Abstract
Background
The role of immune checkpoint inhibitors (ICIs) in NSCLC patients with EGFR mutations are controversial. In this study, we aim to investigate the therapeutic efficacy of ICIs alone or in combination in patients with EGFR mutated NSCLC in late-line settings, and explore the factors that may predict the efficacy of ICIs.
Patients and Methods
We retrospectively collected the clinical and pathological information of 75 patients with confirmed EGFR mutations. All patients have developed acquired resistance to EGFR-TKIs, and were treated with ICIs in late line settings from January 2019 to January 2021, at Shandong Caner Hospital and Institute. Therapeutic efficacy was evaluated by tumor response and survival.
Results
The median follow-up period was 7.3months (range 1.8-31.8 months). The overall response rate (ORR) was 8.0%, and the disease control rate (DCR) was 78.7%. The median PFS for all patients was 3.9 months (95% CI, 2.7-5.0), while the median OS was 9.9 months (95% CI, 5.3-14.6). We found that patients with longer response duration to EGFR-TKIs (≥10 months) showed a longer PFS when treated with immunotherapy compared with patients with shorter PFS-TKI (<10 months), the median PFS in two groups were 5.2 months [95%CI 4.2-6.2] and 2.8 months [2.0-3.6]) respectively (HR, 0.53, 95%CI, 0.31-0.91, P=0.005). In exploratory analysis, we found that concurrent extracranial radiotherapy and higher body mass index (BMI) are associated with longer PFS (P values are 0.006 and 0.021 respectively).
Conclusions
We found that combination regimen of immunotherapy plus chemotherapy plus antiangiogenetic agents may yield longer survival in patients with EGFR mutated NSCLC. We also found that patients with longer PFS-TKI, concurrent extracranial radiotherapy and higher BMI may benefit more from immunotherapy.
Keywords: epidermal growth factor receptor (EGFR), immune checkpoint inhibitor (ICI), non-small cell lung cancer (NSCLC), efficacy, prognosis
Introduction
Epidermal growth factor receptor (EGFR) mutations remains the most common driver mutations in patients with lung adenocarcinomas (LUAD), with an incidence of 50% in Asians and 9.8% in Caucasian Europeans (1, 2). The development of EGFR tyrosine kinase inhibitors (EGFR-TKIs) have significantly prolonged the survival of patients harboring EGFR mutations. The progression-free survival (PFS) of first- and second-generation EGFR-TKIs, including Gefitinib, Erlotinib, Icotinib, and Afatinib, is usually around 9-13 months, while the third generation EGFR-TKI, Osimertinib, yielded a PFS of 18.9 months. Despite these progress, drug resistance and disease progression are inevitable. Hence, disease management after TKI-resistance has become a critical issue, and require further studies.
Immune checkpoint inhibitors (ICIs) are group of monoclonal antibodies targeting immune checkpoints including PD-1/PD-L1 and CTLA-4 etc. By blocking the interaction between immune checkpoints and their partners, ICIs mediate tumor killing effects through unleashing the “breaks” of immune system (3). Although ICIs, especially those targeting PD-1 and PD-L1 has been proven to be effective in patients with advanced NSCLC, their roles in patients harboring EGFR mutations are still in debate. Pre-clinical evidences suggested that activation of EGFR would up-regulate the expression of PD-L1 through numerous signaling pathways, including p-ERK1/2/p-c-Jun and JAK/STAT3, and blockade of PD-1 would improve the survival of murine models with EGFR mutated lung adenocarcinomas by promoting T cell infiltration and down-regulating pro-tumorigenic cytokines (4–6). However, it is well-demonstrated that ICIs alone or in combination with TKIs could not yield improvements in survival in patients with EGFR mutations compared with general unselected NSCLC patients (7–10).
Luckily, recent studies have shed some light into this area. In PROLUNG trial, which compared the therapeutic efficacy of pembrolizumab plus docetaxel versus docetaxel alone in pretreated NSCLC patients, 25 patients with EGFR mutations were enrolled. The PFS in patients received pembrolizumab plus docetaxel was significantly prolonged compared with patients received docetaxel alone. The more recent IMpower150 study further uncover the value of anti-angiogenic therapy in EGFR mutated NSCLC. Patients received atezolizumab and bevacizumab plus chemotherapy had better clinical outcome with an ORR of 73.5% (versus 40.9% in bevacizumab plus chemotherapy group) and median PFS of 10.2 months (versus 7.1 months in bevacizumab plus chemotherapy group) (11, 12). However, real-world efficacy of ICIs in pretreated EGFR mutated NSCLC patients remains limited (13, 14).
In this retrospective study, we aim to examine the therapeutic efficacy of ICIs alone or in combination in patients with EGFR mutated NSCLC in real-world setting. We also explore the potential clinical and pathological characteristics that may predict the efficacy of ICIs.
Patients and Methods
Study Design and Patients
In this retrospective study, we aim to study NSCLC patients with EGFR mutations treated with immune checkpoint inhibitors after receiving EGFR-TKIs. All patients are enrolled at Shandong Cancer Hospital and Institute, form January 2019 to January 2021. The inclusion criteria were as follows: 1) stage IIIB-IVB NSCLC with confirmed EGFR activating mutations, 2) treated with immune checkpoint inhibitors after disease progression with EGFR-TKIs. Patients without measurable tumour lesions or treated with immune checkpoint inhibitors in front line settings were excluded. We retrospectively reviewed the electric medical records of enrolled patients, and collected their detailed clinicopathologic characteristics and clinical responses. This study was approved by the institutional review board of Shandong Cancer Hospital and Institute and was performed in accordance with the Declaration of Helsinki.
Treatment Procedures
All patients were treated with first-, second-, or third-generation EGFR-TKIs prior to immune checkpoint inhibitors. For immunotherapy, patients were treated with one of the following anti-PD-1 or PD-L1 agents until disease progression, or unacceptable toxicity: pembrolizumab (Merck & Co., USA), nivolumab (Bristol-Myers Squibb, USA), sintilimab (Innovent Biologics, China), toripalimab (Shangha Merck & Co.), camrelizumab (Jiangsu Hengrui Medicine, China), tislelizumab (BeiGene, China), durvalumab (AstraZeneca, USA), or atezolizumab (Roche, USA). This study was approved by the institutional review board of Shandong Cancer Hospital and Institute and was performed in accordance with the Declaration of Helsinki. Individual consent for this retrospective analysis was waived.
Outcomes
Radiological assessments of primary and metastatic lesions were performed every 6 weeks during treatment. Therapeutic responses were evaluated with RECIST 1.1. Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Progression-free survival (PFS) was defined as the time interval from the first-time administration of anti-PD-1 or PD-L1 agents to confirmed disease progression or mortality from any cause. Overall survival (OS) was defined as the time interval from the first-time administration of anti-PD-1 or PD-L1 agents to mortality from any cause or the last follow-up. Safety profiles were evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
All statistical analyses were performed using Statistical Product and Service Solutions (SPSS) version 26.0. For survival analyses, Kaplan-Meier analysis were performed, and log-rank test was used for comparison of survival times. Uni- and multi-variate Cox regression model were employed to analyse factors that may associated with treatment response and prognosis. Variates with P value <0.1 in univariate analyses were then subjected for multivariate analysis. In all analyses, differences were significant when P < 0.05.
Results
Patient Characteristics
In total, 75 patients were enrolled in this retrospective study. The relevant clinical and pathological characteristics are included in Table 1. Among the included patients, the distribution of male and female is relatively equal (49.3% vs 50.7%). The median age of patients were 52 years old, ranges from 36 to 81 years old. The majority of patients have stage IV disease. EGFR exon 21 L858R (40%) and 19 exon del (49.3%) are the most common mutation types. Gefitinib was the most commonly used EGFR-TKI in first-line setting (57.3%). During TKI treatment, 22 patients (29.3%) achieved partial regression, and 18 patients acquired subsequent T790M mutation. Most patients received immunotherapy in late line (≥3) setting. Thirty-one patients (41.3%) received immunotherapy concurrent with chemotherapy, 16 (21.3%) are treated together with antiangiogenic therapy, 24 patients (32%) received immunotherapy alongside with chemotherapy and antiangiogenic therapy, while only 4 patients (5.3%) received monotherapy. However, since immunotherapy was applied in late-line settings, PD-L1 expression was not reported in the majority (80%) of enrolled patients. During immunotherapy, 11 patients (14.7%) received concurrent radiotherapy, while 8 (10.7%) received extracranial radiotherapy.
Table 1.
Patient characteristics.
| Factor | N (%) |
|---|---|
| Gender | |
| Male | 37 (49.3%) |
| Female | 38 (50.7%) |
| Age (median, range) (y) | 52.0 (36.0-81.0) |
| Smoking history (n) | |
| Never-smoker | 63 (84.0%) |
| Former/current smoker | 12 (16.0%) |
| T stage in naïve (n) | |
| 1-2 | 40 (53.3%) |
| 3-4 | 35 (46.7%) |
| TNM stage (n) | |
| IIIB-IIIC | 4 (5.3%) |
| IVA-IVB | 71 (94.7%) |
| Type of mutation (n) | |
| EGFR exon 19 del | 30 (40%) |
| EGFR exon 21 L858R | 37 (49.3%) |
| Others* | 8 (10.7%) |
| Acquired T790M mutation | |
| No | 57 (76.0%) |
| Yes | 18 (24.0%) |
| Type of EGFR-TKI (n) | |
| Gefitinib | 43 (57.3%) |
| Erlotinib | 11 (14.7%) |
| Icotinib | 10 (13.3%) |
| Afatinib | 8 (10.7%) |
| Osimertinib | 3 (4.0%) |
| Best response to EGFR-TKIs (n) | |
| PR | 22 (29.3%) |
| SD/PD | 53 (70.7%) |
| Number of immunotherapy lines (n) | |
| 2 | 24 (32.0%) |
| ≥3 | 51 (68.0%) |
| Treatment regimen (n) | |
| Anti-PD-1/PD-L1 monotherapy | 4 (5.3%) |
| Anti-PD-1/PD-L1 + Chemo | 31 (41.3%) |
| Anti-PD-1/PD-L1+Anti-angiogenesis | 16 (21.3%) |
| Anti-PD-1/PD-L1+Chemo+anti-angiogenesis | 24 (32.0%) |
| PD-L1 expression | |
| Negative | 3 (4.0%) |
| 1-49% | 6 (8.0%) |
| ≥50% | 6 (8.0%) |
| Not reported | 60 (80.0%) |
*Others include EGFR exon 20INS (n=3), EGFR exon 18 G719A (n=2), EGFR exon 20 S768I (n=1), EGFR exon 21 L861Q (n=1), EGFR exon 21 G863D (n=1).
EGFR, Epidermal growth factor; TKI, Tyrosine kinase inhibitor; PR, Partial response; SD, Stable disease; PD, Progression disease.
Efficacy of Immunotherapy
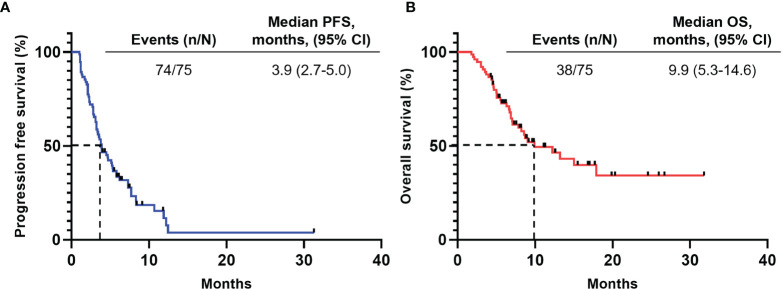
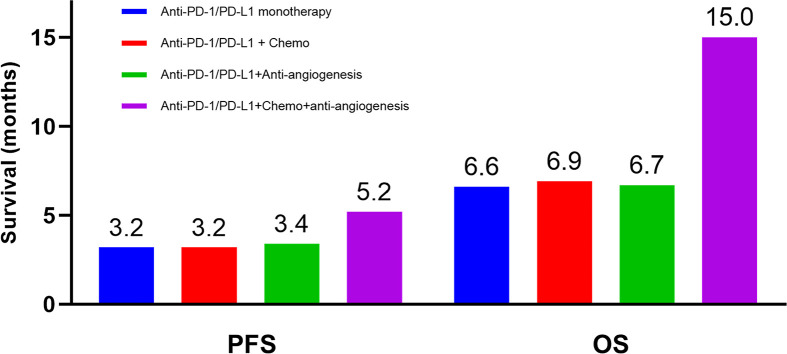
The median follow-up period was 7.3 months (range 1.8-31.8 months). During treatment, 6 patients (8.0%) achieved partial regression (PR), 53 patients (70.7%) experienced stable disease (SD), and 16 patients (21.3%) developed progression disease (PD), yielding an overall response rate (ORR) of 8.0%, and a disease control rate (DCR) of 78.7%. As shown in Figure 1, the median PFS for all patients was 3.9 months (95% CI, 2.7-5.0), while the median OS was 9.9 months (95% CI, 5.3-14.6). The comparison of the PFS and OS of different therapeutic regimen are displayed in Figure 2. Although did not reach statistical significance, patients received immunotherapy plus chemotherapy plus anti-angiogenic agents showed longest survival compared to other groups (median PFS 5.2 months, median OS 15.0 months), while patients received immune-monotherapy showed the shortest survival (median PFS 3.2 months, median OS 6.6 months).
Figure 1.
Survival outcomes. The median PFS for all patients was 3.9 months (95% CI, 2.7-5.0), while the median OS was 9.9 months (95% CI, 5.3-14.6). (A) Progression-free survival. (B) Overall survival. CI, Confidence interval.
Figure 2.
Median survival of patients with different therapeutic regimen. Blue: anti-PD-1/PD-L1 monotherapy, Red: anti-PD-1/PD-L1 plus chemotherapy, Green: anti-PD-1/PD-L1 plus anti-angiogenic agents, Purple: anti-PD-1/PD-L1 plus chemotherapy plus anti-angiogenic therapy. PFS, progression-free survival; OS, overall survival.
As different type of primary EGFR mutation may interfere patient’s response to immunotherapy, we further analyzed the impact of different mutation types on survival. In general, we found no difference in PFS nor OS among different EGFR mutant (all P-value >0.05). However, across all treatment settings, although reach no statistical significance, longer OS were observed in patients bear L858R mutant, suggesting patients bear EGFR L858R mutant may benefit more from immunotherapy. For acquired resistance, no significant difference was found in both PFS and OS between patients with or without acquired T790M mutant (all P-value >0.05) (Table 2).
Table 2.
Efficacy of immunotherapy in patients with different types of EGFR mutations.
| Mutation type | General (95%CI), mo | ICI alone (95%CI), mo | ICI+Chemo (95%CI), mo | ICI+Anti-angiogenesis (95%CI), mo | ICI+Chemo+Anti-angiogenesis (95%CI), mo | |
|---|---|---|---|---|---|---|
| Primary | 19del | PFS:3.87 (1.823-5.917) OS: 7.07 (4.394-9.746) |
PFS:3.2 OS:6.63 |
PFS: 3.1 (1.396-4.806) OS: 9.93 (0.652-19.208) |
PFS: 3.42 (1.829-5.011) OS: 4.67 (4.311-5.029) |
PFS: 7.3 (3.839-10.761) OS: 7.83 (4.546-11.114) |
| 21 L858R | PFS: 3.9 (2.619-5.181) OS: 13.2 (5.497-20.903) |
PFS: 4.7 (1.499-7.901) OS: 17.9 (0-43.073) |
PFS: 5.31 (2.621-7.999) OS: 13.2 (7.018-19.382) |
PFS: 3.1 (1.991-4.209) OS: 6.73 (3.958-9.502) |
PFS: 3.9 (3.608-4.192) OS: 11.42 (8.971-13.874) |
|
| Other | PFS: 2.3 (1.092-3.508) OS: 9.03 |
/ | PFS: 1.4 (0.322-2.478) OS: 8.7 (3.957-13.443) |
PFS: 5.8 OS: NR |
PFS: 2.3 (0.22-4.380) OS: NR |
|
| Acquired | T790M- | PFS: 3.6 (2.129-5.071) OS: 8.7 (3.789-13.611) |
PFS: 2.7 OS: 2.17 |
PFS: 3.1 (1.566-4.634) OS: 9.02 (5.354-12.706) |
PFS: 3.1 (2.048-4.152) OS: 6.73 (1.593-3.607) |
PFS: 4.67 (1.312-8.028) OS: NR |
| T790M+ | PFS: 3.9 (2.276-5.524) OS: 15 (4.884-25.116) |
PFS: 3.2 OS: 6.63 |
PFS: 3.2 (1.16-5.24) OS: NR |
PFS: 3.9 (0-9.453) OS: 5 |
PFS: 5.2 (2.436-7.964) OS: 15.0 (5.727-24.273) |
CI, Confidence interval; ICI, Immune checkpoint inhibitor; PFS, Progression free survival; OS, Overall survival; NR, Not reached.
Factors Associated With Therapeutic Efficacy of Immunotherapy
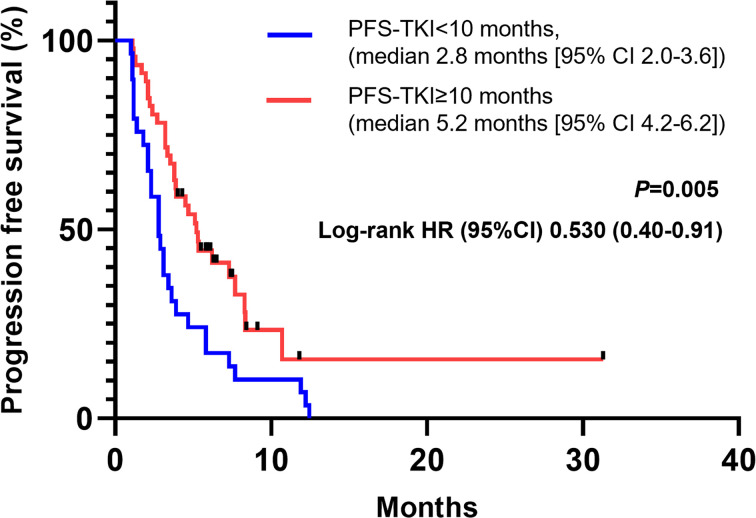
According to previous report, the PFS of front-line EGFR-TKI may predict the therapeutic efficacy of posterior immunotherapy. In our study cohort, the median PFS of EGFR-TKI was 10 months (95% CI, 8.69-11.31). Similar to previous report, we found a cut-off value at 10 months would achieve most statistical differences in predicting the PFS of immunotherapy. As displayed in Figure 3, patients with longer PFS-TKI (≥10 months) showed a longer PFS when treated with immunotherapy compared with patients with shorter PFS-TKI (<10 months), the median PFS in two groups were 5.2 months [95%CI 4.2-6.2] and 2.8 months [2.0-3.6]) respectively (HR, 0.53, 95%CI, 0.31-0.91, P=0.005).
Figure 3.
Patients with PFS-TKI longer than 10 months showed longer PFS during immunotherapy. CI, confidence interval; HR, hazard ratio.
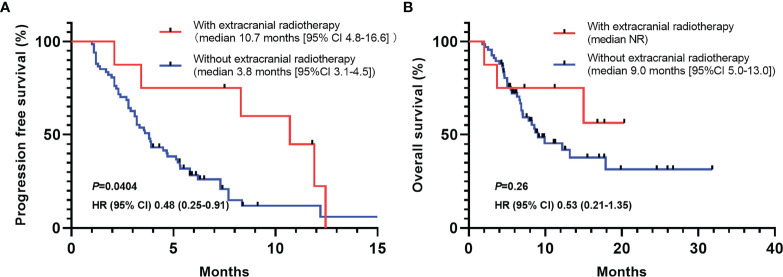
As more and more studies have demonstrated that concurrent radiotherapy during immunotherapy plays an essential role in inflaming immune microenvironment and improve the efficacy of immunotherapy, we perform a sub-group analysis to study the effect of extracranial radiotherapy on the efficacy of immunotherapy. We found that concurrent extracranial radiotherapy is associated with longer PFS (median PFS, 10.7 months [95% CI 4.8-16.6] vs 3.8 months [3.1-4.5]) (HR, 0.48, 95%CI 0.25-0.91, P=0.0404) (Figure 4A). Although have not reached statistical significance, patients with concurrent extracranial radiotherapy also showed longer OS (median OS, NR vs 9.0 months [95%CI 5.0-13.0]) (HR, 0.53, 95%CI 0.21-1.35, P=0.26) (Figure 4B).
Figure 4.
PFS and OS of patients with or without concurrent extracranial radiotherapy during immunotherapy. (A) Patients received concurrent extracranial radiotherapy showed significantly longer PFS (HR, 0.48, 95%CI 0.25-0.91, P=0.0404). (B) Overall survival of patients with or without concurrent extracranial radiotherapy. HR, hazard ratio; CI, confidence interval.
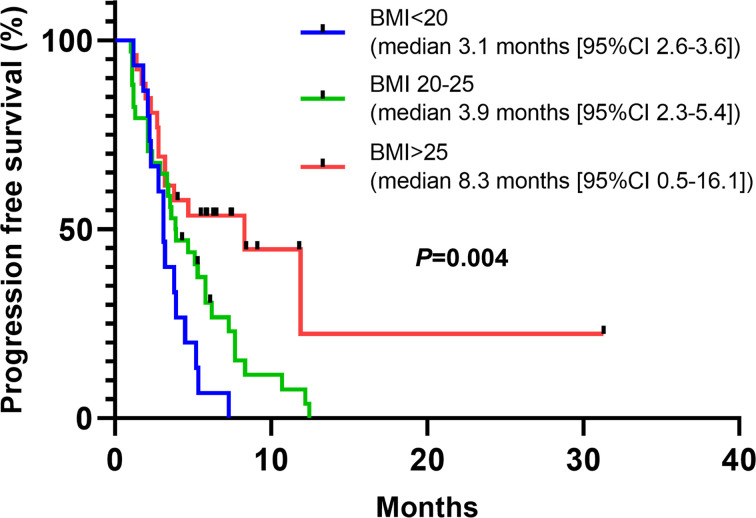
We further performed uni- and multi-variate Cox Regression Analysis to explore the potential clinical and pathological parameters that may be associated with PFS or OS. In consistent with our previous results, in multivariate cox analysis, we found that PFS-TKI and concurrent extracranial radiotherapy are associated with PFS (P values are 0.006 and 0.021 respectively) (Table 3). No parameters were found related to OS (Table 4). Interestingly, we found body mass index was associated with PFS in univariate cox analysis. Linear regression revealed that larger BMI is associated with longer PFS (r=0.4, P=0.005), further subgroup analysis also indicated that patients with BMI over 25 is associated with longer PFS (P=0.004) (Figure 5).
Table 3.
Uni- and multivariate Cox regression analysis of factors associated with PFS.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender | ||||
| Male | ||||
| Female | 1.061 (0.638-1.765) | 0.818 | ||
| Age | ||||
| <60 | ||||
| ≥60 | 1.473 (0.833-2.605) | 0.183 | ||
| Body mass index | ||||
| ≤20 | 0.007 | 0.126 | ||
| 20-25 | 3.202 (1.509-6.793) | 0.002 | 2.780 (0.957-8.074) | 0.060 |
| ≥25 | 1.97 (1.046-3.711) | 0.036 | 2.461 (0.947-6.396) | 0.065 |
| T stage | ||||
| T1-2 | ||||
| T3-4 | 0.945 (0.569-1.570) | 0.828 | ||
| Presence of liver metastasis | ||||
| Yes | ||||
| No | 1.433 (0.723-2.842) | 0.303 | ||
| Presence of brain metastasis | ||||
| Yes | ||||
| No | 0.939 (0.536-1.647) | 0.827 | ||
| Types of mutation | ||||
| Exon 19 del | 0.374 | |||
| Exon 21 L858R | 0.562 (0.247-1.277) | 0.169 | ||
| Others | 0.605 (0.272-1.347) | 0.605 | ||
| Types of EGFR-TKI | ||||
| Gefitinib | 0.444 | |||
| Erlotinib | 1.450 (0.347-6.051) | 0.611 | ||
| Icotinib | 1.838 (0.395-8.549) | 0.438 | ||
| Afatinib | 1.373 (0.289-6.518) | 0.690 | ||
| Osimertinib | 2.891 (0.601-13.911) | 0.185 | ||
| Acquired T790M mutation | ||||
| Yes | ||||
| No | 0.893 (0.421-1.894) | 0.797 | ||
| PFS to EGFR-TKIs | ||||
| <10 months | ||||
| ≥10 months | 1.934 (1.155-3.237) | 0.012 | 5.279 (1.629-17.114) | 0.006 |
| Previous extracranial radiotherapy | ||||
| Yes | ||||
| No | 0.944 (0.558-1.595) | 0.828 | ||
| Previous thoracic radiotherapy | ||||
| Yes | ||||
| No | 0.981 (0.557-1.729) | 0.948 | ||
| Number of immunotherapy lines (n) | ||||
| 2 | ||||
| ≥3 | 1.352 (0.823-2.220) | 0.234 | ||
| Treatment regimen (n) | ||||
| Anti-PD-1/PD-L1 monotherapy | 0.488 | |||
| Anti-PD-1/PD-L1 + Chemo | 0.807 (0.233-2.793) | 0.734 | ||
| Anti-PD-1/PD-L1+Anti-angiogenesis | 1.379 (0.747-2.546) | 0.304 | ||
| Anti-PD-1/PD-L1+Chemo+anti-angiogenesis | 1.572 (0.788-3.138) | 0.199 | ||
| Concurrent extracranial radiotherapy | ||||
| Yes | ||||
| No | 2.251 (0.936-5.417) | 0.070 | 4.694 (1.266-17.406) | 0.021 |
CI, Confidence interval; PFS, Progression free survival; EGFR, Epidermal growth factor; TKI, Tyrosine kinase inhibitor. Bold: P value < 0.05.
Table 4.
Uni- and multivariate Cox regression analysis of factors associated with OS.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender | ||||
| Male | ||||
| Female | 0.955 (0.501-1.822) | 0.890 | ||
| Age | ||||
| <60 | ||||
| ≥60 | 0.715 (0.353-1.449) | 0.352 | ||
| Body mass index | ||||
| ≤20 | 0.354 | |||
| 20-25 | 1.359 (0.515-3.586) | 0.535 | ||
| ≥25 | 1.745 (0.815-3.739) | 0.152 | ||
| T stage | ||||
| T1-2 | ||||
| T3-4 | 1.226 (0.639-2.354) | 0.540 | ||
| Presence of liver metastasis | ||||
| Yes | ||||
| No | 0.592 (0.260-1.353) | 0.214 | ||
| Presence of brain metastasis | ||||
| Yes | ||||
| No | 0.593 (0.304-1.156) | 0.125 | ||
| Types of mutation | ||||
| Exon 19 del | 0.657 | |||
| Exon 21 L858R | 1.588 (0.462-5.458) | 0.463 | ||
| Others | 1.231 (0.362-4.182) | 0.739 | ||
| Acquired T790M mutation | ||||
| Yes | ||||
| No | 1.242 (0.585-2.640) | 0.573 | ||
| PFS to EGFR-TKIs | ||||
| <10 months | ||||
| ≥10 months | 1.610 (0.841-3.083) | 0.150 | ||
| Previous extracranial radiotherapy | ||||
| Yes | ||||
| No | 0.948 (0.489-1.838) | 0.875 | ||
| Previous thoracic radiotherapy | ||||
| Yes | ||||
| No | 0.723 (0.368-1.421) | 0.347 | ||
| Number of immunotherapy lines (n) | ||||
| 2 | ||||
| ≥3 | ||||
| Treatment regimen (n) | ||||
| Anti-PD-1/PD-L1 monotherapy | 0.727 | |||
| Anti-PD-1/PD-L1 + Chemo | 1.583 (0.420-5.970) | 0.498 | ||
| Anti-PD-1/PD-L1+Anti-angiogenesis | 1.424 (0.623-3.255) | 0.402 | ||
| Anti-PD-1/PD-L1+Chemo+anti-angiogenesis | 1.658 (0.666-4.124) | 0.277 | ||
| Concurrent extracranial radiotherapy | ||||
| Yes | ||||
| No | 1.919 (0.578-6.371) | 0.287 | ||
CI, Confidence interval; PFS, Progression free survival; EGFR, Epidermal growth factor; TKI, Tyrosine kinase inhibitor.
Figure 5.
Patients with higher BMI are associated with better response to immunotherapy. Patients with BMI higher than 25 showed longer PFS compared with patients with lower BMI (P=0.004). BMI, body mass index.
Discussion
In this retrospective study, we examine the therapeutic efficacy of ICIs alone or in combination in patients with EGFR mutated NSCLC, founding that a combination of ICIs and anti-angiogenic agents plus chemotherapy would lead to longer PFS and OS, while safety profile is tolerable. For the first time, we also found that concurrent extracranial radiotherapy would significantly prolong the PFS of ICI treatment. We also found that longer PFS-TKI and larger BMI could be a predictor for better response to immunotherapy.
Patients bear EGFR mutations has long been associated with inferior response to second and late line chemotherapy after resistance to EGFR-TKI. In IMPRESS trial, median PFS for patients receiving chemotherapy after resistant to first line gefitinib was 5.4 months, while in AURA3 study, a median PFS of 4.4 months were reported in patients receiving platinum-based chemotherapy after acquired T790M mutation following gefitinib resistance (15, 16). In our study, the median PFS is 3.9 months, lower compared to historical controls. However, as the majority of enrolled patients were treated with immunotherapy in late (3+) line settings, these results could not be compared directly. In recent study, Yu et al. showed that after fail to EGFR-TKI treatment, patients received ICIs plus chemotherapy as second-line therapy had higher response rate compared to anti-angiogenesis plus chemotherapy (ORR 29.5% vs. 13.0%, P=0.018), but no significant difference in patient’s prognosis (median PFS 7.59 vs. 6.90 months, P=0.552) (17). Hence, the value of second-line immunotherapy in patients fail EGFR-TKI treatment requires further investigation.
Studies in EGFR mutated murine models and cell lines suggested that the activation of EGFR would up-regulate PD-L1 expression, and anti-PD-1 therapy could improve the survival of mice with EGFR mutated tumors. However, further studies of clinical samples imply that patients harbor EGFR mutations are associated with fewer infiltrated immune cells and lower PD-L1 expression level, therefore an “immune-cold” microenvironment (18, 19). In practice, patients with EGFR mutations are usually associated with inferior response to anti-PD-1/PD-L1 monotherapy. Although pre-clinical studies suggest that treatment of EGFR-TKIs would inflaming immune microenvironment via improve T cell infiltration and decrease the infiltration of CD4+ regulatory T cells (6, 20, 21), further clinical trials showed that combination of EGFR-TKIs and anti-PD-1 therapy lead to an unacceptable occurrence rate of adverse events, especially interstitial pneumonitis (10, 22).
In consistent with our study, clinical trials and real-world data indicated that combination of ICIs with chemotherapy plus anti-angiogenic agents would yield longer survival comparing to ICI monotherapy or ICIs plus either chemotherapy or anti-angiogenic therapy alone. In IMpower150 study, 58 patients with sensitizing EGFR mutation were enrolled, and the median overall survival were significantly prolonged in patients received ABCP (atezolizumab plus bevacizumab plus chemotherapy), compared to patients received ACP (atezolizumab plus chemotherapy) regimen (11). Numbers of clinical trials have demonstrated that combination of bevacizumab and EGFR-TKI would provide clinical benefits to patients with sensitive EGFR mutations, comparing to EGFR-TKI alone, indicating the value of anti-angiogenic therapy in this patient cohort (23, 24). In mechanism, activation of EGFR signaling pathway in tumor would up-regulate VEGF expression, hence sensitize to anti-angiogenic therapies (25).
Although did not met statistical significance, our study suggested that patients bear EGFR L858R mutation may benefit more from immunotherapy, both alone and in combination. This result is consisted with previous reports that patients bear EGFR L858R mutation have a higher response rate compared to patients bear 19del mutation (13). In mechanism, tumors with L8585R mutation have higher level of tumor mutation burden (TMB) compared to tumors with 19del mutation. Recent study also suggested that tumor with EGFR L858R mutation have higher level of PD-L1 expression and are positively associated with inflammatory phenotype (26).
The relationship between the therapeutic efficacy of EGFR-TKIs in front lines and ICIs in late lines are still in debate. In our study, we found that patients with longer response duration to EGFR-TKIs tends to have longer PFS in immunotherapy, with median PFS of 5.2 months vs 2.8 months. In contrast with our finding, a retrospective study by Liu et al. demonstrated that patients with shorter PFS to EGFR-TKI are associated with better response to late line immunotherapies (14). As treatment of EGFR-TKIs may inflame immune microenvironment by promoting the release of neoantigens, infiltration of effector T cells, and production of pro-inflammatory cytokines, we hypothesis that longer TKI treatment period may transform the original “cold” immune microenvironment to a “hotter” one, therefore more suitable for ICIs. However, the inconsistency of results may also cause by limited sample size and tumor heterogeneity, and requires further investigation in larger patient populations.
Radiotherapy may work synergistically with immunotherapy, preclinical evidences indicated that radiotherapy may re-programme tumor microenvironment by promoting the release of tumor neoantigen, activating innate immune response via cGAS/STING pathway and improve immune cell infiltration. A reanalysis of KEYNOTE-001 trial indicated that patients with advanced NSCLC who received previous radiotherapy would yield longer progression-free survival and overall survival with pembrolizumab treatment comparing to patients without prior radiotherapy (27). In a recent pooled analysis of 2 major clinical trials that evaluate the therapeutic efficacy of pembrolizumab with or without radiotherapy in patients with metastatic NSCLC, Theelen et al. found that the patients treated concurrently with pembrolizumab and radiotherapy result in higher response rate as well as longer PFS and OS (28). However, the role of radio-immunotherapy in patients with EGFR mutations is still unclear (29). In our study, for the first time, we reported that concurrent extracranial radiotherapy during immunotherapy is associated to longer PFS (10.7 months vs 3.8 months), and although did not meet statistical significance, patients received radiotherapy plus immunotherapy also showed longer OS (NR vs 9.0 months). However, due to very limited sample size, the role of radio-immunotherapy in patients with EGFR mutation still requires further investigation.
Interestingly, in multivariate Cox regression, we found that larger BMI is associated with longer PFS. There is a complicated relationship between obesity and cancer prognosis, obesity may increase the risk of cancer development, but can also protect patients with advanced NSCLC from worse outcomes, such as wasting (30). In a pooled analysis of 4 clinical trials that compared the efficacy of atezolizumab versus docetaxel in patients with advanced NSCLC, Kichenadasse et al. found that higher BMI is independently associated with better prognosis with atezolizumab, especially in patients with high expression of PD-L1 (31). In mechanism, obese adipose tissue is regarded as chronically inflammation tissues, through expressing inflammatory cytokines such as IL-1beta, IL-6, IL-10, and TNF-alpha, cancer-associated obese adipocyte recruits macrophages, neutrophils and other immune cells into tumour microenvironment (32). Moreover, by producing leptin, obesity also impairs the function of T cells, increasing the proportion of exhausted PD-1 positive T cell (33, 34). Hence, the association between higher BMI and better response to immunotherapy is probably due to the existence of exhausted PD-1 positive T cells in adipose tissue.
In conclusion, in our study, we found that combination regimen of immunotherapy plus chemotherapy plus anti-angiogenic therapy would yield better PFS and OS in patients with EGFR mutations. We also found that longer response duration to EGFR-TKIs, concurrent extracranial radiotherapy, and higher BMI are independently associated with better response to immunotherapy. However, due to a relatively low sample size (n=75), these conclusions still require further validation in a larger patient population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional review board of Shandong Cancer Hospital and Institute. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
MB, conceptualization, methodology, data curation, visualization, and writing - original draft. WW, data curation and visualization. XG and PJ, visualization and investigation. LW, data curation, writing- reviewing and editing. HW, data curation. JY, conceptualization and supervision. XM, conceptualization, writing- reviewing and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (81972864), Academic Promotion Program of Shandong First Medical University (2019RC002), Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001), Science and Technology Plan of Jinan (201907113), Medical Science and Technology Project of Henan Province (SB201901112).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Gahr S, Stoehr R, Geissinger E, Ficker JH, Brueckl WM, Gschwendtner A, et al. EGFR Mutational Status in a Large Series of Caucasian European NSCLC Patients: Data From Daily Practice. Br J Cancer (2013) 109:1821–8. doi: 10.1038/bjc.2013.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, et al. EGFR Mutation Testing Practices Within the Asia Pacific Region: Results of a Multicenter Diagnostic Survey. J Thorac Oncol (2015) 10:438–45. doi: 10.1097/JTO.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma P, Allison JP. Dissecting the Mechanisms of Immune Checkpoint Therapy. Nat Rev Immunol (2020) 20:75–6. doi: 10.1038/s41577-020-0275-8 [DOI] [PubMed] [Google Scholar]
- 4. Lastwika K, Wilson W, Dennis PA. Abstract 4981: PI3K/AKT/Mtor Pathway Activation Drives Expression of the Immune Inhibitory Ligand PD-L1 in NSCLC. Cancer Res (2013) 73(8_Supplement):4981. doi: 10.1158/1538-7445.am2013-4981. [DOI] [Google Scholar]
- 5. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients With EGFR Mutation. J Thorac Oncol (2015) 10:910–23. doi: 10.1097/JTO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 6. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discovery (2013) 3:1355–63. doi: 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated non–Small Cell Lung Cancer—a Meta-Analysis. J Thorac Oncol (2017) 12:403–7. doi: 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 8. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4:210–6. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol (2018) 13:1138–45. doi: 10.1016/j.jtho.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oxnard GR, Yang JCH, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: A Multi-Arm, Phase Ib Trial of Osimertinib Combined With Selumetinib, Savolitinib, or Durvalumab in EGFR-Mutant Lung Cancer. Ann Oncol (2020) 31:507–16. doi: 10.1016/j.annonc.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab Plus Bevacizumab and Chemotherapy in Non-Small-Cell Lung Cancer (Impower150): Key Subgroup Analyses of Patients With EGFR Mutations or Baseline Liver Metastases in a Randomised, Open-Label Phase 3 Trial. Lancet Respir Med (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 12. Reck M, Mok T, Socinski MA, Jotte RM, Lim D-T, Cappuzzo F, et al. 1293P Impower150: Updated Efficacy Analysis in Patients With EGFR Mutations. Ann Oncol (2020) 31:S837–8. doi: 10.1016/j.annonc.2020.08.1607 [DOI] [Google Scholar]
- 13. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR Mutation Subtypes and Response to Immune Checkpoint Blockade Treatment in Non-Small-Cell Lung Cancer. Ann Oncol (2019) 30:1311–20. doi: 10.1093/annonc/mdz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu S, Wu F, Li X, Zhao C, Jia Y, Jia K, et al. Patients With Short PFS to EGFR-Tkis Predicted Better Response to Subsequent Anti-PD-1/PD-L1 Based Immunotherapy in EGFR Common Mutation NSCLC. Front Oncol (2021) 11:639947. doi: 10.3389/fonc.2021.639947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, et al. Gefitinib Plus Chemotherapy Versus Placebo Plus Chemotherapy in EGFR-Mutation-Positive Non-Small-Cell Lung Cancer After Progression on First-Line Gefitinib (IMPRESS): A Phase 3 Randomised Trial. Lancet Oncol (2015) 16:990–8. doi: 10.1016/S1470-2045(15)00121-7 [DOI] [PubMed] [Google Scholar]
- 16. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N Engl J Med (2017) 376:629–40. doi: 10.1056/nejmoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu X, Li J, Ye L, Zhao J, Xie M, Zhou J, et al. Real-World Outcomes of Chemo-Antiangiogenesis Versus Chemo-Immunotherapy Combinations in EGFR-Mutant Advanced Non-Small Cell Lung Cancer Patients After Failure of EGFR-TKI Therapy. Transl Lung Cancer Res (2021) 10:3782–92. doi: 10.21037/tlcr-21-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR Mutation Correlates With Uninflamed Phenotype and Weak Immunogenicity, Causing Impaired Response to PD-1 Blockade in Non-Small Cell Lung Cancer. Oncoimmunology (2017) 6:e1356145. doi: 10.1080/2162402X.2017.1356145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamberti G, Spurr LF, Li Y, Ricciuti B, Recondo G, Umeton R, et al. Clinicopathological and Genomic Correlates of Programmed Cell Death Ligand 1 (PD-L1) Expression in Nonsquamous Non-Small-Cell Lung Cancer. Ann Oncol (2020) 31:807–14. doi: 10.1016/j.annonc.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 20. Jia Y, Zhao S, Jiang T, Li X, Zhao C, Liu Y, et al. Impact of EGFR-Tkis Combined With PD-L1 Antibody on the Lung Tissue of EGFR-Driven Tumor-Bearing Mice. Lung Cancer (2019) 137:85–93. doi: 10.1016/j.lungcan.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 21. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR Improves Responsiveness to PD-1 Blockade in EGFR-Mutated Non-Small Cell Lung Cancer. Sci Immunol (2020) 5(43):eaav3937. doi: 10.1126/sciimmunol.aav3937 [DOI] [PubMed] [Google Scholar]
- 22. Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, et al. 136O: Osimertinib Combined With Durvalumab in EGFR-Mutant Non-Small Cell Lung Cancer: Results From the TATTON Phase Ib Trial. J Thorac Oncol (2016) 11:S115. doi: 10.1016/S1556-0864(16)30246-5 [DOI] [Google Scholar]
- 23. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib Alone or With Bevacizumab as First-Line Therapy in Patients With Advanced Non-Squamous Non-Small-Cell Lung Cancer Harbouring EGFR Mutations (JO25567): An Open-Label, Randomised, Multicentre, Phase 2 Study. Lancet Oncol (2014) 15:1236–44. doi: 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 24. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib Plus Bevacizumab Versus Erlotinib Alone in Patients With EGFR-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (NEJ026): Interim Analysis of an Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet Oncol (2019) 20:625–35. doi: 10.1016/S1470-2045(19)30035-X [DOI] [PubMed] [Google Scholar]
- 25. Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol (2020) 16:205–15. doi: 10.1016/j.jtho.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 26. Jin R, Liu C, Zheng S, Wang X, Feng X, Li H, et al. Molecular Heterogeneity of Anti-PD-1/PD-L1 Immunotherapy Efficacy Is Correlated With Tumor Immune Microenvironment in East Asian Patients With Non-Small Cell Lung Cancer. Cancer Biol Med (2020) 17:768–81. doi: 10.20892/j.issn.2095-3941.2020.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol (2017) 18:895–903. doi: 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab With or Without Radiotherapy for Metastatic Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomised Trials. Lancet Respir Med (2020) 2600:1–9. doi: 10.1016/s2213-2600(20)30391-x [DOI] [PubMed] [Google Scholar]
- 29. Welsh JW, Heymach JV, Guo C, Menon H, Klein K, Cushman TR, et al. Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation Therapy for Limited-Stage SCLC. J Thorac Oncol (2020) 15:1919–27. doi: 10.1016/j.jtho.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azvolinsky A. Cancer Prognosis: Role of BMI and Fat Tissue. J Natl Cancer Inst (2014) 106:6–7. doi: 10.1093/jnci/dju177 [DOI] [PubMed] [Google Scholar]
- 31. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Y. Adipocyte and Lipid Metabolism in Cancer Drug Resistance. J Clin Invest (2019) 129:3006–17. doi: 10.1172/JCI127201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy KA, James BR, Sjaastad FV, Kucaba TA, Kim H, Brincks EL, et al. Cutting Edge: Elevated Leptin During Diet-Induced Obesity Reduces the Efficacy of Tumor Immunotherapy. J Immunol (2018) 201:1837–41. doi: 10.4049/jimmunol.1701738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical Effects of Obesity on T Cell Function During Tumor Progression and PD-1 Checkpoint Blockade. Nat Med (2019) 25:141–51. doi: 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.