ABSTRACT
The striatum is a central regulator of behavior and motor function through the actions of D1 and D2 medium-sized spiny neurons (MSNs), which arise from a common lateral ganglionic eminence (LGE) progenitor. The molecular mechanisms of cell fate specification of these two neuronal subtypes are incompletely understood. Here, we found that deletion of murine Meis2, which is highly expressed in the LGE and derivatives, led to a large reduction in striatal MSNs due to a block in their differentiation. Meis2 directly binds to the Zfp503 and Six3 promoters and is required for their expression and specification of D1 and D2 MSNs, respectively. Finally, Meis2 expression is regulated by Dlx1/2 at least partially through the enhancer hs599 in the LGE subventricular zone. Overall, our findings define a pathway in the LGE whereby Dlx1/2 drives expression of Meis2, which subsequently promotes the fate determination of striatal D1 and D2 MSNs via Zfp503 and Six3.
KEY WORDS: Meis2, Six3, Zfp503, Dlx1/2, Striatum, LGE, Mouse
Summary: Expression of Meis2, which promotes striatal MSN fate determination, is dependent on Dlx1/2 in the lateral ganglionic eminence, and this regulation is at least partially mediated by the hs599 enhancer.
INTRODUCTION
The striatum is the first component of the basal ganglia that receives cortical input; it participates in computations that mediate complex movements, reward learning, decision making, emotion and cognition (Hikida et al., 2010; Hintiryan et al., 2016; Valjent and Gangarossa, 2020; Xiao et al., 2020). In mammals, the majority of striatal neurons are GABAergic medium-sized spiny neurons (MSNs), which can be subdivided into two subpopulations according to their transcriptional profile and target innervation (Doyle et al., 2008; Gerfen and Surmeier, 2011; Lobo et al., 2006). Striatonigral neurons (D1 MSNs) are characterized by the transcription factors (TFs) Zfp503 and Isl1 and specifically express the dopamine D1 receptor (DRD1) and the neuropeptide TAC1 (known as substance P) (Chen et al., 2020; Ehrman et al., 2013; Gerfen et al., 1990; Gerfen and Surmeier, 2011; Lu et al., 2014; Soleilhavoup et al., 2020). In contrast, striatopallidal neurons (D2 MSNs) are characterized by the Sp8/9 and Six3 TFs, and specifically express the dopamine D2 receptor (DRD2) and the neuropeptide enkephalin (ENK; also known as PENK) (Gerfen et al., 1990; Gerfen and Surmeier, 2011; Gokce et al., 2016; Lobo et al., 2006; Stanley et al., 2019; Xu et al., 2018; Zhang et al., 2016). Both cell types originate from a common pool of progenitor cells located in the lateral ganglionic eminence (LGE) and express TFs such as Gsx1/2, Ascl1 and Dlx1/2 (Anderson et al., 1997; Bocchi et al., 2021; Kelly et al., 2018; Long et al., 2009b; Pei et al., 2011; Wang et al., 2013; Yun et al., 2002). The molecular mechanisms that specify D1 and D2 MSNs remain to be fully elucidated.
Homeodomain TFs comprise a large family of evolutionarily conserved DNA binding proteins that have a myriad of roles in controlling animal development (Gehring et al., 1994). The Meis1/2/3 homeodomain TFs belong to the three amino acid loop extension (TALE) family of homeodomain proteins, that include the PBX and PKNOX families; together they play important roles in development and disease in part through modulating the function of HOX TFs (Golonzhka et al., 2015; Moens and Selleri, 2006; Mukherjee and Bürglin, 2007). In non-neuronal tissues, Meis2 regulates the proliferation, differentiation and survival in the limbs, craniofacial skeleton and heart (Cunningham and Duester, 2015; Machon et al., 2015; Wang et al., 2020; Zha et al., 2014). Furthermore, Meis2 plays pivotal roles in the gene regulatory network, driving the differentiation of human embryonic stem cells towards cardiovascular cell types (Paige et al., 2012). Meis2 loss-of-function causes a triad of palatal defects, congenital heart defects and intellectual disability in humans (Giliberti et al., 2020; Verheije et al., 2019). Although the expression of Meis2 in the central nervous system was reported 20 years ago (Larsen et al., 2010; Toresson et al., 1999), its functions in the forebrain remain to be investigated. Furthermore, investigation of the molecular mechanisms by which Meis2 regulates the development of MSNs may provide a theoretical and experimental basis for clinical applications to disorders of the basal ganglia.
The present study was undertaken to uncover the molecular mechanisms of Meis2 in regulating the development of striatal MSNs. In the Meis2-conditional knockout (CKO) (Dlx5/6-CIE; Meis2F/F) mouse, LGE precursor cells failed to fully differentiate into MSNs, leading to an expansion of the subventricular zone (SVZ). As a result, the majority of MSNs were not generated. Analysis of the results of RNA-sequencing (RNA-seq), Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) and Cleavage Under Targets and Tagmentation with sequencing (CUT&Tag-seq) experiments revealed that Six3 and Zfp503 are crucial direct downstream targets of Meis2 in controlling the specification of the D1 and D2 MSNs. Furthermore, the results of single-cell ATAC-seq and chromatin immunoprecipitation with sequencing (ChIP-seq) (Lindtner et al., 2019) showed that DLX1/2 directly bind to the three Meis2 distal enhancer elements (hs355, hs599 and a 3rd putative enhancer) to promote Meis2 expression in the LGE. Finally, deletion of hs599 led to a significant decrease in the expression of Meis2 in the LGE SVZ. Taken together, our findings suggest that Meis2 promotes the precursor specification in D1 and D2 MSNs by directly promoting the expression of Six3 and Zfp503, respectively. Moreover, Meis2 expression is dependent on Dlx1/2 in the LGE SVZ, and this regulation is at least partially mediated by the hs599 enhancer.
RESULTS
Meis2 is highly expressed in the LGE and striatum
To study the distribution of Meis2 in the ganglionic eminences, we performed a detailed analysis of Meis2 mRNA using in situ hybridization (ISH). We observed a high level of Meis2 in the LGE SVZ and a moderate level in the ventricular zone (VZ). In contrast, Meis2 expression was nearly absent from the globus pallidus, a major nucleus generated by the medial ganglionic eminence (MGE) (Fig. 1A; Fig. S1A). At embryonic day (E)16.5, postnatal day (P)0 and in the adult, Meis2 continued to be strongly expressed in the entire striatum (Fig. S1B-D). ASCL1/MEIS2/BCL11B triple immunofluorescence staining at E14.5 found that very few ASCL1-positive cells were colabeled with MEIS2 in the LGE, suggesting that MEIS2 is not strongly expressed in dividing cells of the SVZ (Fig. S1E,F). Most striatal MSNs express BCL11B (Arlotta et al., 2008; Zhang et al., 2019). Nearly all BCL11B-positive LGE cells also expressed MEIS2, providing evidence that MEIS2 is expressed in striatal neurons (Fig. S1E,F). Finally, we found that all MEIS2-positive cells also expressed the striatal pan-neuronal marker FOXP1 at P30 (Fig. S1G-J). Together, our results showed that Meis2 is principally expressed in MSNs from embryonic to adult stages.
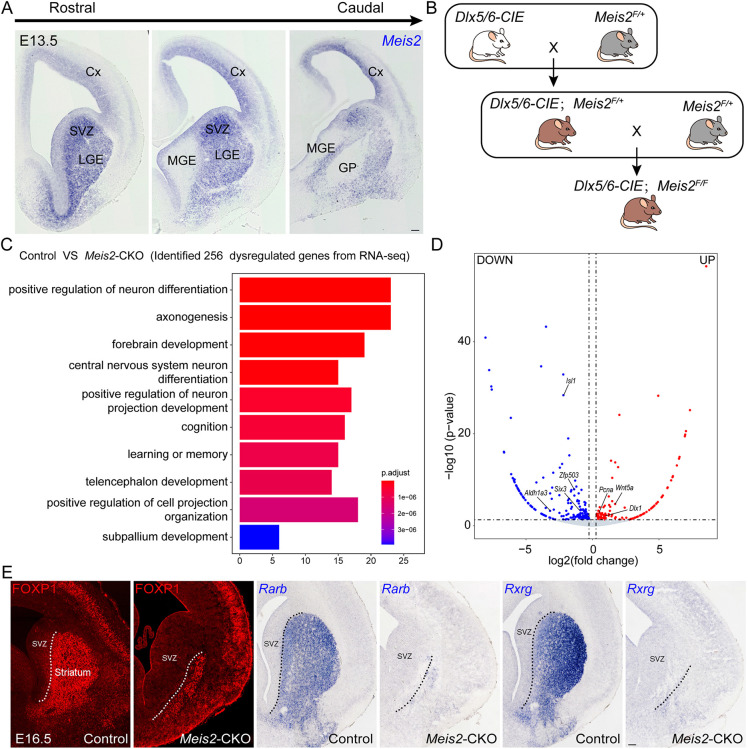
Fig. 1.
The majority of striatal MSNs are lost in Meis2-CKO mice. (A) Meis2 mRNA expression in a series of rostral-to-caudal sections at E13.5. Its expression is strongest in the LGE and septal progenitors and neurons (striatal primordium) and is remarkably low in the MGE and its derivatives. (B,C) Schematic showing crosses (B) and functional enrichment analysis (C) of DEGs in the LGE of Meis2-CKO versus control mice at E14.5. (D) Volcano plots showing the DEGs in the LGE at E14.5 between control and Meis2-CKO mice. (E) The expression of FOXP1, Rarb and Rxrg (three pan-neuronal markers of the MSNs) was significantly decreased in Meis2-CKO mice compared with control mice at E16.5. Dotted lines mark the border of the LGE SVZ and striatum. N=4 mice per group. Cx, cortex; GP, globus pallidus; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; SVZ, subventricular zone. Scale bar: 100 µm for E.
Nearly all striatal MSNs are lost in Meis2-CKO mice
To investigate the role of Meis2 in the LGE (striatal primordium), we crossed a Dlx5/6-CIE mouse with a Meis2F/+ line to eliminate Meis2 expression in the SVZ and mantle zone (MZ) of the LGE (Fig. 1B). We referred to the conditional mutant as Meis2-CKO. First, we identified differentially expressed genes (DEGs) between Meis2-CKO mice and control mice via RNA-seq analysis from the LGE at E14.5. We identified 161 genes with downregulated RNA expression and 95 genes with upregulated RNA expression (Table S1). Next, we performed gene ontology (GO) analysis on the 256 dysregulated genes. This analysis suggests that Meis2 is mainly involved in the positive regulation of neuron differentiation, axonogenesis and forebrain development (Fig. 1C). Genes expressed in immature and/or mature MSNs, such as Aldh1a3, Zfp503 and Six3 were downregulated. In contrast, the genes expressed in proliferating cells, such as Wnt5a, Pcna and Dlx1 were upregulated (Fig. 1D and Table S1). Finally, we selected 8 genes that are highly expressed in MSNs (Foxp1, Rarb, Rxrg, Ikzf1, Ebf1, Foxp2, Penk and Bcl11a) and examined expression in the Meis2-CKO via ISH or immunofluorescence (Fig. 1E; Fig. S2A-J). Our results showed that the expression of these genes was greatly reduced in the Meis2-CKO mice compared with the control mice. Overall, these findings showed that Meis2 plays a central transcriptional role in the generation of striatal MSNs.
Accumulation of progenitors in the LGE SVZ of Meis2-CKO mice
Next, we explored the mechanism underlying the failure of the Meis2-CKO mutants to generate MSNs. First, we tested whether Meis2 affects the proliferation of LGE progenitors, and thus examined the expression of markers of LGE cell proliferation: GSX2, ASCL1 and PCNA (Anderson et al., 1997; Castro et al., 2011; Chapman et al., 2013) at E16.5 (Fig. 2A-F). GSX2 and ASCL1 were expressed in the radial glia cells and intermediate progenitors in the LGE (Chapman et al., 2013; Wang et al., 2013; Zhang et al., 2020) and we found that the number of GSX2- and ASCL1-positive cells was increased in Meis2-CKO mutants (Fig. 2A-D,K). Analysis of the number of cycling cells at E16.5 in Meis2-CKO and control mice showed that the total number of proliferating cells in the SVZ was increased, inducing an enlargement of the proliferative area stained with PCNA (Fig. 2E,F,K). This increase results in an enlarged SVZ and a very small striatum. Dlx1/2 and Dlx5/6 are essential for the MSN differentiation in the LGE (Anderson et al., 1997; Fazel Darbandi et al., 2016; Lindtner et al., 2019; Long et al., 2009a; Wang et al., 2013, 2011; Yun et al., 2002). The Dlx genes are highly expressed in LGE progenitor cells (largely in the SVZ) (Liu et al., 1997; Zhang et al., 2016). Our results showed that the number of cells expressing DLX2, CRE (driven by a Dlx5/6 enhancer), and Dlx5 (mRNA) were increased in the Meis2-CKO LGE SVZ compared with control mice (Fig. 2G-K; Fig. S2K,L).
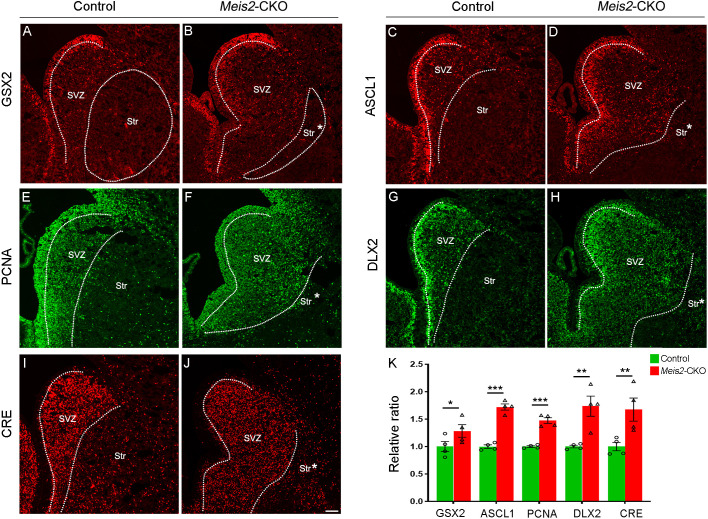
Fig. 2.
Neural progenitors accumulate in the LGE SVZ of Meis2-CKO mice. (A-F) The number of cells expressing ventricular zone and SVZ markers, GSX2 (A,B), ASCL1 (C,D) and PCNA (E,F), were increased in a greatly expanded SVZ of Meis2-CKO mice at E16.5. Note that the mantle zone was smaller in Meis2-CKO mice than in control mice. (G-J) Likewise, the number of cells expressing SVZ and neuronal markers, DLX2 (G,H) and CRE (Dlx5/6) (I,J), were greatly increased in SVZ of the Meis2-CKO mice at E16.5. (K) The number of GSX2-, ASCL1-, PCNA-, DLX2- and CRE (Dlx5/6)-positive cells was significantly increased in Meis2-CKO mice compared with control mice. *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA followed by Tukey–Kramer post-hoc test). n=4 mice per group, mean±s.e.m. Dotted lines mark the border of the lateral ganglionic eminence SVZ and striatum. Str, striatum; Str*, presumptive striatum; SVZ, subventricular zone. Scale bar: 200 µm in J for A-J.
To further confirm that Meis2 affects the proliferation of LGE precursors, we used another Cre line (Gsx2-Cre) to conditionally knock out Meis2 in the VZ and SVZ of the LGE. Using KI67, BrdU incorporation (2 h) and PH3, we confirmed that Meis2 mutants accumulated progenitors in the LGE SVZ at E14.5 or E16.5 (Fig. S3A-D). Taken together, these results indicated that Meis2 is required for LGE progenitors to leave the cell cycle, and as a result Meis2 mutants accumulate proliferating cells in the LGE SVZ.
Abnormal accumulation of the immature MSNs in the LGE SVZ of Meis2-CKO mice
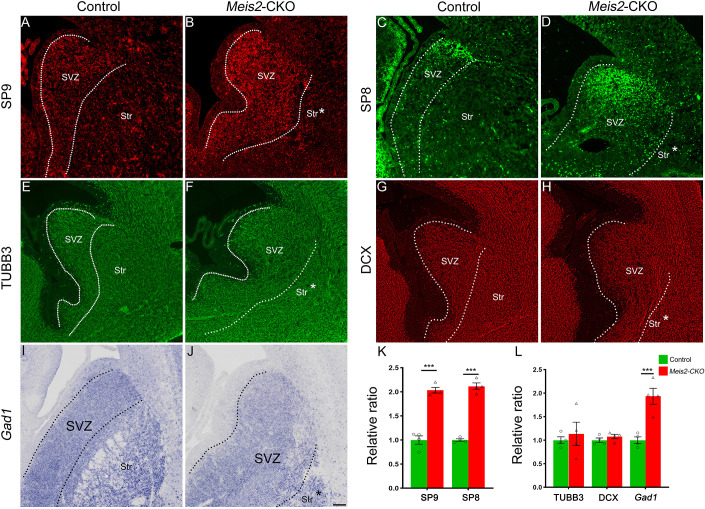
Previously, we have shown that the Sp8 and Sp9 TFs are required for the differentiation of the D2 MSNs in the ventral LGE (vLGE) (Xu et al., 2018; Zhang et al., 2016). These TFs are highly expressed in the LGE SVZ and striatum. Here, we found that the SP8- and SP9-positive cells were increased in the Meis2-CKO LGE SVZ (Fig. 3A-D,K; Fig. S2O,P). Because Sp8/9 are expressed in progenitors and MSNs, we explored whether these cells were proliferating or postmitotic by examining the expression of markers of immature neurons, TUBB3 and doublecortin (DCX). There was robust TUBB3 and DCX expression in the expanded LGE SVZ of the Meis2-CKO (Fig. 3E-H,L). In addition, we found that many immature neurons which expressed Gad1 were located in the LGE SVZ of Meis2-CKO mice (Fig. 3I,J,L). Next, we wanted to further confirm these phenotypes at a later stage. Because the Meis2-CKO mice only survive several hours after birth, we analyzed the Meis2-CKO mice at P0. Meis2-CKO P0 mice had an enlarged ventricle and a small striatum (Fig. 4A,B). In the rostral LGE the Sp8 and Bcl11b TFs are mainly expressed in the SVZ (neuroblast) and MSNs, respectively (Arlotta et al., 2008; Li et al., 2017; Waclaw et al., 2006; Xu et al., 2018). We found some BCL11B-positive cells were generated, but they were significantly reduced in the Meis2-CKO striatum at P0 (Fig. 4A,B). We also found that the SP8-positive cells were present in the enlarged SVZ (Fig. 4C,D). Combined with our previous study (Yang et al., 2021b), we speculated that the cells were gradually depleted in the LGE owing to abnormal differentiation. Taken together, these results suggested that, in the absence of Meis2, progenitor cells and immature neurons cannot differentiate well into striatal mature MSNs.
Fig. 3.
The abnormal differentiation MSNs accumulate in the LGE SVZ of Meis2-CKO mice. (A-D) The expression of the transcription factors SP8 (C,D) and SP9 (A,B) are increased in the LGE SVZ of the Meis2-CKO mice compared with the controls. (E-H) There are many immature neurons which express the pan-neuronal markers TUBB3 (E,F) and DCX (G,H) located in the LGE SVZ of Meis2-CKO and control mice. (I,J) The expression of Gad1 is increased in the LGE SVZ of the Meis2-CKO mice compared with the controls. (K,L) The quantification data of the above markers in the LGE SVZ. ***P<0.001 (one-way ANOVA followed by Tukey–Kramer post-hoc test). n=4 mice per group, mean±s.e.m. Dotted lines mark the border of the LGE SVZ and striatum. LGE, lateral ganglionic eminence; Str, striatum; Str*, presumptive striatum; SVZ, subventricular zone. Scale bar: 200 µm in J for A-J.
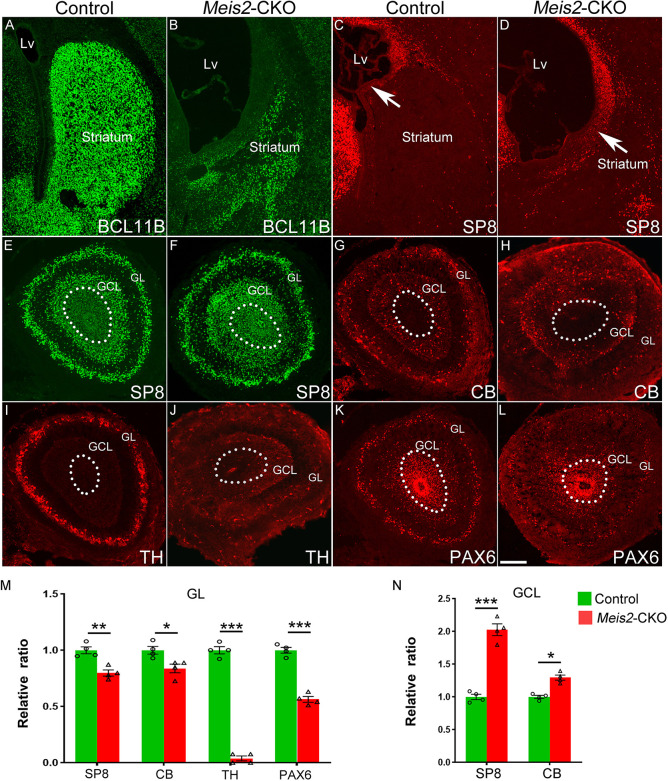
Fig. 4.
Meis2 is required for the development of olfactory bulb interneurons. (A,B) Compared with controls, the number of BCL11B-positive cells was reduced in Meis2-CKO mice at P0 in the striatum. (C,D) More SP8-positive cells were observed in the LGE SVZ of Meis2-CKO mice (arrows). (E-H) The cell number of the SP8- and CB-positive cells was increased in the GCL and decreased in the GL. (I,J) There was a significant reduction in TH-positive cells in the GL of the Meis2-CKO mice at P0. (K,L) The expression of PAX6 was significantly reduced in the GL. (M,N) Quantification of above experiments. *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA followed by Tukey–Kramer post-hoc test). n=4 mice per group, mean±s.e.m. Dotted lines mark the border of the olfactory bulb core. GCL, granule cell layer; GL, glomerular layer. Scale bar: 200 µm in L for A-L.
Meis2 is required for the differentiation of the olfactory bulb interneuron
The LGE consists of subdomains including the dorsal LGE (dLGE) and vLGE (Wen et al., 2021; Yun et al., 2001). The progenitors located in the dLGE contribute to the olfactory bulb (OB) interneurons, whereas vLGE progenitors mainly generate striatal MSNs (Li et al., 2017, 2021; Song et al., 2021; Waclaw et al., 2006, 2009; Xu et al., 2018; Yang et al., 2021a; Zhang et al., 2016). In the Meis2-CKO at E16.5 the dLGE marker Sp8 was expanded ventrally, but the expression of Etv1 (another dLGE marker) was not significantly altered (Fig. 3C,D; Fig. S2M-P). Therefore, these results indicated that the expansion of the SP8-positive cells in the LGE are likely to be the immature MSNs of abnormal differentiation. Next, we wondered whether the OB interneurons and striatal MSNs were affected in the Meis2-CKO mice.
We studied SP8, CB, TH and PAX6 expression in the OB at P0 in the Meis2-CKO mice compared with control mice. We found that the expression of SP8 had increased in the granule cell layer (GCL) (slight increase of CB as well), but decreased in the glomerular layer (GL) (Fig. 4E-H,M,N). Almost all TH-positive OB interneurons were lost in the GL in the Meis2-CKO mice compared with their controls (Fig. 4I,J,M). Moreover, the expression of the Pax6, which is required for the differentiation of the OB TH-positive interneurons, was also significantly reduced in the GL in the Meis2-CKO mice compared with their controls (Fig. 4K-M), consistent with a previous study showing that Meis2 is required for the differentiation of the TH-positive OB interneurons in adult neurogenesis. Taken together, Meis2 is required for the differentiation of OB periglomerular interneurons.
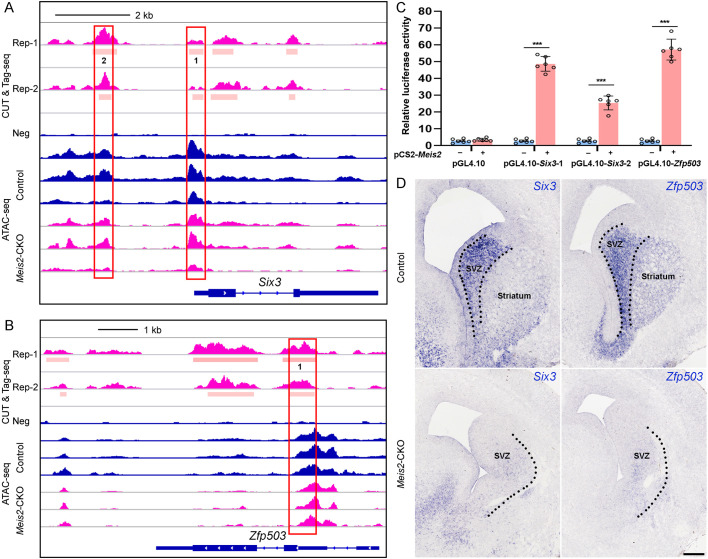
Meis2 controls MSN fate through promoting Six3 and Zfp503 expression
To gain insights into the mechanism of Meis2 in regulating the differentiation of striatal MSNs, we focused on our RNA-seq. The TF genes Six3, Zfp503, Isl1, and the Aldh1a3 gene, caught our attention because their RNA expression was significantly reduced in Meis2-CKO mice. Furthermore, MEIS2 binds to the putative promoters [<3 kb of the transcription start site (TSS)] of Six3, Zfp503, Aldh1a3 and Isl1 (Fig. 5A,B; Fig. S4A). As expected, the expression of these genes, detected by ISH or immunostaining, was significantly decreased in Meis2-CKO mice (Fig. 5D; Fig. S4B). Moreover, our results showed that the number of D2 MSNs (specifically expressing Drd2 and Adora2a) and D1 MSNs (specifically expressing Ebf1 and Foxp2) (Gokce et al., 2016; Lobo et al., 2006, 2008; Stanley et al., 2019; van Rhijn et al., 2018; Zhang et al., 2016) were greatly decreased in Dlx5/6-CIE; Six3F/F (Six3-CKO) and Dlx5/6-CIE; Zfp503F/F (Zfp503-CKO) mice, respectively (Figs S4C,D and S6A-D). In other words, the TFs Six3 and Zfp503 are crucial for the generation of D2 and D1 MSNs, respectively. These phenotypes are very similar to those of Meis2-CKO mice and are consistent with previous reports (Chen et al., 2020; Soleilhavoup et al., 2020; Song et al., 2021; Xu et al., 2018).
Fig. 5.
Meis2 is required for the differentiation of striatal MSNs. (A,B) Genomic regions of the Six3 (A) and Zfp503 (B) loci in the CUT&Tag-seq and ATAC-seq datasets. (C) MEIS2-activated transcription from the Six3 and Zfp503 promoters in a dual-luciferase assay of N2a cells. ***P<0.001 (unpaired two-tailed Student's t-test). n=6, mean±s.e.m. (D) In situ RNA hybridization analysis of Six3 and Zfp503 in control and Meis2-CKO mice at E16.5. Dotted lines mark the border of the lateral ganglionic eminence subventricular zone (SVZ) and striatum. Scale bar: 200 µm in D.
Finally, to test the ability of MEIS2 to regulate transcription of putative promoters of Six3 and Zfp503, we performed a dual-luciferase transcription activation assay using the N2a cell line. MEIS2 robustly activated transcription from these promoters (Fig. 5C). Overall, our findings suggest that the TF Meis2 promotes fate determination of striatal MSNs by directly promoting Six3 and Zfp503 expression.
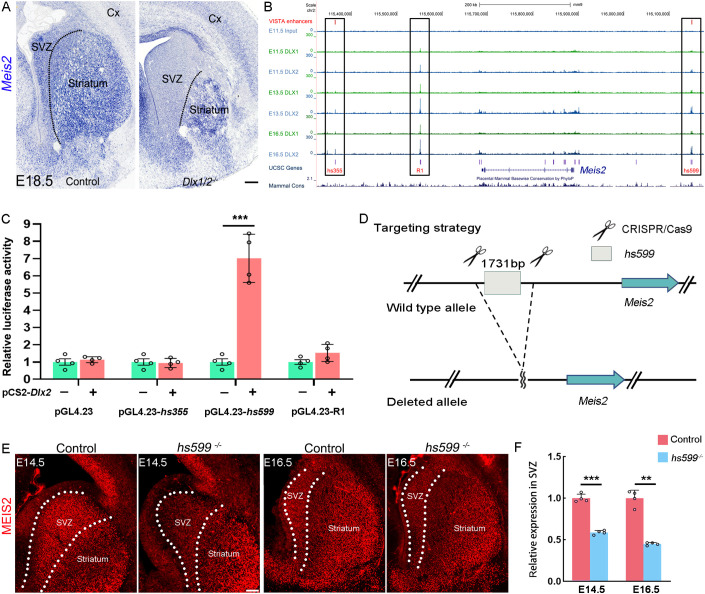
Dlx1/2 are required for the expression of Meis2 in the LGE
Meis2 is highly expressed in the LGE and its derivatives and is a key factor for the normal development of MSNs. Therefore, we wondered which genes can promote the expression of Meis2 in the striatum. A previous study showed that Dlx1/2 were required for the normal development of the striatal SVZ and the differentiation of MSNs (Anderson et al., 1997). Furthermore, the phenotypes of the Dlx1/2 mutant mice were very similar to those of Meis2-CKO mice; these mutant mice had an enlarged LGE SVZ (containing many proliferating cells and undifferentiated cells) and a very small striatum (lacking mature MSNs) (Anderson et al., 1997). Therefore, we analyzed Dlx1/2 mutant mice and found that the expression of Meis2 was greatly reduced in the LGE SVZ of the Dlx1/2 mutant mice (Fig. 6A) (Long et al., 2009b). Next, to test whether Dlx1/2 directly regulate Meis2 expression, we analyzed the Dlx1/2 ChIP-seq data of E11.5, E13.5 and E16.5 LGE (Lindtner et al., 2019). This gave us a chance to systematically analyze the downstream targets of Dlx1/2 in the LGE. As expected, we observed three Dlx1/2 binding regions near the Meis2 gene body (Fig. 6B). Two of the three regions are named hs355 and hs599. Then, we performed a luciferase reporter assay in N2a cells. Cotransfection of cells with a DLX2 expression vector (pCS2-Dlx2) with an enhancer-reporter construct (pGL4.23-Meis2-hs355, R1 or hs599) resulted in an ∼7-fold increase in pGL4.23-Meis2-hs599 luciferase activity compared with that of the pGL4.23 empty luciferase control (Fig. 6C). As in previous reports, hs599 showed enhancer activity in the developing striatum (McGregor et al., 2019; Silberberg et al., 2016; Visel et al., 2013). Thus, we focused on the function of hs599 in regulating Meis2 expression. To examine the in vivo function of hs599, we used CRISPR/Cas9 genome editing in fertilized eggs to generate hs599 heterozygous mice (Fig. 6D; Fig. S6E-H). We then analyzed the expression of Meis2 in hs599 mutant mice at E14.5 and E16.5. Our results showed that the expression of Meis2 was reduced in the LGE SVZ (Fig. 6E,F). This provides functional evidence that hs599 is a Meis2 enhancer in the LGE SVZ. Surprisingly, the development of the LGE appears to be normal in hs599 mutant mice (Fig. S5A-F). We speculated that there needs to be a loss of a specific threshold of Meis2 expression to yield a striatal phenotype, and the hs599 mutant mice have reduced Meis2 expression but are not at the level necessary for the striatal phenotype. Taken together, our findings suggested that Dlx1/2 promote Meis2 expression in the LGE, at least through hs599.
Fig. 6.
Dlx1/2 directly binds hs599 to promote Meis2 expression. (A) Meis2 expression was greatly reduced in the LGE SVZ of Dlx1/2 mutant mice. (B) DLX1/2 ChIP-seq showed that DLX1/2 directly bound to three distal regions near the Meis2 gene. (C) The dual-luciferase assay showed that DLX1/2 promoted the activity of hs599. (D) The CRISPR/Cas9 strategy was used to generate the hs599 mutant allele. (E,F) Meis2 expression was reduced in the LGE SVZ of the hs599 mutant mice at E14.5 and E16.5. N=4 mice per group. Dotted lines mark the border of the LGE SVZ and striatum. **P<0.01, ***P<0.001 (unpaired two-tailed Student's t-test). n=4, mean±s.e.m. Cx, cortex; LGE, lateral ganglionic eminence; SVZ, subventricular zone. Scale bar: 200 µm in A; 100 µm in E.
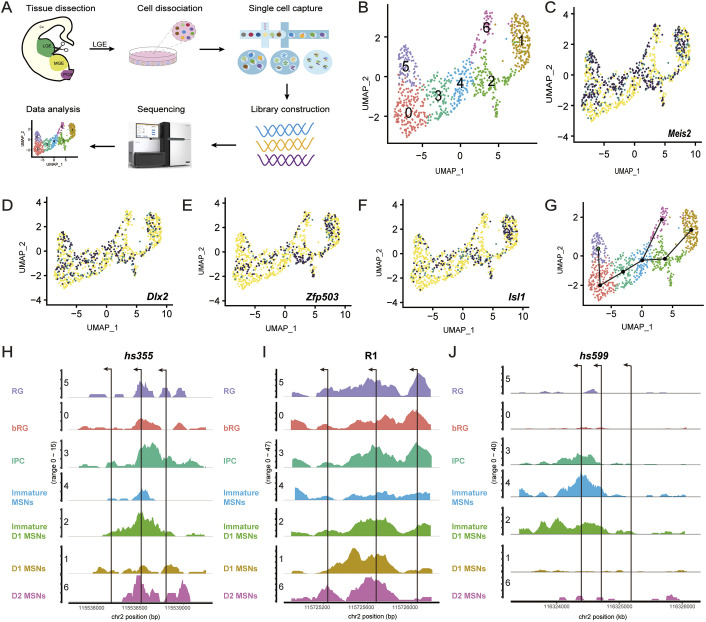
scATAC-seq further supported findings that Dlx1/2 regulate Meis2 expression through three enhancers
To gain further insights into the mechanism by which Dlx1/2 regulates Meis2 expression, we performed single-cell ATAC-seq (scATAC-seq) of the LGE at E14.5 (Fig. 7A). First, we generated an scATAC-seq dataset from 849 cells passing quality control criteria (see Materials and Methods). Through integration of the promoter and gene body accessibility in scATAC-seq, we identified seven clusters (C0-C6), including radial glial cells (C5), basal radial glial cells (C0), intermediate progenitor cells (C3), immature MSNs (C4), immature D1 MSNs (C2), D2 MSNs (C6) and D1 MSNs (C1) (Fig. 7B). The identity of cell clusters was further confirmed by assessing a set of cell-type-specific genes ascertained by differential gene expression analysis (Fig. 7C-F; Fig. S7A-C). According to chromatin accessibility changes, we constructed developmental trajectories of all cell populations (Fig. 7G). Our results suggest that D1 and D2 MSNs are derived from common progenitors. According to a recent study, the same progenitors give rise to both D1 and D2 MSNs in humans (Bocchi et al., 2021). Enhancers are often found in chromatin accessible regions where TFs preferentially bind (Nord et al., 2015). Therefore, we attempted to reveal the chromatin accessibility of the DLX1/2 binding regions near Meis2. Our results showed that the enhancer hs355 and putative enhancer R1 had high chromatin accessibility in all cell populations (Fig. 7H,I). In contrast, the chromatin accessibility of the enhancer hs599 was high in intermediate progenitor (SVZ) cells and immature MSNs but low in radial glial cells and mature MSNs (Fig. 7J). These results indicate that hs599 might exert its function mainly in differentiating cells rather than in LGE VZ radial glia and mature MSNs. This is consistent with our above result – the expression of Meis2 is mainly reduced in the LGE SVZ of the hs599 mutant mice. Finally, we identified multiple loci featuring DLX binding in those regions (Fig. 7H-J), which are consistent with previous DLX ChIP-seq studies (Lindtner et al., 2019). Altogether, our findings provide strong evidence that Dlx1/2 directly promote Meis2 expression in the LGE through multiple enhancers.
Fig. 7.
scATAC-seq revealed that hs599 exerted its function in a subpopulation of striatal MSNs. (A) Workflow of scATAC-seq of mouse LGE at E14.5. (B) Seven clusters (C0-C6) were identified and annotated according to marker gene accessibility. (C-F) UMAP plots show distinct chromatin accessibility profiles of marker genes. (G) The developmental trajectory construction scheme. (H-J) Genome tracks show normalized accessibility of three DLX1/2 binding regions with cluster-specific accessibility. Arrows represent the binding sites of DLX1/2. bRG, basal radial glial cells; Cx, cortex; IPC, intermediate progenitor cell; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; MSN, medium-sized spiny neurons; POA, preoptic area; RG, radial glial cells.
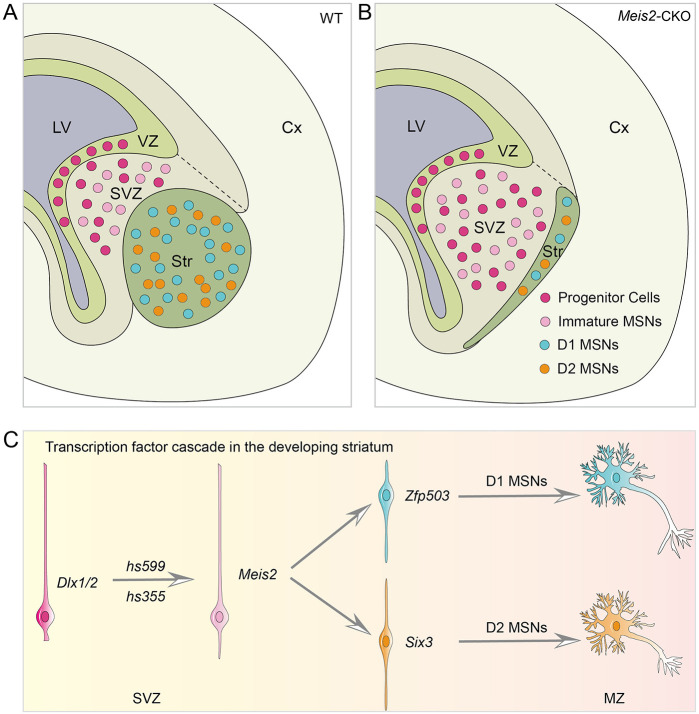
DISCUSSION
In all studied vertebrates, there are two types of striatal MSNs (D1 and D2) that have distinct roles in motor control, reinforcement learning and motivation (Dudman and Krakauer, 2016; Grillner et al., 2013; Peters et al., 2021). They are derived from common LGE progenitors (Bocchi et al., 2021; Kelly et al., 2018). However, the molecular mechanisms that specify MSNs, let alone D1 versus D2 MSNs, are incompletely understood. Here, we found that Meis2 plays a central role in the generation of MSNs. In Meis2-CKO mice, there is a block in the differentiation of LGE progenitors, resulting in the accumulation of proliferating and immature cells in an enlarged LGE (Fig. 8A,B), similar to the phenotype of Dlx1/2 mutants (Anderson et al., 1997). Integration of our RNA-seq, ATAC-seq and CUT&Tag-seq analyses provides evidence that Meis2 directly regulates the expression of Six3 and Zfp503 to control the specification of D2 and D1 MSNs, respectively (Fig. 8C). DLX1/2 directly binds to three putative Meis2 enhancers, including hs599, which are implicated in promoting Meis2 expression (Lindtner et al., 2019; McGregor et al., 2019; Silberberg et al., 2016). We generated a deletion of hs599; in its absence, there is reduced Meis2 expression in the LGE SVZ. Consistent with this, our scATAC-seq data provides evidence for hs599 activity in the LGE intermediate SVZ progenitors. Overall, our findings provided evidence that Meis2 promotes the fate determination of striatal MSNs by promoting Six3 and Zfp503 expression and that DLX1/2 drive Meis2 expression through enhancer hs599.
Fig. 8.
A model of the mechanism by which Meis2 regulates striatal MSN development. (A,B) Proliferating and immature cells accumulated in the lateral ganglionic eminence SVZ in Meis2-CKO mice, compared with control mice. Notably, the Meis2-CKO mice have an enlarged SVZ and a small striatum. (C) The main transcription factor network regulating the fate determination of striatal MSNs. First, Dlx1/2 promote the expression of Meis2 by enhancers such as hs599 and hs355. Then, Meis2 promotes Zfp503 or Six3 expression to further determine precursor cell fate (D1 or D2 MSNs). Cx, cortex; LV, lateral ventricle; MSN, medium-sized spiny neurons; MZ, mantle zone; Str, striatum; SVZ, subventricular zone; VZ, ventricular zone.
Meis2 is a key factor for generating mature MSNs
Elucidating the transcriptional networks in the developing striatum requires the knowledge of both the cellular and developmental functions and the genome-wide molecular functions of individual TFs. Twenty years ago, the homeobox genes Dlx1/2 were reported to be required for the generation of striatal MSNs. In its absence, the number of MSNs was greatly reduced owing to a block in differentiation (Anderson et al., 1997). Subsequently, many other factors were reported to be essential for the development of striatal MSNs, such as Gsx2, Ascl1, Ebf1 and Isl1 (Chapman et al., 2013; Corbin et al., 2000, 2003; Ehrman et al., 2013; Garel et al., 1999; Horton et al., 1999; Lobo et al., 2006, 2008; Long et al., 2009b; Lu et al., 2014; Waclaw et al., 2009; Wang et al., 2013, 2009; Yun et al., 2002). Meis2 is a Hox gene co-factor which is highly expressed in the LGE and its derivatives from embryonic to adult stages. CKO of Meis2 in the LGE greatly reduced the number of striatal MSNs due to a block in differentiation (Figs 1E and 3A-J). Recent studies have shown that the TFs Six3 and Zfp503 are crucial for the generation of the D2 and D1 MSNs, respectively (Chen et al., 2020; Soleilhavoup et al., 2020; Song et al., 2021; Xu et al., 2018). CUT&Tag-seq technology revealed that Meis2 directly binds to the promoters of Six3 and Zfp503 (Fig. 5A,B). Consistent with this, Six3 and Zfp503 expressions were greatly reduced in Meis2-CKO mice (Fig. 5D). Therefore, our findings suggest that Meis2 is a central factor in the specification of D1 and D2 MSNs by promoting Zfp503 and Six3 expressions, respectively. Thus, Meis2 is a crucial step in determining the fate of MSN precursors on their path to become D1 or D2 MSNs. Although our previous study showed that Meis2 was involved in striatal MSN survival (Yang et al., 2021b), this study highlighted the mechanism by which Meis2 regulates the development of striatal MSNs. Finally, another possible result in loss of striatal MSNs in the Meis2-CKO mice is that Meis2 regulates dorsal versus ventral LGE identity. Because we observe the expansion of Sp8 expression but not Etv1 and Tshz1 (Table S1), we speculated that the blockage of generating striatal MSNs in the Meis2-CKO mice was mainly due to a failure in expressing Zfp503 and Six3.
Meis2 regulates genes involved in retinoic acid production in the LGE SVZ
Retinoic acid (RA), a small molecule derived from vitamin A, is a ligand for nuclear RA receptors, and has been proposed to play important roles in striatal neurogenesis (Berenguer and Duester, 2021; Chatzi et al., 2011; Liao et al., 2008; Oulad-Abdelghani et al., 1997; Toresson et al., 1999; Urban et al., 2010; Waclaw et al., 2004). RA is synthesized by Raldh3 (Aldh1a3) in LGE progenitors at E12.5 (Molotkova et al., 2007; Toresson et al., 1999), and the Gsx2 TF is required for Aldh1a3 expression (Waclaw et al., 2004). Aldh1a3 is essential for the differentiation of striatal MSNs (Chatzi et al., 2011). Based on our findings, we hypothesize that Aldh1a3 LGE expression is directly promoted by Meis2. The reasons are as follows: (1) RNA-seq showed that the expression of Aldh1a3 was significantly decreased at E14.5 in Meis2-CKO mice; (2) ISH showed that Aldh1a3 expression was abolished at E12.5 and E16.5; (3) CUT&Tag-seq revealed that Meis2 directly binds to the Aldh1a3 promoter region; (4) there were many immature neurons in the LGE SVZ of Meis2-CKO mice, but they did not express Aldh1a3. Hence, we speculate that Aldh1a3, at least as a part of Meis2, is a direct downstream target that regulates the differentiation of striatal MSNs.
Dlx1/2 regulate Meis2 expression in the LGE SVZ through the enhancer hs599
Our findings demonstrated that proliferating cells and immature neurons accumulated in the LGE SVZ of Meis2-CKO mice due to abnormal differentiation. These phenotypes are very similar to those in the LGE of Dlx1/2 mutant mice (Anderson et al., 1997). Furthermore, the expression of Dlx1/2 was increased in the LGE SVZ of Meis2-CKO mice, and the expression of Meis2 was greatly reduced in the LGE SVZ of Dlx1/2 mutant mice. Therefore, we hypothesize that Dlx1/2 are upstream factors that promote Meis2 expression in the LGE SVZ. Analysis of recently published data showed that Dlx1/2 directly bound to three enhancer regions (hs355, putative enhancer region 1 and hs599) near Meis2 (Lindtner et al., 2019). The luciferase assays also supported our hypothesis. Because hs599 is active during development of the LGE (McGregor et al., 2019; Silberberg et al., 2016; Visel et al., 2013), we wondered whether hs599 can promote Meis2 expression. CRISPR/Cas9 genome editing generated a deletion of hs599. Analysis of hs599−/− mice showed that the expression of Meis2 was reduced in the LGE SVZ (Fig. 6E,F). Considering that an enhancer might show different activity in distinct populations of cells, we performed scATAC-seq to determine hs599 chromatin in different populations of LGE cells at E14.5. The chromatin of enhancer hs599 was mainly open in immature neurons (Fig. 7J). Altogether, our findings indicated that Dlx1/2 directly regulate the expression of Meis2 in the LGE SVZ, at least partially, via the enhancer hs599.
In sum, our findings clearly elucidated the mechanism by which Meis2 regulates the development of striatal MSNs. This study will provide a solid theoretical basis for further research on striatum development and related diseases.
MATERIALS AND METHODS
Animals
All experiments conducted in this study were in accordance with guidelines from Fudan University, China. Meis2F/+ (Yang et al., 2021b), Six3F/+ (Xu et al., 2018), Dlx5/6-CIE (Zhang et al., 2019), Gsx2-Cre (Wei et al., 2019) and Dlx1/2+/− (Anderson et al., 1997) mice have been previously described (see Table S2 for more details). Zfp503F/+ mice were generated via the CRISPR/Cas9 strategy (Banan, 2020). LoxP sites flanked the coding region of exon 3 (Fig. S6A-D). Genotyping of the Zfp503 constitutive knockout (KO) allele was performed by PCR using the following primers: F4: 5′-GCCACACAAACTTGTCTCCTTTT-3′; R4: 5′-CAGCACACACTCCTTGATTCTAAAG-3′; and R5: 5′-TAAACTTTCTTTTCCCTTCCGTAGC-3′. These primers yield bands of 253 and 305 bp for the wild-type and mutant alleles, respectively. hs599+/− mice with deletion of the hs599 enhancer were generated by CRISPR/Cas9 techniques (Fig. 6D; Fig. S6E-H). Genotyping of the hs599 mutant allele was performed by PCR using the following primers: WT-F: 5′-GGAGAGATGTTGCTGCTAGTGAGGC-3′; WT-R: 5′-CGCTTGAGTCATTAACAGTGTGCCC-3′; and Mut-F: 5′-AACCATTAACATCTGTGAGATGCTGTC-3′. These primers yield bands of 455 and 583 bp for the wild-type and mutant alleles, respectively. All mice were maintained in a mixed genetic background of C57BL/6J and CD1. The day of vaginal plug detection was considered E0.5, and the day of birth was defined as P0. Both male and female mice were used in all experiments.
BrdU labeling
A single intraperitoneal injection of BrdU (50 mg/kg) was administered at E14.5 or E16.5. BrdU incorporation was analyzed 2 h after BrdU injection.
Tissue preparation
Tissue preparation was performed as previously described (Liu et al., 2018).
Immunohistochemistry
In this study, 6-μm thick frozen sections were used for immunostaining. The sections were washed with 0.05 M Tris buffered saline (TBS) for 10 min, incubated in Triton X-100 (0.5% in 0.05 M TBS) for 30 min at room temperature (RT), and then incubated with blocking solution (10% donkey serum +0.5%; Triton-X-100 in 0.05 M TBS, pH 7.2) for 2 h at RT. The primary antibodies (Table S2) were diluted in 10% donkey serum blocking solution, incubated overnight at 4°C and then rinsed three times with 0.05 M TBS. Secondary antibodies (Table S2) matching the appropriate species were added and incubated for 2-4 h at RT. Fluorescently stained sections were then washed three times with 0.05 M TBS. This was followed by 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, 200 ng/ml) staining for 5 min, and the sections were then cover-slipped with Gel/Mount (Biomeda).
In situ RNA hybridization
ISH was performed on 20-μm cryosections using digoxigenin riboprobes as previously described (Liu et al., 2018). Probes were made from P0 wild-type mouse brain cDNA amplified by PCR (see Table S3).
RNA-seq
RNA-seq analysis was performed as previously described (Liu et al., 2018). The LGEs of E14.5 Meis2-CKO and littermate control mice were dissected (n=4). Gene expression levels were reported as fragments per kilobase of exon per million fragments mapped (FPKM). Genes with a P-value <0.05 were considered to be differentially expressed.
Bulk ATAC-seq
The ATAC assay was performed using dissected E14.5 LGEs of wild-type mice and Meis2-CKO mice (n=3 per group). The tissues were harvested and the cells were counted. Then, 5×104 cells were centrifuged for 5 min at 500 g at 4°C. The supernatant was removed and discarded. The cells were washed once with 50 μl of cold PBS buffer and centrifuged for 5 min at 500 g at 4°C. The supernatant was removed and discarded. A pipet was used to gently resuspend the cell pellet in 50 μl of cold lysis buffer [10 mM Tris Cl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% (v/v) Igepal CA-630, stored for up to 1 week at 4°C]. The mixture was centrifuged immediately for 10 min at 500 g at 4°C. The supernatant was discarded and the transposition reaction was immediately continued. The cell pellet was placed on ice. To make the transposition reaction mix, the following reagents were combined: 10 μl 5× TTBL (Vazyme TD501 kit), 5 μl TTE Mix V50 (Vazyme TD501 kit) and 35 μl nuclease-free H2O. The nuclear pellet was resuspended in the transposition reaction mix and incubated at 37°C for 30 min. Immediately following transposition, the samples was purified using VAHTS DNA Cleans Beads. The transposed DNA was eluted in 25 μl ddH2O. To amplify transposed DNA fragments, the following reagents were combined in a 0.2 ml PCR tube: 24 μl transposed DNA; 10 μl 5× TAB (Vazyme TD501 kit); 5 μl PCR Primer Mix (PPM) (Vazyme TD501 kit); 5 μl P5 Primer X (Vazyme TD202 kit); 5 μl P7 Primer X (Vazyme TD202 kit); 1 μl TAE (Vazyme TD501 kit). The reaction was performed with a thermal cycler as follows: 72°C for 3 min; 98°C for 30 s; 12 cycles of 98°C for 15 s, 60°C for 30 s and 72°C for 30 s; 72°C for 5 min and hold at 4°C. To purify the PCR products, 1.2× volumes of VAHTS DNA Clean Beads (Vazyme) were added and incubated at RT for 10 min. The libraries were washed twice with 80% ethanol and eluted in 22 μl of ddH2O. The libraries were sequenced on an Illumina NovaSeq platform, and 150-bp paired-end reads were generated.
scATAC-seq
Nuclei were isolated and washed according to the instructions supplied by 10x Genomics [Nuclei Isolation for Single Cell ATAC Sequencing (CG000169)]. The isolated nuclei were resuspended in chilled diluted nuclei buffer (10x Genomics; 2000153) at a volume based on the number of starting cells and the final target concentration of nuclei. A Countstar instrument (Rigel S2) was used to count the nuclei, which were immediately used to generate single-cell ATAC-seq libraries. Following the 10x Genomics instructions for the scATAC solution, the Chromium Chip E Single Cell Kit (1000156) and the Chromium Single Cell ATAC Library and Gel Bead Kit (1000110), the nuclei in the bulk sample were partitioned into nanoliter-scale gel beads-in-emulsion; a pool of ∼750,000 109 barcodes was sampled to separately and uniquely index the transposed DNA of each individual nucleus, and libraries were generated (by CapitalBio Technology). The libraries were sequenced using an Illumina Nova-seq sequencer with a sequencing depth of at least 25,000 read pairs per nucleus with a paired-end 50-bp reading strategy. The Cell Ranger ATAC 1.2.0 pipeline and the mm10 reference genome were downloaded from the 10x Genomics website (https://support.10xgenomics.com/single-cell-atac/software/downloads/latest). Raw sequencing data were converted to fastq format using cell rangeratac mkfastq.6806 cells. Cells with pct_reads_in_peaks >40, peak_region_fragments >3000 and <80,000, TSS.enrichment >2.5, blacklist_ratio <0.01 and nucleosome_signal <4 were also filtered out. Peak calling, peak annotation, clustering visualization, TF motif enrichment analysis and differential accessibility analysis were performed using the Signac package (https://satijalab.org/signac/index.html). We generated an scATAC-seq dataset from 1219 cells passing quality control criteria from four embryos of one litter. Next, we removed the striatal interneurons (markers: Nkx2.1, Lhx6 and Sst), septum neurons (markers: Zic1, Zic2 and Zic4) and cortical projection neurons (Tbr1, Pax6 and Neurod1) according to the mRNA expression profile. We finally obtained 849 cells. Dimensionality was reduced using Latent Semantic Indexing (LSI), and the top 2-30 principal components were used to generate clusters by calculating k-nearest neighbors and constructing the shared nearest neighbor (SNN) graph, which was visualized via Uniform Manifold Approximation and Projection (UMAP).
For analysis of cell lineages trajectory, we used a cell lineage inference algorithm, Slingshot (version 1.2.0, https://bioconductor.org/packages/slingshot/), to predict lineage trajectories and bifurcations by ordering cells along trajectories. Slingshot takes as input a matrix of gene activity from Signac. Lineages are defined by ordered sets of clusters beginning with the root node and terminating in the most distal cluster(s) with only one connection. Potential fitting curves are drawn to the subsets of cells that potentially make up each lineage. The ordering provided by Slingshot, analogous to pseudodevelopmental time points, is referred to here as developmental order. The cluster representing radial glial cells (RGCs) was chosen as the starting root node. The most variable genes among all single cells were identified by Seurat. A pseudodevelopmental timeline of single cells was calculated using the Slingshot package, using the most variable genes as time-ordering genes. We mainly used the Signac package code described in the supplementary Materials and Methods.
CUT&Tag-seq
The CUT&Tag assay was performed using E14.5 LGEs dissected from wild-type CD1 mice and a MEIS2 mouse polyclonal antibody as described previously (Kaya-Okur et al., 2020). Briefly, the cells were isolated from the LGE. Then, 1×105 cells were washed with 500 μl of wash buffer (Vazyme, TD901) [10× wash buffer (-), 50× protease inhibitor cocktail, ddH2O] and centrifuged at 600 g for 3 min at RT. The cell pellets were resuspended in 100 μl of wash buffer. Concanavalin A-coated magnetic beads were washed twice with binding buffer (Vazyme, TD901) (10× binding buffer, ddH2O). Next, 10 μl of activated beads were added and incubated at RT for 10 min. Bead-bound cells were resuspended in 50 μl of antibody buffer [10× wash buffer (-), 50× protease inhibitor cocktail, ddH2O, 5% digitonin, 0.5 M EDTA, 30% bovine serum albumin]. Then, 1 μg of primary antibody (mouse polyclonal anti-MEIS2 antibody; Table S2) or nothing was added and incubated at RT for 2 h. The primary antibody was removed using a magnet stand. Secondary antibody (1 μg, goat anti-mouse; Vazyme, S602-01) was diluted in 50 μl of Dig-wash buffer [10× wash buffer (-), 50× protease inhibitor cocktail, ddH2O, 5% digitonin] and cells were incubated at RT for 1 h. Cells were washed three times with Dig-wash buffer to remove unbound antibodies. The Hyperactive pG-Tn5 Transposase adaptor complex (TTE mix, 4 μM, Vazyme) was diluted 1:100 in 100 μl of Dig-300 buffer [10× Dig-300 buffer (-), 5% digitonin, 50× protease inhibitor cocktail, ddH2O]. The cells were incubated with 0.04 μM TTE mix at room temperature for 1 h. The cells were washed three times with Dig-300 buffer to remove unbound TTE mix. The cells were then resuspended in 300 μl of tagmentation buffer (10 mM MgCl2 in Dig-300 buffer) and incubated at 37°C for 1 h. To terminate tagmentation, 10 μl of 0.5 M EDTA, 3 μl of 10% SDS and 2.5 μl of 20 mg ml−1 Proteinase K were added to 300 μl of sample and incubated overnight at 37°C. DNA was purified using phenol-chloroform-isoamyl alcohol extraction and ethanol precipitation with RNase A treatment. For library amplification, 24 μl of DNA was mixed with 1 μl of TruePrep Amplify Enzyme (TAE, Vazyme), 10 μl of 5× TruePrep Amplify Enzyme Buffer and 5 μl of ddH2O, and 5 μl of uniquely barcoded i5 and i7 primers from TruePrep Index Kit V2 for Illumina (Vazyme). A total of 50 μl of sample was placed in a thermocycler and subjected to the following program: 72°C for 3 min; 98°C for 30 s; 17 cycles of 98°C for 15 s, 60°C for 30 s and 72°C for 30 s; 72°C for 5 min and hold at 4°C. To purify the PCR products, 1.2× volumes of VAHTS DNA Clean Beads (Vazyme) were added and incubated at RT for 10 min. The libraries were washed twice with 80% ethanol and eluted in 22 μl of ddH2O. The libraries were sequenced on an Illumina NovaSeq platform, and 150-bp paired-end reads were generated.
All raw sequence data were quality trimmed to a minimum Phred score of 20 using Trimmomatic. Apparent PCR duplicates were removed using Picard MarkDuplicates vl.107. All reads produced by CUT&Tag of MEIS2 were aligned to the mm10 mouse genome using Bowtie2 version 2.3.4. Sequence tags were aligned to the genome and then subsequently analyzed by MACS2 software version 2.1.4 to detect genomic regions enriched for multiple overlapping DNA fragments (peaks) that we considered to be putative binding sites. Peaks with a false discovery rate lower than 5% were saved to detect chromosomal regions for further analyses. Visualization of peak distribution along genomic regions of genes of interest was performed with the Integrative Genomics Viewer (IGV).
Dual-luciferase assays
The DNA fragments of the hs355, hs599 and R1 enhancers near the Meis2 gene were created by PCR and subsequently cloned into the pGL4.23 firefly luciferase vector (Promega) upstream of the Luc2 gene. Cells from the mouse embryonal carcinoma cell line N2a were grown in medium MEMa (Gibco, 12571063) supplemented with 10% fetal bovine serum (FBS) (Gibco, 10099141). For the luciferase assay, N2a cell transfections were performed in triplicate in 24-well plates using Fugene HD transfection reagent according to the manufacturer's protocol (Promega, E2311). Luciferase activity was quantified by a microplate luminometer (Turner BioSystems, Modulus microplate reader).
The primers used for amplifying the putative Meis2 enhancers were as follows: hs599 Fwd 5′-AGCAACATCTCTCCAAGCCACCTGT-3′; hs599 Rev 5′-TCAGGGACCACCTTCTTGACACCGA-3′; hs355 Fwd 5′-GCACCTCTTTGTACACAGATATTTAAACATGGC-3′; hs355 Rev 5′-ATACGCACAGAGGTCATTTCAAAAACCCTTATAG-3′; R1 Fwd 5′-AAGCACTCCAGCCTGAATCCACAGTCCTCAGGAT-3′; and R1 Rev 5′-CCCAAGCTTGTTCTGTACTCCTTCAGGGTTTACAGGGA-3′.
The Six3 promoter and Zfp503 promoter were amplified by PCR and cloned into the pGL4.10 firefly luciferase vector (Promega) upstream (U) of the Luc2 gene. The primers used for amplifying the putative promoter were as follows: Six3 promoter 1: Fwd 5′-TTCTGTTTCGCCCTCTTCTCCCTC-3′, Rev 5′-TGGAAAGGAGGGGGGAGCAGAAG-3′; Six3 promoter 2: Fwd 5′-CACTGTGGATTTAGGGGAGATATTATG-3′, Rev 5′-AGGAGGAAGGACGTAAGGGACA-3′; Zfp503 promoter: Fwd 5′-AGGCCTTTGGGCTGGCCTGGGTTCCT-3′, Rev 5′-CCCCTCCTCCGCCTCTCAGCCACTCT-3′.
Image acquisition and statistical analysis
Images for quantitative analyses were acquired using an Olympus VS 120 microscope using a 20× objective. Bright-field images were acquired using an Olympus VS 120 microscope using a 10× objective. Images were merged, cropped and optimized in Adobe Photoshop CC without distorting the original information. Analyses were performed using GraphPad Prism 6.0, Microsoft Excel and R language. Unpaired two-tailed t-test was used to determine statistical significance. All quantification results are presented as the mean±s.e.m. Differences with P-values <0.05 were considered significant. For quantification of GSX2+, ASCL1+, PCNA+, DLX2+, CRE+ (DLX5/6), SP9+ and SP8+ cells in the LGE SVZ at E16.5, four anatomically matched 6-μm thick coronal sections were selected (n=4 mice per group). We counted GSX2+, ASCL1+, PCNA+, DLX2+, CRE+ (DLX5/6), SP8+ and SP9+ cells in the LGE SVZ under a 20× objective lens. The LGE SVZ was delineated by DAPI staining. The data of GSX2+, ASCL1+, PCNA+, DLX2+, CRE+ (DLX5/6), SP9+ and SP8+ cells per section for each LGE SVZ were presented as the relative ratio of the control group.
For quantification of TUBB3+, DCX+ and MEIS2+ cells in the LGE SVZ at E16.5, four anatomically matched 6-μm thick coronal sections were selected (n=4 mice per group). We used the histogram tool in Adobe Photoshop CC to calculate the fluorescence intensity of TUBB3+ and DCX+ in the LGE SVZ. Data were presented in proportion to the control group. For quantitative analysis of in situ hybridization with Gad1 probes, the Gad1 signal intensity in the LGE SVZ was analyzed using ImageJ software.
For quantification of Sp8+, CB+, TH+ and Pax6+ cells in the control and Meis2-CKO mice, 20 μm sections from the OB were analyzed. Between two and five random regions (500×500 pix) per OB section and four sections per animal were counted using 20× objective on an Olympus VS120 microscope. We analyzed four mice per group.
For quantification of FOXP1+, MEIS2+ and FOXP1/MEIS2 double-positive cells in the mouse striatum at P30, four 20-μm thick coronal sections from rostral, intermediate and caudal levels of the striatum were selected (n=4 mice per group). We used ImageJ to count the number of FOXP1+, MEIS2+ and FOXP1/MEIS2 double-positive cells in each striatum. Brain regions were identified using a mouse brain atlas, and sections equivalent to the following bregma coordinates were taken (in mm): the most-rostral section, 1.18; and the most-caudal section, −0.10. For quantification of KI67+, PH3+ and BrdU+ cells in the mouse LGE SVZ at E16.5, four anatomically matched 10-μm thick coronal sections were selected (n=4 mice per group). We counted KI67+, PH3+ and BrdU+ cells in the LGE SVZ under a 20× objective lens. The LGE SVZ was delineated by DAPI staining.
Supplementary Material
Acknowledgements
We are grateful to Kenneth Campbell at University of Cincinnati College of Medicine for the Dlx5/6-CIE mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Z.Z.; Methodology: Z. Su., Z.W., Z.Z.; Software: L.Y.; Validation: Z. Su, Z.W., Z. Shang, Y.T., R.G., Y.Y., W.Z.; Data curation: L.Y., R.G., Y.Y.; Writing - review & editing: Z.Z., J.L.R., Z.Y.; Visualization: S.L.; Supervision: Z.Z., J.L.R., Z.Y. Project administration: S.L., L.Y., Z. Shang., Y.T., R.G. and Y.Y.; Funding acquisition: J.L.R., Z.Y.
Funding
This work was supported by research grants to Z.Y. from National Key Research and Development Program of China (2018YFA0108000), National Natural Science Foundation of China (31630032 and 31820103006) and Science and Technology Commission of Shanghai Municipality Major Project (2018SHZDZX01), and a research grant to J.L.R. (R01 MH049428) from the National Institute of Mental Health. Deposited in PMC for release after 12 months.
References
- Anderson, S. A., Qiu, M., Bulfone, A., Eisenstat, D. D., Meneses, J., Pedersen, R. and Rubenstein, J. L. R. (1997). Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19, 27-37. 10.1016/S0896-6273(00)80345-1 [DOI] [PubMed] [Google Scholar]
- Arlotta, P., Molyneaux, B. J., Jabaudon, D., Yoshida, Y. and Macklis, J. D. (2008). Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J. Neurosci. 28, 622-632. 10.1523/JNEUROSCI.2986-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banan, M. (2020). Recent advances in CRISPR/Cas9-mediated knock-ins in mammalian cells. J. Biotechnol. 308, 1-9. 10.1016/j.jbiotec.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Berenguer, M. and Duester, G. (2021). Role of retinoic acid signaling, FGF signaling and Meis genes in control of limb development. Biomolecules 11, 80. 10.3390/biom11010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi, V. D., Conforti, P., Vezzoli, E., Besusso, D., Cappadona, C., Lischetti, T., Galimberti, M., Ranzani, V., Bonnal, R. J. P., De Simone, M.et al. (2021). The coding and long noncoding single-cell atlas of the developing human fetal striatum. Science 372, eabf5759. 10.1126/science.abf5759 [DOI] [PubMed] [Google Scholar]
- Castro, D. S., Martynoga, B., Parras, C., Ramesh, V., Pacary, E., Johnston, C., Drechsel, D., Lebel-Potter, M., Garcia, L. G., Hunt, C.et al. (2011). A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25, 930-945. 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, H., Waclaw, R. R., Pei, Z., Nakafuku, M. and Campbell, K. (2013). The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 140, 2289-2298. 10.1242/dev.091090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi, C., Brade, T. and Duester, G. (2011). Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol. 9, e1000609. 10.1371/journal.pbio.1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.-Y., Lu, K.-M., Ko, H.-A., Huang, T.-H., Hao, J.-H., Yan, Y.-T., Chang, S. L.-Y., Evans, S. M. and Liu, F.-C. (2020). Parcellation of the striatal complex into dorsal and ventral districts. Proc. Natl. Acad. Sci. USA 117, 7418-7429. 10.1073/pnas.1921007117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin, J. G., Gaiano, N., Machold, R. P., Langston, A. and Fishell, G. (2000). The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 127, 5007-5020. 10.1242/dev.127.23.5007 [DOI] [PubMed] [Google Scholar]
- Corbin, J. G., Rutlin, M., Gaiano, N. and Fishell, G. (2003). Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development 130, 4895-4906. 10.1242/dev.00717 [DOI] [PubMed] [Google Scholar]
- Cunningham, T. J. and Duester, G. (2015). Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 16, 110-123. 10.1038/nrm3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, J. P., Dougherty, J. D., Heiman, M., Schmidt, E. F., Stevens, T. R., Ma, G., Bupp, S., Shrestha, P., Shah, R. D., Doughty, M. L.et al. (2008). Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749-762. 10.1016/j.cell.2008.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman, J. T. and Krakauer, J. W. (2016). The basal ganglia: from motor commands to the control of vigor. Curr. Opin. Neurobiol. 37, 158-166. 10.1016/j.conb.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Ehrman, L. A., Mu, X., Waclaw, R. R., Yoshida, Y., Vorhees, C. V., Klein, W. H. and Campbell, K. (2013). The LIM homeobox gene Isl1 is required for the correct development of the striatonigral pathway in the mouse. Proc. Natl. Acad. Sci. USA 110, E4026-E4035. 10.1073/pnas.1308275110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel Darbandi, S., Poitras, L., Monis, S., Lindtner, S., Yu, M., Hatch, G., Rubenstein, J. L. and Ekker, M. (2016). Functional consequences of I56ii Dlx enhancer deletion in the developing mouse forebrain. Dev. Biol. 420, 32-42. 10.1016/j.ydbio.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Garel, S., Marin, F., Grosschedl, R. and Charnay, P. (1999). Ebf1 controls early cell differentiation in the embryonic striatum. Development 126, 5285-5294. 10.1242/dev.126.23.5285 [DOI] [PubMed] [Google Scholar]
- Gehring, W. J., Affolter, M. and Bürglin, T. (1994). Homeodomain proteins. Annu. Rev. Biochem. 63, 487-526. 10.1146/annurev.bi.63.070194.002415 [DOI] [PubMed] [Google Scholar]
- Gerfen, C. R. and Surmeier, D. J. (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441-466. 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., Monsma, F. J., Jr and Sibley, D. R. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429-1432. 10.1126/science.2147780 [DOI] [PubMed] [Google Scholar]
- Giliberti, A., Currò, A., Papa, F. T., Frullanti, E., Ariani, F., Coriolani, G., Grosso, S., Renieri, A. and Mari, F. (2020). MEIS2 gene is responsible for intellectual disability, cardiac defects and a distinct facial phenotype. Eur. J. Med. Genet. 63, 103627. 10.1016/j.ejmg.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Gokce, O., Stanley, G. M., Treutlein, B., Neff, N. F., Camp, J. G., Malenka, R. C., Rothwell, P. E., Fuccillo, M. V., Südhof, T. C. and Quake, S. R. (2016). Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Rep. 16, 1126-1137. 10.1016/j.celrep.2016.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golonzhka, O., Nord, A., Tang, P. L. F., Lindtner, S., Ypsilanti, A. R., Ferretti, E., Visel, A., Selleri, L. and Rubenstein, J. L. R. (2015). Pbx regulates patterning of the cerebral cortex in progenitors and postmitotic neurons. Neuron 88, 1192-1207. 10.1016/j.neuron.2015.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner, S., Robertson, B. and Stephenson-Jones, M. (2013). The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J. Physiol. 591, 5425-5431. 10.1113/jphysiol.2012.246660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida, T., Kimura, K., Wada, N., Funabiki, K. and Nakanishi, S. (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896-907. 10.1016/j.neuron.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Hintiryan, H., Foster, N. N., Bowman, I., Bay, M., Song, M. Y., Gou, L., Yamashita, S., Bienkowski, M. S., Zingg, B., Zhu, M.et al. (2016). The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100-1114. 10.1038/nn.4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, S., Meredith, A., Richardson, J. A. and Johnson, J. E. (1999). Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol. Cell. Neurosci. 14, 355-369. 10.1006/mcne.1999.0791 [DOI] [PubMed] [Google Scholar]
- Kaya-Okur, H. S., Janssens, D. H., Henikoff, J. G., Ahmad, K. and Henikoff, S. (2020). Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 15, 3264-3283. 10.1038/s41596-020-0373-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. M., Raudales, R., He, M., Lee, J. H., Kim, Y., Gibb, L. G., Wu, P., Matho, K., Osten, P., Graybiel, A. M.et al. (2018). Radial Glial lineage progression and differential intermediate progenitor amplification underlie striatal compartments and circuit organization. Neuron 99, 345-361.e344. 10.1016/j.neuron.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, K. B., Lutterodt, M. C., Laursen, H., Graem, N., Pakkenberg, B., Møllgård, K. and Møller, M. (2010). Spatiotemporal distribution of PAX6 and MEIS2 expression and total cell numbers in the ganglionic eminence in the early developing human forebrain. Dev. Neurosci. 32, 149-162. 10.1159/000297602 [DOI] [PubMed] [Google Scholar]
- Li, J., Wang, C., Zhang, Z., Wen, Y., An, L., Liang, Q., Xu, Z., Wei, S., Li, W., Guo, T.et al. (2017). Transcription factors Sp8 and Sp9 coordinately regulate olfactory bulb interneuron development. Cereb. Cortex 28, 3278-3294. 10.1093/cercor/bhx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Liu, G., Yang, L., Li, Z., Zhang, Z., Xu, Z., Cai, Y., Du, H., Su, Z., Wang, Z.et al. (2021). Decoding cortical glial cell development. Neurosci. Bull. 37, 440-460. 10.1007/s12264-021-00640-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, W.-L., Tsai, H.-C., Wang, H.-F., Chang, J., Lu, K.-M., Wu, H.-L., Lee, Y.-C., Tsai, T.-F., Takahashi, H., Wagner, M.et al. (2008). Modular patterning of structure and function of the striatum by retinoid receptor signaling. Proc. Natl. Acad. Sci. USA 105, 6765-6770. 10.1073/pnas.0802109105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindtner, S., Catta-Preta, R., Tian, H., Su-Feher, L., Price, J. D., Dickel, D. E., Greiner, V., Silberberg, S. N., McKinsey, G. L., McManus, M. T.et al. (2019). Genomic resolution of DLX-orchestrated transcriptional circuits driving development of forebrain GABAergic neurons. Cell Reports 28, 2048-2063.e2048. 10.1016/j.celrep.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. K., Ghattas, I., Liu, S. Y., Chen, S. and Rubenstein, J. L. R. (1997). Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dynam 210, 498-512. [DOI] [PubMed] [Google Scholar]
- Liu, Z., Zhang, Z., Lindtner, S., Li, Z., Xu, Z., Wei, S., Liang, Q., Wen, Y., Tao, G., You, Y.et al. (2018). Sp9 regulates medial ganglionic eminence-derived cortical interneuron development. Cereb. Cortex 29, 2653-2667. 10.1093/cercor/bhy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, M. K., Karsten, S. L., Gray, M., Geschwind, D. H. and Yang, X. W. (2006). FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat. Neurosci. 9, 443-452. 10.1038/nn1654 [DOI] [PubMed] [Google Scholar]
- Lobo, M. K., Yeh, C. and Yang, X. W. (2008). Pivotal role of early B-cell factor 1 in development of striatonigral medium spiny neurons in the matrix compartment. J. Neurosci. Res. 86, 2134-2146. 10.1002/jnr.21666 [DOI] [PubMed] [Google Scholar]
- Long, J. E., Cobos, I., Potter, G. B. and Rubenstein, J. L. R. (2009a). Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex 19 Suppl. 1, i96-i106. 10.1093/cercor/bhp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J. E., Swan, C., Liang, W. S., Cobos, I., Potter, G. B. and Rubenstein, J. L. R. (2009b). Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J. Comp. Neurol. 512, 556-572. 10.1002/cne.21854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, K.-M., Evans, S. M., Hirano, S. and Liu, F.-C. (2014). Dual role for Islet-1 in promoting striatonigral and repressing striatopallidal genetic programs to specify striatonigral cell identity. Proc. Natl. Acad. Sci. USA 111, E168-E177. 10.1073/pnas.1319138111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon, O., Masek, J., Machonova, O., Krauss, S. and Kozmik, Z. (2015). Meis2 is essential for cranial and cardiac neural crest development. BMC Dev. Biol. 15, 40. 10.1186/s12861-015-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor, M. M., McKinsey, G. L., Girasole, A. E., Bair-Marshall, C. J., Rubenstein, J. L. R. and Nelson, A. B. (2019). Functionally distinct connectivity of developmentally targeted striosome neurons. Cell Rep. 29, 1419-1428.e1415. 10.1016/j.celrep.2019.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens, C. B. and Selleri, L. (2006). Hox cofactors in vertebrate development. Dev. Biol. 291, 193-206. 10.1016/j.ydbio.2005.10.032 [DOI] [PubMed] [Google Scholar]
- Molotkova, N., Molotkov, A. and Duester, G. (2007). Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 303, 601-610. 10.1016/j.ydbio.2006.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, K. and Bürglin, T. R. (2007). Comprehensive analysis of animal TALE homeobox genes: new conserved motifs and cases of accelerated evolution. J. Mol. Evol. 65, 137-153. 10.1007/s00239-006-0023-0 [DOI] [PubMed] [Google Scholar]
- Nord, A. S., Pattabiraman, K., Visel, A. and Rubenstein, J. L. R. (2015). Genomic perspectives of transcriptional regulation in forebrain development. Neuron 85, 27-47. 10.1016/j.neuron.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulad-Abdelghani, M., Chazaud, C., Bouillet, P., Sapin, V., Chambon, P. and Dollé, P. (1997). Meis2, a novel mouse Pbx-related homeobox gene induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Dev. Dyn. 210, 173-183. [DOI] [PubMed] [Google Scholar]
- Paige, S. L., Thomas, S., Stoick-Cooper, C. L., Wang, H., Maves, L., Sandstrom, R., Pabon, L., Reinecke, H., Pratt, G., Keller, G.et al. (2012). A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151, 221-232. 10.1016/j.cell.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z., Wang, B., Chen, G., Nagao, M., Nakafuku, M. and Campbell, K. (2011). Homeobox genes Gsx1 and Gsx2 differentially regulate telencephalic progenitor maturation. Proc. Natl Acad. Sci. USA 108, 1675-1680. 10.1073/pnas.1008824108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, A. J., Fabre, J. M. J., Steinmetz, N. A., Harris, K. D. and Carandini, M. (2021). Striatal activity topographically reflects cortical activity. Nature 591, 420-425. 10.1038/s41586-020-03166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg, S. N., Taher, L., Lindtner, S., Sandberg, M., Nord, A. S., Vogt, D., McKinsey, G. L., Hoch, R., Pattabiraman, K., Zhang, D.et al. (2016). Subpallial enhancer transgenic lines: a data and tool resource to study transcriptional regulation of GABAergic cell fate. Neuron 92, 59-74. 10.1016/j.neuron.2016.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleilhavoup, C., Travaglio, M., Patrick, K., Garcao, P., Boobalan, E., Adolfs, Y., Spriggs, R. V., Moles-Garcia, E., Dhiraj, D., Oosterveen, T.et al. (2020). Nolz1 expression is required in dopaminergic axon guidance and striatal innervation. Nat. Commun. 11, 3111. 10.1038/s41467-020-16947-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., Chen, H., Shang, Z., Du, H., Li, Z., Wen, Y., Liu, G., Qi, D., You, Y., Yang, Z.et al. (2021). Homeobox gene Six3 is required for the differentiation of D2-type medium spiny neurons. Neurosci. Bull. 37, 985-998. 10.1007/s12264-021-00698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, G., Gokce, O., Malenka, R. C., Südhof, T. C. and Quake, S. R. (2019). Continuous and discrete neuron types of the adult murine striatum. Neuron 105, 688-699.e688. 10.1016/j.neuron.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Toresson, H., Mata de Urquiza, A., Fagerstrom, C., Perlmann, T. and Campbell, K. (1999). Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development 126, 1317-1326. 10.1242/dev.126.6.1317 [DOI] [PubMed] [Google Scholar]
- Urban, N., Martin-Ibanez, R., Herranz, C., Esgleas, M., Crespo, E., Pardo, M., Crespo-Enriquez, I., Mendez-Gomez, H. R., Waclaw, R., Chatzi, C.et al. (2010). Nolz1 promotes striatal neurogenesis through the regulation of retinoic acid signaling. Neural Dev. 5, 21. 10.1186/1749-8104-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent, E. and Gangarossa, G. (2020). The tail of the striatum: from anatomy to connectivity and function. Trends Neurosci. 44, 203-214. 10.1016/j.tins.2020.10.016 [DOI] [PubMed] [Google Scholar]
- van Rhijn, J.-R., Fisher, S. E., Vernes, S. C. and Nadif Kasri, N. (2018). Foxp2 loss of function increases striatal direct pathway inhibition via increased GABA release. Brain Struct. Funct. 223, 4211-4226. 10.1007/s00429-018-1746-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheije, R., Kupchik, G. S., Isidor, B., Kroes, H. Y., Lynch, S. A., Hawkes, L., Hempel, M., Gelb, B. D., Ghoumid, J., D'Amours, G.et al. (2019). Heterozygous loss-of-function variants of MEIS2 cause a triad of palatal defects, congenital heart defects, and intellectual disability. Eur. J. Hum. Genet. 27, 278-290. 10.1038/s41431-018-0281-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel, A., Taher, L., Girgis, H., May, D., Golonzhka, O., Hoch, R. V., McKinsey, G. L., Pattabiraman, K., Silberberg, S. N., Blow, M. J.et al. (2013). A high-resolution enhancer atlas of the developing telencephalon. Cell 152, 895-908. 10.1016/j.cell.2012.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw, R. R., Wang, B. and Campbell, K. (2004). The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development 131, 4013-4020. 10.1242/dev.01272 [DOI] [PubMed] [Google Scholar]
- Waclaw, R. R., Allen, Z. J., II, Bell, S. M., Erdelyi, F., Szabo, G., Potter, S. S. and Campbell, K. (2006). The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron 49, 503-516. 10.1016/j.neuron.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Waclaw, R. R., Wang, B., Pei, Z., Ehrman, L. A. and Campbell, K. (2009). Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron 63, 451-465. 10.1016/j.neuron.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Waclaw, R. R., Allen, Z. J., II, Guillemot, F. and Campbell, K. (2009). Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev. 4, 5. 10.1186/1749-8104-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Lufkin, T. and Rubenstein, J. L. R. (2011). Dlx6 regulates molecular properties of the striatum and central nucleus of the amygdala. J. Comp. Neurol. 519, 2320-2334. 10.1002/cne.22618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Long, J. E., Flandin, P., Pla, R., Waclaw, R. R., Campbell, K. and Rubenstein, J. L. R. (2013). Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J. Comp. Neurol. 521, 1561-1584. 10.1002/cne.23242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Tang, Q., Xu, J., Li, H., Yang, T., Li, L., Machon, O., Hu, T. and Chen, Y. (2020). The transcriptional regulator MEIS2 sets up the ground state for palatal osteogenesis in mice. J. Biol. Chem. 295, 5449-5460. 10.1074/jbc.RA120.012684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S., Du, H., Li, Z., Tao, G., Xu, Z., Song, X., Shang, Z., Su, Z., Chen, H., Wen, Y.et al. (2019). Transcription factors Sp8 and Sp9 regulate the development of caudal ganglionic eminence-derived cortical interneurons. J. Comp. Neurol. 527, 2860-2874. 10.1002/cne.24712 [DOI] [PubMed] [Google Scholar]
- Wen, Y., Su, Z., Wang, Z., Yang, L., Liu, G., Shang, Z., Duan, Y., Du, H., Li, Z., You, Y.et al. (2021). Transcription factor VAX1 regulates the regional specification of the subpallium through repressing Gsx2. Mol. Neurobiol. 58, 3729-3744. 10.1007/s12035-021-02378-x [DOI] [PubMed] [Google Scholar]
- Xiao, X., Deng, H., Furlan, A., Yang, T., Zhang, X., Hwang, G.-R., Tucciarone, J., Wu, P., He, M., Palaniswamy, R.et al. (2020). A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183, 211-227.e220. 10.1016/j.cell.2020.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Liang, Q., Song, X., Zhang, Z., Lindtner, S., Li, Z., Wen, Y., Liu, G., Guo, T., Qi, D.et al. (2018). Sp8 and Sp9 coordinately promote D2-type medium spiny neuron production by activating Six3 expression. Development 145, dev165456. 10.1242/dev.165456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., Li, Z., Liu, G., Li, X. and Yang, Z. (2021a). Developmental origins of human cortical oligodendrocytes and astrocytes. Neurosci. Bull. 38, 47-68. 10.1007/s12264-021-00759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., Su, Z., Wang, Z., Li, Z., Shang, Z., Du, H., Liu, G., Qi, D., Yang, Z., Xu, Z.et al. (2021b). Transcriptional profiling reveals the transcription factor networks regulating the survival of striatal neurons. Cell Death Dis. 12, 262-262. 10.1038/s41419-021-03552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, K., Potter, S. and Rubenstein, J. L. (2001). Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128, 193-205. 10.1242/dev.128.2.193 [DOI] [PubMed] [Google Scholar]
- Yun, K., Fischman, S., Johnson, J., Hrabe de Angelis, M., Weinmaster, G. and Rubenstein, J. L. R. (2002). Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development 129, 5029-5040. 10.1242/dev.129.21.5029 [DOI] [PubMed] [Google Scholar]
- Zha, Y., Xia, Y., Ding, J., Choi, J.-H., Yang, L., Dong, Z., Yan, C., Huang, S. and Ding, H.-F. (2014). MEIS2 is essential for neuroblastoma cell survival and proliferation by transcriptional control of M-phase progression. Cell Death Dis. 5, e1417. 10.1038/cddis.2014.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Zhang, Y., Wang, C., Xu, Z., Liang, Q., An, L., Li, J., Liu, Z., You, Y., He, M.et al. (2016). The zinc finger transcription factor Sp9 Is required for the development of striatopallidal projection neurons. Cell Rep. 16, 1431-1444. 10.1016/j.celrep.2016.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Wei, S., Du, H., Su, Z., Wen, Y., Shang, Z., Song, X., Xu, Z., You, Y. and Yang, Z. (2019). Zfhx3 is required for the differentiation of late born D1-type medium spiny neurons. Exp. Neurol. 322, 113055. 10.1016/j.expneurol.2019.113055 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Liu, G., Guo, T., Liang, X. G., Du, H., Yang, L., Bhaduri, A., Li, X., Xu, Z., Zhang, Z.et al. (2020). Cortical neural stem cell lineage progression is regulated by extrinsic signaling molecule Sonic Hedgehog. Cell Rep. 30, 4490-4504.e94. 10.1016/j.celrep.2020.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.