Abstract
Background: This study aimed to assess the stability of pressure derived fractional flow reserve (FFR) measurement and the handling performance of the OptoWire Deux with an optical pressure sensor relative to the PressureWire X with piezo resistive pressure sensors. Methods: This multicenter centre observational study included 50 patients between June 2017 and November 2018 undergoing a diagnostic coronary angiography with FFR measurement of moderate to severe lesions. The reliability of FFR measurement measured with the OptoWire Deux relative to the PressureWire X in each lesion was assessed by the presence of drift. Handling characteristics for both pressure wires were assessed by a 5-point scale and by comparing the time between equalization and crossing the distal target lesion. Results: Hundred and sixteen measurements in 50 patients were performed. Very stable and reliable FFR measurements with the optical sensors were registered, relative to the piezo resistive pressure sensors. There is statistically significant difference in favor of the OptoWire Deux over the PressureWire X (P=0.001). However, the differences are small, when drift values were compared as continuous variables, no statistically significant difference was found for both directional (P=0.435) as for absolute drift (P=0.058). Conclusions: In patients undergoing FFR measurement, both optical sensor pressure wires (Optowire Deux) as piezo resistive sensor pressure wires (PressureWire X) generate stable and reliable pressure and thus FFR measurement. The optical pressure sensor is less susceptible for drift relative to the piezo resistive pressure sensor, but the difference is within an acceptable range.
Keywords: Fractional flow reserve, drift, optical pressure sensor, piezo resistive pressure sensor
Introduction
Pressure derived fractional flow reserve measurement (FFR) is an objective and quantitative assessment of the functional severity of a coronary artery stenosis during cardiac catheterization using a hyperemic stimulus. The concept was first described by Pijls et al. [1]. In the decades following the DEFER, FAME 1 and FAME 2 studies provide strong evidence that systematic use of FFR for evaluation of angiographically intermediate lesions to guide revascularization improves outcome and is desirable from economical point of view [2-5]. It is safe to defer treatment with an FFR value >0.80 with an excellent long-term prognosis reflected in a rate of death and myocardial infarction (MI) of 0.6%/year [2-4,18]. The results of the Define-Flair and Swede-Heart studies showed a non-inferiority of the non-hyperemic resting index iFR versus FFR [6,7,16,17]. These large and well conducted trials only add up the amount of evidence in favor of using the physiological assessment of moderate coronary stenoses. Since 2010, FFR has a Class Ia recommendation of the European society of Cardiology for the severity assessment of intermediate coronary lesions.
Hence, a reliable pressure measurement is the corner stone for assessing physiological severity of intermediate lesions both for FFR as for non-hyperemic resting indices. Nonetheless, there are a few pitfalls for a reliable measurement: procedural-related (variability of the aortic pressure transducer height, inappropriate pressure wire calibration, not removing the needle guidewire introducer, contrast medium in the catheter, excessive intubation of the guide catheter in the coronary ostium…) and pressure wire-related (temperature-mediated or temporal-mediated). These are all potentially causes of the pressure measuring error phenomenon we call drift [15,19,20]. During the last 20 years, efforts have been made by the manufacturers of electronic pressure wires to decrease drift. Despite improvements, this phenomenon still occurs. Therefore, there is a clinical need for a pressure wire providing reliable pressure measurements with minimal pressure drift and whose performance would approximate a standard angioplasty workhorse wire. This becomes even more important with the increasing use of resting indices. Opsens Inc. has developed a pressure wire with an optical pressure sensor: the OptoWire deux. It has been hypothesized that this design would lead to less undesired drift [12,14]. To date, a systematic study to monitor drift for different pressure wires has never been tested to the best of our knowledge. Therefore, we prospectively studied both the drift phenomenon and the handling characteristics of the optical sensor pressure wire (OptoWire Deux) versus the most frequently used piezo resistive electrical sensor pressure wire (PressureWire X).
Methods
Study population
We performed a multicenter prospective study of FFR measurements in 50 patients. A total of 48 lesion assessments were included in the analysis. Each lesion was assessed by both the piezo resistive sensor pressure wire (PressureWire X) and the optical sensor pressure wire (OptoWire Deux). All measurements were performed by four different operators. Each operator tested the two devices for handling characteristics, presence of drift and disconnection/reconnection capability. A FFR measurement was performed on intermediate lesions in native coronary arteries. Patients with type C lesions, prior-coronary artery bypass grafting, total occlusions, (expected) need for atherectomy device and lesions with angiographic haziness or suspected thrombus were excluded.
The study was approved by the local ethics committee and conformed to the Declaration of Helsinki on human research. Written informed consent was obtained from all study patients.
Procedure
All coronary angiography procedures were performed using radial or femoral route with 6Fr guiding catheters for the physiological assessment. Heparin was administered (70 units/kg). All patients received intracoronary nitrates (isosorbide dinitrate 1-2 mg). The first type of pressure wires was randomly assigned to the patient, followed by a second measurement by the second type of pressure wire. Fifty pressure wires with an optical pressure sensor (OptoWire Deux-Opsens medical, Quebec, Canada) and 50 pressure wires with a piezo resistive pressure sensor (PressureWire X-Abbott, Illinois, United States) were used. Each of the four operators (each operator has a minimal volume of >300 percutaneous coronary interventions per year as a primary operator) equally tested the two devices for presence of drift, disconnection/reconnection capability, and handling characteristics.
A FFR measurement was performed on intermediate lesions in native coronary arteries. The electrical sensor pressure wires were flushed with saline and stabilized on a flat surface 3 minutes before use. Pressure wire equalization was performed with the pressure sensor 1 mm distal to the guiding catheter. A stabilization interval of 30 seconds was admitted and a Pd/Pa value afterwards of 1.0 was mandatory. Following equalization, the wire was advanced and positioned distal to the target lesion. The measurement of FFR was performed with intravenous adenosine (140 μg/min/kg). After FFR measurement a pullback was performed and Pd/Pa was measured again with the pressure sensor 1 mm distal to the guiding catheter tip; at the location of the initial equalization with the same baseline conditions as described above. Pressure drift was defined in two different ways: the Pd/Pa post stabilization minus the Pd/Pa after pullback being >0.02 and Pa minus Pd after pullback being >3 mmHg. Thereafter, successful disconnection/reconnection was tested.
All measurements were performed after withdrawal of the needle and flushing the guiding catheter with saline to avoid interference of blood/contrast in the catheter and contrast induced hyperemia.
The handling performance was assessed by scoring each wire relative to the same investigator’s own perception on a five-point scale: superior, better, equal, minor or inferior with respect to the other wire. The following characteristics were scored: overall, torquability, steerability, pushability and support. The time needed to position the pressure wire from the equalization position till the distal region of the target lesion was recorded as well.
Statistical analysis
Data are expressed as mean (± standard deviation, SD) or median with interquartile range as appropriate. Student’s T test or the Mann-Whitney U test were used for comparison of continuous variables as appropriate. For comparison of categorical data the Chi-square test was used. A P-value less than 0.05 was considered statistically significant, and applicable tests were always two-sided. All analyses were conducted using SPSS 25 software (IBM corporation, Armonk, NY).
Results
Baseline characteristics
Fifty patients underwent a physiological study at the hospital Ziekenhuis Oost-Limburg, Belgium and at the hospital‘s Hertogenbosch, the Netherlands, between June 2017 and November 2018. The study population was predominantly male (78%) and almost all measurements were performed in patients planned for elective coronary angiography for stable angina or silent ischemia on their non-invasive stress test (82%). All 50 patients had at least one cardiovascular risk factor and 12% of the population had diabetes (Table 1). The characteristics of the different interrogated lesions are illustrated in Table 2.
Table 1.
Demographics of the study population
| Study patients (n=50) | |
|---|---|
| Age (years) | 66±9 |
| Male | 39 (78) |
| Tobacco use | 12 (24) |
| Familial history | 25 (50) |
| Hypertension | 35 (70) |
| Dyslipidemia | 40 (80) |
| Diabetes mellitus* | 6 (12) |
| Atrial fibrillation | 1 (2) |
| Congenital Heart Failure | 1 (2) |
| Previous MI | 9 (18) |
| Previous CABG | 1(2) |
| Previous PCI | 18 (36) |
| Previous TIA/stroke | 3 (6) |
| Clinical presentation | |
| Stable angina/silent ischaemia | 41 (82) |
| Unstable angina | 2 (4) |
| N-STEMI | 3 (6) |
| STEMI | 1 (2) |
| Other∑ | 3 (6) |
Values expressed as n (%) or mean ± standard deviation. Data was missing for a number of variables: previous TIA/stroke (n=1), atrial fibrillation (n=1), congenital heart failure (n=2), previous CABG (n=2), familial history (n=3).
Diabetes mellitus was treated in 6 patients using either oral hypoglycemic treatment (n=3) or insulin (n=3).
Patients were treated for decompensation (n=1), asymptomatic left ventricular dysfunction (n=1), and pre-operatively for aortic valve replacement (n=1).
Abbreviations: CABG, coronary artery bypass graft surgery; MI, myocardial infarction; N-STEMI, Non-STEMI; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TIA, transient ischaemic attack.
Table 2.
Characteristics of different coronary vessels measured using pressure wire technology
| First vessel (n=50) | Second vessel (n=7)∑ | Third vessel (n=1)∑ | |
|---|---|---|---|
| Coronary vessel involved | |||
| Left Anterior Descending artery | 35 (70) | 4 (57) | 1 (100) |
| Circumflex artery | 6 (12) | 1 (14) | - |
| Right coronary artery | 8 (16) | 2 (29) | - |
| Other* | 1 (2) | - | - |
| Diameter stenosis (visual estimation) | 60±8 (60) | 72±6 (70) | - |
| Vessel tortuosity | |||
| None | 13 (28) | - | - |
| Mild | 26 (55) | 6 (86) | 1 (100) |
| Moderate | 7 (15) | 1 (14) | - |
| Severe | 1 (2) | - | - |
| Calcification | |||
| None | 9 (19) | 2 (29) | 1 (100) |
| Mild | 26 (55) | 3 (43) | - |
| Moderate | 10 (21) | 2 (29) | - |
| Severe | 2 (4) | - | - |
| Catheter size (French) | 6±0 (6) | 6±0 (6) | 6±0 (6) |
Values expressed as n (%) or mean ± standard deviation (median). Abbreviations: LAD, left anterior descending coronary artery; CX, circumflex artery; RCA, right coronary artery.
Other vessel specified as AL/FB (n=1).
In seven patients the same pressure wire was used in a second vessel. In one patient, the same pressure wire was used in a third vessel.
Results of the pressure study
A total of 58 coronary arteries in 50 patients undergoing coronary angiography were prospectively included and analyzed in this study. In 42 of the patients only 1 vessel was measured, 2 vessels were measured in 7 patients, and 3 vessels were measured in 1 patient.
Overall initial Pd/Pa ratio before equalization was 1.02±0.06 and after equalization Pd/Pa had a median value of 1.01±0.03 (Tables 3, 4).
Table 3.
Overall pressure wire measurements with both devices
| Total measurements (n=116) | |
|---|---|
| Pd/Pa prior to equalization | 1.02±0.06 (1.02) |
| Pd prior to equalization | 88.81±12.64 (88.0) |
| Pd/Pa post stabilization | 1.01±0.02 (1.0) |
| Pd post stabilization | 88.12±12.80 (87.0) |
| Time from equalization to desired destination (sec) | 39±43 (25) [13-45] |
| FFR measurement possible | 116 (100) |
| FFR | 0.81±0.11 (0.83) |
| Pd post adenosine | 66.21±14.85 (66.5) |
| Post Pd/Pa | 1.00±0.02 (1.00) |
| Post Pd | 83.44±14.31 (84.00) |
| Post Pa | 83.88±14.34 (84.58) |
Values expressed as n (%) or mean ± standard deviation (median) or n (%) or interquartile range [iQR].
Table 4.
Pressure measurements in the different devices
| Pressure wire 1 (n=58) | Pressure wire 2 (n=58) | |
|---|---|---|
| Pd/Pa prior to equalization | 1.04±0.05 (1.05) | 1.00±0.06 (0.99) |
| Pd prior to equalization | 90.16±12.22 (89.0) | 87.47±13.02 (88.00) |
| Pd/Pa post stabilization | 1.01±0.03 (1.00) | 1.01±0.02 (1.00) |
| Pd post stabilization | 86.77±13.73 (86.00) | 89.45±11.77 (88.50) |
| Time from equalization to desired destination (sec) | 35±38 (26) [13-45] | 43±48 (25) [15-47] |
| FFR measurement possible | 58 (100) | 58 (100) |
| FFR | 0.81±0.10 (0.83) | 0.82±0.11 (0.83) |
| Pd post adenosine | 65.81±13.00 (63.00) | 66.60±16.61 (68.50) |
| Post Pd/Pa | 1.00±0.01 (1.00) | 0.99±0.03 (1.00) |
| Post Pd | 83.59±14.23 (85.00) | 83.29±14.53 (83.00) |
| Post Pa | 83.74±14.45 (85.00) | 84.03±14.35 (84.02) |
| Pd minus Pa | 0.55±0.67 (0.00) | 1.76±2.43 (0.70) |
| Pd/Pa post stabilization minus Pd/Pa post | 0.01±0.02 (0.01) | 0.03±0.04 (0.01) |
Values expressed as n (%) or mean ± standard deviation (median) or n (%) or interquartile range [iQR].
For the primary study endpoint, the pressure drift phenomenon was defined in two ways with cut-off points. For both definitions of drift, there is a statistically significant difference between the two wires in favor of the Optowire Deux. Pd/Pa post stabilization minus the Pd/Pa after pullback being different from 1.00±0.02 was observed in 24% of the measurements (Optowire Deux 6/57 or 11%-Pressurewire X 21/57 or 37%; P=0.001) or Pa minus Pd after pullback >3 mmHg was observed in 11% of the measurements (Optowire Deux 0%-PressureWire X 21%; P=0.001) (Table 5). However, when we consider the pressure drift as a continuous variable, there is no statistically significant difference between the two wires both. Directional drift (positive and negative drift) did not differ significantly between the 2 wires. Absolute drift showed no statistically significant difference (Figures 1, 2).
Table 5.
Drift analyses with cut-off points. Drift was either defined as the PdPa post-stabilization minus the post PdPa (drift definition 1) or as post Pd minus post Pa with a cut-off value of 3 mmHg (drift definition 2)
| Total (n) | Pressure wire 1 (n) | Pressure wire 2 (n) | P-value | |
|---|---|---|---|---|
| Drift definition 1 | 0.001 | |||
| Drift | 27 | 6 | 21 | |
| No drift | 87 | 51 | 36 | |
| Total | 114 | 57 | 57 | |
| Drift definition 2 | <0.001 | |||
| Drift | 12 | 0 | 12 | |
| No drift | 102 | 58 | 44 | |
| Total | 114 | 58 | 56 |
Values expressed as n. Drift was defined either as the PdPa post-stabilization minus the post PdPa (drift definition 1) or as post Pd minus post Pa with a cut-off value of 3 mmHg (drift definition 2).
Figure 1.
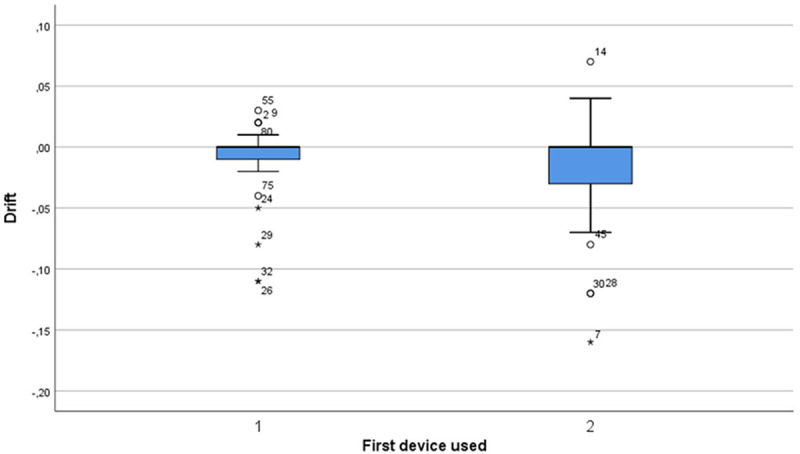
Directional drift comparison between pressure wire 1 and 2 for measurements performed in the first vessel. No statistically significant difference (median pressure wire 1: 0.0 [IQR; -0.01 to 0.0], median pressure wire 2: 0.0 [IQR; -0.04 to 0.0], P=0.435) was found for directional drift between the two wires.
Figure 2.
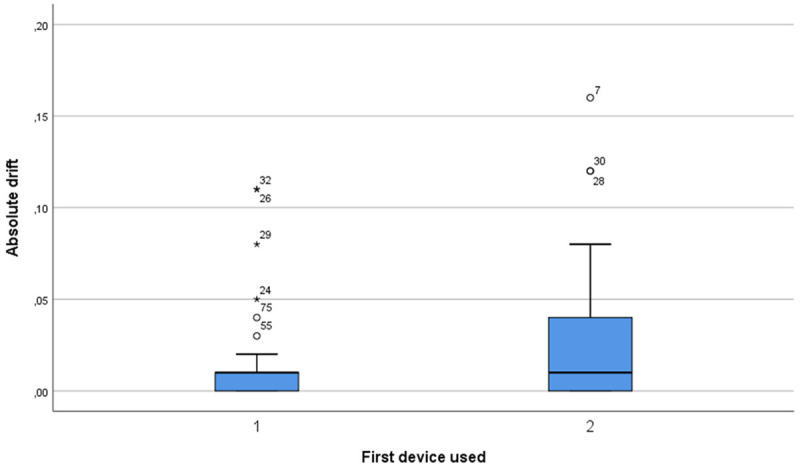
Absolute drift comparison between pressure wire 1 and 2 for measurements performed in the first vessel. No statistically significant difference (median pressure wire 1: 0.01 [IQR; 0.0 to 0.01], median pressure wire 2: 0.01 [IQR; 0.0 to 0.04], P=0.058) was found for absolute drift between the two wires.
For the secondary endpoint, the median time to bring the pressure sensor from the tip of the guiding to the target zone after crossing the first target was not statistically different between the two pressure wires was (Figure 3). We have comparable results for the second vessel measurement (Figure 4). After equalization, the wire was successfully advanced in all cases. The mean FFR value was 0.81±0.11 (0.83).
Figure 3.
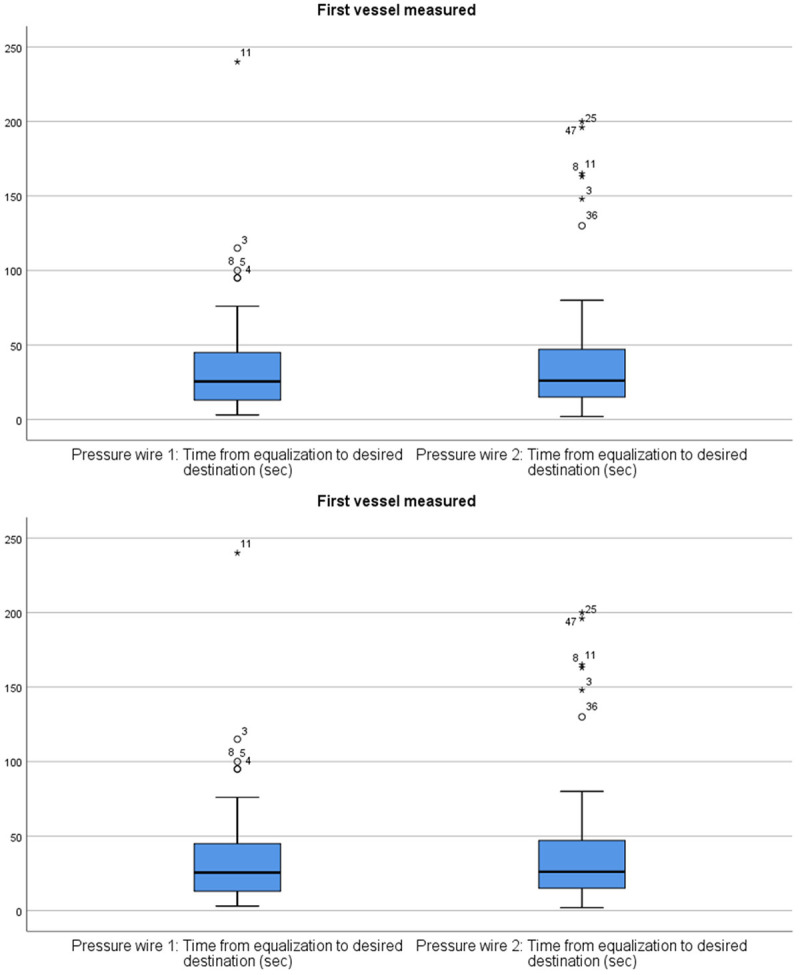
Comparison of the time required from equalization to the desired destination between the two pressure wires in the first coronary vessel measured. No statistically significant difference (median pressure wire 1: 13.0 [IQR; 6.0 to 33.0], median pressure wire 2: 14.0 [IQR; 9.5 to 22.5], P=0.418) for the time it takes to reach and cross the lesion was found between the two pressure wires.
Figure 4.
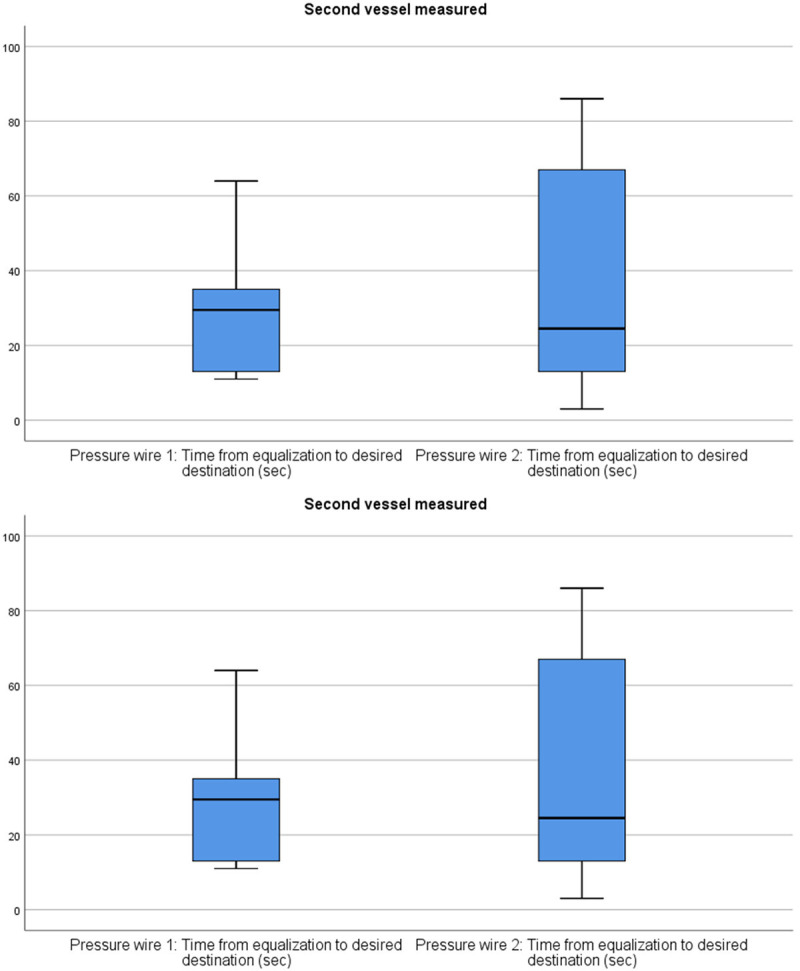
Comparison of the time required from equalization to the desired destination between the two pressure wires in the second coronary vessel measured. No statistically significant difference (median pressure wire 1: 31.0 [IQR; 13.0 to 35.0], median pressure wire 2: 24.5 [IQR; 10.5 to 71.8], P=0.943) for the time it takes to reach and cross the lesion was found between the two pressure wires.
For the handling characteristics, we refer to Figure 5. No statistically significant differences were found for pushability (P=0.141) and support (P=0.276). On the other hand, torquability (P=0.001), steerability (P=0.001) and the overall performance (P=0.018) did differ significantly in favor of PressureWire X.
Figure 5.
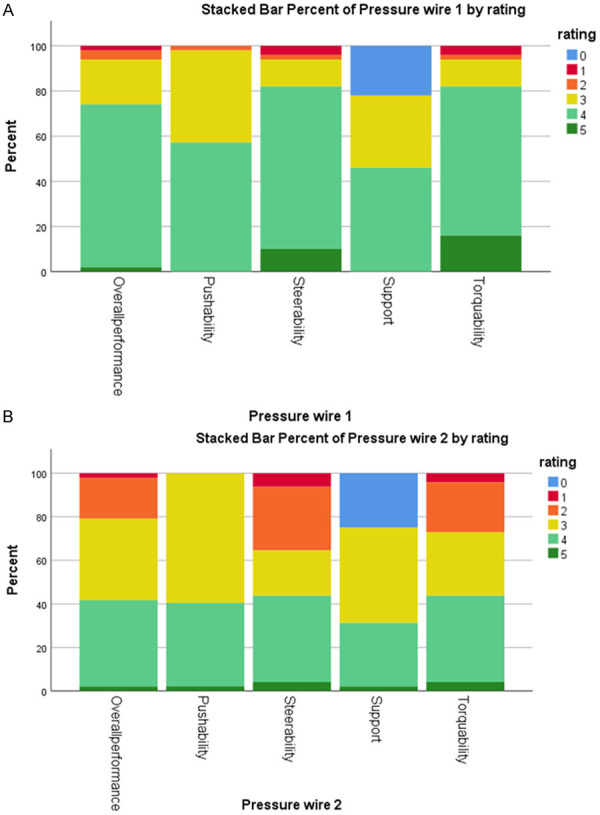
Comparison of the performance of pressure wire 1 (A) and 2 (B), evaluated according to several characteristics: overall performance, pushability, steerability, support, torqueability. No statistically significant differences were found for pushability (P=0.141) and support (P=0.276). On the other hand, torqueability (P=0.001), steerability (P=0.001) and the overall performance (P=0.018) did differ significantly in favor of pressure wire 1.
The number of pressure wires used (P=0.558) and the number of re-equalizations (P=0.13) did not differ significantly.
Discussion
The main study findings are: (1) the optical sensor pressure wire OptoWire Deux gives reliable results with no significant difference in absolute or directional pressure drift in comparison to the conventional piezo resistive pressure sensor wires and significant less drift phenomenon when defined as a cutoff value; (2) the handling characteristics of both wires are equally performant with no significant difference in time from equalization to reach the target distal vessel after crossing the; and (3) non-blinded handling performance scoring of the different wires showed no difference for pushability and support and a slight better score for torquability and steerability in favor of the Optowire Deux.
Pressure drift (PD) is, even with the impressive technical developments over the last 20 years, still a common phenomenon. It is often defined as a mean aortic pressure minus mean distal pressure measured after pullback at the tip of the guiding of >3 mmHg. A recent large study with the St. Jude Medical piezo resistive pressure sensor Pressure Wire Certus reported a significant drift phenomenon (≥4 mmHg) in 11.8% of the cases and a small drift (PD ≤3 mmHg) frequency in 39.3% of the cases [8]. It is generally accepted that a PD >4 mmHg makes a re-measurement mandatory. The phenomenon of small drift does not have a significant impact. A recent study showed that it does not changed the decision when FFR value is <0.76 or >0.82, it only changes for the FFR in between the classification in 18.7% of the cases but not in a single patient did the value shift from above the upper limit of the grey zone (0.76-0.80) to below the lower limit or vice versa [8]. The small PD phenomenon does have a more important impact when you measure resting gradients, because the signal to noise ratio during hyperemia is 2.5 to 3.0-fold higher compared to resting gradients. As a consequence, it can be expected to be more vulnerable to the influence of drift [9]. It may cause stenosis misclassification, especially when indices values are close to their cutoff values. A recent study showed that a pressure wire drift of ±2 mmHg causes stenosis misclassification in all contemporary-used pressure-derived indices, in particular when close to the cutoff value. The effect of drift originating from changes in distal pressure resulted in reclassification in 21%, 25% and 33% with FFR, iFR, and whole-cycle Pd/Pa, respectively. FFR and iFR are reported to be less susceptible to drift than whole-cycle Pd/Pa [11,13].
Despite a very strict protocol to prevent the procedure-related causes of pressure drift phenomenon (rigorous flushing of the piezo resistive pressure sensors before procedure, adequate zero, 30 s interval to see a stable value after equalizing, extraction of introducer needle when checking for drift and flushing the guiding catheter with water) and thus be able to see the pressure-wire related causes of drift, we observed PD in 11% to 24% of all our measurements (according definition of binary cutoff respectively Pd minus Pa after pullback >3 mmHg vs Pd/Pa after stabilization minus Pd/Pa after pullback). These results are in line with the previously observed pressure drift in the Certus wire [8]. Most of the cases were just borderline significant drifts which did not lead to a re-measurement (especially since FFR was <0.76 or >0.82. The observed differences in pressure drift are attributable to small differences just beyond the 3 mmHg treshold. We observed significantly more drift in the piezo resistive sensor systems (21/57-37%) compared to the optical sensors (6/57-11%; P=0.004) using the Pd/Pa after stabilization minus Pd/Pa after pullback >0.02 (Table 5). Theoretically, it is suggested that the optical signal is not affected by the presence of moisture. The design of the OptoWire deux minimizes the impact of moisture induced stress that may otherwise lead to undesired PD.
Overall, the OptoWire deux scores better with respect to steering- and torque characteristics than the piezo resistive sensor pressure wires and equally with respect to pushability and support characteristics. As opposed to electric pressure sensors, the OptoWire deux uses an optical pressure sensor that requires only a single fiber, which runs along the central axis of the hollow tubing. This allows a 1:1 transmission of torque. On the contrary, the traditional pressure wires consist of 3 small electrical wires that offset the core-wire from the center, in turn limiting the torquability. Consequently, it is believed that the OptoWire deux has handling characteristic that almost mimic a standard angioplasty wire. The disadvantage of the Optowire deux is a wired connector (Pressurewire X has a wireless connection) which causes build-up of counter torque, to overcome that in practice, the operators often tend to disconnect the wire from the connector before wiring the distal vessel (we did not correct for this disadvantage in the assessment of the torquability). We have to emphasize that the scoring of the wire was not blinded for the operator, so there is potentially a bias. When we have a look at the time needed to get the wire from the guiding catheter to the distal target zone, there is no significant difference between the two pressure wires, which means that objectively there is not a major difference in handling characteristics between the two devices. The pressureWire X has a thermal sensor which allows thermodilution measurements and open the possibility of measuring microvasculature (IMR or index of microcirculatory resistance) in one procedure with one single wire possible.
These results are promising. In daily clinical practice, the availability of a wire that provides reliable measurement outcomes (i.e. limited drift) and a pressure wire with good handling characteristics is of the utmost clinical significance. Despite the high level of recommendation, the measurement of FFR and other resting indices currently remain underused techniques to interrogate intermediate coronary lesions. With the advent of recent technical developments, the threshold for using these devices will potentially be reduced [10].
Study limitations
The presented study has a number of limitations that merit consideration. First, the rather small number of patients represents a limitation in the statistical evaluation. Second, the assessment of the handling characteristics of the two types of pressure wires was not blinded so potentially generating a bias.
Conclusion
In patients undergoing a physiological coronary measurement, the reliability of the novel OptoWire (Opsens Medical) with an optical pressure sensor in regard to the electrical pressure sensor pressure wires (PressureWire X) assessed by drift phenomenon was statistically significant different in favor of the optical pressure sensor wire. However the differences are small and within a clinical acceptable range.
Acknowledgements
We would like to thank Jo Zelis and Marcel van ‘t Veer from the Department of Biomedical Engineering of the University of Technology Eindhoven for his statistical support.
Disclosure of conflict of interest
Jo Dens receives grants from TopMedical (Distributor of OptoWire Deux) Asahi Intecc co. materials) for teaching courses and proctoring.
References
- 1.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 2.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 3.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 5.Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert U Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) Study InvestigatorsI. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122:2545–2550. doi: 10.1161/CIRCULATIONAHA.109.925396. [DOI] [PubMed] [Google Scholar]
- 6.Davies JE, Sen S, Escaned J. Instantaneous wave-free ratio versus fractional flow reserve. N Engl J Med. 2017;377:1597–1598. doi: 10.1056/NEJMc1711333. [DOI] [PubMed] [Google Scholar]
- 7.Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Todt T, Venetsanos D, James SK, Kåregren A, Nilsson M, Carlsson J, Hauer D, Jensen J, Karlsson AC, Panayi G, Erlinge D, Fröbert O iFR-SWEDEHEART Investigators. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–1823. doi: 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- 8.Wakasa N, Kuramochi T, Mihashi N, Terada N, Kanaji Y, Murai T, Lee T, Yonetsu T, Kobashi K, Miyamoto K, Tobata H, Kakuta T. Impact of pressure signal drift on fractional flow reserve-based decision-making for patients with intermediate coronary artery stenosis. Circ J. 2016;80:1812–1819. doi: 10.1253/circj.CJ-15-1195. [DOI] [PubMed] [Google Scholar]
- 9.Pijls NH, Bruyne BD. Fractional flow reserve, coronary pressure wires, and drift. Circ J. 2016;80:1704–1706. doi: 10.1253/circj.CJ-16-0623. [DOI] [PubMed] [Google Scholar]
- 10.Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, Rusinaru D, Di Gioia G, Pellicano M, Barbato E, Van Mieghem C, Heyndrickx GR, De Bruyne B, Wijns W. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014;7:751–759. doi: 10.1161/CIRCINTERVENTIONS.114.001608. [DOI] [PubMed] [Google Scholar]
- 11.Cook CM, Ahmad Y, Shun-Shin MJ, Nijjer S, Petraco R, Al-Lamee R, Mayet J, Francis DP, Sen S, Davies JE. Quantification of the effect of pressure wire drift on the diagnostic performance of fractional flow reserve, instantaneous wave-free ratio, and whole-Cycle Pd/Pa. Circ Cardiovasc Interv. 2016;9:e002988. doi: 10.1161/CIRCINTERVENTIONS.115.002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawase Y, Tanigaki T, Hirakawa A, Omori H, Hirata T, Okamoto S, Ota H, Kikuchi J, Okubo M, Kamiya H, Kawasaki M, Suzuki T, Matsuo H. Frequency of a large drift caused by pressure wire using optical fibers. Cardiovasc Interv Ther. 2018;33:270–276. doi: 10.1007/s12928-017-0481-x. [DOI] [PubMed] [Google Scholar]
- 13.Casadonte L, Piek JJ, VanBavel E, Spaan JAE, Siebes M. Discordance between pressure drift after wire pullback and intracoronary distal pressure offset affects stenosis physiology appraisal. Int J Cardiol. 2019;277:29–34. doi: 10.1016/j.ijcard.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 14.Ulacia P, Rimac G, Lalancette S, Belleville C, Mongrain R, Plante S, Rusza Z, Matsuo H, Bertrand OF. A novel fiber-optic based 0.014’’ pressure wire: designs of the OptoWire, development phases, and the O2 first-in-man results. Catheter Cardiovasc Interv. 2020 doi: 10.1002/ccd.29321. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Shlofmitz E, Busch J. Recognition of drift: a key to success with invasive physiology. Cardiovasc Revasc Med. 2022;35:57–58. doi: 10.1016/j.carrev.2021.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Sen S, Ahmad Y, Dehbi HM, Howard JP, Iglesias JF, Al-Lamee R, Petraco R, Nijjer S, Bhindi R, Lehman S, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Harle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Silva PC, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J, Davies JE. Clinical events after deferral of LAD revascularization following physiological coronary assessment. J Am Coll Cardiol. 2019;73:444–453. doi: 10.1016/j.jacc.2018.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DEFINE-FLAIR Trial Investigators. Lee JM, Choi KH, Koo BK, Dehbi HM, Doh JH, Nam CW, Shin ES, Cook CM, Al-Lamee R, Petraco R, Sen S, Malik IS, Nijjer SS, Mejia-Renteria H, Alegria-Barrero E, Alghamdi A, Altman J, Baptista SB, Bhindi R, Bojara W, Brugaletta S, Silva PC, Di Mario C, Erglis A, Gerber RT, Going O, Harle T, Hellig F, Indolfi C, Janssens L, Jeremias A, Kharbanda RK, Khashaba A, Kikuta Y, Krackhardt F, Laine M, Lehman SJ, Matsuo H, Meuwissen M, Niccoli G, Piek JJ, Ribichini F, Samady H, Sapontis J, Seto AH, Sezer M, Sharp ASP, Singh J, Takashima H, Talwar S, Tanaka N, Tang K, Van Belle E, van Royen N, Vinhas H, Vrints CJ, Walters D, Yokoi H, Samuels B, Buller C, Patel MR, Serruys P, Escaned J, Davies JE. Comparison of major adverse cardiac events between instantaneous wave-free ratio and fractional flow reserve-guided strategy in patients with or without type 2 diabetes: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4:857–864. doi: 10.1001/jamacardio.2019.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrom T, Kaab S, Dambrink JH, Rioufol G, Toth GG, Piroth Z, Witt N, Frobert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbuhler M, Juni P, De Bruyne B FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379:250–259. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 19.Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49:1–16. doi: 10.1002/(sici)1522-726x(200001)49:1<1::aid-ccd1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Aminian A, Dolatabadi D, Lefebvre P, Khalil G, Zimmerman R, Michalakis G, Lalmand J. Importance of guiding catheter disengagement during measurement of fractional flow reserve in patients with an isolated proximal left anterior descending artery stenosis. Catheter Cardiovasc Interv. 2015;85:595–601. doi: 10.1002/ccd.25568. [DOI] [PubMed] [Google Scholar]


