Dear Editor,
Coronavirus disease 2019 (COVID-19)-infected pregnant women are at higher risk of intensive care unit (ICU) admission and mechanical ventilation [1, 2]. Because reports describing the clinical course and management of critically ill COVID-19 pregnant women remain scarce, it is important to better grasp the trajectory and management of these women to improve decision making and allocation of resources. We therefore describe the trajectory, the ICU treatment adapted to pregnancy, and maternal outcomes among critically ill COVID-19 pregnant women admitted to the ICU of a larger tertiary referral center in the Netherlands.
We identified all pregnant women who were consecutively admitted to the ICU of our hospital with COVID-19 between February 27, 2020 and October 31, 2021. To date (reference: December 07, 2021), our ICU admitted 32% of all critically ill pregnant women with COVID-19 in the Netherlands [3]. The study was approved by our local institutional review board (number: MEC-2020-0381) and the need for written informed consent was waived. All patients were treated according to our local protocol (eMethods). Descriptive statistics were used to summarize the data; results are reported as medians and interquartile ranges or minimum–maximum or means and standard deviations, as appropriate. Categorical variables were summarized as counts and percentages. Analyses were performed using SPSS (version 25.0; SPSS Inc., Chicago, IL).
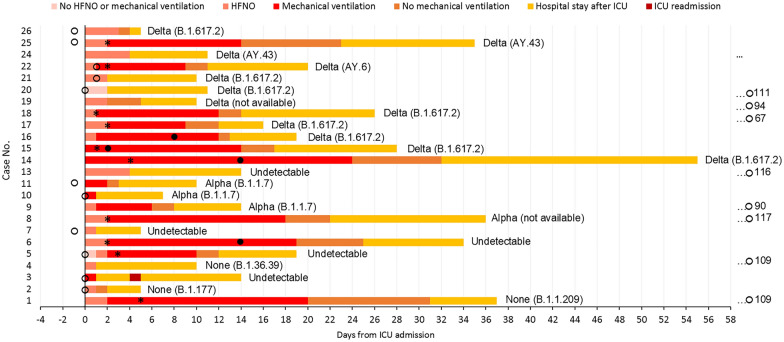
We identified 26 critically ill pregnant women, 2 were excluded. The trajectories are presented in Fig. 1. None were vaccinated and the majority (83%) was non-Caucasian. The median age was 33 (interquartile range, IQR, 30–36) years and the women had a median Sequential Organ Failure Assessment (SOFA) score of 4 (IQR 3–7) and a median Acute Physiologic Assessment and Chronic Health Evaluation IV (APACHE IV) score of 53 (IQR 44–61) at admission, eTable 1. All women were treated with a set scheme of corticosteroids, tocilizumab, neutralizing monoclonal antibodies in case off undetectable antibodies against SARS-CoV-2, and a high dose of prophylactic low-molecular-weight heparin. 15 (63%) women were mechanically ventilated for a median duration of 11 days (IQR 5–16) with a median P/F ratio of 182 (IQR 77–281) mmHg on the day of admission. Ten women (42%) were ventilated in prone positioning with a median duration of 6 (5–7) days of whom four were/remained pregnant throughout proning. After recovery, three of them delivered at term and one was still pregnant at database closure. Eight women delivered prior to ICU admission, and six women (6/16, 38%) were delivered during ICU admission by cesarean section because of maternal respiratory deterioration, eTable 2. During ICU, all deliveries occurred preterm (< 37 weeks gestation) with a median of 27 + 2 (IQR 25 + 1 − 34 + 5) weeks of gestational age at delivery, of which three (13%) were extreme preterm deliveries (< 28 weeks gestation). All women survived ICU treatment and the median length of ICU stay was 6 days (IQR 1–15), eTables 3/4.
Fig. 1.
Trajectory for individual patients included in the case series. Days are presented at the X-axis where day 0 is the start of ICU admission. Patient cases are presented on the Y-axis. The bar charts are color coded and pink represents no HFNO or mechanical ventilation, pale orange represents HFNO, red represents mechanical ventilation, orange represents ICU stay after mechanical ventilation cessation, yellow represents hospital stay after ICU discharge, and dark red represents ICU readmission. Open circles, delivery not during mechanical ventilation; closed circles, delivery during mechanical ventilation; asterisk, initiation of prone positioning. Case no. 3, 5, 7, 10, 11, 20, 25 and 26 delivered before ICU admission. Case no. 2, 6, 14, 15, 16 and 22 delivered during ICU admission. Case no. 1, 4, 8, 9, 13, 17, 18, 19, 21 delivered after ICU admission. Case no. 24: still pregnant at database closure. Two cases were excluded: case 12 because she delivered vaginally and was admitted to our ICU more than 1 week later after falling ill with COVID-19; case 23 was transferred to our ICU 4 weeks after the first admission to an ICU elsewhere, and she was extubated and discharged within 24 h after transfer to our ICU
In summary, proven therapies from the non-pregnant critically ill COVID-19 patient population can be extrapolated to the critically ill pregnant patient, resulting in good maternal ICU survival and limited extreme premature delivery. In the very premature period (< 32 weeks of gestation), continuation of pregnancy during mechanical ventilation, and prone positioning, can certainly be attempted with good fetal monitoring.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
COVPREG study group members: Davy van de Sande, Departments of Intensive Care, Erasmus MC, Rotterdam, The Netherlands; Jasper van Bommel, Departments of Intensive Care, Erasmus MC, Rotterdam, The Netherlands; Jérôme Cornette, Departments of Obstetrics and Gynecology, Erasmus MC, Rotterdam, The Netherlands and Jeroen J.A. van Kampen, Departments of Viroscience, Erasmus MC, Rotterdam, The Netherlands.
Author contributions
MvG drafted the manuscript. MvG and EU collected the data, and analyzed and interpreted the data. MvG participated in the study design, interpreted the data and drafted the manuscript. JvB and DvdS participated in its design and reviewed the manuscript. JC interpreted the data and drafted the manuscript. JD and DG reviewed the manuscript. All authors read and approved the final manuscript for publication.
Funding
No funding fees.
Availability of data and materials
The anonymized dataset analyzed during the current study is available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interest. DG has received speaker’s fees and travel expenses from Dräger, GE Healthcare (medical advisory board 2009–12), Maquet, and Novalung (medical advisory board 2015–18). All other authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the local medical ethics committees and the need for written informed consent was waived.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michel E. van Genderen, Email: m.vangenderen@erasmusmc.nl
on behalf of the COVPREG study group:
Davy van de Sande, Jasper van Bommel, Jérôme Cornette, and Jeroen J. A. van Kampen
References
- 1.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, Coler B, Walker CL, Sheng JS, Al-Haddad BJS, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225(1):77 e71–77 e14. doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinn J, Sedighim S, Kirby KA, Hohmann S, Hameed AB, Jolley J, Nguyen NT. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open. 2021;4(8):e2120456. doi: 10.1001/jamanetworkopen.2021.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutch national registration of COVID-19 pregnant woman. https://www.nvog.nl/actueel/registratie-van-covid-19-positieve-zwangeren-in-nethoss/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized dataset analyzed during the current study is available from the corresponding author upon reasonable request.