Abstract
Macrophages, first discovered for their phagocytic ability, are a complicated and heterogeneous cell type. The unique properties of macrophages allow them to perform a vast array of functions, including phagocytosis, cytokine production, antigen presentation, and wound healing. Some macrophage populations are derived from monocytes and are induced into specific phenotypes by the local tissue microenvironment, while other macrophages form during early embryonic development. The exposure of the host to local pathogens and/or traumatic injury alters the tissue microenvironment and, in turn, influences changes in macrophage phenotype and function. Perhaps the most significant change in the local tissue microenvironment and subsequent macrophage phenotype occurs after thermal injury, which causes localized tissue damage and a massive systemic inflammatory response. However, few studies have explored the influence of burn injury on the host macrophages and macrophage function in burn wounds. Furthermore, the literature is scant regarding the impact macrophage function has on outcomes in thermal injury. This review will focus on the current knowledge of macrophage function in burn wounds and the phenotypic changes in macrophages during thermal injury while identifying knowledge gaps.
Keywords: Macrophages, thermal injury, wound healing, macrophage dysfunction, macrophage phenotype
Introduction
Macrophages were initially discovered for their phagocytic ability by Elie Metchnikoff in the late 19th century [1]. However, we now know that these cells display remarkable phenotypic and functional diversity having a role in almost every aspect of an organism’s biology to regulate tissue homeostasis [2-6]. Macrophages are discretely positioned to monitor their local environment and equipped with a vast array of sensory molecules for an encounter with environmental challenges, such as thermal injury or pathogens [7,8]. As such, macrophages are the most prominent cells found in tissues within the first few days after thermal injury [9]. Since the primary defense against pathogens, the skin, is compromised after thermal injury, macrophages serve a pivotal role in the host defense [9]. Their unique properties allow them to perform a variety of functions in all aspects of healing, including antigen presentation [10], cytokine secretion [11], phagocytosis, and wound healing [12]. Macrophages are altered after thermal injury leading to dysfunction and are linked to severe complications. Thus, macrophages are an ideal cell population to study in thermal injury to improve burn care research.
Macrophage function
Macrophages’ primary function is to maintain homeostasis through host defense, clearance of apoptotic debris, and wound healing following injury. These operations can be simplified into four basic innate functions: sensing, chemotaxis, phagocytosis, and repair [13].
Macrophages are equipped with broad-range pattern-recognition receptors used to sense two groups: pathogen/danger signals from the environment (exogenous stimuli) or apoptotic cell debris/modified host proteins (endogenous stimuli) [13]. When bound to a given ligand, intracellular signaling pathways are activated to initiate the appropriate macrophage response. For example, a bacterial infection can activate macrophages to promote a Th1 type response, which secretes several specific chemokines (e.g., CCL2, CCL5, CXCL8, and CXCL11) that attract monocytes, macrophages, neutrophils, and natural killer cells to resolve the infection [14].
To return the injured tissue to homeostasis, the damaged/unwanted materials (e.g., microbes and dying/necrotic cells) must be phagocytized. Efferocytosis, a term used to describe phagocytosis of apoptotic cells by macrophages, is an essential function for resolving inflammation. The injured tissue is protected from exposure to toxic contents of dying cells and stimulates the production of anti-inflammatory mediators, which, in turn, prevents further tissue damage [15-17]. Specifically, the well-characterized clearance of apoptotic neutrophils is theorized to evoke the switch in macrophages from an inflammatory phenotype to a growth-promoting phenotype initiating the healing process [15,17]. Macrophages also clear other types of apoptotic cells, such as fibroblasts, suggesting the importance of macrophage function throughout the entire wound healing process [17]. Finally, macrophages promote repair through extracellular matrix remodeling, fibrogenesis, and angiogenesis [18].
Therefore, macrophages have a plethora of different functional capabilities that are all necessary to fulfill tissue-specific functions to maintain homeostasis. Understanding these phenotypic differences between different subsets of macrophages is key to understanding their roles in thermal injury.
Origin of macrophages
In 1968, as an initial attempt to classify macrophages, van Furth and Cohn determined that macrophages were derived from blood monocytes [19]. This discovery led to the concept of the mononuclear phagocyte sequence (MPS), a linear model describing how adult tissue-resident macrophages rely on circulating monocytes derived from hematopoietic cells in the bone marrow. The local tissue microenvironment then induces these cells into their specific phenotypes. However, in the 2000s, studies demonstrated a separate lineage of tissue-resident macrophages are generated during development, persist into adulthood, and maintain themselves via self-renewal [20,21]. Research studies have demonstrated that certain embryonic macrophage populations, such as microglia and Langerhan cells, are derived from the yolk sac and therefore do not have a monocytic progenitor [6,8]. Thus, a revised concept of the MPS now accommodates two distinct origins for macrophages: 1) tissue-resident macrophages established prenatally, and 2) macrophages that develop in adulthood from monocytes [7] (Figure 1).
Figure 1.
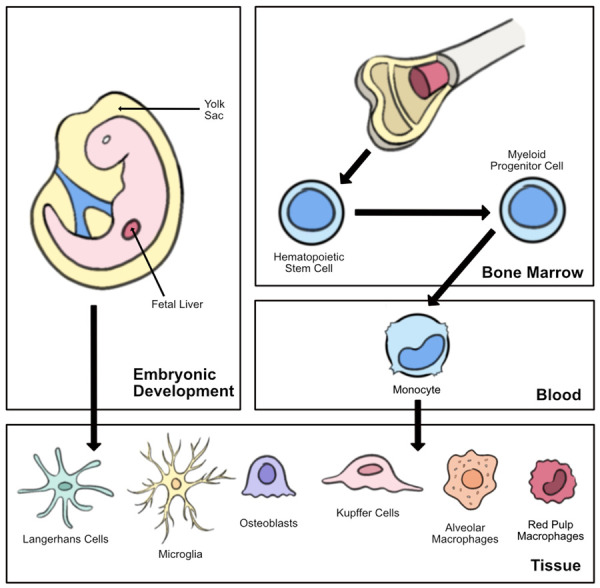
Development of tissue-resident macrophages. Macrophages can be derived from early embryonic development (the fetal liver or yolk sac) or from monocytes. Circulating monocytes develop from hematopoietic cells in the bone marrow and are induced into their specific phenotypes by the local tissue microenvironment.
Interestingly, skin tissue contains both embryonic macrophages in the epidermis (i.e., Langerhans cells) as well as monocyte-derived macrophages in the dermis. The inflammatory response relies heavily on monocyte-derived macrophages as tissue-resident macrophages are rapidly lost during the initial inflammation phase [22]. This phenomenon, referred to as the “disappearance reaction”, occurs specifically in Langerhans cells [23,24]. Therefore, after initial injury, a small pool of monocytes can enter the wound through the leaky vasculature and differentiate into macrophages [25]. Simultaneously, the injury initiates the production of the myeloid progenitor cell (the monocyte precursor cell) in the bone marrow, leading to the eventual increase in circulating monocytes that must actively cross the endothelium and differentiate into macrophages [26] (Figure 1). Tissue-resident macrophages rely on their capacity for self-renewal to repopulate during the resolution of the inflammatory process. Therefore, thermal injury involves both an acute inflammatory phase and a delayed wound healing phase in which both embryonic and monocyte-derived macrophages have a role.
Macrophage phenotype
An additional binary classification separates macrophages based on their inflammatory states in response to environmental signals: activated macrophages (M1) with pro-inflammatory features versus alternatively activated macrophages (M2) with reparative features that contribute to resolving inflammation [27]. In vitro, interferon (IFN)-γ and toll-like receptors (TLRs) activate M1 macrophages producing pro-inflammatory cytokines [e.g., tumor necrosis factor (TNF)-α and interleukin (IL)-1, -6, -12] as a crucial response to host protection followed by the production of nitric oxide or reactive oxygen species [6,21,28]. Conversely, in vitro, IL-4 and IL-13 stimulate M2 macrophages, which triggers IL-10, transforming growth factor (TGF)-β, and glucocorticoid (GC) production to help dampen inflammation and initiate tissue remodeling [8,28].
However, such a simplistic binary classification does not accurately represent the complexity of the in vivo environment [6]. Instead, studies have demonstrated that macrophages can develop mixed M1 and M2 phenotypes, switching their phenotype between pro- and anti-inflammatory states to stimulate growth after the initial period of inflammation [28-30]. Tissue-resident macrophages are commonly classified as “M2-like”, whereas monocyte derived macrophages differentiate into M1 or M2 depending on the microenvironment [21]. Interestingly, it has been proposed that M2 macrophages can convert into M1 phenotype, but generally the reverse does not occur because M1 macrophages are “end-stage killer cell[s]” that likely die during the inflammatory response [21,31].
Overall, multiple theories propose how macrophages differentiate, originate, and determine their phenotypes, but few studies reveal the role of macrophages with respect to wound healing in thermal injury. Here, we aim to review the available literature on macrophages and their function in thermal injuries, which will allow for identification of further areas of research needed to improve outcomes for patients affected by burn injuries.
Macrophages and thermal injury
As a highly dynamic cell, macrophages are crucial to all aspects of wound healing. Although the wound healing process after thermal injury is lengthy, it generally comprises three interrelated and overlapping phases: inflammation, proliferation, and remodeling (Figure 2). This review will discuss the role of macrophages in the initial inflammatory process after burn injury, which involves the release of inflammatory cytokines, antigen presentation, and phagocytosis that starts immediately upon burn injury and can last approximately 5-7 days [26]. The proliferation phase, covering about 3-10 days post-injury, involves angiogenesis and granulation tissue being formed through the combined aid of macrophages, granulocytes, and keratinocytes [26]. Finally, the effects of macrophage presence on remodeling, which can take up to a year to complete after injury, will be discussed regarding scar formation [26] (Figure 2). As macrophages are implicated in all aspects of the wound healing process, this cell’s dysfunction can lead to multiple complications spanning the spectrum of severe sepsis or death to functional complications such as hypertrophic scarring and contractures. Therefore, reviewing the current literature involving the roles macrophages have in wound healing is key to understanding how to treat burn injuries and avoid complications.
Figure 2.
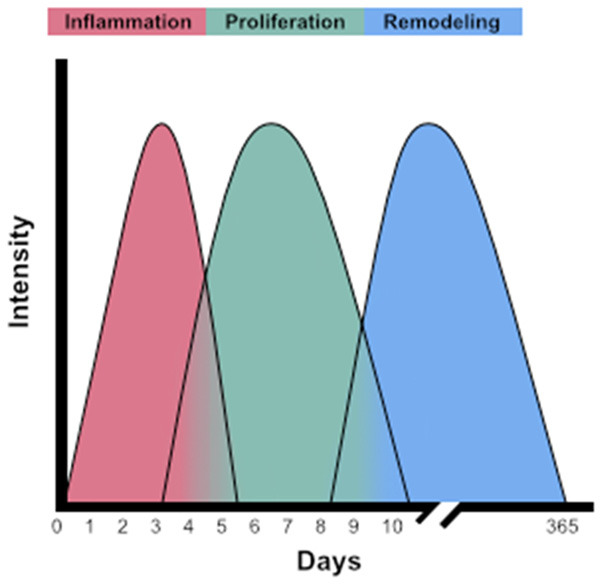
Three phases of wound healing after thermal injury. The wound healing cascade is described by three interrelated and overlapping phases: inflammation (red), proliferation (green), and remodeling (blue). Inflammation begins immediately upon burn injury and can last approximately 5-7 days. Proliferation covers 3-10 days post-injury, while remodeling can take up to a year to complete after injury. While macrophages are implicated in all aspects of the wound healing process, macrophage dysfunction can lead to severe complications such as sepsis or death or cosmetic complications such as hypertrophic scarring and contractures.
Macrophage cytokine production in burn injury
Burn injury induces an inflammatory cytokine response initiated by macrophages immediately following injury. Macrophages have a decisive role in supporting the differential expression of Th1 or Th2 and the subsequent cytokine response by producing IL-12 and IL-10, respectively. Th1 cytokines (e.g., IL-2, IFN-γ) support the cell-mediated immune response and generally support activation, whereas Th2 cytokines (e.g., IL-4, IL-10) support the humoral immune response and are suppressive [32,33]. Macrophages can also act as a predominant and continuous source for TNF-α, IL-1, and IL-6, which can induce a secondary cascade of cytokines and intracellular signaling that assist in the early inflammatory phase of wound healing [9,32]. Our group and others have revealed that burn injury alone induces numerous pro- (IL-1, -6, -8, and TNF-α) and anti-inflammatory (IL-4, -10) cytokines to regulate the acute phase response, usually peaking in the first week and then decreasing over time [34,35]. However, the cytokine profiles differ significantly between pediatric and adult burn patients [36].
While macrophages are pivotal in the immune response to tissue homeostasis, burn injury can also alter the macrophages’ response and initiate several physiological parameters resulting in a massive inflammatory response, hypermetabolism, and immune dysfunction, which ultimately contribute to increased susceptibility to sepsis and multisystem organ failure [37]. Specifically, thermal injury increases the productive capacity of macrophages for pro-inflammatory mediators (e.g., prostaglandin E2, nitric oxide, TNF-α, IL-6) leading to macrophage hyperactivity, which has been implicated in post-burn immune dysfunction and the development of immunosuppression [32,37,38]. This dysfunction led to the concept of the “two-hit” phenomenon explained by Dietrich (1992): the initial insult (i.e., burn) “primes” the host to exhibit an abnormal response (e.g., increased pro-inflammatory cytokines) so that on the second hit (i.e., infection) the host’s response is greatly amplified and can ultimately lead to multisystem organ failure and death [39]. Specifically, increased cytokine production of TNF-α, IL-6, macrophage migration inhibitory factor (MIF), and TGF-β and decreased production of IL-12 and IFN-γ have been linked to immunosuppression resulting in an increased risk of infections after thermal injury [33,40-45].
As such, infection-related complications remain high, but macrophage hyperactivity has recently gained attention as a potential implication to deleterious outcomes. However, there is still limited research in understanding macrophage function and its role in inflammatory dysfunction following burn injuries, especially in pediatrics who are noted to have different cytokine profiles than adults. Understanding this relationship is critical to developing immunomodulatory therapies that can either augment macrophage function or alter their phenotype to improve outcomes related to thermal injury. For example, recent research has suggested granulocyte macrophage colony-stimulating factor (GM-CSF) as a topical therapy to help with burn wound healing [46-48]. The use of GM-CSF could potentially target macrophages to augment its phenotype or function to improve outcomes.
Phagocytosis in burn injury
As previously mentioned, the ability of macrophages to phagocytize infectious agents and senescent or dying cells is a key function for adequate wound repair [17]. Specifically, in burn injury, macrophages aim to uptake and remove infectious organisms (e.g., predominantly bacteria, but also yeast, fungi, or viruses) as a critical role in the first line of defense against infection [49]. Bacteria, once bound to the surface of macrophages, are rapidly internalized into phagosomes, which gradually acquire the characteristics of terminal phagolysosomes resulting in the microbe’s death [50]. However, some pathogens (e.g., Legionella spp. and Mycobacteria spp.) can be resistant to the phagosome contents or escape the phagosome into the cytosol [50]. Additionally, a 2021 review of the role of macrophages in the clearance of Staphylococcus aureus infection notes that the presence of the M1 or M2 phenotype of macrophages leads to either an appropriate phagocytic response or ineffective clearance, respectively [51].
The inability to appropriately remove cellular debris and infectious organisms can ultimately lead to chronic inflammation [15]. Interestingly, both adult and animal studies have exhibited reduced phagocytic function following thermal injury, which may eventually lead to infections throughout the body, not just the injury site [52-54]. Accordingly, data from the National Burn Repository from the American Burn Association states burn patients’ top two complications (pneumonia and urinary tract infection) are separate from the site of injury, further suggesting systemic immune dysfunction following thermal injury [35,55,56]. Additionally, six of the top ten complications following thermal injury are infection-related, further stressing the importance of understanding phagocytic activity and the systemic immune response in burn patients [55].
Future research elucidating the receptor mechanisms used by macrophages to identify cells for phagocytosis may ultimately enhance our knowledge about infections associated with burn injury [16]. Importantly, research has suggested that different receptor-mediated phagocytosis in macrophages initiates varying levels of pro-inflammatory cytokines, therefore linking phagocytosis dysfunction and the massive inflammatory response [57]. To date, no research has been done on phagocytotic dysfunction in pediatric thermal injury, indicating a knowledge gap. Understanding the phagocytosis and cytokine relationship can further assist immunomodulator therapy development. Specifically, GM-CSF has shown to enhance phagocytic activity and could potentially serve as a stimulant to prevent infection following burn injury [58]. However, immunomodulator therapies have not been pursued in pediatric burn patients, indicating a research gap with potential implications of improving outcomes for patients with thermal injury.
Antigen presentation in burn injury
Macrophages represent the most prominent antigen-presenting cell type during inflammation [59]. Simply, macrophages ingest the antigen via phagocytosis and break it down into fragments in the lysosome. A fragment then binds to the class II major histocompatibility complex (MHC II) proteins on the surface of macrophages for presentation to T cells. However, the precise phenotype and access to antigen vary extensively from tissue to tissue [10,60].
Lipopolysaccharide (LPS) stimulation is a commonly used assay to explore antigen presentation on macrophages and its influence on T cell activation [61]. Specifically, LPS stimulation can influence T cell activation by releasing inflammatory cytokines (e.g., TNF-α) on healthy macrophage antigen presenting cells. However, after burn injury, several studies have indicated increased anti-inflammatory cytokines (e.g., IL-10) inhibit macrophage antigen presentation while decreasing ex vivo LPS-stimulated TNF-α production [62,63]. This impairment of LPS-stimulated cytokine production is associated with an increased risk for infection and mortality in both adult and pediatric populations [62-72].
Concurrently, levels of MHC class II expression and antigen presentation decrease after a burn injury [73-78]. Specifically, human leukocyte antigen-DR (HLA-DR), an MHC class II cell surface receptor, commonly decreases following burn injury in both adults and pediatrics [63,77,79]. A delayed return to normal HLA-DR expression has been linked to developing infections and correlates with increased mortality [62,63,79,80].
Our previous research in pediatric thermal injury has indicated that both percent HLA-DR expression on CD14+ monocytes and ex vivo LPS-induced TNF-α production capacity are better predictors of the development of nosocomial infections than plasma cytokines [63]. Interestingly, decreases in antigen presentation capacity were observed as early as 72 hours after injury, with the median time to diagnosis of infection at 7 days, indicating a potential window to intervene with immunomodulator therapy. Additional research is also needed to understand the responsible mechanisms of immune suppression perhaps through the investigation of apoptotic signaling pathways.
Macrophage phenotype on healing burn tissue
As previously mentioned, wound macrophages can exhibit both M1 and M2 markers. In general, tissue-resident macrophages are “M2-like” and reparative, whereas cells expressing the M1 phenotype are pro-inflammatory and formed from monocyte-derived macrophages [17,21] (Figure 3). In normal human skin, the M2 macrophage is dominant, but in response to burn injuries, the initial inflammatory response is primarily dominated (~85% of macrophages) by the pro-inflammatory M1 phenotype [81,82]. M1 macrophages secrete mediators such as nitric oxide, TNF-α, and IL-6, which serve as cytotoxins against infected cells and mediate resistance against infections [83-85]. However, while pro-inflammatory mediators can subside quickly, M1 macrophages can persist for weeks following burn injury [78].
Figure 3.
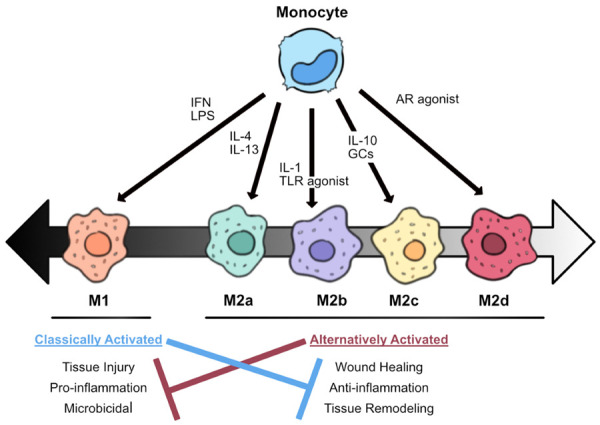
Macrophage polarization and function. Monocyte-derived macrophages can differentiate into activated macrophages (M1) or alternatively activated macrophages (M2) depending on environmental signals. Interferon (IFN) and lipopolysaccharide (LPS) are common stimulators of M1 polarization. M2 macrophages are further divided into four subgroups that are differentially activated: M2a is stimulated by interleukin (IL)-4 or IL-13; M2b by IL-1 or toll-like receptor (TLR) agonists; M2c by glucocorticoids (GC) or IL-10; and M2d by TLR agonists through the adenosine receptor (AR). Classically activated macrophages are associated with pro-inflammation and microbicidal activity and appear immediately after tissue injury. However, persistence can block appropriate wound healing. Conversely, alternatively activated macrophages appear at later time points and are associated with anti-inflammation and tissue remodeling as part of the wound healing process.
The macrophage population then shifts to a less inflammatory, proliferative, and remodeling profile dominated by the M2 phenotype (~80% of macrophages) at later time points [78,82]. M2 macrophages debride the injury site by phagocytosis, down-regulate inflammation, and release growth factors to promote proliferation and angiogenesis [84]. M2 macrophages can further be divided into four subgroups, differentiated based on the activating stimulus: M2a is stimulated by IL-4 or IL-13; M2b by IL-1 or TLR agonists (e.g., LPS); M2c by GCs or IL-10; and M2d by TLR agonists through the adenosine receptor (AR) [85] (Figure 3). However, it has been theorized that M2 macrophages in wound tissues are primarily M2c being activated and maintained through a positive IL-10 feedback loop: M2c releases IL-10 and TGF-β which in turn stimulates macrophages to polarize into more M2c [85,86].
To date, no human studies have explored macrophage phenotypes after thermal injury alone. However, one study by Chen et al. (2019) observed macrophage phenotype in different stages of wound healing post-injury (including trauma and burn patients). In this work, M1 markers increased early, peaking between 7 to 14 days post-injury (trauma and burn), then rapidly declining between 14 to 28 days. Conversely, M2 markers were elevated two days after injury but remained high up to 28 days later until decreasing during scar formation (up to 6 weeks later) [86,87]. While this study does provide insight into the macrophage phenotypes after burn injury, additional research is needed to further delineate these results in burn injury alone and explore the functional response of these macrophages in pediatric thermal injuries.
Macrophages and adverse wound healing after burn injury
The delicate balance between the pro- and anti-inflammatory response mediated by macrophages is an extreme paradox for researchers studying post-burn wound healing. While an active immune response is crucial to control infection, excessive activation of macrophage production of pro-inflammatory cytokines (e.g., IL-6, TNF-α) can lead to a systemic inflammatory response syndrome (SIRS) and result in multisystem organ failure [32,38]. A compensatory anti-inflammatory response syndrome (CARS) also occurs alongside systemic inflammation, which results in post-burn immunosuppression and is linked to the progression of infections [88]. Along the same lines, increased macrophage production of IL-10, peaking within the first 3-4 days, has been associated with sepsis and death after burn injury [89,90]. Therefore, the dysregulation of the macrophage inflammatory response poses a serious risk to morbidity and mortality after thermal injury and warrants further investigation.
Despite the roles macrophages have in the inflammatory cascade of SIRS or CARS, macrophage dysfunction can also pose additional issues in terms of wound healing, including skin graft failure and fibrosis (e.g., hypertrophic scars) [91]. Although the functional nature of macrophages in allografts is poorly understood, macrophages have been implicated as a detrimental component to allograft failure [92,93]. Rodent studies have best explored allograft failures where depleting macrophage infiltration improves graft survival in animal rejection models [94]. Interestingly, in a study by Schwacha et al. (2000), burn wound excision and grafting in a rodent model decreased the hyperactive macrophage phenotype [95]. Therefore, while the optimal timing of excision and grafting post-burn injury remains controversial, the macrophage phenotype could well determine its success [96].
Contrary to the limited research on macrophages in graft failure, many animal studies have linked increased macrophages to improper wound healing, including scar formation. For example, Martin et al. used a PU.1 gene knockout mouse (deficient in both macrophages and neutrophils) and observed no apparent scar formation with similar wound repair times to normal mice [97]. Similarly, wounds in the oral mucosa have significantly lower levels of macrophages than dermal wounds and generally heal faster with less scarring [98]. In another study, when diphtheria toxin ablated macrophages, these mice had less scarring, delayed re-epithelialization, impaired angiogenesis, and decreased cell proliferation [99]. An additional study conducted to determine how the exact timing of macrophage depletion affects the healing process found that depleting macrophages during the early inflammatory phase significantly reduced the formation of vascularized granulation tissue and resulted in less scar formation [100]. However, depleting macrophages during the late stage (tissue maturation) did not significantly impact scar formation [100]. Therefore, it is hypothesized that excessive pro-inflammatory mediators and the presence of M1 macrophages stall/impair the healing process and ultimately contribute to scarring [101-104] (Figure 3). Nevertheless, the M2 macrophage cytokine production of TGF-β has also been associated with collagen deposition and been detected in post-burn scars [105-108]. Therefore, the spatiotemporal diversity of the M1 and M2 macrophages has a crucial role in the formation and degree of scarring in post-burn injury [109].
Unfortunately, animal models (e.g., mice, rabbits) comprise most of the current literature involving macrophages, but the wound healing process in these species differs significantly from humans [110]. Particularly, healing in mouse skin primarily occurs through contraction and not re-epithelialization, and mice have an enriched pool of progenitor cells expediting their healing process [78]. Thus, the inflammatory processes surrounding wound healing are likely to lead to different clinical outcomes [86]. Only a few human studies describe macrophage function in burn wound healing exists [103,111], therefore studies fully delineating the regulation of macrophage phenotypes in wounds are urgently needed. An additional area of research, with great translational importance, is the use of immunomodulator therapy, that can either control inflammation or augment macrophage phenotype to reduce the risk of macrophage dysfunction and reduce infectious complications while assisting in optimal wound healing and functional outcomes.
Conclusion
Burn injuries are a serious health problem, affecting approximately two million people in the United States per year; roughly half of these occur in children [112]. Macrophages represent a highly heterogeneous population of immune cells with many different functional capabilities (phagocytosis, cytokine production, antigen presentation, and wound healing). This review highlights the complexity of the macrophages through the healing cascade in burn injury and suggests their role in post burn immune dysfunction. Macrophages serve as a double-edged sword in which hyperactive macrophages can lead to increased inflammation, slowed wound healing, and increased scar formation. In contrast, decreased macrophage function can lead to immunosuppression and subsequent risk of infection. Studies following thermal injury have displayed prolonged dysregulation of the macrophage response is linked to an increase risk of morbidity and mortality. Unfortunately, literature is lacking on the impact macrophage function has on outcomes in human thermal injury, especially in pediatrics. Current research investigating the complete mechanisms of macrophage dysfunction after thermal injury are limited and needs further evaluation in both adults and pediatrics. Through this more complete understanding of macrophages, the development of appropriate therapeutics in treating burn wounds that can simultaneously help control inflammation, prevent infection, assist in wound healing, and reduce scar formation, is an absolute necessity for burn care research.
Acknowledgements
The authors would like to acknowledge Madeline Nelson and Will Ray, PhD, from the Abigail Wexner Research Institute Graphics Support Resource at Nationwide Children’s Hospital for their assistance in designing the figures.
Disclosure of conflict of interest
None.
References
- 1.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 3.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 5.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 8.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 11.Ariasnegrete S, Keller K, Chadee K. Proinflammatory cytokines regulate cyclooxygenase-2 MRNA expression in human macrophages. Biochem Biophys Res Commun. 1995;208:582–589. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- 12.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verschoor CP, Puchta A, Bowdish DME. The macrophage. In: Ashman R, editor. Leucocytes. Methods in molecular biology (methods and protocols) Humana Press; 2012. pp. 139–156. [DOI] [PubMed] [Google Scholar]
- 14.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 15.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 17.DiPietro LA, Wilgus TA, Koh TJ. Macrophages in healingwounds: paradoxes and paradigms. Int J Mol Sci. 2021;22:950. doi: 10.3390/ijms22020950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 19.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Italiani P, Boraschi D. From Monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barth MW, Hendrzak JA, Melnicoff MJ, Morahan PS. Review of the macrophage disappearance reaction. J Leukoc Biol. 1995;57:361–367. doi: 10.1002/jlb.57.3.361. [DOI] [PubMed] [Google Scholar]
- 24.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature Reviews Immunology. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 25.Rodero MP, Licata F, Poupel L, Hamon P, Khosrotehrani K, Combadiere C, Boissonnas A. In vivo imaging reveals a pioneer wave of monocyte recruitment into mouse skin wounds. PLoS One. 2014;9:e108212. doi: 10.1371/journal.pone.0108212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohl J, Zaharia A, Rudolph M, Murray RZ. The role of inflammation in cutaneous repair. Wound Practice and Research. 2015;23:8–15. [Google Scholar]
- 27.Xuan W, Qu Q, Zheng B, Xiong S, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61–69. doi: 10.1189/jlb.1A0314-170R. [DOI] [PubMed] [Google Scholar]
- 28.Lee KY. M1 and M2 polarization of macrophages: a mini-review. Medical Biological Science and Engineering. 2019;2:1–5. [Google Scholar]
- 29.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 33.Toliver-Kinsky TE, Varma TK, Lin CY, Herndon DN, Sherwood ER. Interferon-γ production is suppressed in thermally injured mice: decreased production of regulatory cytokines and corresponding receptors. Shock. 2002;18:322–330. doi: 10.1097/00024382-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 35.Devine RA, Diltz Z, Hall MW, Thakkar RK. The systemic immune response to pediatric thermal injury. Int J Burns Trauma. 2018;8:6–16. [PMC free article] [PubMed] [Google Scholar]
- 36.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG Investigators of the Inflammation and the Host Response Glue Grant. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 38.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators (review) Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 39.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Chaudry IH, Choudhry MA. ERK and not p38 pathway is required for IL-12 restoration of T cell IL-2 and IFN-γ in a rodent model of alcohol intoxication and burn injury1. J Immunol. 2009;183:3955–3962. doi: 10.4049/jimmunol.0804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drost AC, Burleson DG, Cioffi WG Jr, Mason AD Jr, Pruitt BA Jr. Plasma cytokines after thermal injury and their relationship to infection. Ann Surg. 1993;218:74–78. doi: 10.1097/00000658-199307000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Wang H, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol. 2007;292:H2408–2416. doi: 10.1152/ajpheart.01150.2006. [DOI] [PubMed] [Google Scholar]
- 44.Gurfinkel R, Czeiger D, Douvdevani A, Shapira Y, Artru AA, Sufaro Y, Mazar J, Shaked G. Ketamine improves survival in burn injury followed by sepsis in rats. Anesth Analg. 2006;103:396–402. doi: 10.1213/01.ane.0000226140.84281.3e. [DOI] [PubMed] [Google Scholar]
- 45.Grieb G, Simons D, Piatkowski A, Bernhagen J, Steffens G, Pallua N. Macrophage migration inhibitory factor-a potential diagnostic tool in severe burn injuries? Burns. 2010;36:335–342. doi: 10.1016/j.burns.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Yan D, Liu S, Zhao X, Bian H, Yao X, Xing J, Sun W, Chen X. Recombinant human granulocyte macrophage colony stimulating factor in deep second-degree burn wound healing. Medicine (Baltimore) 2017;96:e6881. doi: 10.1097/MD.0000000000006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chi YF, Chai JK, Luo HM, Zhang QX, Feng R. Safety of recombinant human granulocyte-macrophage colony-stimulating factor in healing pediatric severe burns. Genet Mol Res. 2015;14:2735–2741. doi: 10.4238/2015.March.31.3. [DOI] [PubMed] [Google Scholar]
- 48.Yuan L, Minghua C, Feifei D, Runxiu W, Ziqiang L, Chengyue M, Wenbo J. Study of the use of recombinant human granulocyte-macrophage colony-stimulating factor hydrogel externally to treat residual wounds of extensive deep partial-thickness burn. Burns. 2015;41:1086–1091. doi: 10.1016/j.burns.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Jeschke MG, Van Baar ME, Choudhry MA, Chung KK, Gibran N, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6:11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 51.Pidwill GR, Gibson JF, Cole J, Renshaw SA, Foster SJ. The role of macrophages in staphylococcus aureus infection. Front Immunol. 2021;11:620339. doi: 10.3389/fimmu.2020.620339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt K, Bruchelt G, Kistler D, Koslowski L. Phagocytic activity of granulocytes and alveolar macrophages after burn injury measured by chemiluminescence. Burns. 1983;10:79–85. doi: 10.1016/0305-4179(83)90002-5. [DOI] [PubMed] [Google Scholar]
- 53.Loose LD, Turinsky J. Macrophage dysfunction after burn injury. Infect Immun. 1979;26:157–162. doi: 10.1128/iai.26.1.157-162.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoilova YD, Haidushkal IA, Murdjeval MA, Traikov IZ, Popova TA, Kevorkyan AK. Immunological and microbiological investigations of patients with burn injuries. Folia Med (Plovdiv) 2007;49:49–58. [PubMed] [Google Scholar]
- 55.American Burn Association, National Burn Repository 2019 Update: Report of Data from 2009-2018. 2019. https://sk75w2kudjd3fv2xs2cvymrg-wpengine.netdna-ssl.com/wp-content/uploads/2020/05/2019-ABA-Annual-Report_FINAL.pdf.
- 56.Engelich G, Wright DG, Hartshorn KL. Acquired disorders of phagocyte function complicating medical and surgical illnesses. Clin Infect Dis. 2001;33:2040–2048. doi: 10.1086/324502. [DOI] [PubMed] [Google Scholar]
- 57.Fu YL, Harrison RE. Microbial phagocytic receptors and their potential involvement in cytokine induction in macrophages. Front Immunol. 2021;12:662063. doi: 10.3389/fimmu.2021.662063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roilides E, Walsh TJ, Pizzo PA, Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163:579–583. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 59.Barker RN, Erwig LP, Hill KSK, Devine A, Pearce WP, Rees AJ. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol. 2002;127:220–225. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muntjewerff EM, Meesters LD, van den Bogaart G. Antigen cross-presentation by macrophages. Front Immunol. 2020;11:1276. doi: 10.3389/fimmu.2020.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T cell immunity. Crit Rev Immunol. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muszynski JA, Nofziger R, Greathouse K, Nateri J, Hanson-Huber L, Steele L, Nicol K, Groner JI, Besner GE, Raffel C, Geyer S, El-Assal O, Hall MW. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock. 2014;42:313–321. doi: 10.1097/SHK.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thakkar RK, Devine R, Popelka J, Hensley J, Fabia R, Muszynski JA, Hall MW. Measures of systemic innate immune function predict the risk of nosocomial infection in pediatric burn patients. J Burn Care Res. 2021;42:488–494. doi: 10.1093/jbcr/iraa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, Tran T, Keisling S, Randolph AG Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network PICFlu Study Investigators. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ploder M, Pelinka L, Schmuckenschlager C, Wessner B, Ankersmit HJ, Fuerst W, Redl H, Roth E, Spittler A. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25:129–134. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- 67.Johnson NB, Posluszny JA, He LK, Szilagyi A, Gamelli R, Shankar R, Muthumalaiappan K. Perturbed MafB/GATA1 axis after burn trauma bares the potential mechanism for immune suppression and anemia of critical illness. J Leukoc Biol. 2016;100:725–736. doi: 10.1189/jlb.1A0815-377R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De A, Laudanski K, Miller-Graziano C. Failure of monocytes of trauma patients to convert to immature dendritic cells is related to preferential macrophage-colony-stimulating factor-driven macrophage differentiation. J Immunol. 2003;170:6355–6362. doi: 10.4049/jimmunol.170.12.6355. [DOI] [PubMed] [Google Scholar]
- 69.Oswald IP, Wynn TA, Sher A, James SL. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc Natl Acad Sci U S A. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 71.Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992;89:4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sachse C, Prigge M, Cramer G, Pallua N, Henkel E. Association between reduced human leukocyte antigen (HLA)-DR expression on blood monocytes and increased plasma level of interleukin-10 in patients with severe burns. Clin Chem Lab Med. 2005;37:193–8. doi: 10.1515/CCLM.1999.036. [DOI] [PubMed] [Google Scholar]
- 73.Valvis SM, Waithman J, Wood FM, Fear MW, Fear VS. The immune response to skin trauma is dependent on the etiology of injury in a mouse model of burn and excision. J Invest Dermatol. 2015;135:2119–2128. doi: 10.1038/jid.2015.123. [DOI] [PubMed] [Google Scholar]
- 74.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sayed S, Bakry R, El-Shazly M, El-Oteify M, Terzaki S, Fekry M. Effect of major burns on early and late activating markers of peripheral blood T lymphocytes. Ann Burns Fire Disasters. 2012;25:17–21. [PMC free article] [PubMed] [Google Scholar]
- 76.Stephan RN, Ayala A, Harkema JM, Dean RE, Border JR, Chaudry IH. Mechanism of immunosuppression following hemorrhage: defective antigen presentation by macrophages. J Surg Res. 1989;46:553–556. doi: 10.1016/0022-4804(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 77.Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443–450. doi: 10.1097/00024382-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Lateef Z, Stuart G, Jones N, Mercer A, Fleming S, Wise L. The cutaneous inflammatory response to thermal burn injury in a murine model. Int J Mol Sci. 2019;20:538. doi: 10.3390/ijms20030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venet F, Tissot S, Debard AL, Faudot C, Crampe C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- 80.Palojarvi A, Petaja J, Siitonen S, Janer C, Andersson S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr Res. 2013;73:469–475. doi: 10.1038/pr.2012.199. [DOI] [PubMed] [Google Scholar]
- 81.Klar AS, Michalak-Micka K, Biedermann T, Simmen-Meuli C, Reichmann E, Meuli M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int. 2017;34:129–135. doi: 10.1007/s00383-017-4179-z. [DOI] [PubMed] [Google Scholar]
- 82.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis. 2011;24:230–234. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- 84.Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov Today. 2017;22:186–193. doi: 10.1016/j.drudis.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 86.Chen L, Wang J, Li S, Yu Z, Liu B, Song B, Su Y. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int Wound J. 2019;16:360–369. doi: 10.1111/iwj.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Broek LJ, Van der Veer WM, de Jong EH, Gibbs S, Niessen FB. Suppressed inflammatory gene expression during human hypertrophic scar compared to normotrophic scar formation. Exp Dermatol. 2015;24:623–629. doi: 10.1111/exd.12739. [DOI] [PubMed] [Google Scholar]
- 88.Muszynski JA, Thakkar R, Hall MW. Inflammation and innate immune function in critical illness. Curr Opin Pediatr. 2016;28:267–273. doi: 10.1097/MOP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 89.Pileri D, Palombo AA, D’Amelio L, D’Arpa N, Amato G, Masellis A, Cataldo V, Mogavero R, Napoli B, Lombardo C, Conte C. Concentrations of cytokines Il-6 and Il-10 in plasma of burn patients: their relationship to sepsis and outcome. Ann Burns Fire Disasters. 2008;21:182–185. [PMC free article] [PubMed] [Google Scholar]
- 90.Yeh FL, Shen HD, Fang RH. Deficient transforming growth factor β and interleukin-10 responses contribute to the septic death of burned patients. Burns. 2002;28:631–637. doi: 10.1016/s0305-4179(02)00113-4. [DOI] [PubMed] [Google Scholar]
- 91.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mannon RB. Macrophages: contributors to allograft dysfunction, repair or innocent bystanders? Curr Opin Organ Transplant. 2012;17:20–25. doi: 10.1097/MOT.0b013e32834ee5b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixit S, Baganizi DR, Sahu R, Dosunmu E, Chaudhari A, Vig K, Pillai SR, Singh SR, Dennis VA. Immunological challenges associated with artificial skin grafts: available solutions and stem cells in future design of synthetic skin. J Biol Eng. 2017;11:49. doi: 10.1186/s13036-017-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao W, Topham PS, King JA, Smiley ST, Csizmadia V, Lu B, Gerard CJ, Hancock WW. Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Invest. 2000;105:35–44. doi: 10.1172/JCI8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14:623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 96.Miroshnychenko A, Kim K, Rochwerg B, Voineskos S. Comparison of early surgical intervention to delayed surgical intervention for treatment of thermal burns in adults: a systematic review and meta-analysis. Burns Open. 2021;5:67–77. [Google Scholar]
- 97.Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 98.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 99.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 101.Watanabe N, Suzuki Y, Inokuchi S, Inoue S. Sepsis induces incomplete M2 phenotype polarization in peritoneal exudate cells in mice. J Intensive Care. 2016;4:6. doi: 10.1186/s40560-015-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M, Hou Q, Zhong L, Zhao Y, Fu X. Macrophage related chronic inflammation in non-healing wounds. Front Immunol. 2021;12:681710. doi: 10.3389/fimmu.2021.681710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge following burn injury. Lancet. 2016;388:1427–1436. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strudwick XL, Cowin AJ. The role of the inflammatory response in burn injury. In: Kartal SP, Bayramgurler D, editors. Hot topics in burn injuries. IntechOpen; 2017. [Google Scholar]
- 106.Feng Y, Sun Z, Liu S, Wu J, Zhao B, Lv G, Du Y, Yu S, Yang M, Yuan F, Zhou X. Direct and indirect roles of macrophages in hypertrophic scar formation. Front Physiol. 2019;10:1101. doi: 10.3389/fphys.2019.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams H, Suda S, Dervish S, Yap YT, Holland A, Medbury HJ. Monocyte M1/M2 profile is altered in paediatric burn patients with hypertrophic scarring. Wound Repair Regen. 2021;29:996–1005. doi: 10.1111/wrr.12960. [DOI] [PubMed] [Google Scholar]
- 108.Wong J, Lin W, Ding J, Tredget EE. Prevention and management of scarring after thermal injury. Plast Aesthet Res. 2021;8:9. [Google Scholar]
- 109.Xu X, Gu S, Huang X, Ren J, Gu Y, Wei C, Lian X, Li H, Gao Y, Jin R, Gu B, Zan T, Wang Z. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8:tkaa006. doi: 10.1093/burnst/tkaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramos MLC, Gragnani A, Ferreira LM. Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res. 2008;29:363–368. doi: 10.1097/BCR.0b013e3181667557. [DOI] [PubMed] [Google Scholar]
- 111.Liu H, Ding J, Ma Z, Zhu Z, Shankowsky HA, Tredget EE. A novel subpopulation of peripheral blood mononuclear cells presents in major burn patients. Burns Incl Therm Inj. 2015;41:998–1007. doi: 10.1016/j.burns.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez R, Shanti CM. Overview of current pediatric burn care. Semin Pediatr Surg. 2015;24:47–49. doi: 10.1053/j.sempedsurg.2014.11.008. [DOI] [PubMed] [Google Scholar]


