Abstract
Purpose:
To report a rather rare entity of facial palsy due to chondromyxoid fibroma (CMF). We present a case along with clinico-pathological features, management, treatment options and follow up.
Methods :
We present a case of a 29-year-old male who suffered from right facial weakness and numbness for a period of 6-months. Imaging studies demonstrated a soft, locally invasive tumor, located mainly in the right temporal bone and extended extracranially.
Results:
A surgical procedure of local excision followed by cross-facial nerve grafting was performed.
Conclusion:
Diagnosis should be based on combination of histopathologic and radiographic findings, because of its histological similarities to chondrosarcoma.
Keywords: chondromyxoid fibroma, temporal bone, differential diagnosis, chondrosarcoma, histopathologic features, cross-facial nerve grafting
Introduction
Chondromyxoid fibroma (CMF) represents a rare benign cartilaginous bone tumor comprising approximately 0,5% of all primary bone tumors. Chondrosarcoma and chondroid chordoma are similar entities although they present with different natural history and prognosis (6). Recurrence rate of 7–27% has been reported in the literature. Usually a CMF locates to the extremities and mandible. Temporal bone location is rather unusual. We describe clinical and imaging characterictics, along with the treatment options.
Case description
History and examination
We present a case of a 29-year-old male with a 6 month history of right hemifacial weakness and numbness of the upper and lower face. Neurological examination revealed a severe peripheral facial weakness. Total paralysis of orbicularis oris muscle in the affected side resulted in mouth corner assymetry. No synkinesis was present. Facial appearance was flat and expressionless; lack of grimace or movement in frontalis muscle. (House-Brackmann grade VI).
Asymmetry, obvious weakness and reduced muscle tone was noticeable on inspection a rest. Ofthalmology revealed complete disfunction of irritating the conjunctiva and drying the right cornea due to incomplete closure of the eye at maximum effort Audiometry revealed revealed normal hearing. The patient had no pathologic reflexes. Tunning fork test: Weber was the same as in normal hearnig and Rinne test at 256, 512 Hz showed airconduction greater than bone conduction on the right. The patient’s laboratory values were normal. Initially the patient was treated as in cases of Bell’s palsy with high dose of corticosteroid for two months. There was no sign of recovery. Due to progressive neurologic deficit, we used a computered tomography (CT) scan and a magnetic resonance imaging (MRI) to exclude an infarction or a mass and to investigate the cause of this condition. CT of temporal bone revealed partial occupation of the right mastoid air cells with corrosion of the trabeculae of bone.(FIGURE 1b,c) There was no suspicious for cholosteatoma. The eardrum and the auditory ossicles of middle ear were intact. Subsequent MRI of internal acoustic meatus demonstrated an atypical lobulated soft tissue mass 1,8 cm occupying the inner – lower part of mastoid process of the right petrous bone.(FIGURE 1a) The lesion was predominantly hyperintense on T2 -weighted imaging and hypointense on T1 -weighted imaging. The lesion showed heterogenous enhancement on post gadolinium contrast images. The sigmoid sinus had no diffusion defect and the meninx-sigmoid corner and the cerebellopontine angle were free. A facial nerve electroneurography (ENoG), an electromyography (EMG) and the blink reflex test followed, which were repeated two months and one year later in order to estimate the facial function outcome. The site of facial nerve injury was central to the stylomastoid foramen. There were no composite action potentials in the right facial muscles and any type of responses from the right sphincter muscle recorded. EMG showed total denervation of frontal and sphincter muscle and a bit of spontaneous activity in the foursquare lower lip muscle. After one year it seemed a small conductance of regenarating nerve fibers by the irritation of the right facial nerve. Moreover there was an obvious reinnervation of sphincter and fouesquare lower lip muscle from the sublingual nerve and from the opposite left facial nerve respectively. The patient was operated. A known retroauricular C-shaped incicion was performed. An invasive lesion of the mastoid cavity was identified. The tumor removal required extensive drilling of the right temporal bone. Biopsy was sent to the histopathology department. The tumor had eroded the wall of the facial canal and invaded the vertical portion of the facial nerve. A cross-facial nerve grafting procedure with an anastomosis between the amputeted fascial nerve trunk -the major auricular n.-sublingual nerve was performed. Total removal of the mass was unable. The surgical approach was extremely difficult due to infiltration of important vital stuctures (VII nerve). The tumor had not penetrated the dura. The wall of the sigmoid sinus was intact and jujular bulb was free of tumor. The wound was closed in a multilayered fashion.
FIGURE 1.
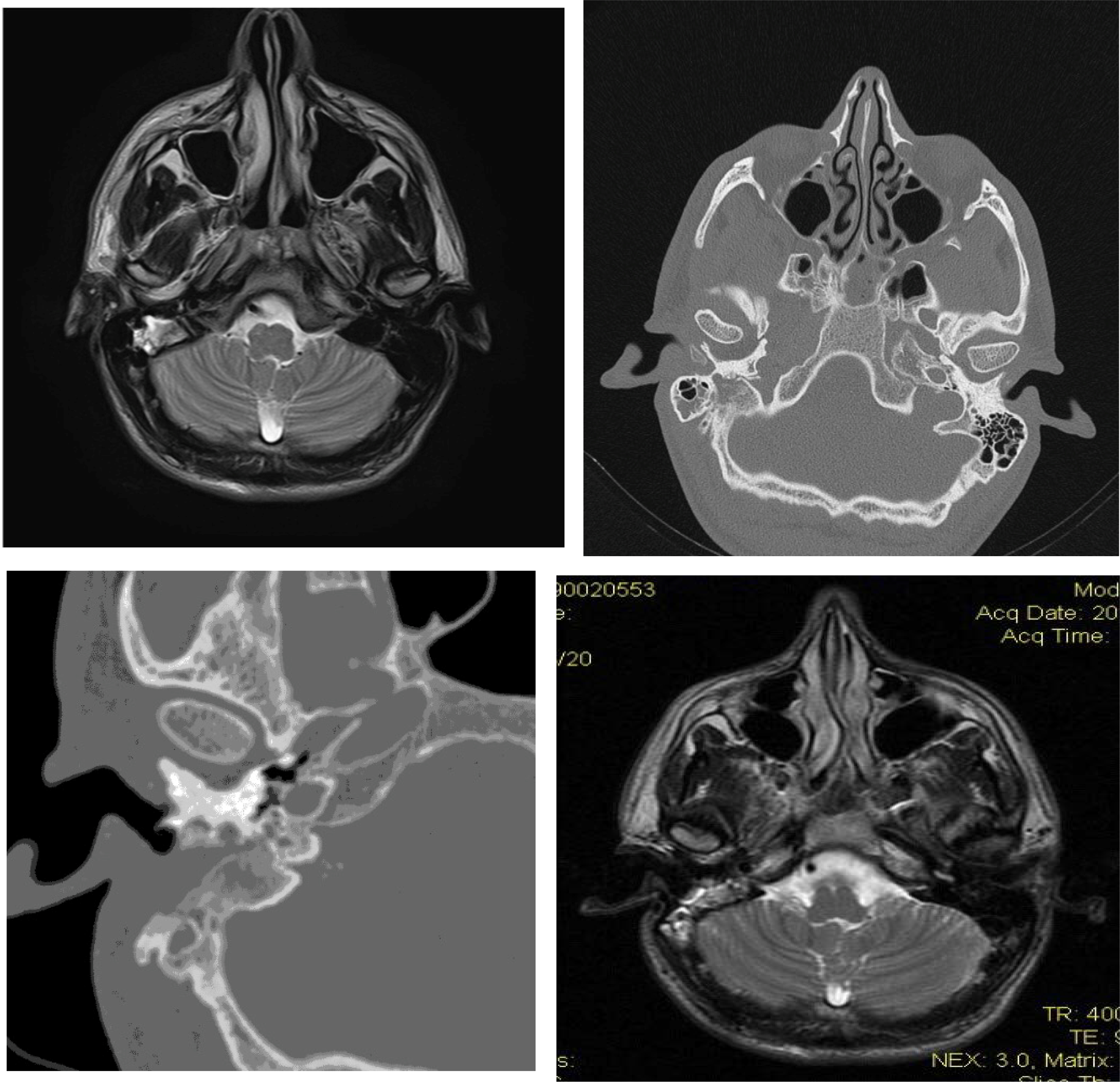
a: Axial MRI of visceral cranium: Solid-tissue mass of the right temporal bone with hyperintense pathological magnetic signal and peripheral areas of enhancement after gadolinium administration
b,c: CT of the temporal bone. An invasive soft tissue density lesion arising from the right temporal bone occupying partially the right mastoid cavity-with corrosion of the trabeculae of bone
d: Axial MRI of visceral cranium (2 years later): Hyperintense signal in the resection cavity with clear reduction of the pathological signal and of the intake of gadolinium in the right temporal bone
HISTOPATHOLOGIC EXAMINATION
The biopsy showed a pseudolobulated tumour consisting of myxoid and chondroid regions. Lobules tended to be more celullar at the periphery and were seperated by fibrous bands. The tumor cells were spindle shaped or stellate. There were some pleomorphic and binucleate tumor cells, but rarely contained mitosis. There was scattered calcification and no mature hyaline cartilage was found. Tumor cells expressed S-100 protein. The histologic features were consistent with chondomyxoid fibroma.
POSTOPERATIVE COURSE
The postoperative visceral cranium MRI demonstrated residual tumor with clearly reduced dimensions. We can distinguish a clear reduction in the area of the pathological signal and of the intake of paramagnetic substance in the left temporal bone. The patient an improvement of VII nerve function (House-Brackmann III). There had been no recurrence till the recent follow-up two years later. (FUGURE 1d)
Discussion
CMF of mastoid bone is extremely rare. In our literature review, we found 67 published cases of cranial CMF (1).
Of the cases affecting the cranium, the sinonasal structures were most commonly involved. There are only five CMF of mastoid region described in the literature till date, as shown is Supplememental Table 1. Because of its rarity in this region, its diagnosis may be easily delayed or it may be misdiagnosed as another disease of connective tissue or bone tumor.
Our case is the fourteenth reported CMF developing in temporal bone.
Kitamura K first in 1989 reported a case of CMF of mastoid region, eroding the posterior bony wall of the external auditory canal, destroying the petrous portion and extending into the occipital bone, the foramen magnum and jugular foramen.
Resection of the tumor via subtemporo-occipital approach was performed.
The vertical portion of the facial nerve was surrounded by the tumor and the stylomastoid foramen was destroyed. Due to bleeding, resection of the tumor around the jugular foramen was unable.
The tumor had reccurence one year after the initial excision and reexcision was performed in order to achieve complete cure (4).
LeMay DR, Sun JK et al. referred a second case of CMF arising from the left temporal mastoid region. A left temporal craniotomy and extensive drilling of the bone was performed. He concluded that complete resection without radiation is the main treatment of skull base CMFs. Recurrence rates depend on the degree of initial resection and reoperation is required in such cases (2).
Dr Jose Ignacio Patino-Cordoba presented a third patient with CMF arising from the mastoid area and abutting the cerebellum without penetrating the dura, the sigmoid sinus and the jugular bulb. An infratemporal fossa approach with neck dissection with blind sac closure of the external auditory canal was carried out for total tumor excision. He concluded that wide local excision (en – block complete resection) using skull base techniques is the ideal treatment for CMF of skull base. Radiotherapy is not recommended because of the benign nature of CMF.
Recently, Otto et al. reported the fourth case of CMF within the mastoid tip and the retrofascial air cells. An intact-canal wall mastoidectomy was performed. There was no evidence of recurrence six months after operation.(6)
Oh N et al. presented the fifth case of expansile destructive CMF of the mastoid portion of the temporal bone. The patient underwent a postauricular infratemporal fossa approach with a suboccipital craniectomy/cranioplasty. Skeletonization of the facial nerve and decompression of the sigmoid sinus and jugular bulb was performed. Removal of the middle ear structures and the tympanic bone was necessary, in order to achieve control of the lower cranial nerves.(10)
In our case the surgical approach was extremely difficult due to infiltration of VII nerve. Thus, the tumor resection required extensive drilling of the right mastoid region combined with neck dissection. A cross-facial nerve grafting procedure with an anastomosis between the amputated facial nerve trunk -the major auricular nerve –sublingual nerve was performed.
Thompson AL, Bharatha A et al. presented a similar case of chondromyxoid fibroma of the mastoid portion of the facial nerve canal, also destroying the facial nerve. The patient complained of progressive facial nerve paralysis. The imaging findings were consistent with a facial nerve schwannoma, but surgery revealed an irregular, cystic lesion and an histological diagnosis of CMF(5). Thus, surgical exploration should be considered in all cases with remaining peripheral facial palsy in patients with unclear clinically and radiologically findings of the ear.
There is no doubt that CMF is a benign tumour. Sometimes it can be confused with a malignant tumour because of its possibility of recurrence and the existence of nuclear atypia occasionally. It can be misdiagnosed as chondrosarcoma. This can result in over-treatment of radical resection and amputation.
All CMF of bone described in the literature have common histological and imaging features. There are many typical characteristics: The tumors are arranged in lobules, which are macrolobular, tend to be more celullar at the periphery, hypocelluler centrally and are seperated by fibrous bands. The tumor cells are spindle shaped or stellate with. There is occasional nuclear atypia (some pleomorphic and binucleate tumor cells are found), but rarely contained mitosis. There is scattered calcification and hyaline cartilage and chondroblastoma -like areas. Tumor cells and myxoid stroma express S-100 protein.
The radiological features of CMF are the following: CT scan shows isodense, soft tissue mass with calcific foci suggestive of chondroid neoplasm.
MRI shows hypointense lesion on T1-weighted images, hyperintense on T2 -weighted images with intense contrast enhancement. These findings are sentisive but not specific for CMF.
Differential diagnosis especially in cases of calcified lesions includes chordoma, chondroid chondroma and chondrosarcoma.
Histologically, chondrosarcoma can be differentiated from CMF by its invasive pattern of growth and the presence of mitosis, mature hyaline-matrix and absence of fibrous component. Chondrosarcomas infiltrate the adjacent bony trabeculae. They are more uniform than CMF, lack giant cells at the periphery of the lobules, the lobules are larger and also contain myxoid areas (8). It is important to take a large piece of tissue for biopsy in order to achieve the correct diagnosis. Chordoma cells express epithelial antigens, such as keratin, whereas CMF doesn’t stain with antibodies to this potein.
Immunohistochemistry cannot distinguish the tumors as both of them express S-100 protein and vimentin. Recent studies report a strong correlation between clinicopathological features and karyotype among cartilaginous and chondroid tumours recently. There are alterations of chromosome arm 6q in CMF and 17p1 in chondrosarcoma which are associated with malignancy, tumor grade and specific diagnosis, and are valuable for correct diagnosis.
There are no many long-term follow –up data on patients with post therapeutic CMF. There are described some cases of recurrences in patients with incomplete resection after conservative procedures, especially in those treated with intra-lesional cutturage and/or enucleation of the tumor versus those treated with en-block wide resection and marginial excision (7).
There is controversy surrounding the doctor’s decision to postoperative radiation therapy in CMF. Radiotherapy after subtotal resection or recurrence cannot be definitively recommended and needs further investigation. Roentgen or gamma-ray therapy with heavy doses was associated with the development of sarcoma in irratiated bone since 1945 (9). Thus, for some authors, radiotherapy is contraindicated due to a risk of malignant transformation (2). Other authors believe that postoperative irradiation reduces the risk of recurrence(3). Generally it is known, that radiation therapy alone is not a successful treatment in cases of sarcomas.
Factors that should be measured before take the decision for adjuvant radiotherapy are age and histological subtype, partial resection due to vicinity with critical neurological region, and/or high-risk surgery in case of recurrent tumor (3).
Feuvret L, Noël G, Calugaru V described such two cases of incompletely excised tumors required postoperative radiation -proton -therapy with no recurrence and complication. The tumors compressed cranial nerves of the skull base, thus reoperation was contraindicated (3).
Proton therapy is found to more effective in reduction of late effects of radiation than other models of radiotherapy (3).
Conclusion
Chondromyxoid fibroma of the temporal bone represents a diagnostic and therapeutic challenge. There are not specific pathognomonic characteristics of CMF. Diagnonis should be based on combination of histopathologic and radiographic findings. Clinical behaviour of CMF may be aggressive with high recurrence rate 7–27% (2). Malignant transformation of CMF has been observed in very rare cases 1–2% (8). Complete surgical excision is required for total cure and long term control. Because of its clinical and histopathological rarity CMF is difficult to diagnose as there is an absence of typical diagnostic features in each case. It is also necessary to exclude the possibility of a delayed presentation of a malignant tumor such as chondrosarcoma because of a false initial diagnosis as benign CMF. Thus, patients with initial diagnosis of CMF should undergo close and long-term clinical monitoring.
Supplementary Material
Supplemental Table 1: Summary of published cases of chondromyxoid fibromas of mastoid region of temporal bone
Footnotes
There is no conflict of interest among authors.
The patient is consent with the publication of the article.
References
- 1).Yaghi NK, DeMonte F, Chondromyxoid Fibroma of the Skull Base and Calvarium: Surgical Management and Literature Review. J Neurol Surg Rep. 2016. Mar [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).LeMay DR, Sun JK, Mendel E, et al. Chondromyxoid fibroma of the temporal bone. Surg Nerol 1997. Aug;48(2):148–52. [DOI] [PubMed] [Google Scholar]
- 3).Feuvret L, Noël G, Calugaru V, Terrier P, Habrand JL, Chondromyxoid fibroma of the skull base: differential diagnosis and radiotherapy: two case reports and a review of the literature. Acta Oncol.2005. [DOI] [PubMed] [Google Scholar]
- 4).Kitamura K, Nibu K, Asai M, Shitara N, Niki T, Chondromyxoid fibroma of the mastoid invading the occipital bone. Arch Otolaryngol Head Neck Surg. 1989. Mar [DOI] [PubMed] [Google Scholar]
- 5).Thompson AL, Bharatha A, Aviv RI, Nedzelski J, Chen J, Bilbao JM, Wong J, Saad R, Symons SP. Chondromyxoid fibroma of the mastoid facial nerve canal mimicking a facial nerve schwannoma. Laryngoscope. 2009. Jul. [DOI] [PubMed] [Google Scholar]
- 6).Otto BA, Jacob A, Klein MJ, Welling DB,Chondromyxoid fibroma of the temporal bone: case report and review of the literature. Ann Otol Rhinol Laryngol. 2007. Dec [DOI] [PubMed] [Google Scholar]
- 7).Baujat B, Attal P, Racy E, Quillard J, Parker F, Evennou A, Bobin S. Chondromyxoid fibroma of the nasal bone with extension into the frontal and ethmoidal sinuses: report of one case and a review of the literature. 2001. Mar–Apr Am J Otolaryngol. [DOI] [PubMed] [Google Scholar]
- 8).Desai SS, Jambhekar NA, Samanthray S, Merchant NH, Puri A, Agarwal M. Chondromyxoid fibromas: a study of 10 cases. J Surg Oncol. 2005. Jan [DOI] [PubMed] [Google Scholar]
- 9).Kikuchi F, Dorfman HD, Kane PB. Recurrent chondromyxoid fibroma of the thoracic spine 30 years after primary excision: case report and review of the literature. Int J Surg Pathol 2001Oct;9(4):323–9. [DOI] [PubMed] [Google Scholar]
- 10).Oh N, Khorsandi AS, Scherl S, Wang B, Wenig BM, Manolidis S, Jacobson A. Chondromyxoid fibroma of the mastoid portion of the temporal bone: MRI and PET/CT findings and their correlation with histology. Ear Nose Throat J. 2013. Apr–May [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Summary of published cases of chondromyxoid fibromas of mastoid region of temporal bone


