Abstract
Background
Microbial dysbiosis in critically ill patients is a leading cause of mortality and septic complications. Probiotics and synbiotics have emerged as novel therapy on gut microbiota to prevent septic complications. However, current evidence on their effects is conflicting. This work aims to systematically review the impact of probiotics or synbiotics in critically ill adult patients.
Methods
A comprehensive search of the PubMed, CBM, Embase, CENTRAL, ISI, and CNKI databases was performed to identify randomized controlled trials that evaluate probiotics or synbiotics in critically ill patients. The quality assessment was based on the modified Jadad's score scale and the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The major outcome measure was mortality. Secondary outcomes included incidence of septic complications, sepsis incidence, length of intensive care unit (ICU) stay, incidence of non-septic complication, and ventilator day. Data synthesis was conduct by Review Manager 5.4.
Results
A total of 25 randomized controlled trials reporting on 5049 critically ill patients were included. In the intervention group, 2520 participants received probiotics or synbiotics, whereas 2529 participants received standard care or placebo. Pooling data from randomized controlled trials demonstrated a significant reduction in the incidence of ventilator-associated pneumonia (VAP) in the treatment group [(risk ratio (RR) 0.86; 95% confidence interval (CI): 0.78–0.95; p < 0.003, I2 = 85%)]. However, in the subgroup analysis, the reduction of incidence of VAP was only significant in patients receiving synbiotics (RR = 0.61, 95% CI: 0.47–0.80, p = 0.0004, I2 = 40%) and not significant in those receiving only probiotics (RR = 0.91, 95% CI: 0.82–1.01, p = 0.07, I2 = 65%). Moreover, sepsis incidence of critically ill patients was only significantly reduced by the addition of synbiotics (RR = 0.41; 95% CI: 0.22–0.72, p = 0.005, I2 = 0%). The incidence of ICU-acquired infections was significantly reduced by the synbiotics therapy (RR = 0.72; 95% CI: 0.58–0.89, p = 0.0007, I2 = 79%). There was no significant difference in mortality, diarrhea, or length of ICU stay between the treatment and control groups.
Conclusions
Synbiotics is an effective and safe nutrition therapy in reducing septic complications in critically ill patients. However, in such patients, administration of probiotics alone compared with placebo resulted in no difference in the septic complications.
Keywords: Critically ill patients, Probiotics, Synbiotics, Meta-analysis, Systematic review, Mortality, Ventilator-associated pneumonia
Highlights.
Synbiotics are an effective and safe therapy in reducing septic complications in critically ill patients.
Among critically ill patients, the administration of probiotics alone, compared with placebo, resulted in no difference in septic complications.
The effect of a mixture of probiotics is better than a single probiotic species.
Background
The gut is one of the most important organs in the human immune system. Moreover, it is also a leading target organ during stress, especially in burn, trauma and shock patients. The gut is known as the ‘motor’ of multiple organ dysfunction and bacterial translocation [1]. The latter is a major cause of mortality in critically ill patients [2,3]. Owing to the limitations of septic control strategies, the frequency of sepsis is increasing in intensive care units (ICUs). Protecting the commensal microbiota and gut function is becoming a novel strategy to reduce the risk of septic complications in critically ill patients in Europe [4].
Probiotics are live microorganisms that are beneficial to the host when administered in adequate quantities. Commensal microbiota are a vital barrier component of the intestine, helping prevent the spread of pathogens. Intestinal resistance could decrease because of deteriorating commensal microbiota in critically ill patients. Probiotics are commonly used as microbial nutritional supplements to maintain the balance of the intestinal microbiota [5,6]. On the other hand prebiotics are non-digestible foods that can benefit the host by stimulating the activity of selective dominant bacteria in the colon [7,8]. Synbiotics are a combination of probiotics and prebiotics in a single preparation. In 1990, supplementation with probiotics or synbiotics emerged as a potential therapy geared towards reducing the incidence of septic complications in critically ill patients.
The efficacy of probiotics and synbiotics has been demonstrated in elective surgery [9,10]. They are effective in treating diarrhea in patients with systemic inflammatory response syndrome (SIRS) [11,12]. Beneficial microbiota continue to decrease while pathogens increase in the intestines of patients with SIRS, which is the leading cause of decreased short-chain fatty acids (SCFAs). The pH of the gut mucosa can increase, which could further deteriorate the gut microbiota. This vicious cycle promotes the progression of SIRS and septic complications [11,13]. The hypothesized mechanism of probiotics or synbiotics breaks the vicious circle by increasing beneficial microbiota and altering the gut environment. Many randomized controlled trials (RCTs) have evaluated the effects of probiotics or synbiotics in reducing septic complications in critically ill patients, but their conclusions have been mixed. Some studies have shown that probiotics or synbiotics are more effective than placebo treatments in reducing mortality and the incidence of ventilator-associated pneumonia (VAP) [14,15]. However, other studies find probiotics useless or even engendering adverse effects [16–19]. Clinicians are puzzled by the conflicting nature of the evidence. A systematic review and meta-analysis to synthesize the current evidence is needed for practitioners faced with the decision of using probiotics or synbiotics. In this systematic review of RCTs, we examined the effects of probiotics or synbiotics on mortality and septic complications in critically ill patients.
Methods
Literature retrieval strategy
RCTs were identified from PubMed (US National Library of Medicine 1990–2021), Web of Science, The Cochrane Library (2021, Issue 11), China Knowledge Resource Integrated Database (CNKI), World Health Organization (WHO) Global Index Medicus and Chinese Biomedicine Database (CBM). Search terms were connected by AND/OR and included patients (adult critically ill patients and those with trauma), interventions (probiotic, prebiotic and synbiotic) and comparisons (placebo and standard treatment). References from RCTs were browsed and corresponding authors were consulted for any further information that may have been acquired by them but not been reported publicly. Ongoing RCTs were checked using clinical trial registers. The complete terms and strategies for identifying the articles (Table S1, see online supplementary material).
Inclusion and exclusion criteria
RCTs evaluating probiotics or synbiotics in adult critically ill patients (APACHE II scores >10) were included. Non-RCTs and RCTs that included pregnant women or patients younger than 18 years of age were excluded. Studies that did not address any primary or secondary outcomes, as previously mentioned, were excluded.
The intervention groups received probiotics or synbiotics through any approach, preparation or duration. Participants with severe acute pancreatitis were excluded from the study. Control groups administered standard care or placebo did not receive any synbiotics or probiotics.
Outcome measures
The primary outcome was mortality in critically ill patients. Secondary outcomes included the incidence of VAP, incidence of septic complications, incidence of sepsis, length of ICU stay and non-septic complications.
Selection of studies
Two reviewers (KW and QZ) independently performed electronic literature searches and evaluated the eligibility of the studies based on the inclusion criteria. Relevant studies were initially screened using titles and abstracts. The potential articles were then assessed independently, and any disagreements were adjudicated by a third reviewer (HJ).
Data extraction and analysis
Data were extracted independently by two investigators (KW and QZ) from the full text of the studies and compiled into shared sheets. We collected the following information from the included studies: study identifier (first author and year of publication), duration of treatment, study design, inclusion and exclusion criteria, intervention and number of subjects, and primary and secondary outcomes. Data were validated by a third reviewer (HJ) using a standardized method. The methodological quality assessment shown in Table 1 was based on the Cochrane Reviewers’ Handbook [20] and the modified Jadad scale [21,22]. The risk ratio (RR) was used to report discrete numerical variables along with 95% confidence intervals (CIs). The mean difference (MD) was reported to estimate the continuous outcomes. The I2 statistic was used to quantify the heterogeneity, and forest plots were generated and double-checked by two reviewers (KXL and MWS). If I2 < 25%, the pooled outcomes were considered to have low statistical heterogeneity, and if I2 > 75%, the pooled outcomes were considered to have high statistical heterogeneity. Data synthesis was conducted by the Review Manager (RevMan) 5.4 and R (R package version 3.7–0.) software [23]. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement to report the research protocol, outcome and relevant items in this systematic review [24].
Table 1.
Methodological characteristics of studies included
| Study | Population | PE (n) | PA(n) | Groups | Concealment | Blinding | Probiotic | Prebiotic | Synbiotic | Placebo/SC | Route | DoT | M.J.S. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alberda et al., 2007 [25] | Critically ill patients MV>2d | 19 | 28 | 3 | Identical packaging | Double blinded | Lactobacillus casei, L. plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii subsp. Bulgaricus, Bifidobacterium longum, B. breve and B. Infantis, Streptococcus salivarius subsp. T | − | − | Placebo | NG/NJ | 7d | 7/7 |
| Arruda et al., 2004 [26] | TBI, GCS5–12 | 20 | 20 | 2 | NR | NR | Lactobacillus johnsonii | − | − | SC | NG/NJ | 5d | 4/7 |
| Barraud et al., 2010 [27] | Critically ill patients MV>2d | 167 | 167 | 2 | Envelopes | Double blinded | Mainly Lactobacillus rhamnosus GG, but also L. casei, L. acidophilus and Bifidobacterium bifidum | − | − | Placebo | NG/NJ | 7d | 7/7 |
| Bleichner et al., 1997 [28] | Critically ill patients | 128 | 128 | 2 | NR | Double blinded | S. boulardii | − | − | Placebo | NG/NJ | 21d | 5/7 |
| Ferrie et al., 2011 [29] | Critically ill patients | 27 | 27 | 2 | Identical packaging | Double blinded | L. rhamnosus GG | − | − | Placebo | NG | 7d | 7/7 |
| Forestier et al., 2008 [30] | Critically ill patients | 208 | 208 | 2 | Envelopes | Double blinded | Lactobacillus | − | − | Placebo | NG | 7d | 7/7 |
| Frohmader et al., 2010 [31] | Critically ill patients | 45 | 45 | 2 | Envelopes | Double blinded | Lactic acid bacteria, lyophilized Bifidobacterium breve, B. longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L. casei, L bulgaricus, Streptococcus thermophilus | − | − | Placebo | NG/NJ | 7d | 7/7 |
| Giamarellors-Bourboulis et al., 2009 [32] | Severe multiple injuries MV>2d | 72 | 72 | 2 | Envelopes | Double blinded | − | − | Synbiotic 2000Forte | Placebo | NG | 15d | 7/7 |
| Jain et al., 2004 [33] | Critically ill patients | 90 | 90 | 2 | Identical packaging | Double blinded | − | − | (L. acidophilus La5, Bifidobacterium lactis Bb-12, S. thermophilus and L. bulgaricus) and prebiotic oligofructose | Placebo | Oral or NG | 10d | 7/7 |
| Johnstone et al., 2021 [18] | Critically ill patients MV>3d | 1318 | 1332 | 2 | Envelopes | Double blinded | L. rhamnosus GG | − | − | Placebo | Oral | 60d | 7/7 |
| Klarin et al., 2005 [34] | Critically ill patients | 15 | 15 | 2 | Envelopes | Double blinded | L. plantarum 299 (Lp299) | − | − | Placebo | NG | 7d | 6/7 |
| Klarin et al., 2008 [35] | Critically ill patients MV>1d | 44 | 44 | 2 | Envelopes | Double blinded | L. plantarum 299 (Lp299) | − | − | SC | NG | 12d | 6/7 |
| Knight et al., 2009 [36] | Critically ill patients MV>2d | 259 | 259 | 2 | NR | Double blinded | − | − | Synbiotic 2000 Forte | Placebo | NG | 10d | 5/7 |
| Kotzampassi et al., 2006 [37] | Trauma patients | 65 | 65 | 2 | NR | Double blinded | − | − | Synbiotic 2000 Forte | Placebo | NG | 15d | 5/7 |
| Litton, 2021 [38] | Critically ill patients | 110 | 108 | 2 | Unblinded pharmacist | Double blinded | L. plantarum 299 (Lp299) | − | − | Placebo | Oral or NG | 60d | 7/7 |
| Lopez de Toro et al., 2014 [39] | Multi-organ failure | 89 | 89 | 2 | Envelopes | Double blinded | − | − | Synbiotic drink | Placebo | NG | 7d | 7/7 |
| Mahmoodpoor et al., 2019 [19] | Critically ill patients MV>2d | 100 | 100 | 2 | NR | Double blinded | Lactobacillus species (casei, acidophilus, rhamnosus, bulgaricus), Bifidobacterium species (breve, longum) and S. thermophilus | − | − | Placebo | NG/NJ | 14d | 7/7 |
| Mcnaught et al., 2005 [40] | Critically ill patients | 103 | 103 | 2 | NR | Double blinded | L. plantarum 299v | − | − | SC | Oral or NG | 9d | 5/7 |
| Morrow et al., 2010 [41] | Critically ill patients MV>3d | 138 | 138 | 2 | NR | Double blinded | L. rhamnosus GG | − | − | Placebo | NG | 14d | 5/7 |
| Rongrungruang et al., 2015 [42] | Critically ill patients MV>3d | 150 | 150 | 2 | NR | NR | L. casei (Shirota strain) | − | − | Placebo | Oral or NG | 12d | 4/7 |
| Sanaie et al., 2014 [43] | Critically ill patients | 40 | 40 | 2 | NR | Double blinded | Lactic acid bacteria, lyophilized B. breve, B. longum, B. infantis, L acidophilus, L. plantarum, L. casei, L bulgaricus, S. thermophilus | − | − | Placebo | NG | 7d | 5/7 |
| Shimizu et al., 2018 [14] | Critically ill patients | 72 | 72 | 2 | NR | Single blinded | − | − | B. breve strain, L. casei strain and galactooli- gosaccharides | SC | NG | 20d | 5/7 |
| Spindler-Vesel et al., 2007 [44] | Multiple injured patients | 52 | 113 | 4 | NR | NR | − | − | Synbiotic 2000 Forte | SC | NG | 14d | 4/7 |
| Tan et al., 2011 [45] | TBI, GCS5–8 | 43 | 43 | 2 | NR | Single blinded | B. longum, Lactobacillus bulgaricus, S. thermophilus. | − | − | Placebo | NG | 21d | 5/7 |
| Zeng et al., 2016 [15] | Critically ill patients MV >2d | 235 | 235 | 2 | Envelopes | Double blinded | live Bacillus subtilis and Enterococcus faecalis (Medilac-S) | − | − | SC | NG | 14d | 7/7 |
PA participants analyzed, PE participants enrolled, DoT duration of treatment, NG nasogastric, NJ nasojejunal, NR not reported, SC standard care, M.J.S. modified Jadad score, TBI Traumatic brain injury, GCS Glasgow Coma Scale, MV mechanical ventilation
Results
Studies included
A total of 186 potential studies were identified in the initial literature retrieval. The initial screening resulted in 45 candidate studies. The PRISMA diagram shows the details of the selection process and exclusion criteria (Figure 1). Finally, 25 RCTs [14,15,18,19,25–45] were deemed appropriate for full analysis. The characteristics of the included studies and their designs are listed in Table 1. Of the 25 studies included in the final meta-analysis, 7 used synbiotics as the intervention and the other 18 used probiotic therapy. In total, 5049 patients were included in this meta-analysis, of whom 2520 were randomly treated with probiotic or synbiotic therapy, whereas the remaining 2529 received placebo or standard care.
Figure 1.
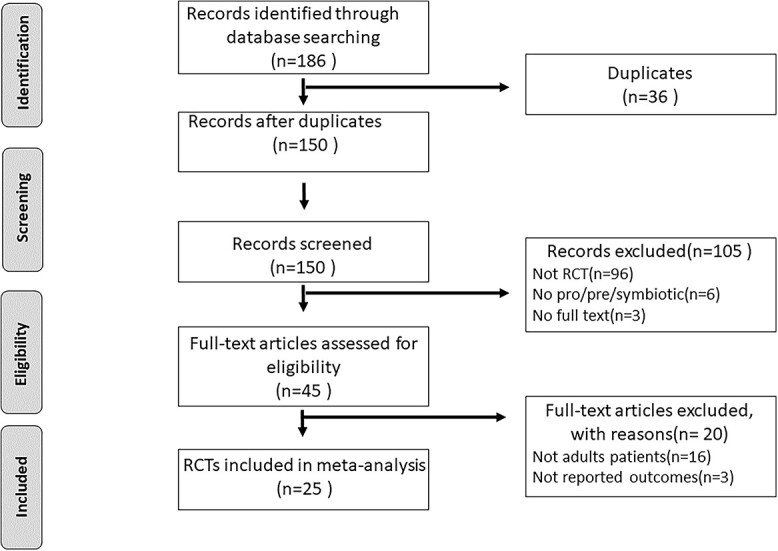
PRISMA diagram detailing the literature search and the study selection/exclusion process. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-analyses, RCT randomized controlled trials
Patients and interventions
The mean (standard deviation) age of patients who received probiotics or synbiotic treatment was 58.2 (16.8) years and the mean age for those in the control group was 58.8 (17.5) years. A variety of diagnostic categories were included: respiratory, cardiac, neurological, sepsis, trauma, thoracic, acute illnesses and surgery. Of the 18 studies receiving probiotics alone, only 5 used a mixture of probiotics, while the remainder received a single probiotic species (Lactobacillus or Saacharomyces boulardii). Seven studies administered synbiotics using a mixture of probiotics. Eleven studies reported side effects or complications associated with the intervention [14,18,19,25,27–29,31,37,41,42]. There were two serious adverse events reported in a randomized clinical trial [18]. Compared to the placebo or standard treatment group, rates of diarrhea, vomiting, abdominal bloating and abdominal pain were not significantly increased in participants in the treatment group (Table 2).
Table 2.
Summary of side effects and complications associated with probiotics or synbiotics in the randomized controlled trials (RCTs) included. Serious adverse events were Lactobacillus isolates resulting in persistent or significant disability or incapacity or life-threatening situations or resulting in death
| Study | Probiotics | Synbiotics | Placebo or standard care | P |
|---|---|---|---|---|
| Alberda et al., 2007 [25] | Diarrhea:1/10 | − | Diarrhea:2/9 | − |
| Arruda et al., 2004 [26] | Not stated | − | Not stated | − |
| Barraud et al., 2010 [27] | Diarrhea:48/87 | − | Diarrhea:42/80 | − |
| Bleichner et al., 1997 [28] | Diarrhea:18/36 | − | Diarrhea:24/36 | 0.26 |
| Ferrie et al., 2011 [29] | Diarrhea:2/13 | − | Diarrhea:2/14 | 0.08 |
| Forestier et al., 2008 [30] | Not stated | Not stated | − | |
| Frohmader et al., 2010 [31] | Diarrhea:5/20 | Diarrhea:3/25 | 0.03 | |
| Giamarellors-Bourboulis et al., 2009 [32] | − | Not stated | Not stated | − |
| Jain et al., 2004 [33] | − | Not stated | Not stated | − |
| Johnstone et al., 2021 [18] | Diarrhea:836/1318 | − | Diarrhea:855/1332 | − |
| Adverse events: 13/1318 | − | Adverse events: 1/1332 | 0.001 | |
| Serious adverse events: 2/1318 | Serious adverse events: 0/1318 | 0.001 | ||
| Klarin et al., 2005 [34] | Not stated | − | Not stated | − |
| Klarin et al., 2008 [35] | Not stated | − | Not stated | − |
| Knight et al., 2009 [36] | − | Not stated | Not stated | − |
| Kotzampassi et al., 2006 [37] | − | Diarrhea:5/35 | Diarrhea:10/30 | 0.34 |
| − | severe constipation: 4/35 | Severe constipation: 6/35 | 0.04 | |
| − | Gastric residuals: 7/35 | Gastric residuals: 15/35 | 0.01 | |
| Litton, 2021 [38] | Not stated | − | Not stated | − |
| Lopez de Toro et al., 2014 [39] | − | Not stated | Not stated | − |
| Mahmoodpoor et al., 2019 [19] | Diarrhea:7/48 | − | Diarrhea:15/54 | 0.08 |
| Gastric residuals: 14/48 | − | Gastric residuals: 31/54 | 0.26 | |
| Gastric bacterial colonization: 14/48 | − | Gastric bacterial colonization: 20/54 | ||
| Oropharyngeal bacterial colonization: 23/48 | Oropharyngeal bacterial colonization: 34/54 | 0.11 | ||
| Mcnaught et al., 2005 [40] | Not stated | − | Not stated | − |
| Morrow et al., 2010 [41] | Clostridium difficile diarrhea:4/68 | − | Clostridium difficile diarrhea:13/70 | − |
| Rongrungruang et al., 2015 [42] | Diarrhea:19/75 | − | Diarrhea:14/75 | − |
| Sanaie et al., 2014 [43] | Not stated | − | Not stated | − |
| Shimizu et al., 2018 [14] | − | Enteritis:2/35 | Enteritis:10/37 | − |
| Spindler-Vesel et al., 2007 [44] | − | Not stated | Not stated | − |
| Tan et al., 2011 [45] | Not stated | − | Not stated | − |
| Zeng et al., 2016 [15] | Not stated | − | Not stated | − |
Major outcome: mortality
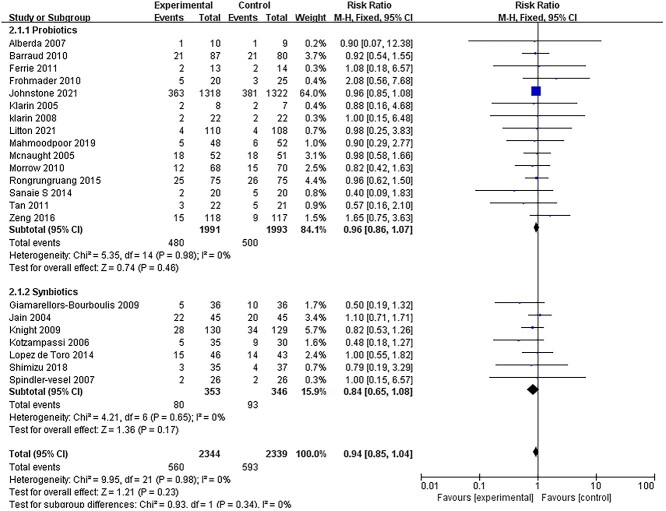
Among all the included studies, 22 reported on the primary outcome (mortality) [14,15,18,19,25,27,29,31–45]. There was no heterogeneity among the 22 studies and a fixed model was used for meta-analysis. The mortality of patients receiving probiotics or synbiotics was not significantly reduced compared to those who received placebo treatment or standard care (RR = 0.94, 95% CI: 0.85–1.04, p = 0.23, I2 = 0%) (Figure 2). In the subgroup analysis, there were no significant differences in mortality between patients receiving probiotics and those who received synbiotics.
Figure 2.
Forest plot of pooled weighted mean difference from RCTs evaluating the effect on risk ratio for mortality with probiotics and synbiotics therapy. RCTs randomized controlled trials, CI confidence intervals
Septic complications
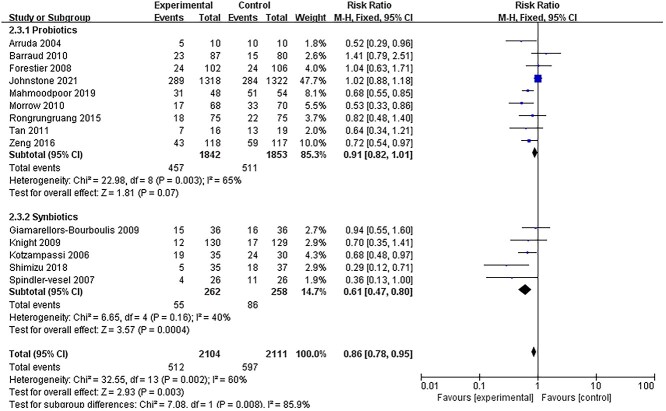
The incidence of VAP
Fourteen studies [14,15,18,19,26,27,30,32,36,37,41,42,44,45] reported data on the incidence of VAP. Quantitative pooling of data revealed a significant reduction in the incidence of VAP in patients receiving probiotics or synbiotics (Figure 3). The risk of developing VAP was reduced in the intervention group (RR = 0.86, 95% CI: 0.78–0.95; p = 0.003; I2 = 85%). However, in the subgroup analysis, the reduction in the incidence of VAP was only significant in patients receiving synbiotics (RR = 0.61, 95% CI: 0.47–0.80, p = 0.0004, I2 = 40%) and not significant in those receiving only probiotics (RR = 0.91, 95% CI: 0.82–1.01, p = 0.07, I2 = 65%).
Figure 3.
Forest plot of randomized controlled trials evaluating the efficacy for reducing the incidence of VAP. VAP ventilator-associated pneumonia, CI confidence intervals
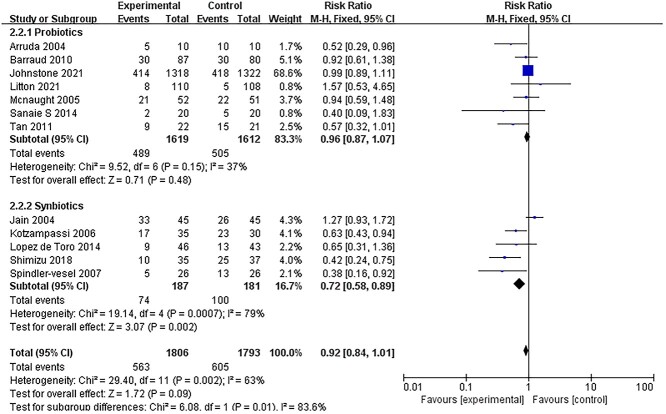
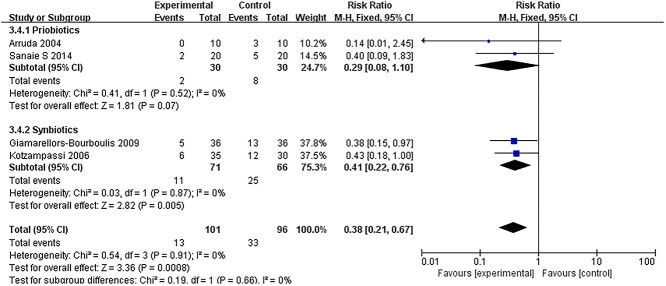
All ICU-acquired infections and sepsis incidence
Twelve RCTs [14,18,26,27,33,37–40,43–45] reported data on the incidence of ICU-acquired infections. Pooling data from RCTs demonstrated that there was no significant difference in the incidence of ICU-acquired infections between the treatment and control groups (RR = 0.92; 95% CI: 0.84–1.01, p = 0.09, I2 = 63%) (Figure 4). The I2 test revealed a significantly high heterogeneity. We then conducted a subgroup analysis that found that studies using synbiotics might be the source of heterogeneity. Furthermore, in the subgroup analyses of the 5 RCTs [14,33,37,39,44] that administered synbiotics, there was a significant reduction in the incidence of ICU-acquired infections (RR = 0.72; 95% CI: 0.58–0.89, p = 0.0007, I2 = 79%). However, in 7 trials [18,26,27,38,40,43,45] that administered probiotics alone, there was no effect on infections (RR = 0.96; 95% CI: 0.87–1.07, p = 0.48; I2 = 37%). These results also confirmed the incidence of sepsis. In 2 studies [32,37] that administered synbiotics, there was a significant reduction in the incidence of sepsis (RR = 0.41; 95% CI: 0.22–0.72, p = 0.005, I2 = 0%) (Figure 5).
Figure 4.
Forest plot of pooled data form RCTs demonstrating the reduction in risk of ICU-acquired infections. RCTs randomized controlled trials, CI confidence intervals, ICUs intensive care units
Figure 5.
Forest plot of pooled data form RCTs demonstrating the reduction in risk of sepsis. RCTs randomized controlled trials, CI confidence intervals
Length of ICU stay
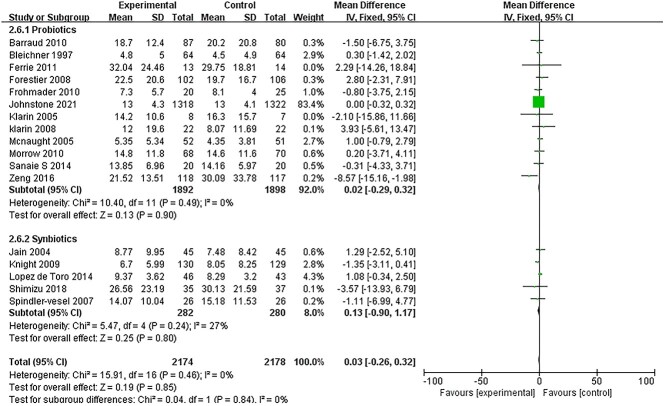
Data on the length of ICU stay was reported in only 17 studies [14,15,18,27–31,33–36,39–41,43,44]. There was no significant difference in the length of ICU stay between the intervention and control groups (MD: 0.03; 95% CI: −0.26 to 0.32, p = 0.85, I2 = 0%) (Figure 6). The same result was obtained in subgroup analysis.
Figure 6.
Forest plot of pooled weighted mean difference from RCTs evaluating the risk ratio for length of ICU stay. RCTs randomized controlled trials, CI confidence intervals, ICUs intensive care units
Non-septic complications
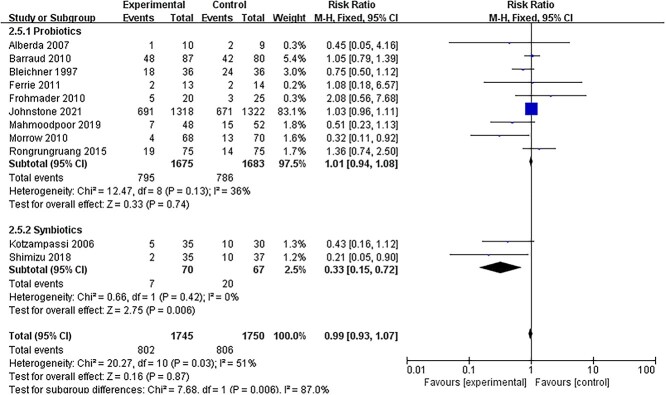
Some RCTs reported data on non-septic complications. Eleven studies [14,18,19,25,27–29,31,37,41,42] provided a count of the number of patients with diarrhea. Pooling data from RCTs showed no significant difference between the intervention and control groups (RR = 0.99; 95% CI: 0.93–1.07, p = 0.87, I2 = 51%) (Figure 7). However, in the subgroup analysis, the reduction in the incidence of diarrhea was significant in the synbiotic group (RR = 0.33; 95% CI: 0.15–0.72, p = 0.006, I2 = 0%).
Figure 7.
Effect on the incidence of diarrhea with probiotics or synbiotics therapy. CI confidence intervals
Subgroup analysis depending on probiotics
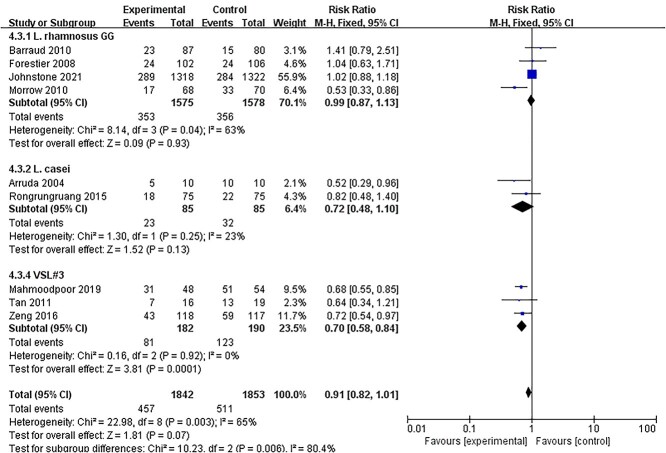
Considering that different bacterial species may have different effects on critically ill patients, we performed a subgroup analysis depending on probiotics such as Lactobacillus rhamnosus GG, Lactobacillus casei, Lactobacillus plantarum 299 and VSL#3 (a specific mixture of different bacterial species). It may be a better strategy to distinguish between beneficial and unbeneficial probiotics. Subgroup analyses showed that the risk of developing VAP was reduced only by VSL#3 (RR = 0.70, 95% CI: 0.58–0.84, p = 0.0001, I2 = 0%) (Figure 8). L. rhamnosus GG and L. casei did not reduce the incidence of VAP. None of the other outcomes, including mortality, diarrhea, sepsis, other ICU-acquired infections or length of ICU stay, showed a significant difference among these four types of probiotics.
Figure 8.
Subgroup analysis: effects of different bacterial species on incidence of VAP. VSL#3 is a specific mixture of different bacterial species, consisting of four strains of Lactobacillus, three strains of Bifidobacterium and Streptococcus salivarius subsp. CI confidence intervals, VAP ventilator-associated pneumonia
Publication bias and sensitivity analysis
It is well known that studies with positive outcomes are easier to publish. Consequently, all valid studies cannot be truly represented merely by those studies that end up being published. A funnel plot was used to assess publication bias. The evaluation of publication bias based on mortality demonstrated no asymmetry in favor of positive studies (Figure 9). Moreover, the risk of the included RCTs is shown in Figure 10. To evaluate the stability of the results, a sensitivity analysis was conducted by excluding one study at a time. The combined RR of risks was confirmed to be consistent and without apparent fluctuations.
Figure 9.
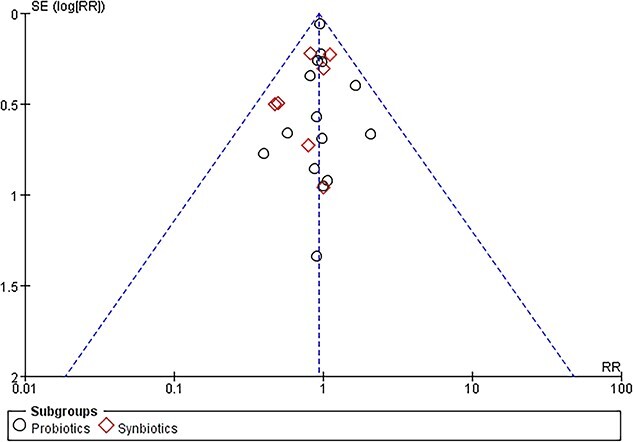
Funnel plot of included randomized controlled trials. RR risk ratio, SE standard error
Figure 10.
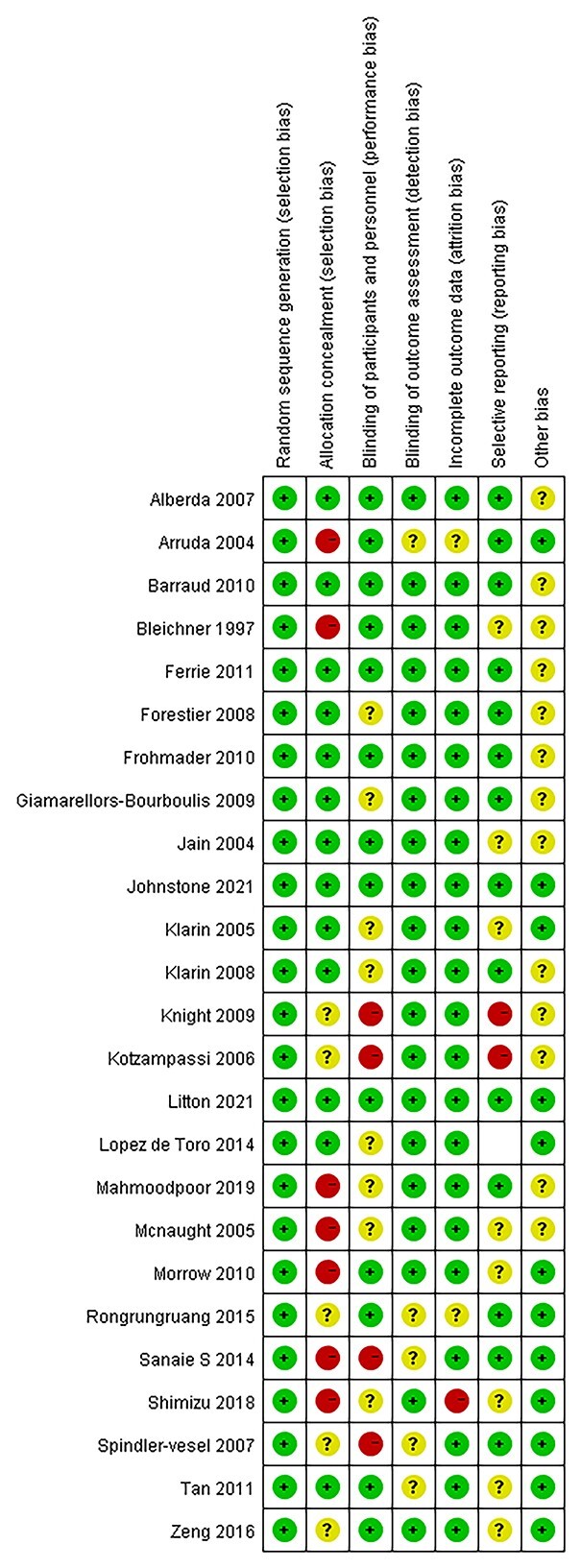
Risk of bias assessment for the randomized controlled trials (RCTs) included
Discussion
This meta-analysis of pooled data from 25 RCTs revealed that synbiotic therapy significantly reduced the risk of septic complications. Furthermore, the incidence of sepsis in critically ill patients is significantly reduced by the administration of synbiotics. In contrast, probiotic or synbiotic administration had no effect on mortality, length of ICU stay or non-septic complications. The probiotic or synbiotic therapy duration of the included studies was not uniform. The mean (standard deviation) duration was 11.59 (4.75) days. However, the reduction risk of septic complications remained whether the duration was more than 11.59 days or less. Thus, it was difficult to infer the optimal duration of therapy from this meta-analysis. The reduction in septic complications revealed in this study is consistent with the results of other meta-analyses [10,46]. In addition, Chowdhury et al. [9] demonstrated that synbiotics are more effective than probiotics in reducing infection risks and length of hospital stay.
On the whole, probiotics and synbiotics are safe and well tolerated [9,14,47]. Whelan and Myers systematically evaluated the safety issue and adverse effects of probiotics in patients receiving nutritional support [47]. They indicated that in some specific patient groups (e.g. severe acute pancreatitis or liver transplantation), the adverse events may increase after probiotic intervention. Besselink et al. reported that bowel ischemia may occur after probiotic administration in patients with severe acute pancreatitis, although the occurrence was found to be relatively low (6%) [48]. Lactobacillus- and S. boulardii-related sepsis have also been reported in some studies, especially in ICU patients who have inserted central venous catheters (CVCs). However, the researchers indicated that these kinds of infections may also be associated with environmental contamination with the probiotic (for example, S. boulardii products may be introduced into CVC lines by unintentional hand contamination) [47]. Thus, the relationship between probiotic products and sepsis in the ICU is weak and requires more evidence. This meta-analysis revealed that synbiotics were better tolerated than probiotics. This is consistent with a recent study by Johnstone et al. that reported that serious adverse events occurred in patients receiving L. rhamnosus GG [18].
Due to the variable species of probiotics used in the studies included, it is difficult to evaluate which one was most effective. We performed a subgroup analysis based on probiotics such as L. rhamnosus GG, L. casei, L. plantarum 299 and VSL#3. The analysis showed that the risk of developing VAP was reduced only with VSL#3. Administration of L. rhamnosus GG, L. casei or L. plantarum 299 alone could not reduce the incidence of VAP, mortality, diarrhea, sepsis or other ICU-acquired infections. VSL#3 is a mixture of different bacterial species. The use of a complex mixture of different bacterial species promotes the balance of the microbial composition of the intestines and stomach through the synergistic actions of the different strains. The same may improve microbial dysbiosis in order to lower septic complications in critically ill patients, create a better balance by adding good (probiotic) bacteria to help control the bad bacteria, help to protect and strengthen the intestinal barrier, and prevent bad bacteria from sticking to and irritating the gastrointestinal tract. Therefore, it ultimately reduces the immune response and inflammation caused by the bacteria. However, the same probiotic therapy may have different effects in different patient groups [49].
The results of this meta-analysis are consistent with the proposed theory that the gut is the ‘motor’ of multiple organ dysfunction and the origin of sepsis. Gut motility is often decreased by ischemia, fluid overload and opioids in critically ill patients. Consequently, the mucosal permeability for bacteria and SIRS incidence could increase due to motor stasis and gut intolerance [50]. Montejo [51] found that the complication of intolerance to enteral feeding was significantly increased in critically ill patients. Probiotics and synbiotics alter the gut microbiota and environment to lower septic complications in patients with severe SIRS [12]. Although evidence for the mechanism of probiotic and synbiotic therapy was not provided by this meta-analysis, one of the hypotheses is that the increasing levels of Lactobacillus and Bifidobacterium lead to increased production of SCFAs in the gut. The gut microbiota and environment maintained by SCFAs that increase the pH in the gut may decrease mucosal permeability and septic complications.
Limitations of the study
There are some limitations to this study that need further attention. First, the duration and preparation of probiotics or synbiotic therapy was different among studies included [25,26,28,30,31,41,42]. There were no uniform standards. Second, some studies did not use a placebo to reduce unintentional physical cues and prejudice [14,15,26,35,40,44]. Although there are many published studies regarding the use of probiotics or synbiotics in critically ill adult patients, few of them meet the high-quality standards of evidence-based medicine. Large high-quality multicenter RCTs are needed to reduce heterogeneity and influence.
Conclusions
Synbiotics are an effective and safe nutrition therapy that can be used to reduce septic complications in critically ill patients. However, in critically ill patients, administration of probiotics alone compared with placebo resulted in no difference in septic complications. The effect of a mixture of probiotics was better than that of a single probiotic species.
Supplementary Material
Acknowledgements
The authors express their thanks to Yu-wen Zhou and Yi-tao Liu, who provided enormous help with English language editing and figure processing, and Dr Chi Zhang and Guang-yu Yang of the Institute for Emergency and Disaster Medicine for their assistance in retrieving the relevant literature.
Abbreviations
APACHEII scores:Acute Physiology and Chronic Health Evaluation II scores; CI: Confidence interval; CVC: Central venous catheter; GCS:Glasgow Coma Scale; ICU: Intensive care unit; MD: Mean difference; MV:Mechanical ventilation; RCT: Randomized controlled trial; RR: Risk ratio; SCFA: Short-chain fatty acid; SIRS: Systemic inflammatory response syndrome; TBI:Traumatic brain injury; VAP: Ventilator-associated pneumonia.
Authors’ contributions
KW, QZ and HJ performed the study design and conceptualization. KXL, YW, LW, KW and QZ completed the literature retrieval and data extraction. Mathematical modeling and meta-analysis were conducted with the help of KW and MWS. KW and QZ drafted the manuscript. JZ and HJ contributed to the visualization and edited the final version of the manuscript. All authors approved the manuscript.
Conflicts of Interest
None declared.
Funding
This work was supported by grants from the Sichuan Provincial Department of Science and Technology (No. 2020YFS0006 to HJ and No. 2018JY0050 to QZ), National Natural Science Foundation of China (No. 71974200 to HJ), Sichuan Provincial People's Hospital (No. 2021ZX01 to KW), Health Commission of Sichuan Province (No. 20PJ102 to KW), and Education Department of Sichuan Province (No. 18ZA0155). Funders played no role in the study design, conduct or manuscript writing.
References
- 1. Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the ``motor'' of critical illness. Shock. 2007;28:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bateman BT, Schmidt U, Berman MF, Bittner EA. Temporal trends in the epidemiology of severe postoperative sepsis after elective surgery: a large, nationwide sample. Anesthesiology. 2010;112:917–25. [DOI] [PubMed] [Google Scholar]
- 3. Martin GS, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. [DOI] [PubMed] [Google Scholar]
- 4. Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–23. [DOI] [PubMed] [Google Scholar]
- 5. Biancone L, Monteleone I, Del Vecchio BG, Vavassori P, Pallone F. Resident bacterial flora and immune system. Dig Liver Dis. 2002;34:S37–43. [DOI] [PubMed] [Google Scholar]
- 6. Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr. 2002;88:S39–49. [DOI] [PubMed] [Google Scholar]
- 7. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–63. [DOI] [PubMed] [Google Scholar]
- 8. Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative probiotics or Synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020;271:1036–47. [DOI] [PubMed] [Google Scholar]
- 10. Wu XD, Liu MM, Liang X, Hu N, Huang W. Effects of perioperative supplementation with pro−/synbiotics on clinical outcomes in surgical patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Clin Nutr. 2018;37:505–15. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu K, Ogura H, Asahara T, Nomoto K, Morotomi M, Tasaki O, et al. Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig Dis Sci. 2013;58:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, et al. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci. 2009;54:1071–8. [DOI] [PubMed] [Google Scholar]
- 13. Chakraborty RK, Burns B. Systemic Inflammatory Response Syndrome. Treasure Island (FL): StatPearls, 2021, StatPearls [Internet]. [PubMed] [Google Scholar]
- 14. Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng J, Wang CT, Zhang FS, Qi F, Wang SF, Ma S, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42:1018–28. [DOI] [PubMed] [Google Scholar]
- 16. Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011;200:1–645. [PMC free article] [PubMed] [Google Scholar]
- 17. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169:240–7. [DOI] [PubMed] [Google Scholar]
- 18. Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmoodpoor A, Hamishehkar H, Asghari R, Abri R, Shadvar K, Sanaie S. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double-blind randomized controlled trial. Nutr Clin Pract. 2019;34:156–62. [DOI] [PubMed] [Google Scholar]
- 20. JPT H. Cochrane Handbook for systematic reviews of Interventions version 5.0.1.www.Cochrane-handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Min G, Liu F, Zhai S, Zhu JS, Zhang H, Wang T. Salbutamol in preventing preterm labour: a systematic review. Chin J Evid-based Med. 2007;7:591–600. [Google Scholar]
- 23. Schwarzer G. Meta: Meta-Analysis with R. R package version 3.7–0. http://CRAN.R-project.org/package=meta. 2014. [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–23. [DOI] [PubMed] [Google Scholar]
- 26. Falcao de Arruda IS, Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106:287–92. [DOI] [PubMed] [Google Scholar]
- 27. Barraud D, Blard C, Hein F, Marcon O, Cravoisy A, Nace L, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36:1540–7. [DOI] [PubMed] [Google Scholar]
- 28. Bleichner G, Blehaut H, Mentec H, Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. A multicenter, randomized, double-blind placebo-controlled trial. Intensive Care Med. 1997;23:517–23. [DOI] [PubMed] [Google Scholar]
- 29. Ferrie SDM. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness: randomized controlled trial. JPEN J Parenter Enteral Nutr. 2011;35:43–9. [DOI] [PubMed] [Google Scholar]
- 30. Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12:R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frohmader TJ, Chaboyer WP, Robertson IK, Gowardman J. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: a pilot trial. Am J Crit Care. 2010;19:e1–11. [DOI] [PubMed] [Google Scholar]
- 32. Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67:815–21. [DOI] [PubMed] [Google Scholar]
- 33. Jain PK, McNaught CE, Anderson AD, MacFie J, Mitchell CJ. Influence of synbiotic containing lactobacillus acidophilus La5, Bifidobacterium lactis bb 12, Streptococcus thermophilus, lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr. 2004;23:467–75. [DOI] [PubMed] [Google Scholar]
- 34. Klarin B, Johansson ML, Molin G, Larsson A, Jeppsson B. Adhesion of the probiotic bacterium lactobacillus plantarum 299v onto the gut mucosa in critically ill patients: a randomised open trial. Crit Care. 2005;9:R285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klarin BMG, Jeppsson B. Use of the probiotic lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit Care. 2008;12:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knight DJ, Gardiner D, Banks A, Snape SE, Weston VC, Bengmark S, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–61. [DOI] [PubMed] [Google Scholar]
- 37. Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30:1848–55. [DOI] [PubMed] [Google Scholar]
- 38. Litton E, Anstey M, Broadhurst D, Chapman A, Currie A, Ferrier J, et al. Early and sustained lactobacillus plantarum probiotic therapy in critical illness: the randomised, placebo-controlled, restoration of gut microflora in critical illness trial (ROCIT). Intensive Care Med. 2021;47:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ismael Lopez de Toro MC, Marcelino SC, SB MJP-P, Torrijos PL-R, Pilar SR, Ana RC, et al. Influencia de los simbio’ticos en la disfuncio’n multiorga’nica: ensayo aleatorizado y controlado. Med Clin (Barc). 2014;143:161–2.24486114 [Google Scholar]
- 40. McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–9. [DOI] [PubMed] [Google Scholar]
- 41. Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rongrungruang Y, Krajangwittaya D, Pholtawornkulchai K, Tiengrim S, Thamlikitkul V. Randomized controlled study of probiotics containing lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thail. 2015;98:253–9. [PubMed] [Google Scholar]
- 43. Sanaie S, Ebrahimi-Mameghani M, Hamishehkar H, Mojtahedzadeh M, Mahmoodpoor A. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: a randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19:827–33. [PMC free article] [PubMed] [Google Scholar]
- 44. Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31:119–26. [DOI] [PubMed] [Google Scholar]
- 45. Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15:R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr. 2010;91:687–703. [DOI] [PubMed] [Google Scholar]
- 48. Besselink MG, Santvoort HC, Buskens E, Boermeester MA, Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9. [DOI] [PubMed] [Google Scholar]
- 49. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–23. [DOI] [PubMed] [Google Scholar]
- 50. Fruhwald S, Holzer P, Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med. 2007;33:36–44. [DOI] [PubMed] [Google Scholar]
- 51. Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The nutritional and metabolic working Group of the Spanish Society of intensive care medicine and coronary units. Crit Care Med. 1999;27:1447–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.