Abstract
To determine the mechanisms of immunomodulating action of fosfomycin (FOF), we examined its effect on the production of inflammatory cytokines in mice injected with lipopolysaccharide (LPS). Treatment with FOF significantly lowered the peak serum levels of tumor necrosis factor alpha and interleukin-1β, indicating that FOF alters inflammatory cytokine production after LPS stimulation.
Fosfomycin (FOF), 1-cis-1,2-epoxypropylphosphoric acid, is a broad-spectrum bactericidal antibiotic not structurally related to other classes of antimicrobial agents. We have recently reported that FOF exerts a protective effect against murine gut-derived sepsis caused by Pseudomonas aeruginosa (6). In that study, we demonstrated that the enantiomer of FOF [FOF(+)], which lacks antimicrobial activity, also exerts a protective effect. FOF(+) significantly suppresses the concentrations of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in sera of mice with gut-derived sepsis. Several studies have indicated that the excessive production of host inflammatory cytokines might be responsible for the morbidity associated with septic shock (1, 9, 10). Previous studies from our laboratory also demonstrated that TNF and IL-1 might facilitate bacterial translocation and result in deterioration of gut-derived sepsis caused by P. aeruginosa in mice (5, 7). These results suggested that the alteration of inflammatory cytokine production by FOF and FOF(+) may potentially affect the pathophysiology of septic shock. However, we could not demonstrate a direct alternative effect of FOF on cytokine production in vivo, because murine gut-derived sepsis is a complex model for the analysis of the protective mechanisms of FOF. Therefore, in this study, we evaluated the effects of FOF on inflammatory cytokine production with a simple animal model induced by inoculation with lipopolysaccharide (LPS).
Specific-pathogen-free male ddY mice (Japan Shizuoka Laboratory Center Co., Shizuoka, Japan) were or were not treated with 200 mg of FOF (Meiji Seika, Tokyo, Japan) per kg by intravenous injection. Ten minutes after FOF or saline injection, 2 mg of LPS (Escherichia coli O55:B5; Difco Laboratories, Detroit, Mich.) per kg was injected intraperitoneally. Mice were then sacrificed at the indicated time intervals (0, 1.5, 3, 6, and 12 h; n = 10 [at each time point]) by inhalation of ether, and cardiac blood samples were immediately obtained. Serum samples were preserved at −80°C until measurements of cytokine concentrations were taken. Concentrations of inflammatory cytokines in serum were determined by using enzyme-linked immunosorbent assay kits (kits for TNF-α, gamma interferon [IFN-γ], and IL-6 were from Endogen Inc., Boston, Mass., and that for IL-1β was from Genzyme Corp., Boston, Mass.). Each experiment was performed twice.
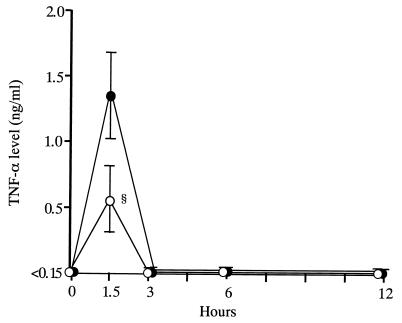
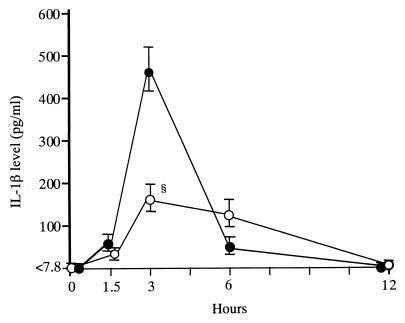
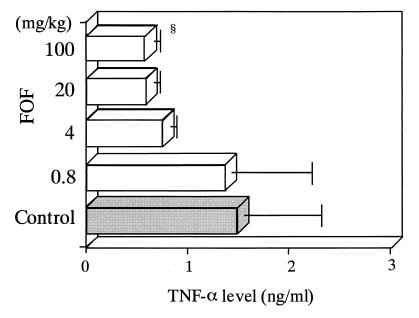
Treatment with FOF significantly lowered the peak concentration of TNF-α in serum, compared with that in saline-treated control mice (0.56 ± 0.26 versus 1.35 ± 0.33 ng/ml; Fig. 1). The peak level of IL-1β was also significantly lowered in FOF-treated mice, compared with that in saline-treated control mice (163.8 ± 34.6 versus 468.6 ± 54.7 pg/ml; Fig. 2). Furthermore, we determined the concentration of TNF-α in serum after treatment of mice with various doses of FOF and noted a dose-response effect of FOF on TNF-α production (Fig. 3).
FIG. 1.
Effect of FOF on serum TNF-α kinetics after LPS stimulation in mice. Mice in groups of 10 were each treated intravenously with 200 mg of FOF per kg (open circles) or with saline alone (solid circles) 10 min before LPS inoculation. Data are means ± standard deviations (error bars). §, P < 0.05.
FIG. 2.
Effect of FOF on serum IL-1β kinetics after LPS stimulation in mice. Mice in groups of 10 were each treated intravenously with 200 mg of FOF per kg (open circles) or with saline alone (solid circles) 10 min before LPS inoculation. Data are means ± standard deviations (error bars). §, P < 0.05.
FIG. 3.
Dose-response effect of FOF on TNF-α production following LPS stimulation in mice. Mice in groups of 8 were each treated intravenously with 0.8, 4, 20, or 100 mg of FOF per kg (white bars) or with saline alone (gray bar) 10 min before LPS inoculation. Data are means ± standard deviations (error bars). §, P < 0.05.
Although the differences in IFN-γ and IL-6 concentrations between FOF-treated and saline-treated mice were rather marginal, FOF significantly lowered IFN-γ concentrations (5.51 ± 1.48 versus 0.35 ± 0.35 ng/ml; P < 0.05) and increased IL-6 concentrations (24.9 ± 1.5 versus 20.4 ± 1.6 ng/ml; P < 0.05) in the initial stage (the first 1.5 h), compared to those in saline-treated control mice. Because there were only weak influences on IFN-γ and IL-6, we speculate that the effects of FOF on changes in inflammatory cytokines are mediated largely through TNF-α and IL-1β.
Concerning the FOF treatment schedule, there were no significant differences in cytokine levels between groups treated with FOF 10 min or 1 day before LPS administration. On the other hand, FOF treatment 1 h after LPS administration did not show a significant effect, compared to saline treatment on the same schedule (data not shown). With regard to the route of treatment, there were no significant differences between groups treated intravenously and those treated intraperitoneally (data not shown).
In a series of preliminary studies, we confirmed that the FOF preparation used in the present study contained an amount of endotoxin below the detection level and that the administration of FOF alone did not produce a rise in concentrations of TNF-α, IL-1β, IL-6, and IFN-γ in mouse sera (data not shown). We also confirmed that all mice survived more than 14 days after treatment with 2 mg of LPS per kg, the dose used in the present study.
Morikawa et al. (8) reported the effects of FOF on cytokine synthesis by LPS-stimulated human monocytes in vitro. An interesting finding in their report was that FOF decreased the rate of synthesis of TNF and IL-1 but increased that of the synthesis of IL-6. Our results in the present study were consistent with these findings. However, the enhancement of IL-6 production in this study was small, and we could not directly demonstrate whether this enhancement influenced the expression of other cytokines.
Although the exact reasons for the contradictory result in a recent study (6) concerning the effect of FOF on IL-6 production are not known at present, we speculate that some possible reasons for these contradictory results might include the pathophysiological differences between endotoxin shock and septic shock. For example, not only LPS but also various types of exotoxins may influence the pathophysiology of septic shock (2–4).
Our results indicate that FOF directly alters the production of inflammatory cytokines, especially TNF-α and IL-1β, in vivo. Further studies are necessary to investigate the mechanisms of this effect. We are currently investigating the effects of FOF on the expression of LPS-induced cellular surface molecules.
Acknowledgments
We are grateful to Shogo Kuwahara for useful advice and to Yasuko Kaneko for expert technical assistance. We also thank F. G. Issa (Word-Medex, Sydney, Australia) for careful reading and editing of the manuscript.
This work was supported by a research grant provided by The Japan Health Sciences Foundation, Tokyo, Japan.
REFERENCES
- 1.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 2.Furuya N, Hirakata Y, Tomono K, Matsumoto T, Tateda K, Kaku M, Yamaguchi K. Mortality rates amongst mice with endogenous septicemia caused by Pseudomonas aeruginosa isolates from various clinical sources. J Med Microbiol. 1993;39:141–146. doi: 10.1099/00222615-39-2-141. [DOI] [PubMed] [Google Scholar]
- 3.Goto S. Recent progress in the identification of pathogenic factors of Pseudomonas aeruginosa. J Infect Chemother. 1996;2:111–116. [Google Scholar]
- 4.Matsumoto T, Tateda K, Furuya N, Miyazaki S, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Efficacies of alkaline protease, elastase, and exotoxin A toxoid vaccines against gut-derived Pseudomonas aeruginosa sepsis in mice. J Med Microbiol. 1998;47:303–308. doi: 10.1099/00222615-47-4-303. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Adverse effects of tumor necrosis factor in cyclophosphamide-treated mice subjected to gut-derived Pseudomonas aeruginosa sepsis. Cytokine. 1997;9:763–769. doi: 10.1006/cyto.1997.0222. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Immunomodulating effect of fosfomycin on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 1997;41:308–313. doi: 10.1128/aac.41.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. Paradoxical synergistic effects of tumor necrosis factor and interleukin-1 on murine gut-derived Pseudomonas aeruginosa sepsis. Cytokine, in press. [DOI] [PubMed]
- 8.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 10.Wallach D, Holtmann H, Engelmann H, Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988;140:2994–2999. [PubMed] [Google Scholar]