Abstract
Introduction
With no treatment for dementia, there is a need to identify high risk cases to focus preventive strategies, particularly in low‐ and middle‐income countries (LMICs) where the burden of dementia is greatest. We evaluated the risk of conversion from mild cognitive ompairment (MCI) to dementia in LMICs.
Methods
Medline, Embase, PsycINFO, and Scopus were searched from inception until June 30, 2020. The search was restricted to observational studies, conducted in population‐based samples, with at least 1 year follow‐up. There was no restriction on the definition of MCI used as long as it was clearly defined. PROSPERO registration: CRD42019130958.
Results
Ten thousand six hundred forty‐seven articles were screened; n = 11 retained. Of the 11 studies, most were conducted in China (n = 7 studies), with only two studies from countries classified as low income. A qualitative analysis of n = 11 studies showed that similar to high‐income countries the conversion rate to dementia from MCI was variable (range 60%–448%; average follow‐up 37 years [standard deviation = 12]). A meta‐analysis of studies using Petersen criteria (n = 6 studies), found a pooled conversion rate to Alzheimer's disease (AD) of 238% (95% confidence interval = 154%–33.4%); approximately one in four people with MCI were at risk of AD in LMICs (over 30–58 years follow‐up). Risk factors for conversion from MCI to dementia included demographic (e.g., age) and health (e.g., cardio‐metabolic disease) variables.
Conclusions
MCI is associated with high, but variable, conversion to dementia in LMICs and may be influenced by demographic and health factors. There is a notable absence of data from low‐income settings and countries outside of China. This highlights the urgent need for research investment into aging and dementia in LMIC settings. Being able to identify those individuals with cognitive impairment who are at highest risk of dementia in LMICs is necessary for the development of risk reduction strategies that are contextualized to these unique settings.
Keywords: dementia, low‐ and middle‐income countries, mild cognitive impairment, risk factors
1. INTRODUCTION
Mild cognitive impairment (MCI) defines an intermediate cognitive state between normal aging and dementia and is a target for dementia prevention and risk reduction research. 1 , 2 Numerous definitions for MCI exist and prevalence estimates vary (range < 1% to > 50%) depending on the population sampled (e.g., the age/sex distribution of participants, clinical‐based sample vs. individuals recruited from population‐based settings), MCI case definition, and operationalization of the component criterion for an MCI case diagnosis. 3 , 4 , 5 , 6 , 7 , 8 Further, within high‐income countries (HICs), the rates of conversion from MCI (across different subtypes) to dementia vary (range 10%–15% annually). 5 , 6 , 7 Although some cases remain stable, others can revert to normal, with studies suggesting reversion ranges of 4% to 15% in clinic‐based samples 8 , 9 , 10 , 11 and 29% to 55% in population‐based samples. 12 , 13 , 14 , 15 In the absence of a cure for dementia, understanding the likelihood of, and risk factors associated with, conversion to dementia among MCI cases is important to help identify strategies for dementia risk reduction and prevention. 16
The definition of MCI can be a difficult concept to disentangle. One of the most widely applied set of criteria, in clinical and research practice, are those defined by Petersen et al., describing patients with subjective memory loss verified by neuropsychological testing, with no significant impairment in other cognitive domains, no functional impairments, and no dementia. 17 Other similar criteria have also been developed and applied including, for example, from the International Working Group, 18 National Institute on Aging–Alzheimer's Association (NIA‐AA), 19 and the American Psychiatric Association (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [DSM‐IV]). 20 MCI is therefore an evolving concept, with varying definitions, which can make cross‐study comparisons challenging. 21
While some have suggested that MCI as a method for classification of prodromal dementia can have a limited role in clinical and epidemiological settings, others argue that MCI could be a pragmatic tool for identifying individuals who could benefit from risk reduction. 22 Modifiable risk factors for MCI and its conversion to dementia include health and lifestyle factors such as an unhealthy diet (e.g., a diet high in saturated fat, sugar, and salt), physical inactivity, smoking, cardiometabolic diseases (e.g., coronary heart disease and diabetes) and their risks including obesity and hypertension. 23 , 30 The literature on risk of conversion to dementia predominantly refers to MCI classified using Petersen criteria. 24 As discussed, with the multiple definitions of MCI available, this can make comparisons challenging. That said, this evidence highlights the potential for risk reduction and possible prevention or delay of dementia onset in MCI cases. Indeed, a recent report indicated that 217% of MCI cases that progress to dementia are potentially preventable, by targeting diet (using obesity as a proxy) (87%), diabetes (15%), and neuropsychiatric symptoms (115%). 23
Compared to HICs, very few studies on MCI have been conducted in low‐ and middle‐income countries (LMICs). 4 Extending the findings from a recent systematic review on MCI prevalence in LMICs, 4 the aim of this systematic review and meta‐analysis was to identify and review longitudinal population‐based studies reporting on the risk of conversion from MCI to dementia in LMICs. The focus was on the rate of conversion and associated risk factors. Given that nearly two‐thirds of people with dementia live in LMICs 25 identifying those individuals at highest risk is important for targeted interventions focused on reducing the burden of dementia in these settings. 26
2. METHODS
This systematic review and meta‐analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (Appendix A.1 in supporting information). 27 The study protocol was registered on the PROSPERO database (registration number CRD42019130958).
2.1. Search strategy and eligibility criteria
Four commonly used, comprehensive medical databases were searched electronically from inception until April 30, 2019. A second electronic database search was conducted from May 1, 2019 to June 30, 2020. The selected databases were: Medline; Embase; PsycINFO, assessed via Ovid (https://ovidsp.ovid.com); and Scopus (https://www.Scopus.com/home.uri). A detailed description of the search strategy is provided in the supporting information.
The search was restricted to observational studies, conducted in population‐based samples, with at least 1 year of follow‐up. Participants were those with a diagnosis of MCI according to internationally accepted and validated classifications. 1 , 17 , 18 , 19 , 28 , 29 Studies which included participants with “memory problems” or “self‐reported memory complaints” and no clear diagnosis of MCI were excluded. To be eligible, MCI participants had to be followed during the study period for risk of dementia; diagnosed according to established criteria, for example, National Institute of Neurological Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria, 30 DSM‐IV criteria, 20 , 31 or Neuro‐epidemiology Branch of the International Workshop of the National Institute of Neurological Disorders and Stroke with support from the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria. 32 All‐cause dementia and its subtypes (e.g., Alzheimer's disease [AD] and vascular dementia) were included. Studies that combined participants with MCI with another level of cognitive status at baseline (e.g., cognitively healthy or dementia), were only included if the MCI group data were analyzed and presented separately. Studies were excluded where the MCI sample was stratified by disease status (e.g., diabetes) and rates of conversion were not reported for the total population. Studies were required to be from a LMIC as per the Organisation for Economic Co‐operation and Development (OECD) criteria and World Bank classification, with inclusion based on the income status of the country at the time the study was conducted.
Research in context
Systematic Review: Medline, Embase, Scopus, and PsycINFO were searched for eligible articles reporting on conversion from mild cognitive impairment (MCI) to dementia in population‐ or community‐based studies from low and middle‐income countries (LMICs). The search strategy included terms that encompassed “dementia,” “mild cognitive impairment,” “incidence,” and “conversion.”
Interpretation: This is the first study to synthesize research on the risk of conversion to dementia in people with MCI in LMIC settings. We found that very few studies on risk of dementia in MCI cases have been undertaken in LMICs. Across the 11 studies, conversion to dementia was high and ranged from 6.0% to 44.8% over an average follow‐up of 3.7 years. A meta‐analysis of studies using Petersen criteria (n = 6 studies), found a pooled conversion rate to Alzheimer's disease (AD) of 23.8% (95% confidence interval = 15.4%–33.4%); approximately one in four people with MCI were at risk of AD in LMICs over 3.0 to 5.8 years follow‐up.
Future Directions: Like findings from high‐income countries, MCI is associated with increased risk of conversion to dementia in LMIC. There was, however, large heterogeneity in study methodology including definition of MCI, operationalization of MCI criterion, follow‐up time, and diagnosis of dementia as well as a scarcity of studies from countries classed as low income. There is an urgent need for LMICs to invest in the collection of robust, population‐based data, to determine the best strategies for identifying those individuals at highest dementia risk to inform the development of dementia risk reduction plans in these settings.
2.2. Screening process
Two reviewers independently assessed potentially relevant articles for eligibility (AMM and EP). The decision to include or exclude studies was hierarchical and initially made based on the study title and abstract to eliminate obviously irrelevant studies (see Figure 1). When a study's title/abstract could not be rejected with certainty, the full text of the article was obtained for evaluation. Discrepancies between reviewers were resolved by a third reviewer (BCMS). Next, full‐text articles were searched. In addition, the reference lists of all included articles were checked for any potentially missing papers.
FIGURE 1.
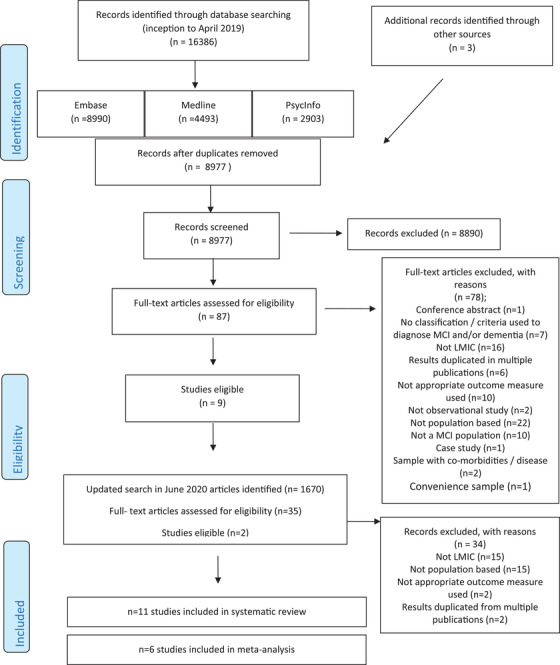
Study selection. LMIC, low‐ and middle‐income countries; MCI, mild cognitive impairment
2.3. Data extraction
A standardized form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted data included information on: (1) author and year of publication, (2) country, (3) age, (4) sample size, (5) follow‐up duration, (6) MCI and dementia diagnostic criteria, (7) analytical method for determining rate of conversion including loss to follow‐up, (8) results of conversion from MCI to dementia, and (9) risk factors for conversion to dementia (including details of all risk factors assessed—both significant and non‐significant). One author extracted data (EP), and a second checked the extraction (AMM). A third author (BCM) reviewed any discrepancies.
2.4. Risk of bias assessment
Quality assessment was guided by the Newcastle–Ottawa Scale for cohort studies. 33 Risk of bias was assessed on three main categories: selection, comparability, and outcome. The maximum possible score was 8; three stars for selection, two stars for comparability, and two stars for outcome. Two authors (AMM and EP) independently assessed risk of bias, with any disagreement resolved by discussion with a third assessor if required.
2.5. Analysis
For each study, we report the proportion of MCI cases that converted to dementia for each definition of MCI separately. This was calculated as the ratio of those who converted to dementia over the total sample size. We also report on the key risk factors significantly associated with an increased risk of dementia. Details of all risk factors assessed are in Table S2 in supporting information.
Where there were multiple studies using the same criteria to diagnosis MCI and dementia, a meta‐analysis was undertaken. This was only possible for studies that diagnosed MCI using Petersen‐type criteria with an outcome of AD (n = 6 studies). The analysis was run in Stata using the Metaprop command to compute the meta‐analysis of pooled proportions. This allows computation of 95% confidence intervals (95% CI) using the score statistic and the exact binomial method and incorporates the Freeman‐Tukey double arcsine transformation of proportions to compute the weighted pooled estimate for normality assumptions. The program also allows the within‐study variability to be modeled using the binomial distribution. 34 Given large differences in the design and sampling across studies, the random effects model was computed. Heterogeneity was assessed using the I2 statistic.
3. RESULTS
3.1. Study selection
From the electronic search, the titles and abstracts of 8977 publications were screened, and the full texts of 87 articles reviewed. The screening and study selection process is illustrated in the PRISMA flow diagram (Figure 1). Nine articles met the eligibility criteria. The most common reasons for exclusion were that the study was not from a LMIC, the sample was not population‐based, and the study did not report incident dementia. A second search conducted in June 2020 identified a further 1670 articles, from which two studies were eligible for inclusion. Thus, 11 articles are included in this review.
3.2. Study characteristics
Of the 11 studies, most were conducted in China (n = 7 studies), 35 , 36 , 37 , 38 , 39 , 40 , 41 followed by Brazil (n = 2 studies), 42 , 43 Nigeria (n = 1 study 44 ), 44 and Tanzania (n = 1 study). 45 Only one study was nationally representative using census data from Rio Grande do Sul, Brazil. 42 At the time of participant recruitment, two studies 44 , 45 were from low‐income countries while the majority (n = 9 studies) 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 were from middle‐income countries. MCI sample size at baseline ranged from n = 21 42 to n = 837, 35 with a mean of n = 370 (standard deviation [SD] = 295). Most studies included participants aged > 60 years (n = 6 studies), 37 , 38 , 39 , 40 , 41 , 42 while others included those > 55 years (n = 2 studies), 35 , 36 > 65 years (n = 1 study), 44 and > 70 years (n = 2 studies). 43 , 45 Duration of follow‐up ranged from 2 years 44 to 58 years, 42 with a mean of 37 years (SD = 1.1 year). At follow‐up, MCI sample size ranged from n = 21 42 to n = 638, 35 with a mean of n = 298 (SD = 238).
3.3. Diagnostic criteria for MCI
Most studies diagnosed MCI using Petersen criteria 17 , 28 , 46 , 47 (n = 5 studies). 35 , 36 , 38 , 40 , 41 Other criteria included the Clinical Dementia Rating scale (CDR 0.5; 48 n = 1 study), 43 Dubois 2004 criteria 49 (n = 1 study), 42 the criteria from the DSM‐IV 31 (n = 2 studies), 37 , 39 and the International Working Group on MCI criteria 18 (n = 1 study). 45 Only 1 study 44 used the Cognitive Impairment No Dementia (CIND) classification. 50 This paper 44 reports that of the n = 87 CIND participants, n = 74 were classed as having “medically unexplained memory loss” (MUML) described as comparable to MCI using Petersen criteria. 51 For this review, the total sample of n = 87 CIND participants were included. Table 1 shows a description of the MCI diagnostic criteria used across the different studies. The majority of studies classified participants as MCI (n = 9 studies), 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 45 while others subtyped MCI into amnestic MCI (aMCI; n = 2 studies), 39 , 41 aMCI single domain (n = 1 study), 41 aMCI multiple domain (n = 1 study), 41 non‐amnestic MCI single domain (n = 1 study), 41 and non‐amnestic MCI multiple domain (n = 1 study). 41
TABLE 1.
Study characteristics (grouped by criteria for MCI diagnosis and ordered by age)
| Sample size | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Population characteristics | Age (years) | Total sample at baseline | MCI sample | MCI sample at follow up | Mean Follow‐up (years) | MCI Type | Dementia criteria | Conversion to dementia |
| Petersen criteria 17 , 28 , 47 , 81 (n = 5 studies) | ||||||||||
| Huang et al. 36 | China | 29 geographically defined communities located within Greater Beijing, China (12 urban and 17 rural) | >55 | 5743 | 175 | 121 | 3 | MCI |
DSM‐IV (all cause) NINCDS‐ADRDA (AD) NINDS‐AIREN (VaD) |
42% (51/121 including n = 29 with AD, n = 18 with VaD and n = 4 with other dementias (after 3 years follow up) |
| Li et al. 35 | China | 10 randomly selected communities in the city of Chongqing | >55 | 26,481 (18,683 screened) | 837 | 638 | 5 | MCI |
DSM‐IV (all cause) NINCDS/ADRDA (AD) |
448% (298/638 dementia including n = 298 AD) (after 5 years follow up) |
| Ding et al. 41 | China | Jingansi community in downtown Shanghai, China | >60 |
3141 |
655 | 362 | 4 |
MCI aMCI aMCI‐SD aMCI‐MD naMCI‐SD naMCI‐MD |
DSM‐IV (all cause) |
MCI 60 (95% CI: 47–73) per 100 person‐years (79/362) aMCI 69 (95% CI 52–86) per 100 person years (59/238) aMCI‐SD 30 (95% CI: 16–44) per 100 person‐years (17/156) aMCI‐MD 142 (95% CI: 102–182) per 100 person years (42/82) naMCI‐SD 27 (95% CI: 10–45) per 100 person‐years (9/91) naMCI‐MD 87 (95% CI: 38–137) per 100 person‐years (11/33) (after 4 years follow up) |
| Yang et al. 40 | China | Chinese community dwelling elder people | >60 | 652 | 465 | 465 | 3 | MCI | DSM‐ IV‐TR (AD) | 168% (78/465) (after 3 year follow up) |
| Yu et al. 38 | China |
8 geographically convenient communities in Taiyuan city, China |
>60 | 6192 | 600 | 518 | 5 | MCI | NINCDS‐ADRDA (AD) | 170% (89/518) (after 5 years follow up) |
| CIND based on Levy and Working Party of the International Psychogeriatric Association in collaboration with the WHO 50 (n = 1 study) | ||||||||||
| Baiyewu et al. 44 | Nigeria |
Idikan community and adjacent wards of the city of Ibadan, Nigeria |
>65 | 2487 | 152 | 87 | 2 | CIND |
DSM III‐R ICD‐10 (all cause) |
161% (14/87) developed dementia (after 2 years follow up) |
| CDR 82 (n = 1 study) | ||||||||||
| Montano et al. 43 | Brazil | A community cohort living in an urban district, in São Paulo city | >70 | 1667 | 80 | 40 | 26 | CDR 05 | NINCDS–ADRDA (all cause) |
375% (145.4 per 1,000 person‐years; 15/40) (after follow up) |
| IWG 18 (n = 1 study) | ||||||||||
| Paddick et al. 45 | Tanzania | 6 randomly selected villages from the Hai District in Northern Tanzania. | >70 | 296 | 46 | 46 | 4 | MCI |
DSM‐IV (all cause) NINCDS‐ADRDA (AD) NINDS‐ AIREN (VaD) |
370% (17/46) all cause (n = 9 AD, n = 4 VaD, n = 2 Parkinson disease dementia & n = 2 mixed aetiology) (after 4 years follow up) |
| DSM‐IV Criteria 31 (n = 2 studies) | ||||||||||
| Yu et al. 37 | China | 26 military cadres’ sanatoriums of Shijiazhuang city | >60 | 2674 | 216 | 209 | 3 | MCI |
DSM‐IV (all cause) NINCDS‐ADRDA (AD) |
244% (51/209) all cause 157% (35/209) AD (per year) |
| Wang et al. 39 | China | 9 densely distributed elderly communities in Taiyuan | >65 | 6152 | 600 | 557 | 3 | aMCI | NINCDS‐ADRDA (AD) | 6% (34/557 per year) |
| Dubois and Albert, 2004 49 (n = 1 study) | ||||||||||
| Godhino et al. 42 | Brazil | Older community dwelling residents in the catchment area of the Hospital de Clinicas de Porto Alegre, Rio Grande do Sul, Brazil | >60 | 245 | 21 | 21 | 58 | MCI | DSM‐IV & NINCDS‐ADRDA (AD) | 381% (8/21) (after 5.8 years follow up) |
Abbreviations: AD, Alzheimer's disease; aMCI, amnestic mild cognitive impairment; CIND, cognitive impairment no dementia; CDR, Clinical Dementia Rating; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; ICD, International Classification of Diseases; IWG, International Working Group; MCI, mild cognitive impairment; NIA‐AA, National Institute on Aging and Alzheimer's Association; NINCDS‐ADRDA, National Institute of Neurological Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association; NINDS‐AIREN, National Institute of Neurological Disorders and Stroke International Workshop with support from the Association Internationale pour la Recherche et l'Enseignement en Neurosciences; VaD, vascular dementia; WHO, World Health Organization.
3.4. Diagnostic criteria for dementia
Most studies (n = 7 studies) 35 , 36 , 37 , 40 , 41 , 42 , 45 defined incident dementia using the DSM‐IV criteria. 20 , 31 This was followed by NINCDS‐ADRDA criteria 49 , 52 (n = 5 studies), 35 , 36 , 37 , 38 , 39 the NINDS‐AIREN criteria 32 (n = 2 studies), 35 , 36 and DSM‐III‐R criteria 20 (n = 1 study 44 ). 44 Studies defined dementia as either all‐cause (n = 7 studies), 35 , 36 , 37 , 41 , 43 , 44 , 45 AD (n = 8 studies), 35 , 36 , 37 , 38 , 39 , 40 , 42 , 45 or vascular dementia (VaD; n = 2 studies). 36 , 45 One study 44 mentioned using International Classification of Diseases (ICD)‐10 criteria from the World Health Organization. 53
3.5. Conversion from MCI to dementia
Rates of conversion from MCI to dementia ranged from 60% 39 to 448% 35 for all‐cause dementia (n = 11 studies) over an average follow‐up of 37 years (SD 12 years); 60% 35 , 39 to 467% 25 for AD (n = 8 studies) over an average follow‐up of 4.0 years (SD 11 years), and 87% 45 to 149% 36 for VaD (n = 2 studies) over an average follow‐up of 35 years (SD 07 years). Rates of conversion to all‐cause dementia were generally higher for those definitions that captured broader impairment; for example, range of conversion for all‐MCI (n = 8 studies), 35 , 36 , 37 , 38 , 40 , 41 , 42 , 45 CIND (n = 1 study), 44 and CDR (n = 1 study) 43 (range 168% to 44.8% over 20–58 years follow‐up) compared to more restricted definitions of single domain MCI; for example, aMCI range 60% to 69% over an average of 35 years follow‐up (n = 2 studies). 39 , 41
3.6. Risk factors for dementia
Risk factors for MCI conversion to dementia were investigated in 10 (out of 11) studies. 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Significant risks included older age (n = 6/10 studies 19 , 35 , 38 , 39 , 40 , 41 , 44 ), poor baseline performance on cognitive tests (n = 5/7 studies 36 , 40 , 41 , 42 , 43 ), sex (being female; n = 3/10 studies 35 , 38 , 44 ), hypertension (n = 3/6 studies 35 , 38 , 39 ), low educational attainment (illiteracy or primary school; n = 2/10 studies 35 , 36 ), anxiety and depression (n = 2/5 studies 39 , 40 ), history of stroke (n = 1/4 studies 36 ), diabetes (n = 2/4 studies 35 , 39 ), and apolipoprotein E (APOE) ε4 status (n = 2/4 studies 35 , 39 ). Full details of all risk factors assessed in each study are presented in Table S2.
3.7. Risk of bias assessment
Full details of the risk of bias assessment can be found in Table S3 in supporting information. The included studies averaged 7.3 stars out of 10 (range 5–8). Eight studies (out of 11) scored the maximum of eight stars.
3.8. Meta‐analysis—risk of conversion to AD
Six studies were included in the meta‐analysis; n = 4 from China 35 , 36 , 38 , 40 and n = 1 each from Tanzania 45 and Brazil. 42 Across the six studies, MCI sample size ranged from n = 21 42 to n = 837 35 and age from > 55 years 35 , 36 to > 70 years. 45 Figure 2 presents the study‐specific proportion of Petersen‐defined MCI cases that converted to AD over time with 95% CIs for each study as well as the Chinese subgroup and overall pooled estimate with 95% Wald confidence interval and the I2 statistics. As shown, significant intra‐group heterogeneity among China‐based studies was observed (P < .0001 with I2 = 96.54%). When pooling the six studies together conversion to dementia was estimated at 238% (95% CI = 154%–33.4%) over a range of 3.0–58 years.
FIGURE 2.
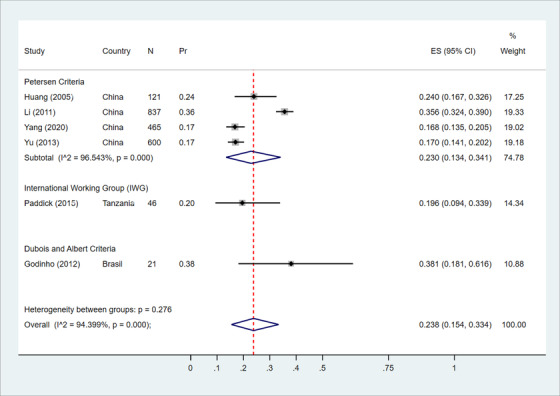
Forest plot showing the meta‐analysis of the proportion of the mild cognitive impairment converting to Alzheimer's disease. CI, confidence interval; Effect Size (ES), xxxxxxxxx
4. DISCUSSION
In this systematic review and meta‐analysis, we found that very few studies on risk of MCI conversion to dementia have been undertaken in LMIC settings. This contrasts to the significant research investment into MCI (and more specially dementia) in HICs. Across the 11 studies, conversion to all‐cause dementia (including all dementia, AD, and VaD) was high and varied, ranging from 60% to 448% over an average follow‐up of 37 years. Similar variability in risk of conversion to dementia has been reported in studies from HICs. 5 , 6 , 7 For studies using Petersen criteria (n = 6), the pooled conversion rate to AD was 238% over 3.0 to 58 years. This suggesting that approximately one in four people with MCI are at risk of developing AD in LMICs.
While risk of dementia in MCI has been studied extensively in HICs, in contrast very little research has been undertaken in LMIC settings. Further, the majority of studies were from China (middle‐income) with data from only three other countries represented including Brazil 42 , 43 (upper middle income), Nigeria 44 (lower middle income), and Tanzania 45 (low income). Limited resources for research, including access to funding, and infrastructure to diagnose MCI/dementia in LMIC settings could be possible reasons for this scarcity. However, this is a significant research gap particularly considering the high burden of cognitive impairment and dementia in low‐income country settings. 25 Of the studies included in this review, there was also variability in terms of outcome measure (all‐cause dementia vs. AD vs. VaD), length of follow‐up, sample size, and diagnostic criteria for MCI. In addition, the examination of MCI conversion to other dementia subtypes was limited as only AD and VaD were investigated. Therefore, there is an urgent need for future studies to attempt to standardize the methodology used to allow for better cross‐study comparisons, aiming for studies to be population representative and generalizable.
Studies from HICs estimate annual conversion from MCI (irrespective of MCI definition used) to dementia at approximately 3% to 10% in community settings and 10% to 15% in clinical settings. 5 , 16 , 17 , 18 , 54 Similar to findings from HCIs, conversion rates were found to be variable across the different LMICs sites ranging from 60% to 448%. Rates of conversion to dementia were generally higher for those definitions that capture broader impairment (e.g., range of conversion for all‐MCI, CIND, and CDR: range 168% to 448% over 2.0–58 years follow‐up) compared to more restricted definitions of single domain MCI (e.g., aMCI range 60% to 69% over an average of 35 years follow‐up). 55 , 56 , 57 , 58 , 59 , 60 , 61 Given the high reported prevalence of MCI in LMIC settings 4 in addition to the high dementia conversion rates reported here, the development of strategies to prevent or delay dementia progression in those individuals with cognitive impairment could have a significant impact on the burden of disease associated with mental health conditions in these settings.
Similar to findings from HICs, non‐modifiable risk factors for progression to dementia from MCI included age 62 , 63 , 64 , 65 and APOE ε4 allele status. 51 , 66 , 67 Regarding sex, while being female has been found to be associated with increased risk of prevalent MCI, 4 and has been associated with higher risk of progression to dementia in HICs, 68 only 2 studies 38 , 44 out of 10 that investigated sex effects found that being female was a risk factor for conversion from MCI to dementia. Research evidence, predominantly from HICs, suggests a putative link between sex and/or educational attainment and cognition. 69 , 70 , 71 , 72 However, methodological weaknesses and potential of reverse causality within these studies adds limitations to their interpretation and warrants longitudinal studies with longer follow‐up. 71 Furthermore, key modifiable risk factors were also similar to those reported in HIC settings, including poor cardiometabolic health, the presence of vascular risk factors, and poor neuro‐psychiatric health such as the presence of depression. 23 , 62 , 73 Targeting these factors could be an early strategy for not only preventing MCI, but also reducing the burden of dementia. Research evidence suggests that up to 40% of dementia cases may be preventable through targeting 12 modifiable risk factors, 74 many of which can be influenced by diet and lifestyle practices. 75 , 76 Emerging evidence also indicates that non‐pharmacological interventions such as cognitive training may reduce dementia risk. 77 Further work is required to identify if these strategies are plausible for those with MCI, and feasible within LMIC settings.
This is the first study to synthesize research on the risk of dementia in people with MCI in LMIC settings. We undertook a wide literature search capturing many of the different definitions of MCI and included studies of all‐cause dementia and its subtypes. While there was variability in how dementia was diagnosed, most (636%) studies used DMS‐IV criteria. However, there are some limitations. The electronic search was undertaken in English and therefore studies published in other languages, including those common in LMICs such as Spanish, Portuguese, and French could have been missed. Although we used a wide search strategy to ensure that we captured all studies on the topic, we did not search the gray literature, which could have resulted in missing non‐published studies highlighting a risk of publication bias. Only a limited number of studies were identified and only one study was from a nationally representative sample. 42 This makes it hard to generalize the results especially across different LMIC settings particularly LMIC countries in Eastern Europe, the Middle East, and Global South where no data are currently available. While most studies were associated with low risk of bias, there was variability in study robustness, for example, in terms of MCI diagnosis (including cognitive test used), sample size, and participant selection. While all studies were population‐ or community‐based, there was large heterogeneity in study methodology including definition of MCI, operationalization of MCI criterion, follow‐up time, reporting of conversion rates (annually vs. after number of years of follow‐up), and diagnosis of dementia, all of which could impact the results. In addition, due to the small number of studies included it was not possible to stratify the meta‐analysis by age or follow‐up duration. Last, we should emphasize that the studies in the meta‐analysis are limited to a few studies from three countries only, with different characteristics and profiles of the population. Indeed, heterogeneity was high and possibly reflects differences in sample selection, sample characteristics (age and sex distribution), sample size, and operationalization of MCI criteria (Figure S1 in supporting information). The results support calls for an urgent need to harmonize methodology in MCI and dementia research to improve cross‐study comparability. 78 Indeed, future studies could draw on current recommendations 78 for harmonization in the methods of conducting dementia and MCI work and intelligent data synthesis in HICs as well as specifically in LMICs including the 10/66 Study protocol 79 and the Harmonized Cognitive Assessment Protocol (HCAP), 80 all of which outline recommendations for cognitive assessment tools and interviewing methods to improve cross‐study comparability. Until there is agreed‐upon methodology for MCI/dementia research globally, evidence synthesis findings, such as the findings here, should be interpreted with caution.
There is an urgent need for research investment into robust, population‐representative studies focused on risk of cognitive impairment and dementia in LMICs using harmonized methodology. 78 This is necessary to make it possible to campaign for prioritization of funding toward cognitive screening and risk reduction. This would also allow investment in better education and development of infrastructure in these settings to improve knowledge of diagnosis and risk factor management, but also facilitate the implementation of more population representative, robust studies, particularly in countries of low income.
Two‐thirds of people with dementia live in LMICs, where resources, services, research, and support for older age care are limited and often non‐existent. While dementia is currently incurable, results from HICs suggest that early interventions focused on reducing risk could lessen the number of people who develop dementia in the future. This would result in major health benefits and reduced public spending. As highlighted by this review little comparative data on MCI exists in LMIC settings. The results suggest that MCI is associated with risk of conversion to dementia in LMICs and may be influenced by demographic (e.g., age) and health (e.g., cardiometabolic disease) factors, but more research is needed particularly in low‐income settings.
CONFLICTS OF INTEREST
Andrea M. McGrattan, Eduwin Pakpahan, Mario Siervo, Daniel D. Reidpath, Matthew Prina, Yueping Zhu, Chen Shulin, and Louise Robinson report no relevant disclosures. Authors who have received grants or contracts from any entity in the past 36 months: Devi Mohan—(1) Dementia toolkit for carers (DeToC): A comprehensive toolkit to support carers of people living with dementia in rural communities. Monash University Malaysia ASEAN grant; Role: Principal investigator. (2) Salivary lactoferrin for early identification of sleep related cognitive decline: A potential target for dementia prevention. Monash University Malaysia, School Strategic Grant; Role: Principal investigator. (3) Pathways of Acquired Epilepsy and their Comorbidities: A Translational Crosstalk between HMGB1 Mechanisms and Gut Microbiome. Monash University Malaysia, School Strategic Grant; Role: Co‐Investigator. (4) Global dementias: Examining structural vulnerability and dementia outcomes. Australian Research Council: Discovery Projects; Role: Partner investigator. (5) Investigating Multimorbidity Through capacity building (MUTUAL). MRC UK‐ GCRF Global Multimorbidity—Seed Funding; Role: Co‐Investigator. (6) Improving early detection and diagnosis of breast cancer among multi‐ethnic rural communities in Malaysia—the implementation of the community education and navigation programme (CENP). Newton Fund Impact Scheme Grant; Role: Co‐investigator. (7) Dementia Prevention and Enhanced Care (DePEC). National Institute of Health Research, UK: Global Health Group (Dementia)‐ (16/137/62); Role: Co‐investigator (all payments made to the institution). Jennifer Yates—NIHR RfPB Co‐investigator Enhance project (all payments made to the institution). Stella‐Maria Paddick—British council science South Asia grant; Broadening our horizons scheme; GCRF funding (Leicester university) for biomarker analysis; all small grants competitively awarded for research costs (all payments made to the institution). All authors who have received funding to travel to attend meetings in the past 36 months: Stella‐Maria Paddick—Cumbria, Northumberland, Tyne and wear NHS foundation trust—travel to Dhaka for a work related meeting; Newcastle University broadening our horizons scheme 2018—reciprocal visit between UK and Chile for one researcher at each site (all payments made to the institution). Blossom C. M. Stephan—NIHR Funded Global Health Research Group on Dementia Prevention and Enhanced care to attend ADI2020 conference (virtual due to COVID‐19; all payments made to the institution). All authors who have been members of external committees (within the past 36 months): Blossom C. M. Stephan—member of the Alzheimer's Society Research Strategy Council (chair the Dementia Prevention Subcommittee); an invited participant to the World Dementia Council, Prevention Workshop, 2021. Stella‐Maria Paddick—on the executive committee of the Royal College of Psychiatrists International Psychiatry and Volunteering Special interest group and attended a number of online meetings. Other financial or non‐financial interests in the past 36 months: Stella‐Maria Paddick—employed by the UK National Health Service.
AUTHOR CONTRIBUTIONS
Blossom C. M. Stephan conceptualized the study. Andrea M. McGrattan and Eduwin Pakpahan undertook screening and data extraction. Blossom C. M. Stephan acted as third reviewer. Eduwin Pakpahan undertook the meta‐analysis. Andrea M. McGrattan drafted the initial manuscript, and Blossom C. M. Stephan and Eduwin Pakpahan critically reviewed and edited. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
This research was funded by the National Institute for Health Research (NIHR) Global Group: DePEC (Grant Number: 16/137/62) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care.
McGrattan AM, Pakpahan E, Siervo M, et al., the DePEC team . Risk of conversion from mild cognitive impairment (MCI) to dementia in low‐ and middle‐income countries (LMICs): A systematic review and meta‐analysis. Alzheimer's Dement. 2022;8:e12267. 10.1002/trc2.12267
Andrea M. McGrattan and Eduwin Pakpahan are joint first authors.
DATA AVAILABILITY STATEMENT
This systematic review was registered via Prospero; PROSPERO registration number: CRD42019130958. A copy of the systematic review protocol is available on request and can be provided by the corresponding author. Requests for access to the data reported in this article will be considered by the corresponding author.
REFERENCES
- 1. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xue J, Li J, Liang J, Chen S. The Prevalence of mild cognitive impairment in China: a systematic review. Aging Dis. 2018;9:706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGrattan AM, Zhu Y, Richardson CD, et al. Prevalence and risk of mild cognitive impairment in low and middle‐income countries: a systematic review. J Alzheimers Dis. 2021;79:743–762. [DOI] [PubMed] [Google Scholar]
- 5. Farias ST, Mungas D, BR Reed, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic‐ vs community‐based cohorts. Arch Neurol. 2009;66:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia ‐ meta‐analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252–265. 10.1111/j.1600-0447.2008.01326.x [DOI] [PubMed] [Google Scholar]
- 7. Matthews FE, Stephan BCM, McKeith IG, Bond J, Brayne C, Medical Research Council Cognitive Function and Ageing Study . Two‐year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56:1424–1433. [DOI] [PubMed] [Google Scholar]
- 8. de Jager CA, Budge MM. Stability and predictability of the classification of mild cognitive impairment as assessed by episodic memory test performance over time. Neurocase. 2005;11:72–79. [DOI] [PubMed] [Google Scholar]
- 9. Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two‐year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81(5):541–546. 10.1136/jnnp.2008.171066 [DOI] [PubMed] [Google Scholar]
- 10. Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallassi R, Oppi F, Poda R, et al. Are subjective cognitive complaints a risk factor for dementia? Neurol Sci. 2010;31:327–336. [DOI] [PubMed] [Google Scholar]
- 12. Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population‐based prospective cohort. Neurology. 2002;59:1594–1599. [DOI] [PubMed] [Google Scholar]
- 13. Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. [DOI] [PubMed] [Google Scholar]
- 14. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. [DOI] [PubMed] [Google Scholar]
- 16. Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–140. [DOI] [PubMed] [Google Scholar]
- 17. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 18. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 19. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Third ed rev. 1897.
- 21. Pírez G, Santabárbara J, Lopez‐Anton R, et al. Different subpopulations of mild cognitive impairment are identified by using Petersen's or DSM‐5 criteria. Eur J Psychiatry. 2017;31(2):80–86. 10.1016/j.ejpsy.2016.11.001 [DOI] [Google Scholar]
- 22. Saunders S, Ritchie K, Russ TC, Muniz‐Terrera G, Ritchie CW. Evolution and future directions for the concept of mild cognitive impairment. Int Psychogeriatr. 2018;30:1431–1434. [DOI] [PubMed] [Google Scholar]
- 23. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet (London, England). 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 24. Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29:873–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prince M, Wimo A, Guerchet M, Ali G‐C, Wu Y‐T & Prina M. World Alzheimer Report . The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015. World Alzheimer's Report.
- 26. Ferri CP, Jacob KS. Dementia in low‐income and middle‐income countries: different realities mandate tailored solutions. PLoS Med. 2017;14:e1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. [DOI] [PubMed] [Google Scholar]
- 29. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 31. Bell CC. DSM‐IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272:828–829. [Google Scholar]
- 32. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43(2):250. 10.1212/wnl.43.2.250 [DOI] [PubMed] [Google Scholar]
- 33. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. 2000. Accessed 31 July, 2021. http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf
- 34. Nyaga NyagaV, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. [DOI] [PubMed] [Google Scholar]
- 36. Huang J, Meyer JS, Zhang Z, et al. Progression of mild cognitive impairment to Alzheimer's or vascular dementia versus normative aging among elderly Chinese. Curr Alzheimer Res. 2005;2:571–578. [DOI] [PubMed] [Google Scholar]
- 37. Yu BC, Tian JL, Ouyang LS, et al. Incidence rate of mild cognitive impairment and the conversion rates into dementia or Alzheimer disease among elderly people: a population‐based cohort study. Neurology. 2006;10:147–150. [Google Scholar]
- 38. Yu HM, Yang SS, Gao JW, Zhou LY, Liang RF, Qu CY. Multi‐state Markov model in outcome of mild cognitive impairments among community elderly residents in Mainland China. Int Psychogeriatr. 2013;25:797–804. [DOI] [PubMed] [Google Scholar]
- 39. Wang YP, Zhai JB, Zhu F, Zhang WW, Yang XJ, Qu CY. A three‐year follow‐up study on the transfer of mild cognitive impairment to Alzheimer's disease among the elderly in Taiyuan city. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:105–109. [PubMed] [Google Scholar]
- 40. Yang AN, Wang XL, Rui HR, Luo H, Pang M, Dou XM. Neuropsychiatric symptoms and risk factors in mild cognitive impairment: a cohort investigation of elderly patients. J Nutr Health Aging. 2020;24:237–241. [DOI] [PubMed] [Google Scholar]
- 41. Ding D, Zhao Q, Guo Q, et al. Progression and predictors of mild cognitive impairment in Chinese elderly: a prospective follow‐up in the Shanghai Aging Study. Alzheimers Dement (Amst). 2016;4:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godinho C, Camozzato AL, Onyszko D, Chaves ML. Estimation of the risk of conversion of mild cognitive impairment of Alzheimer type to Alzheimer's disease in a south Brazilian population‐based elderly cohort: the PALA study. Int Psychogeriatr. 2012;24:674–681. [DOI] [PubMed] [Google Scholar]
- 43. Montaño MBMM, Andreoni S, Ramos LR. Clinical Dementia Rating independently predicted conversion to dementia in a cohort of urban elderly in Brazil. Int Psychogeriatr. 2013;25:245–251. [DOI] [PubMed] [Google Scholar]
- 44. Baiyewu O, Unverzagt FW, Ogunniyi A, et al. Cognitive impairment in community‐dwelling older Nigerians: clinical correlates and stability of diagnosis. Eur J Neurol. 2002;9:573–580. [DOI] [PubMed] [Google Scholar]
- 45. Paddick SM, Kisoli A, Samuel M, et al. Mild cognitive impairment in rural Tanzania: prevalence, profile, and outcomes at 4‐year follow‐up. Am J Geriatr Psychiatry. 2015;23:950–959. [DOI] [PubMed] [Google Scholar]
- 46. Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. [DOI] [PubMed] [Google Scholar]
- 47. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(Suppl 1):65–69. [DOI] [PubMed] [Google Scholar]
- 48. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 49. Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease?. Lancet Neurol. 2004;3:246–248. [DOI] [PubMed] [Google Scholar]
- 50. Levy R. Aging‐associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6:63–68. [PubMed] [Google Scholar]
- 51. Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory‐impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 52. Dubois B, Feldman H, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 53. Orgogozo JM, van Drimmelen‐Krabbe J, Bradley WG, L'Hours A, Sartorius N. The international classification of WHO diseases (ICD‐10) and its application in neurology (ICD‐10 NA). Rev Neurol (Paris). 1994;150:813–822. [PubMed] [Google Scholar]
- 54. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Michaud TL, Su D, Siahpush M, Murman DL. The risk of incident mild cognitive impairment and progression to dementia considering mild cognitive impairment subtypes. Dement Geriatr Cogn Dis Extra. 2017;7:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel‐Heller SG. Mild cognitive impairment: long‐term course of four clinical subtypes. Neurology. 2006;67:2176–2185. [DOI] [PubMed] [Google Scholar]
- 57. Jungwirth S, Zehetmayer S, Hinterberger M, Tragl KH, Fischer P. The validity of amnestic MCI and non‐amnestic MCI at age 75 in the prediction of Alzheimer's dementia and vascular dementia. Int Psychogeriatr. 2012;24:959–966. [DOI] [PubMed] [Google Scholar]
- 58. Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. [DOI] [PubMed] [Google Scholar]
- 59. McGuinness B, Barrett SL, McIlvenna J, Passmore AP, Shorter GW. Predicting conversion to dementia in a memory clinic: a standard clinical approach compared with an empirically defined clustering method (latent profile analysis) for mild cognitive impairment subtyping. Alzheimers Dement. 2015;1:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nordlund A, Rolstad S, Klang O, Edman Å, Hansen S, Wallin A. Two‐year outcome of MCI subtypes and aetiologies in the Göteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81:541. [DOI] [PubMed] [Google Scholar]
- 61. Summers MJ, Saunders NL. Neuropsychological measures predict decline to Alzheimer's dementia from mild cognitive impairment. Neuropsychology. 2012;26:498–508. [DOI] [PubMed] [Google Scholar]
- 62. Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882. [DOI] [PubMed] [Google Scholar]
- 63. Jorm AF, Jolley D. The incidence of dementia: a meta‐analysis. Neurology. 1998;51:728–733. [DOI] [PubMed] [Google Scholar]
- 64. Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amieva H, Letenneur L, Dartigues JF, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population‐based study. Dement Geriatr Cogn Disord. 2004;18:87–93. [DOI] [PubMed] [Google Scholar]
- 66. Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE‐ 4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology, 2003;60(7):1077–1081. 10.1212/01.wnl.0000055875.26908.24 [DOI] [PubMed] [Google Scholar]
- 67. Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer's disease. Alzheimer's Disease Centers Consortium on Apolipoprotein E and Alzheimer's Disease. N Engl J Med. 1998;338:506–511. [DOI] [PubMed] [Google Scholar]
- 68. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (New York, N Y). 2015;1:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paddick S‐M, Longdon A, Gray WK, et al. The association between educational level and dementia in rural Tanzania. Dement Neuropsychol. 2014;8:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Angrisani M, Lee J, Meijer E. The gender gap in education and late‐life cognition: evidence from multiple countries and birth cohorts. J Econ Ageing. 2020;16:100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hasselgren C, Ekbrand H, Halleröd B, et al. Sex differences in dementia: on the potentially mediating effects of educational attainment and experiences of psychological distress. BMC Psychiatry. 2020;20:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bloomberg M, Dugravot A, Dumurgier J, et al. Sex differences and the role of education in cognitive ageing: analysis of two UK‐based prospective cohort studies. Lancet Public Health. 2021;6:e106–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ganguli M, Lee C‐W, Snitz BE, Hughes TF, McDade E, Chang C‐CH. Rates and risk factors for progression to incident dementia vary by age in a population cohort. Neurology. 2015;84:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the LancetCommission. Lancet North Am Ed. 2020;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scarmeas N, Anastasiou CA. Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–1015. [DOI] [PubMed] [Google Scholar]
- 76. Organisation WH. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. WHO; 2019. [PubMed] [Google Scholar]
- 77. Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stephan BCM, Siervo M, Brayne C. How can population‐based studies best be utilized to reduce the global impact of dementia? Recommendations for researchers, funders, and policymakers. Alzheimers Dement. 2020;16:1448–1456. [DOI] [PubMed] [Google Scholar]
- 79. Prina AM, Acosta D, Acosta I, et al. Cohort profile: the 10/66 study. Int J Epidemiol. 2017;46:406–i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weir DRLK & Ryan LH Harmonized cognitive assessment protocol (HCAP): study protocol summary. 2016. Available at: https://hrs.isr.umich.edu/publications/biblio/9950 [Accessed: 31 July 2021].
- 81. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 82. Morris JC. The clinical dementia rating (CDR). Neurology. 1993;43:2412. [DOI] [PubMed] [Google Scholar]
- 83. Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment1 ‐ a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106(6):403–414. 10.1034/j.1600-0447.2002.01417.x [DOI] [PubMed] [Google Scholar]
- 84. Nie H, Xu Y, Liu B, et al. The prevalence of mild cognitive impairment about elderly population in China: a meta‐analysis. Int J Geriatr Psychiatry. 2011;26:558–563. [DOI] [PubMed] [Google Scholar]
- 85. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alexander M, Perera G, Ford L, et al. Age‐stratified prevalence of mild cognitive impairment and dementia in European Populations: a systematic review. J Alzheimers Dis. 2015;48:355–359. [DOI] [PubMed] [Google Scholar]
- 87. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. [DOI] [PubMed] [Google Scholar]
- 88. Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta‐analysis. Int Psychogeriatr. 2017;29:1595–1608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION
Data Availability Statement
This systematic review was registered via Prospero; PROSPERO registration number: CRD42019130958. A copy of the systematic review protocol is available on request and can be provided by the corresponding author. Requests for access to the data reported in this article will be considered by the corresponding author.


