21]. Compared to mouse models, evaluation of progenitor cells of human skeletal muscle origin is still limited. Many researchers have introduced the pre-plate method into the practice and the purity of the isolated cells have been improved in the recent reports but mostly in the mice models [18]. Our results revealed that there were no striking differences observed between the phenotype of distinct myogenic cell populations obtained after human cells isolation using the modified pre-plate techniques. It could be related to the differences between the humans and mice, since in rodents, significant differences were clearly noticed between myoblast subpopulations which were isolated by different pre-plate methods. Although, the discrete discrepancy in myoblasts phenotype was revealed on p6 as the low-density cell populations (Q2 and Q4) showed maintenance of the higher percentage of CD56 positive cells during long-term (up to p6) in vitro culture. Since the cell-based therapies will require propagation of millions or even billions of cells, thus stability of the CD56 marker at the high (p6) passage could be considered as the pivotal asset of our technique.
Assessment of myoblasts proliferation rate revealed no difference between myoblast populations with low and high density and distinct adherence properties. We have confirmed that cells after p3 present shorter PDT which could be associated with entering of the logarithmic growth phase observed in the primary cell culture after isolation and the subsequent passages. This logarithmic growth is observed until the cells reach senescence. The Nehlin et al. showed that over 60% of cells showed SA-beta-Gal expression on p11 and the slowing of the proliferation rate based on the days between the passages was clearly visible after p6 [22].
In addition to different pre-plate techniques implemented by research laboratories, there are several protocols for isolation and growth media design for in vitro culture of the myogenic cells. The goal of our study was to compare two commercially available media, the SkGM-2 Bullet Kit (Lonza) and Skeletal medium (Sigma-Aldrich) with the in-house prepared-DMEM based media-with or without supplementation with chick embryo extract (CEE). The rationale for this approach was to assure the reproducibility of the in vitro cell culture conditions and removal of not defined and xeno-derived supplement from the medium content. We found that the commercially available media have not guaranteed maintenance of high percentage of the CD56 positive myogenic cells, during the long-term in vitro culture. Moreover, the SkGM-2-Bullet Kit medium has not supported attachment of the myogenic cells. It should be mentioned that we used gelatin coated flasks instead of the collagen or ECM-coated flasks as recommended by the commercial media producers. This could potentially explain why we did not observe beneficial impact of commercially available media on the expression of CD56 marker in the isolated and cultured human cells. We have also found that supplementation of culture medium with CEE is not a critical factor for myogenic cell survival and phenotype maintenance. This was a crucial finding since the presence of any components of animal origin could negatively affect the process of regulatory approval of myoblasts based therapies such as the ATMP.
Moreover, we found that myoblasts cultured in the commercially available media showed significantly shorter PDT. Fast proliferation in the in vitro culture resulted in the higher number of cells obtained during a shorter period of time. However, this observation has not corresponded with the maintenance of the CD56+ phenotype on the collected cells which could be used to endorse myogenic potential of cells.
As explained above, the presented myoblast’s evaluation criteria were verified on the human muscle tissues, thus the provided evaluation and assessments criteria have a potential clinical relevance. Moreover, the initial in vitro culture conditions (DMEM medium, 20% FBS, CEE) were applied during preparation of cell population for the intracardiac transplantation in phase I clinical trial and the applied conditions successfully stimulated proliferation of both, the native and genetically modified myoblasts [23,24]. The detailed preclinical study of in vivo cell tracking as well as cell imaging clearly showed that tested conditions guarantee both, the cell survival and therapeutic effect [25,26].
In summary, in this study we have described a fast, simple and high-yield protocol for the isolation and in vitro culture of myoblasts derived from the human skeletal muscle biopsies. Our protocol includes the steps of repeated centrifugation of cells and subsequent plating procedure which allows to maximize the total number of myoblasts isolated from the tissues. To the best of our knowledge, the proposed approach for myoblasts isolation and culture is unique due to application of the cell’s population pooling. This technique was only feasible after detailed characterization of the specific-Q1-Q4 cell subpopulations which was assessed in this study. Thus, the proposed protocol tested under R&D conditions could be applied and tested during myoblast culturing under cGMP conditions in preparation for future clinical application of the myoblast-based cellular therapy.
Acknowledgements
This study was supported by Dystrogen Therapeutics.
Disclosure of conflict of interest
MS is CMO and shareholder of Dystrogen Therapeutics SA, KS, MS, KK, KB, AC are Dystrogen Therapeutics SA employee; NR is scientific consultant of Dystrogen Therapeutics SA.
References
- 1.Nowaczyk M, Malcher A, Zimna A, Łabędź W, Kubaszewski Ł, Fiedorowicz K, Wierzbiński K, Rozwadowska N, Kurpisz M. Transient and stable overexpression of extracellular superoxide dismutase is positively associated with the myogenic function of human skeletal muscle-derived stem/progenitor cells. Antioxidants (Basel) 2020;9:817. doi: 10.3390/antiox9090817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausems CRM, Van Engelen BGM, Van Bokhoven H, Wansink DG. Systemic cell therapy for muscular dystrophies. Stem Cell Rev Rep. 2021;17:878–899. doi: 10.1007/s12015-020-10100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska-Migaj E, Oko-Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M. Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail. 2017;19:148–157. doi: 10.1002/ejhf.700. [DOI] [PubMed] [Google Scholar]
- 4.Romaniszyn M, Rozwadowska N, Malcher A, Kolanowski T, Walega P, Kurpisz M. Implantation of autologous muscle-derived stem cells in treatment of fecal incontinence: results of an experimental pilot study. Tech Coloproctol. 2015;19:685–696. doi: 10.1007/s10151-015-1351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mierzejewski B, Archacka K, Grabowska I, Florkowska A, Ciemerych MA, Brzoska E. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin Cell Dev Biol. 2020;104:93–104. doi: 10.1016/j.semcdb.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Klimczak A, Kozlowska U, Kurpisz M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch Immunol Ther Exp (Warsz) 2018;66:341–354. doi: 10.1007/s00005-018-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein AB, Smith GR, Raue U, Begue G, Minchev K, Ruf-Zamojski F, Nair VD, Wang X, Zhou L, Zaslavsky E, Trappe TA, Trappe S, Sealfon SC. Single-cell transcriptional profiles in human skeletal muscle. Sci Rep. 2020;10:229. doi: 10.1038/s41598-019-57110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottle BJ, Lewis FC, Shone V, Ellison-Hughes GM. Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res Ther. 2017;8:158. doi: 10.1186/s13287-017-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-adipogenic progenitors cross-talk in skeletal muscle: the social network. Front Physiol. 2019;10:1074. doi: 10.3389/fphys.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Charlton CA, Mohler WA, Blau HM. Neural cell adhesion molecule (NCAM) and myoblast fusion. Dev Biol. 2000;221:112–119. doi: 10.1006/dbio.2000.9654. [DOI] [PubMed] [Google Scholar]
- 12.Jarocha D, Stangel-Wojcikiewicz K, Basta A, Majka M. Efficient myoblast expansion for regenerative medicine use. Int J Mol Med. 2014;34:83–91. doi: 10.3892/ijmm.2014.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurner M, Asim F, Garczarczyk-Asim D, Janke K, Deutsch M, Margreiter E, Troppmair J, Marksteiner R. Development of an in vitro potency assay for human skeletal muscle derived cells. PLoS One. 2018;13:e0194561. doi: 10.1371/journal.pone.0194561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 15.Lu SH, Yang AH, Chen KK, Chiang HS, Chang LS. Purification of human muscle-derived cells using an immunoselective method for potential use in urological regeneration. BJU Int. 2010;105:1598–1603. doi: 10.1111/j.1464-410X.2009.09032.x. [DOI] [PubMed] [Google Scholar]
- 16.Rozwadowska N, Kolanowski T, Wiland E, Siatkowski M, Pawlak P, Malcher A, Mietkiewski T, Olszewska M, Kurpisz M. Characterisation of nuclear architectural alterations during in vitro differentiation of human stem cells of myogenic origin. PLoS One. 2013;8:e73231. doi: 10.1371/journal.pone.0073231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Péault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 18.Lavasani M, Lu A, Thompson SD, Robbins PD, Huard J, Niedernhofer LJ. Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol Biol. 2013;976:53–65. doi: 10.1007/978-1-62703-317-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kho AT, Kang PB, Kohane IS, Kunkel LM. Transcriptome-scale similarities between mouse and human skeletal muscles with normal and myopathic phenotypes. BMC Musculoskelet Disord. 2006;7:23. doi: 10.1186/1471-2474-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Charles JP, Akay T, Hutchinson JR, Blemker SS. Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans. Skelet Muscle. 2017;7:26. doi: 10.1186/s13395-017-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nehlin JO, Just M, Rustan AC, Gaster M. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology. 2011;12:349–365. doi: 10.1007/s10522-011-9336-5. [DOI] [PubMed] [Google Scholar]
- 23.Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kałmucki P, Kurpisz M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005;26:1188–95. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 24.Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska-Migaj E, Oko-Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M. Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail. 2017;19:148–157. doi: 10.1002/ejhf.700. [DOI] [PubMed] [Google Scholar]
- 25.Fiedorowicz K, Wargocka-Matuszewska W, Ambrożkiewicz KA, Rugowska A, Cheda Ł, Fiedorowicz M, Zimna A, Drabik M, Borkowski S, Świątkiewicz M, Bogorodzki P, Grieb P, Hamankiewicz P, Kolanowski TJ, Rozwadowska N, Kozłowska U, Klimczak A, Kolasiński J, Rogulski Z, Kurpisz M. Molecular imaging of human skeletal myoblasts (huSKM) in mouse post-infarction myocardium. Int J Mol Sci. 2021;22:10885. doi: 10.3390/ijms221910885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wargocka-Matuszewska W, Fiedorowicz K, Rugowska A, Bednarowicz K, Zimna A, Cheda Ł, Hamankiewicz P, Kilian K, Fiedorowicz M, Drabik M, Rozwadowska N, Rogulski Z, Kurpisz M. Molecular imaging of myogenic stem/progenitor cells with [18F]-FHBG PET/CT system in SCID mice model of post-infarction heart. Sci Rep. 2021;11:19825. doi: 10.1038/s41598-021-98861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


