ABSTRACT
Appropriate DNA double-strand break (DSB) and crossover distributions are required for proper meiotic chromosome segregation. Schizosaccharomyces pombe linear element proteins (LinEs) determine DSB hotspots; LinE-bound hotspots form three-dimensional clusters over ∼200 kb chromosomal regions. Here, we investigated LinE configurations and distributions in live cells using super-resolution fluorescence microscopy. We found LinEs form two chromosomal structures, dot-like and linear structures, in both zygotic and azygotic meiosis. Dot-like LinE structures appeared around the time of meiotic DNA replication, underwent dotty-to-linear-to-dotty configurational transitions and disassembled before the first meiotic division. DSB formation and repair did not detectably influence LinE structure formation but failure of DSB formation delayed disassembly. Recombination-deficient LinE missense mutants formed dot-like, but not linear, LinE structures. Our quantitative study reveals a transient form of LinE structures and suggests a novel role for LinE proteins in regulating meiotic events, such as DSB repair. We discuss the relationship of LinEs and the synaptonemal complex in other species.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Double-strand break formation and repair, Schizosaccharomyces pombe, Chromosome dynamics, Linear elements, Meiosis
Summary: S. pombe linear element proteins, which are important for meiotic DNA breakage and recombination, show dynamic configurations, of which the longest form might also act in DSB repair.
INTRODUCTION
Meiosis is a special type of cell division that allows diploid cells to reduce the chromosome number by half to generate haploid gametes essential for sexual reproduction. In meiotic prophase, homologous chromosomes pair and undergo meiotic recombination to exchange genetic material. The physical reciprocal exchange of portions of homologous chromosomes – formation of a crossover – generates recombinant chromosomes that increase the genetic diversity of the gametes to aid long-term evolution. Of immediate importance, crossovers also form the physical connections (chiasmata) between homologous chromosomes that provide tension to ensure that at their centromeres homologous chromosomes segregate properly from each other in the first meiotic nuclear division (MI) (Zickler and Kleckner, 1999; Petronczki et al., 2003; Marston and Amon, 2004). In the absence of crossovers, chromosomes mis-segregate in most species, leading to inviable or disabled progeny.
Meiotic recombination is initiated by programmed DNA double-strand breaks (DSBs). The topoisomerase-like protein Spo11, first identified in budding yeast, contains the active site for meiotic DSB formation in multiple experimental organisms (Keeney et al., 1997; Dernburg et al., 1998; McKim and Hayashi-Hagihara, 1998; Baudat et al., 2000; Cervantes et al., 2000; Bowring et al., 2006; Hartung et al., 2007). In the few species examined, meiotic DSBs are non-randomly distributed and concentrated in short DNA intervals called DSB hotspots (Pan et al., 2011; Fowler et al., 2014; Lange et al., 2016; Nambiar et al., 2019).
DSB hotspot determination is well documented in the fission yeast Schizosaccharomyces pombe. Linear element (LinE) proteins – Rec10, Rec25, Rec27 and Mug20 – are DSB hotspot determinants (Fowler et al., 2013). The latter three LinE proteins colocalize at most, if not all, DSB hotspots with high specificity (enrichments up to 80× the genome median density), whereas Rec10 is distributed more uniformly along chromosomal DNA (Miyoshi et al., 2012; Fowler et al., 2013). Rec10 is necessary for all DSB formation, and the other three LinE proteins are required for DSB formation at nearly all DSB hotspots by stabilizing or activating the DSB-forming complex containing Rec12 (Spo11 homolog in S. pombe) (Ellermeier and Smith, 2005; Martín-Castellanos et al., 2005; Fowler et al., 2013). The strong correlation (r=0.79–0.88) between the chromosomal distribution of LinE protein binding and DSB hotspots, and the requirement for LinE proteins for DSB formation at most hotspots, makes S. pombe one of the best understood models in DSB hotspot-designation studies.
LinE proteins are distantly related to the synaptonemal complex proteins of other species (Colaiácovo et al., 2003; Lorenz et al., 2004; Loidl, 2006; Fowler et al., 2013), but S. pombe has been considered not to form synaptonemal complex in meiosis. Electron microscopic studies of nuclear spreads show that S. pombe forms transient linear-like nuclear structures (LinE structures) that are much shorter than the Saccharomyces cerevisiae synaptonemal complex, which forms continuous end-to-end chromosomal structures (Dresser and Giroux, 1988; Bähler et al., 1993; Molnar et al., 2003; Lorenz et al., 2006). Fluorescence microscopy studies of LinE proteins in nuclear spreads show that LinE proteins form transient dots or short linear LinE structures similar to the structure seen in electron microscopy studies (Lorenz et al., 2004, 2006; Estreicher et al., 2012), but GFP-tagged LinE structures in fixed cells show dot-like foci (Davis et al., 2008; Fowler et al., 2013). However, most of these studies were performed with spread nuclei or fixed cells, which were cultured at higher temperatures (30°C or 34°C) than 25°C, which is often used for S. pombe genetic analyses (Gutz, 1971; Zahn-Zabal and Kohli, 1996; Smith, 2009; Escorcia and Forsburg, 2018). By contrast, the cohesin subunit Rec8 shows dot-like structures in spread nuclei (Davis et al., 2008) and uniform nuclear distribution in fixed cells (Fowler et al., 2013) at 34°C, but long filamentous structures aligned on the chromosome in live cells at 26°C using super-resolution microscopy (Ding et al., 2016). These results indicate that (1) Rec8 forms different configurations under different culture or examination conditions, and (2) that S. pombe chromosomes may form large but more fragile structures that partially disassemble upon cell lysis, nuclear spreading or fixation. These findings indicate the importance of investigating whether LinE proteins form elongated synaptonemal complex-like chromosomal structures in live cells at lower temperatures.
Because the previous studies noted above were carried out under a variety of conditions (Lorenz et al., 2004, 2006; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013), we conducted experiments using a standard condition most nearly wild type [zygotic meiosis in homothallic (h90) live cells at 25°C]. In many cases, we used other conditions concurrently for comparison with previous reports. We used structured-illumination fluorescence microscopy (SIM) to obtain ultra-high resolution of live cells. We confirmed that LinE proteins formed linear structures, like previous studies reported at 30°C (Lorenz et al., 2004; Estreicher et al., 2012), and they underwent dot-to-line-to-dot transitions, showing that there are at least two different LinE structural configurations. The LinE linear structures were found in synchronized azygotic meiosis at 25°C but not at 34°C, and they were sensitive to 1,6-hexanediol treatment, like the synaptonemal complex in other species (Rog et al., 2017; see Discussion). Moreover, we investigated the genetic requirements for intact LinE structure formation and factors that trigger its disassembly. We also found that some recombination-deficient LinE point mutants failed to form elongated LinE linear structures. Our results reveal sequential transitions of LinE-chromatin structure in live cells and suggest that LinE proteins have a function in addition to DSB hotspot-determination and crossover regulation. They also reveal that S. pombe LinE proteins and structures share properties with the synaptonemal complex of other species.
RESULTS
Using live-cell microscopy, we investigated LinE structures in both zygotic and azygotic meiosis but for different purposes. When incubated on sporulation medium, homothallic strains carrying the h90 mating-type allele switch mating type, mate and undergo zygotic meiosis (i.e. meiosis immediately after mating) (Egel, 1977). Although such cultures are not synchronized for meiosis and thus not ideal for phenotypic quantification, they reflect wild-type S. pombe, which is homothallic and undergoes zygotic meiosis. For most phenotypic quantification, we used synchronized cultures of established diploids artificially induced for meiosis (i.e. azygotic meiosis). We used two alleles, pat1-114 (Iino and Yamamoto, 1985) and pat1-as1 (Guerra-Moreno et al., 2012), to synchronize meiotic cultures by raising the temperature (to 34°C) or by adding an ATP analog [4-amino-1-tert-butyl-3-(3-methylbenzyl)pyrazolo[3,4-d]pyrimidine], respectively, to inactivate the Pat1 repressor of meiosis (Yamamoto, 1996). In this study, we used asynchronous zygotic cultures (h90) for qualitative analyses and synchronized azygotic cultures (pat1-114 or pat1-as1) for most quantitative analyses.
LinE proteins form long linear structures in zygotic meiosis at 25°C
We observed LinE morphology in live zygotic cultures (h90 strains) at 25°C using super-resolution microscopy (SIM) and concentrated on cells with elongated nuclei (i.e. in the horsetail stage of meiotic prophase I; Ding et al., 2004). We found that each LinE protein – Rec10, Rec25, Rec27 and Mug20 – showed long linear nuclear structures (Fig. 1A,D; Fig. S1A). The linear structures were similar to the linear structures of Rec8 (Fig. 1C; Fig. S1A; Ding et al., 2016), which also showed the four chromatids were usually closely juxtaposed as they are in the synaptonemal complex in other organisms. It is not feasible to compare directly the morphology of LinE structures found in previous observations using fixed cells at 30°C or 34°C as no quantification of morphology was reported (Lorenz et al., 2004, 2006; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013), but the linear structures we studied here appeared more elongated. Some of the horsetail nuclei showed more dotty LinE foci, suggesting that LinEs may assume different forms in different meiotic stages, as explored below. Our observations are consistent with elongated linear forms of the four LinE proteins in h90 strains at 26°C (Ding et al., 2021) and indicate that the LinE protein complex may frequently form closely juxtaposed chromosomal structures (Ding et al., 2016).
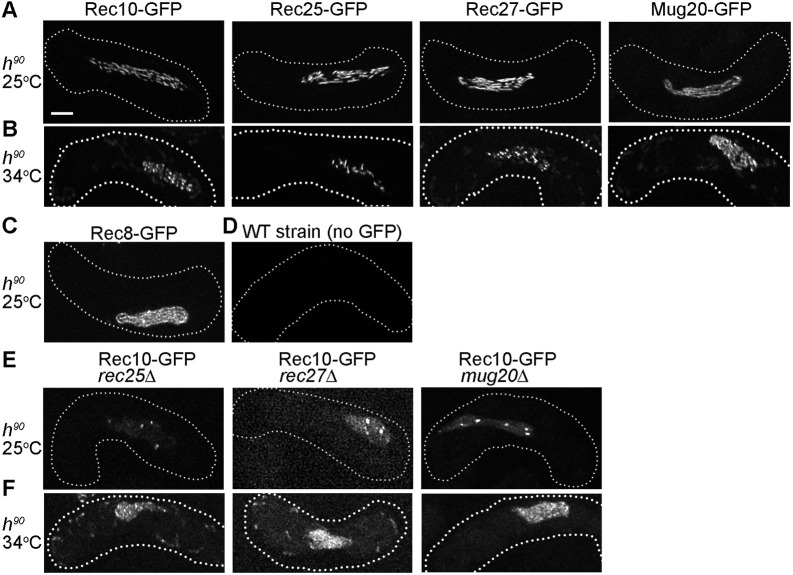
Fig. 1.
LinE proteins form elongated linear structures in live zygotic meiosis. (A-F) Rec10, Rec25, Rec27, Mug20 and Rec8 were observed as GFP fusion proteins in h90 (homothallic) strains using SIM. Images are maximal projections of the z-stack (see Fig. S1). The dotted line represents the cell outline. Each image is representative of ≥20 horsetail stage cells (i.e. meiotic prophase; Ding et al., 2004). (A) All LinE proteins form elongated structures in zygotic meiosis at 25°C. (B) Rec10, Rec27 and Rec25 fail to form linear structures at high temperature (34°C); instead, they form non-continuous structures and often bright nuclear dots. These ‘dotty foci’ differ from the ‘linear structures’ at 25°C (Fig. 1A) but are like previously reported structures in azygotic meiosis at 34°C (Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). Mug20 shows long linear structures at 34°C, like those at 25°C (Fig. 1A; see also Ding et al., 2021). (C) Rec8 forms nearly end-to-end chromosomal structures in zygotic meiosis at 25°C (Ding et al., 2016). (D) Wild-type (WT, no GFP) h90 cells show limited green fluorescence in zygotic meiosis at 25°C. (E) At 25°C in zygotic meiosis of LinE deletion mutants, Rec10 forms a few bright nuclear foci and more nearly uniform nuclear distribution than in wild type (Fig. 1A). Thus, Rec10 still enters the nucleus and forms a few nuclear foci, but not linear structures, in the absence of any other LinE protein. (F) At 34°C in zygotic meiosis of LinE deletion mutants, Rec10 forms a few nuclear foci and nearly uniform nuclear distribution, more prominent than at 25°C. Scale bar: 2 μm.
Previous published studies of LinE structure morphology carried out at 34°C using fixed cells from azygotic cultures (pat1-114 strains) showed that each LinE protein forms only dotty structures (Davis et al., 2008; Fowler et al., 2013). Therefore, we tested whether the LinE linear structures are established at 25°C but not at 34°C in live h90 zygotic cells. In the 34°C cells in the horsetail stage, Rec10, Rec25 and Rec27 showed only dotty structures (Fig. 1B; Fig. S1B). These 34°C dotty structures were dimmer than the linear structures found at 25°C, and they were in non-continuous forms. Mug20 foci still showed elongated linear forms at 34°C but they were fuzzier than those at 25°C. The data indicate that LinE proteins established long linear structures only at 25°C in zygotic meiosis. This outcome contrasts with previously published reports of Rec25 and Rec27, using different conditions noted above (Davis et al., 2008; Fowler et al., 2013), but is consistent with previous studies of Rec10 and Mug20 (Lorenz et al., 2004, 2006; Estreicher et al., 2012; Ding et al., 2021), which formed ‘network’ structures noted below. Our data also showed Rec10, Rec25 and Rec27 linear structures were not found at a high temperature.
Rec10 forms nuclear foci independent of other LinE subunits
Previous studies using fluorescence microscopy of either fixed cells or nuclear spreads showed that nuclear structure-formation by each LinE protein depends on each of the other LinE proteins tested (Davis et al., 2008; Fowler et al., 2013). These studies were carried out in synchronized azygotic cultures (pat1-114 strains) at 34°C. Rec10 nuclear structures were found in mug20Δ but were not as elongated as in mug20+; that study was performed at 30°C (Estreicher et al., 2012). Consequently, we tested this genetic dependence in zygotic cultures (h90 strains) at 25°C. When any other LinE was missing, we found that Rec25, Rec27 and Mug20 were spread throughout the cell but did not form visible nuclear structures (Fig. S2). These results show that formation of visible nuclear structures by a LinE is dependent on each of the other LinE proteins, as previously reported under different conditions, and is not caused by the GFP fusion. However, Rec10 formed a limited number of abnormal dotty nuclear structures in rec25Δ, rec27Δ or mug20Δ strains (Fig. 1E). The Rec10 nuclear structures in these deletion strains were fuzzier and often showed a few bright dots in the nucleus; in addition, there was higher fluorescence spread nearly uniformly throughout the nucleus. This result shows that Rec10 can form nuclear structures in rec25Δ, rec27Δ or mug20Δ, but the Rec10 protein appears more dispersed than in wild type.
These results differ from previous reports that Rec10 does not form visible nuclear structures in rec25Δ or rec27Δ strains (Davis et al., 2008; Fowler et al., 2013). The difference may result from the type of meiotic culture (azygotic versus zygotic), the preparation of the sample (fixed versus live cells) or the temperature (34°C versus 25°C). Therefore, we examined Rec10 morphology in rec25Δ, rec27Δ or mug20Δ in zygotic (h90) strains at 34°C. We found Rec10 still formed a few nuclear structures in rec25Δ, rec27Δ or mug20Δ at 34°C (Fig. 1F). Our results and those of a similar study (Ding et al., 2021) indicate that in live zygotic cultures Rec10 can bind to chromosomes and form limited abnormal dotty structures even without the other LinEs, in contrast to a previously published report with rec25Δ and rec27Δ (Davis et al., 2008). Our results are consistent with Rec10 promoting limited DSB formation and recombination in the absence of the other LinEs (Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013).
LinE structures undergo ‘dotty-to-linear-to-dotty’ transitions in azygotic meiosis at 25°C
LinE proteins may form long linear structures in zygotic meiosis (h90 strains) but not in azygotic meiosis (pat1-114 or pat1-as1 strains). To test this hypothesis, we investigated the morphology of a representative LinE protein (Rec27) under different azygotic and temperature culture conditions (Fig. 2). We found that Rec27 formed dotty nuclear structures in pat1-114 at 34°C (Fig. 2A), which is consistent with previous studies using this condition (Davis et al., 2008; Fowler et al., 2013). Rec27 structure-formation started at 2 h after meiotic induction and ended at ∼4.5 h, before MI (Fig. 3A,B). Rec27 also formed dotty structures in pat1-as1 at 34°C (Fig. 2B). The dotty structures in pat1-as1 were slightly dimmer than those in pat1-114 but the focus number and formation timing were similar (Fig. 2A,B). These results suggest that the LinE structure-formation phenotype is similar in temperature-sensitive and analog-sensitive pat1 mutants at 34°C, as expected from the inactivation of the same regulatory protein (Yamamoto, 1996).
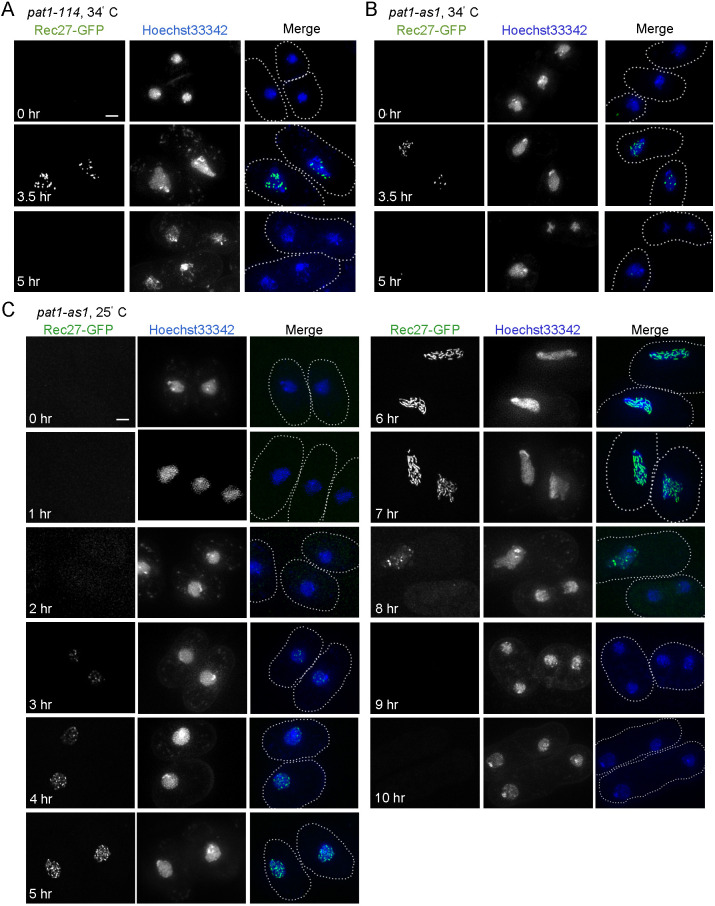
Fig. 2.
LinEs undergo structural transitions during synchronized azygotic meiosis at 25°C but not at 34°C. (A-C) A representative LinE protein (Rec27) was observed in synchronized azygotic meiotic cells in pat1-114 (A) and pat1-as1 (B) strains at 34°C, or in pat1-as1 strains (C) at 25°C. Cells were collected at the indicated times after meiotic induction and observed with SIM. (A,B) Rec27 (green) show ‘dotty’ nuclear foci at 3.5 h after induction, consistent with ‘dots’ reported previously (Lorenz et al., 2004; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). Rec27 foci disassemble at ∼5 h (i.e. before MI). Each image is representative of ≥20 cells in each condition. (C) Rec27 ‘dotty’ foci (green) appear at 3 h after induction and increase in number until 6 h and 7 h when they undergo a conformational transition to ‘linear structures’. The linear structures at 7 h are shorter than those in zygotic meiosis at 25°C (Fig. 1A) but are more nearly continuous than those at 34°C (Fig. 1B, Fig. 2A,B) (Lorenz et al., 2004; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). Each image is representative of ≥50 cells analyzed in each condition. Nuclei are shown by Hoechst 33342 staining (blue). The dotted line represents the cell outline. Scale bars: 2 μm.
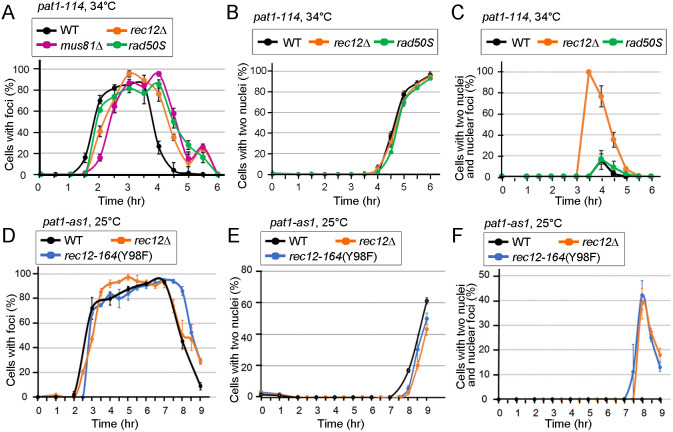
Fig. 3.
LinE structure formation is not affected by DSB formation or repair, but DSB absence delays LinE structure disassembly. (A-F) Quantification of a representative LinE protein (Rec27) in various mutant azygotic pat1 cells. For each condition, ≥250 random cells, in ≥10 random microscopic fields, were examined using an Evos microscope. (A-C) Rec27 foci in pat1-114 meiosis (34°C). (A) Rec27 ‘dotty’ foci appear ∼3 h after induction in wild type (WT), rec12Δ, rad50S and mus81Δ. The foci disappear between 4 h and 5 h. (B) Quantification of cells with ≥2 nuclei (i.e. after MI) in wild type, rec12Δ and rad50S. (C) Quantification of cells with two nuclei but still having Rec27 foci in one or both nuclei in wild type, rec12Δ and rad50S. We observed 2, 33, 67 and 26 cells with two nuclei and Rec27 foci in rec12Δ at 3.5, 4, 4.5 and 5 h, respectively. (D-F) Rec27 foci in pat1-as1 meiosis (25°C). (D) Rec27 structures appear ∼3 h after induction and disappear between 7 h and 8 h in wild type, rec12Δ and rec12-164 (Y98F). The timing of Rec27 structure formation and disassembly is similar in wild type and mutants. (E) Quantification of cells with two or more nuclei in wild type, rec12Δ and rec12-164 (Y98F). (F) Cells with delayed Rec27 structure disassembly. Quantification of cells with two nuclei but still having Rec27 structures in one or both nuclei in wild type, rec12Δ and rec12-164 (Y98F). We observed 0, 15, 73 and 82 cells with two nuclei and Rec27 foci at 7.5, 8, 8.5 and 9 h, respectively, in rec12Δ, and 1, 43, 68 and 78, respectively, in rec12-164 (Y98F). Both rec12Δ and rec12-164 (Y98F) show delay in Rec27 structure disassembly. Data are mean±s.e.m. See also Table S2.
We then investigated Rec27 morphology in azygotic (pat1-as1) strains at 25°C (Fig. 2C). We found approximately half of the population showed a few Rec27 nuclear structures 3 h after meiotic induction, before replication started (Fig. 2C, Fig. 3D; Fig. S3). Over 85% of the cells had Rec27 structures at and after 5 h, and the structures disassembled at ∼8-9 h, before MI (Fig. 2C, Fig. 3D). This result shows that Rec27 structures exist for ∼5 h at 25°C (Fig. 3D) and ∼2.5 h at 34°C (Fig. 3A), as expected from the slower cell cycle progression at 25°C than at 34°C. In addition, most of the cells at 25°C showed dotty structures at 5 h, and the dotty foci turned into linear structures at 6-7 h (Fig. 2C). The linear structures then became dotty again at ∼8-9 h and went away before MI at ∼9 h (Fig. 2C). This linear LinE structure was not found at any time during meiosis at 34°C.
We further quantified the morphology of Rec27 in azygotic (pat1-as1) strains at 25°C. We defined Rec27 structures with fewer than six voxels as short elements (see Materials and Methods; Fig. S4, Table S1), and analyzed Rec27 morphology using three parameters: (1) maximum length; (2) mean length of long elements (≥6 voxels); and (3) proportion of long elements (Fig. 4; Table S3). Rec27 formed short elements at 4-5 h, extended to long linear structures at 5-6 h and then became short elements at 7-8 h (Fig. 4A,B; Table S3). In addition, there were more long Rec27 elements at 6-7 h than at either 4-5 h or 8 h (Fig. 4C; Table S3). This result indicates that LinE proteins first form dotty structures and later expand into linear structures, before returning to the dotty form. In addition, long linear structures were found only at 25°C, not at 34°C, indicating that formation of the linear-expansion structure is temperature sensitive.
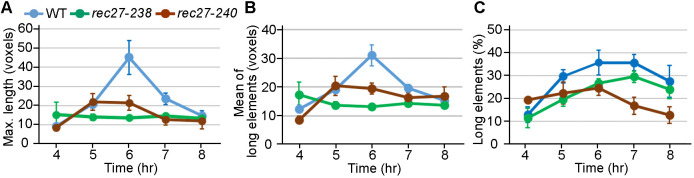
Fig. 4.
LinE structural transitions in wild type but not recombination-deficient mutants during synchronized azygotic meiosis at 25°C. (A-C) Quantification of the length and morphology of Rec27-GFP [in wild type (WT)] or Rec25-GFP [in rec27-238 (K28E) and rec27-240 (K38E)] at the indicated times after induction of azygotic meiosis (pat1-as1) at 25°C observed with SIM. Maximum length (A), mean length (B) and percentage of long elements (C; ≥6 voxels). LinE length and percentage of long elements in wild type and mutants are similar at early timepoints (4 h and 5 h), but LinEs do not elongate in mutants at later times (6 h and 7 h). Data are mean±s.e.m. See also Table S3.
LinE ‘dotty-to-linear-to-dotty’ structural transitions are also found in zygotic meiosis at 25°C
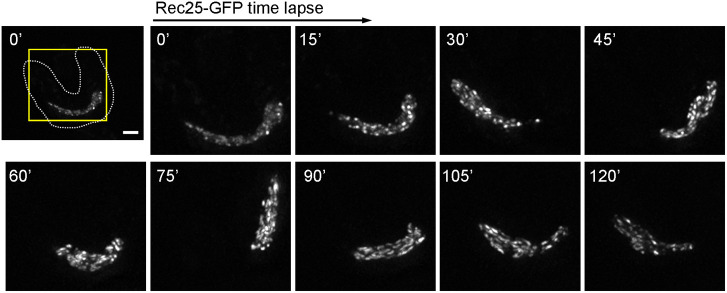
To confirm the LinE structural transitions, we performed time-lapse microscopy of individual zygotic cells (h90 strains) at 25°C (Fig. 5). We focused on cells anchored to the microscope slide at or shortly before karyogamy (nuclear fusion), and monitored them every 15 min through the horsetail stage. In the first image taken (defined as 0 min; ∼20-30 min after karyogamy), LinEs (represented by Rec25-GFP) showed dotty structures at the early horsetail stage, became linear forms in the middle horsetail stage (75-105 min), and then showed dotty structures again at the late horsetail stage (120 min). These data showed unambiguously the ‘dotty-to-linear-to-dotty’ structural transitions of LinEs in both azygotic (h90) and zygotic (pat1-as1) live cells at 25°C. These results indicate two conformations of LinE structures during meiosis in different conditions at 25°C.
Fig. 5.
LinE structures undergo transitions in zygotic meiosis at 25°C. A representative LinE protein (Rec25) was observed in live zygotic meiotic cells (h90) at 25°C using DeltaVision. Over 20 cells were imaged in the experiment, and a single, but representative, cell is shown. Rec25 shows dotty structures during the early horsetail stage; ∼90–105 min after karyogamy, the dotty structures become linear structures, which become dotty again after ∼120 min. The first image shows the whole cell at 0 min, ∼20-30 min after marking the cell as just before or just after karyogamy. The dotted line represents the cell outline. Later images show the enlarged nuclear region (yellow box of first image). Scale bar: 2 μm.
LinE structures are sensitive to 1,6-hexanediol, like the synaptonemal complex in budding yeast, flies and worms
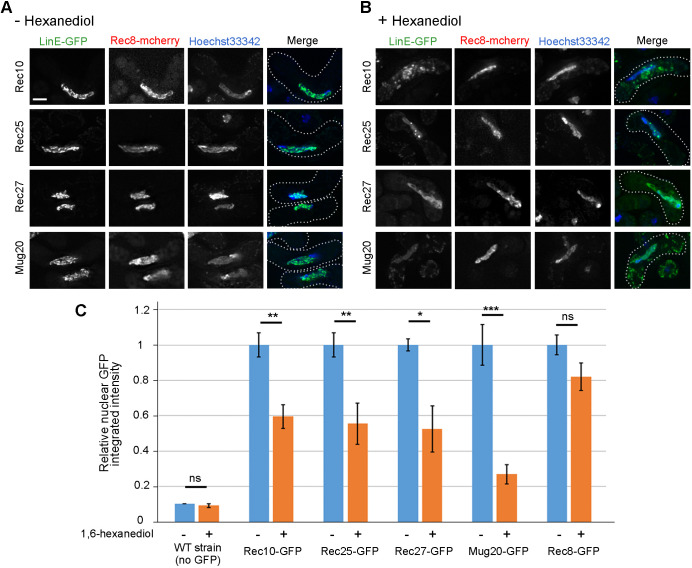
Like the synaptonemal complex, LinEs are needed for meiotic recombination (Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013) and formation of linear structures in meiosis (Fig. 1A; Fig. S1). We thus tested whether LinEs share another notable character of the synaptonemal complex. The synaptonemal complex in budding yeast, flies and worms shows liquid-crystalline properties, as indicated by sensitivity of part of the synaptonemal complex (e.g. central element protein Zip1 or SYP-3), but not an axial protein (e.g. cohesin Rec8), to the chaotropic agent 1,6-hexanediol (Rog et al., 2017). Remarkably, we found that the LinE linear structures were not visible after treating the cells with 10% 1,6-hexanediol for 5 min (Fig. 6A,B; Fig. S5). All four LinE proteins, separately tagged with GFP, showed similar sensitivity to the treatment. Over 90% of treated cells did not show LinE linear structures; rather, they showed nearly uniform nuclear signal after 1,6-hexanediol treatment, and some of them showed a few dots in the nucleus. Ding et al. (2021) reported similar data but with different conclusions (see Discussion).
Fig. 6.
LinE proteins but not cohesins are sensitive to 1,6-hexanediol. (A,B) Rec10-GFP, Rec25-GFP, Rec27-GFP, Mug20-GFP and Rec8-GFP were observed in h90 strains using DeltaVision with (A) and without (B) 1,6-hexanediol treatment. The 1,6-hexanediol (10% in EMM2-N) was added for 5 min at room temperature before observation. After treatment, all four LinEs lose their nuclear localization or become lumpy, but cohesin subunit Rec8 is little changed. Nuclei are shown by Hoechst 33342 staining (blue). Merge panels show the superimposed images of LinE-GFP (green) and Hoechst 33342 staining. The dotted line represents the cell outline. Scale bar: 2 μm. (C) Quantification of nuclear LinE-GFP and cohesin-GFP integrated intensity relative to that of untreated samples. Relative to Rec10-GFP, the untreated values were 1.01 (Rec25-GFP), 0.67 (Rec27-GFP), 1.10 (Mug20-GFP) and 1.03 (Rec8-GFP). For wild type (negative control without GFP), the intensity was plotted relative to that for untreated Rec10-GFP cells. At least 10 cells were quantified in each condition (n≥10). Data are mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001; ns, not significant (two-tailed unpaired Student’s t-test). P=0.04 between untreated Rec8-GFP and untreated Rec10-GFP.
We quantified nuclear GFP intensity of all four LinE proteins, before and after treatment. Nuclear GFP intensity of Rec10, Rec25 and Rec27 was significantly reduced after the treatment to 50-60% of the untreated control, while that of Mug20 was reduced even more, to 30% (Fig. 6C). Some treated cells still showed high nuclear GFP intensity (Fig. 6C) but none of them showed LinE linear structures (Fig. 6B; Fig. S5). In cells with any one of the four LinE-GFPs, Rec8-mCherry, a meiotic cohesin subunit fused to mCherry, maintained, remained nearly the same structure in treated and untreated cells (Fig. 6A,B). Nuclear Rec8-GFP intensity also did not show significant reduction in treated cells (Fig. 6C), indicating that LinE structures, but not cohesin, are sensitive to 1,6-hexanediol treatment, as seen in other species (Rog et al., 2017).
Absence of meiotic DSBs delays LinE structure disassembly
Previous studies using nuclear spreads showed that Rec10 forms nuclear foci in rec12Δ (Lorenz et al., 2004; Davis et al., 2008), indicating that meiotic DSB formation is not needed for LinE structure-formation. To understand which meiotic event(s) is (are) necessary for LinE structure-formation and disassembly, we analyzed the morphology of a representative LinE (Rec27-GFP) in synchronized azygotic cultures in three meiosis-deficient mutants: rec12Δ (DSB formation-deficient; Cervantes et al., 2000), rad50S (DSB resection-deficient; Young et al., 2002) and mus81Δ (Holliday junction resolution-deficient; Boddy et al., 2001; Smith et al., 2003; Cromie et al., 2007) in azygotic pat1-114 at 34°C. The timing of formation, focus number and focus morphology of Rec27 were similar in these mutants and the wild-type control (Fig. 3A), showing that DSB formation and repair are not essential for LinE structure formation.
However, we found that failure of DSB formation leads, curiously, to a LinE structure-disassembly defect (Fig. 3C). In rec12Δ (pat1-114 at 34°C), Rec27 structures formed at the same time as in wild type, but over 50% of the rec12Δ cells with two nuclei (i.e. after MI) showed foci in one or both nuclei at 3.5 h and 4 h (Fig. 3C). Moreover, no Rec27 structures were found in cells with four nuclei (i.e. after the second meiotic division, or MII, which began at 5 h) (Fig. 3C), showing that, in rec12Δ, Rec27 structure-disassembly was delayed in some cells until after MI but happened before MII. This phenotype was also found in rec12Δ pat1-as1 at 25°C (Fig. 3D-F), suggesting the Rec27 structure-disassembly defect is not related to temperature or meiotic induction method differences. To test whether the delayed disassembly of LinE structures is related to DSB formation rather than some other feature of Rec12, we did the same analysis in rec12-164 (Y98F), an active-site mutant (Cervantes et al., 2000), in pat1-as1 cells at 25°C. rec12-164 (Y98F) showed the same delayed disassembly of Rec27 structures as rec12Δ (Fig. 3D-F). These results showed that meiotic DSB formation is necessary for timely LinE structure disassembly but not for their assembly.
We also investigated the LinE structure disassembly in DSB repair-deficient mutants. Rec27 structures assembled and disassembled normally in the rad50S mutant, which is deficient in the removal of Rec12 covalently bound to the DSBs that it forms (Cromie et al., 2007) (Fig. 3A-C). Rec27 structure disassembly was delayed in the mus81Δ Holliday junction resolution-deficient mutant, but the cells were arrested before MI entry (Boddy et al., 2001), precluding determination of the requirement for Holliday junction resolution in LinE-focus disassembly.
Recombination-deficient LinE missense mutants have defective LinE structures
Several studies have shown how LinEs interact with each other and other meiotic proteins using yeast two-hybrid analysis or immunoprecipitation analysis (Lorenz et al., 2006; Spirek et al., 2010; Estreicher et al., 2012; Miyoshi et al., 2012; Sakuno and Watanabe, 2015). We and others have shown that all LinEs are needed for intact LinE structure formation (Fig. 1E; Fig. S2; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). However, how the LinEs interact and how the LinE complex is formed are not well understood. To address this question, we used h90 strains at 25°C to assay LinE morphology in LinE missense mutants that show defects in recombination and meiotic DSB formation (Ma et al., 2017; Fig. 7A). Based on their LinE morphology, we classified the LinE mutants into three groups. In group a, rec27-238 (K28E) showed dot-like Rec25 structures; thus, in this mutant the LinE proteins could enter the nucleus and interact with other LinE proteins but did not form intact linear structures. In group b, rec25-236 (K124I), rec27-239 (R32E), rec27-240 (K36E) and mug20-251 (V78E) showed much more uniform LinE distribution throughout the nucleus, and there were a few nuclear LinE structures in these mutants but fewer than in group a; thus, in these mutants, the LinEs could enter the nucleus but seemed not to interact with other LinE proteins to form abundant foci or linear structures. Finally, in group c, mug20-250 (L52P) showed very few nuclear Rec25 protein signals, suggesting this mutant protein is defective in Rec25 protein binding to chromatin or in LinE complex formation.
Fig. 7.
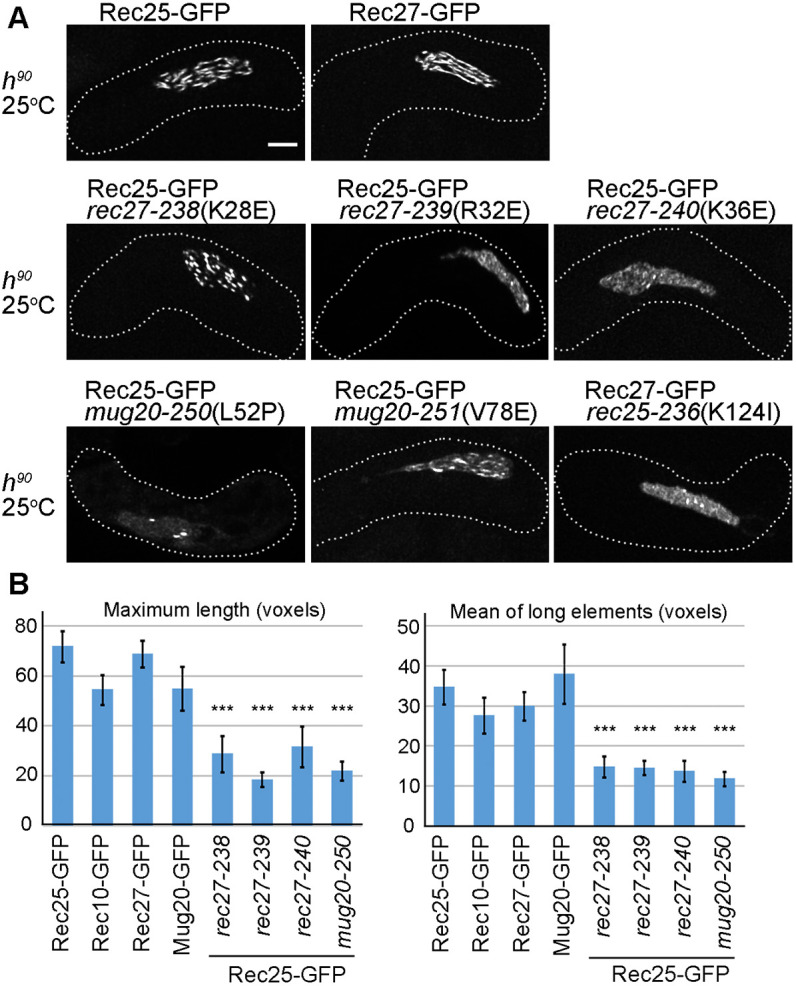
Recombination-deficient LinE missense mutations reduce LinE linear structures. (A) Rec25-GFP [in wild type (WT) and in rec27 or mug20 missense mutants] and Rec27-GFP (in a rec25 missense mutant) were analyzed in live zygotic cultures at 25°C. At least 20 cells for each strain were analyzed using SIM. None of the mutants form intact LinE linear structures. In rec27-238, rec27-239 and mug20-251, Rec25 shows non-continuous dotty foci. Rec25 in rec27-240 and Rec27 in rec25-236 show a few dot-like foci, but Rec25 and Rec27 still entered the nucleus. In mug20-250, Rec25 forms very few, if any, foci, which are dot-like. The dotted line is the cell outline. See also Fig. S6. Scale bar: 2 μm. (B) Quantification of the length of LinE-GFP in wild type and mutants. Mean of ‘maximum length’ of each cell and ‘mean of long elements’ (≥6 voxels) of each cell are shown. Data are mean±s.e.m. ***P<0.001 (two-tailed unpaired Student's t-test). ‘Maximum length’ and ‘mean of long elements’ are not significantly different among the four LinEs in wild-type cells (P>0.07). See also Table S4.
All these LinE protein mutants show a defect in meiotic DSB formation and recombination (Ma et al., 2017). Moreover, group b mutants show more severe defects than group a mutants, and DSBs are undetectable in the group c mutant mug20-250 (Ma et al., 2017). We further quantified LinE structures using the parameters described earlier. We found that in wild-type cells all four LinE proteins showed similar morphology in both maximum length and mean length of long elements (Fig. 7B; Table S4). In contrast, four recombination-deficient LinE missense mutants (rec27-238, rec27-239, rec27-240 and mug20-251) showed significant reductions in both maximum length and mean length of long elements (Fig. 7B; Table S4). We tried to quantify mug20-250 (L52P) and rec25-236 (K124I); however, the methods failed to apply to these two mutants. The nuclear GFP signal of mug20-250 was too dim to pass the background threshold, and the nuclear background of rec25-236 was too high to measure the low level of LinE structures above this background. These data confirmed that these LinE mutations lead to defective LinE structures.
LinEs in recombination-deficient LinE missense mutants fail to undergo ‘dotty-to-linear-to-dotty’ structural transitions
We next tested in time-course experiments whether the defective LinE structures in these recombination-deficient LinE missense mutants show defects in the structural transitions. We assayed Rec25-GFP configuration in rec27-238 (group a) and rec27-240 (group b) in azygotic meiosis (pat1-as1) by the method used in Fig. 2C; linear Rec25 structures were not found through the whole time-course of either mutant (Fig. S6). The quantification data showed LinE morphology was very similar in wild type and both mutants at 4-5 h, suggesting the mutants could form dotty LinE structures and short linear structures (Fig. 4; Table S3). However, the mean length of long LinE structures (≥6 voxels) in both mutants stayed the same throughout the time-course, whereas that in wild type extended 1.5-2-fold at 6-7 h. The results confirmed that wild-type cells had two configurations of LinEs but the second configuration was not fully established in two recombination-deficient mutants. Thus, there is a strong correlation between DSB formation, meiotic recombination and LinE linear structure establishment.
DISCUSSION
Previous studies have not found synaptonemal complex structures in S. pombe (Olson et al., 1978; Egel-Mitani et al., 1982; Bähler et al., 1993). Electron microscopic studies of nuclear spreads in S. pombe found only short linear forms and network chromosomal structures in meiosis (Bähler et al., 1993; Molnar et al., 2003; Lorenz et al., 2004). Our studies here focus on the LinE proteins of S. pombe, which bear some resemblance to the synaptonemal complex of other species: they are meiosis-specific proteins sharing limited homology and predicted coiled-coil regions, form linear structures along the chromosomes, are required for meiotic recombination, and are sensitive to 1,6-hexanediol (Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013; Jumper et al., 2021; Fig. 1A, Fig. 6; Figs S1, S5).
Fluorescence microscope studies of nuclear spreads and fixed cells showed that two of the LinE proteins, Rec25 and Rec27, form dot-like structures in meiosis (Davis et al., 2008; Fowler et al., 2013). The other two LinE proteins, Rec10 and Mug20, also form dotty foci at the early stage and then elongate into threads and network structures, but they were not considered end-to-end chromosomal structures resembling the synaptonemal complex of nearly all other species examined (Egel-Mitani et al., 1982; Bähler et al., 1993; Lorenz et al., 2004; Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). However, the relationship between the silver-stained linear structures found by electron microscopy and the linear Rec10-GFP structures found by fluorescence microscopy is not certain. In addition, the inferred thread-to-network structural transition of Rec10 during meiosis is not clear (Lorenz et al., 2004). Furthermore, in these studies, cells were from azygotic cultures at higher temperatures (30°C or 34°C), and they were mostly chemically fixed or used for nuclear spreads. Spore formation, spore viability and meiotic recombination are sensitive to temperature (Li and Smith, 1997; Brown et al., 2020). Studies of LinE morphology under the conditions most physiologically relevant – live cells from zygotic (homothallic) cultures at the optimal temperature (∼25°C) – are limited (Fowler et al., 2013).
In the current study, we analyzed LinE structures in live cells in zygotic meiotic cultures at 25°C (Fig. 1; Fig. S1) and, for comparison with many previous reports, azygotic meiotic cultures at both 25°C and 34°C (Fig. 2). We found that LinE proteins formed nearly continuous chromosomal structures in wild-type (h90) cultures at 25°C (Fig. 1A), like the meiotic cohesin subunit Rec8, which showed the four chromatids were usually closely juxtaposed (Ding et al., 2016). In addition, linear LinE structures were sensitive to 1,6-hexanediol treatment (Fig. 6; Fig. S5), just like the synaptonemal complex in S. cerevisiae, Drosophila melanogaster and Caenorhabditis elegans (Rog et al., 2017) (also see below). Cohesin subunit Rec8 was resistant to 1,6-hexanediol in S. cerevisiae (Rog et al., 2017) and in S. pombe (Fig. 6). Previous studies also showed features associated with the synaptonemal complex – positive DSB and crossover interference – in S. pombe meiosis (Fowler et al., 2018), as in meiosis of nearly all species examined. In S. pombe, crossover interference is weak but statistically highly significant (0.26±0.051; mean±s.e.m. P<0.01) in the intervals assayed (Fowler et al., 2018). Rec10 has amino acid sequence similarity to S. cerevisiae Red1 (Lorenz et al., 2004), one of the synaptonemal complex axial element components, and Rec27 and Mug20 have amino acid sequence similarities to C. elegans SYP-2 (an synaptonemal complex protein) and DDL-1 (a protein associated with the synaptonemal complex), respectively (Fowler et al., 2013). These data support the view that S. pombe forms structures that may be functionally related to the synaptonemal complex of other species. We also infer that in S. pombe this structure is more fragile and collapses upon fixation, cell lysis or nuclear spreading.
Ding et al. (2021) also tested the sensitivity of linear LinE structures to 1,6-hexanediol under conditions similar to ours (Fig. 6; Fig. S5). From a limited number of cells reported, without quantification, Ding et al. concluded that LinEs are not sensitive to 1,6-hexanediol treatment. However, our quantification of multiple cells for each LinE-GFP strain confirmed that the intensity of nuclear LinE proteins, but not that of nuclear Rec8, decreased significantly upon 1,6-hexanediol treatment (Fig. 6C). Furthermore, to our eyes, many images from Ding et al. (2021) are quite similar to ours (Fig. 6B; Fig. S5): in both reports most of the LinE-GFP structures were disrupted after treatment with 1,6-hexanediol. In addition, washing out the 1,6-hexanediol led to a return of higher fluorescence in several cases (Ding et al., 2021). In the absence of quantification, numerical comparisons between the two studies cannot be made, but qualitative comparisons indicate that the LinE structures are 1,6-hexanediol sensitive (Fig. 6C). Our qualitative and quantitative data suggest that LinE linear structures behave with regard to 1,6-hexanediol-sensitivity like the synaptonemal complex of other species (Rog et al., 2017).
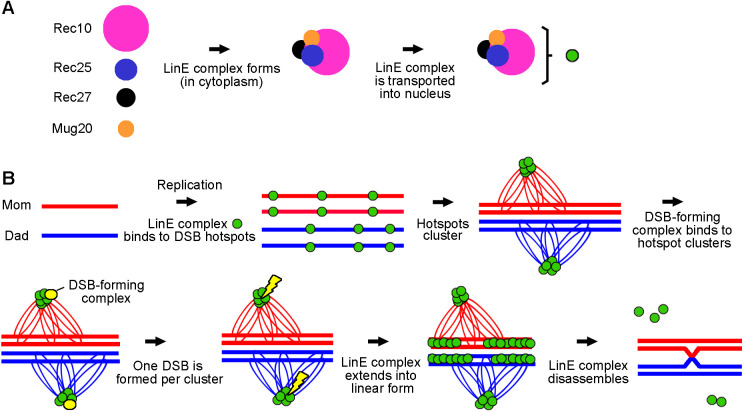
In the absence of other LinEs, we found Rec10 showed a more uniform nuclear distribution with a few nuclear foci (Fig. 1E). This result supports the idea that Rec25, Rec27 and Mug20 enhance the binding of Rec10 to DSB hotspots and is consistent with Rec10 promoting recombination at reduced levels in the absence of other LinE proteins (Ellermeier and Smith, 2005; Davis et al., 2008). We also found that Rec10 entered the nucleus and formed a few nuclear foci in other LinE subunit deletion mutants at either 25°C or 34°C (Fig. 1E,F). Previous studies showed Rec10 forms nuclear foci in mug20Δ (Estreicher et al., 2012; Fowler et al., 2013) but not in rec25Δ or rec27Δ (Fowler et al., 2013). These studies were carried out with fixed azygotic cells at 30°C or 34°C. Our live-cell imaging results showed that Rec10, in the absence of any one of the other LinEs, enters the nucleus and forms nuclear structures but not the elongated linear forms seen in wild type (Fig. 1E). However, we observed that GFP-tagged Rec25, Rec27 and Mug20 were spread throughout the cells and were not detectable as foci in the nucleus in any of the LinE deletion mutants (Fig. S2), even though the proteins are present at normal levels (Davis et al., 2008). These data indicate that the GFP tag did not cause the formation of the linear structures seen in wild-type cells. Previous studies also showed the GFP tag did not significantly change the function of LinEs (Davis et al., 2008; Estreicher et al., 2012; Fowler et al., 2013). In addition, our results suggest that, unlike Rec10, the localizations of Rec25, Rec27 and Mug20 are interdependent with the other LinEs. These results support the model that the four LinE proteins form a multimeric LinE complex in the cytoplasm and are transported into the nucleus by the nuclear localization signal on Rec10, and that Rec10 alone can enter the nucleus but acts differently than the complete LinE complex; alternatively, the other LinE proteins may enter the nucleus on their own and form a complex with Rec10, and stay in the nucleus only if Rec10 is there (Wintrebert et al., 2021).
Our data showed that the linear LinE structures are similar to that of cohesin Rec8, which forms long filamentous structures in live cells (Fig. 1A,C; Fig. S1; Ding et al., 2016). However, the linear LinE structures were sharper than that of Rec8 (Fig. 1A,C; Fig. S1). Previous ‘ChIP-chip’ studies showed that the cohesin subunits Rec8 and Rec11 bind nearly uniformly along chromosomes, whereas Rec25, Rec27 and Mug20 are strongly enriched at DSB hotspots (Fowler et al., 2013). These differences likely account for the contrasting linear structures of LinEs and those of Rec8 seen by microscopy. In addition, Rec10 is more uniformly distributed along chromosomes than Rec25, Rec27 and Mug20, which are highly concentrated at DSB hotspots (Fowler et al., 2013, 2018). However, the morphology and maximum length of Rec10 structures were not significantly different from those of the other LinEs (Fig. 1A, Fig. 7B; Table S4). These results suggest that the LinE linear structures reflect not just the hotspot-bound LinEs but a more complicated nuclear structure, such as extensions of LinE clusters (Fowler et al., 2018).
A conformational transition of meiotic chromosomal structures, without identification of the component proteins, was inferred from previous electron microscopy studies (Bähler et al., 1993; Molnar et al., 2003); the structures, designated ‘linear elements’, were inferred to first form short segments, transform into networks and then turn into bundle-like structures before being disassembled. Immunofluorescence microscopy showed that Rec10 forms similar structures in meiosis (Lorenz et al., 2004), but a sequence of conformational transitions was not clear from that study. Using live azygotic time-course imaging at 25°C, we found two configurations of LinEs: dotty foci and linear structures. We showed LinEs form dotty foci at 3-4 h after meiotic induction, transform into linear structures at 6-7 h and then become dotty again at ∼8 h, before MI (Fig. 2C, Fig. 4; Table S3), consistent with results of a recent publication that included Rec27 (Escorcia et al., 2021). These results support and extend LinEs being involved in the meiotic chromosomal structural transitions reported in electron microscopy studies of nuclear spreads. We suppose the linear structures in our study are the previously reported networks seen in nuclear spreads (Bähler et al., 1993; Lorenz et al., 2004). We observed very few cells with bundle-like structures, which were also rarely observed in previous studies (Bähler et al., 1993; Molnar et al., 2003; Lorenz et al., 2004). The nuclear morphology of these rare cells was abnormal, and they may not complete meiosis. The time-lapse data of live zygotic meiosis unambiguously showed the LinE structural transitions (Fig. 5; Escorcia et al., 2021; Ding et al., 2021). Moreover, we found that several recombination-deficient LinE mutants form only dotty LinE structures, not the fully established linear forms in wild type (Fig. 4, Fig. 7; Fig. S6). The initial dotty forms may be the LinE protein-bound hotspot clusters for DSB formation, and the later linear structures may be involved in another meiotic process, such as DSB repair or crossover regulation.
We found that LinE dotty foci appeared around the time of replication, extended into long linear structures around or soon after the time of DSB formation, and returned to dotty foci before disassembling before MI (Figs 2, 3; Fig. S3; Hyppa et al., 2014). These results indicate that LinE proteins likely remain on the chromosome after DSBs are made, which raises the possibility that LinEs also participate in other meiotic processes, such as chromosome pairing (Molnar et al., 2003) and DSB repair, including chromosomal partner choice for DSB repair (Hyppa and Smith, 2010). Previous studies in budding yeast and mouse also showed proteins required for both meiotic DSB formation and DSB repair (Keeney, 2001; Mahgoub et al., 2020; Wells et al., 2020). Moreover, LinE nuclear structure disassembly was delayed in both rec12Δ and rec12-164 (Y98F), showing that DSB formation is needed for a later event, proper LinE disassembly (Fig. 3D-F). However, those LinE structures remaining in cells with two nuclei went away before MII, which begins at ∼5 h at 34°C or 8-9 h at 25°C (Fig. 3A,D). This result indicates that meiotic DSB formation is one of the factors leading to LinE structure disassembly but other mechanisms can also regulate the process. As LinE proteins determine meiotic DSB hotspots and are necessary for DSB formation at most hotspots (Fowler et al., 2013), we propose that LinEs stay on the chromosomes until they sense that sufficient DSBs have been formed. But another mechanism can remove LinE proteins much later (before MII), allowing the sister chromatids to complete their separation at MII. Several proteins, such as Rec15 and Hop1, interact with both Rec10 and the DSB-forming complex (Kariyazono et al., 2019), and may also be involved in LinE structure disassembly.
Based on our observations and previous studies, we propose a model incorporating the sequential dynamic configurations of LinE structures (Fig. 8). Rec10, Rec25, Rec27 and Mug20 form a LinE protein complex in the cytoplasm and are transported into the nucleus (Fig. 8A; Wintrebert et al., 2021). After DNA replication, LinE protein complexes bind to meiotic DSB hotspots (Fowler et al., 2013), form LinE clusters (Fowler et al., 2018), which appear as dotty foci in light microscopy of live cells (Fig. 2C, 4-5 h), and promote DSB formation (Ellermeier and Smith, 2005; Davis et al., 2008) (Fig. 8B). LinEs then expand along the chromosome (Fig. 2C, 6-7 h), making linear structures that may participate in other meiotic processes, such as chromosome pairing (Molnar et al., 2003) and DSB repair (Lorenz et al., 2006; Hyppa and Smith, 2010). Before MI, the LinE linear structures disassemble and show dotty foci again (Fig. 2C; 8 h). Finally, the LinE proteins dissociate from the chromosomes. Missense mutants of three LinE components, Rec25, Rec27 and Mug20, showed mainly the first configuration (dotty foci) (Figs 4, 7; Fig. S6) but failed to establish the fully elongated linear structures. This result indicates these point mutations interrupt a critical feature of LinE complexes. The recombination-deficient phenotype of these mutants implies that the linear form of the LinE structures is important for proper meiosis. Our results reveal two configurations of LinE structures, ‘dotty’ foci and ‘linear structures’, which may represent two faces of LinE proteins in regulating meiotic recombination.
Fig. 8.
Model for dotty-to-linear-to-dotty configurations of LinE structures. (A) In the cytoplasm, Rec10, Rec25, Rec27 and Mug20 form LinE protein complexes (small green disk), which are transported into the nucleus via the nuclear localization signal on Rec10 (Wintrebert et al., 2021). (B) Transitions of two LinE configurations. LinE protein complexes bind to DSB hotspots around the time of DNA replication, and LinE protein-bound hotspots within a ∼200 kb region form LinE-bound hotspot clusters [green disks on thin loops of separate homologs, which are shown as red and blue lines to indicate maternal (Mom) and paternal (Dad) homologs, respectively] (Fowler et al., 2013, 2018); a few hotspot clusters assemble into a ‘dotty’ structure visible in the microscope. Close alignment of homologs (Ding et al., 2016) may occur separate from the LinE structures. The DSB-forming complex (small yellow disk) is activated by the LinE proteins in a cluster and introduces one meiotic DSB (yellow lightning bolt) in each DSB hotspot cluster; this feature accounts for DSB interference at LinE-bound hotspots and consequent crossover interference (Fowler et al., 2018). After DSB formation, the LinE protein complex extends along the chromosome, forming a ‘linear LinE structure’ visible in the microscope. The linear LinE structures may aid DSB repair, perhaps by regulating partner choice for DSB repair (Hyppa and Smith, 2010). The LinE structure returns to the dotty configuration, and the LinE proteins dissociate from the chromosomes before MI.
MATERIALS AND METHODS
S. pombe strains and meiotic induction methods
S. pombe strains used in this study are listed in Table S5. Media for cell growth and meiosis were as described (Smith, 2009). To generate rec25-303-GFP::hygMX6 and rec27-302-GFP::hygMX6, we replaced the kanMX6 cassette of rec25-204-GFP::kanMX6 or rec27-205-GFP::kanMX6, respectively, with hygMX6 cassette using the method described in Sato et al. (2005). For zygotic meiotic induction (h90 strains), cells from 50-100 µl of fresh overnight culture in rich medium (YEL, yeast extract liquid) were collected, washed three times with water, suspended in water, spotted on sporulation medium (malt extract agar), and incubated at 25°C for 13-15 h or 34°C for 10-17 h. After incubation, cells were collected and suspended in Edinburgh minimal medium without nitrogen (EMM2-N; water for Fig. 3) for microscopic assay. For azygotic meiotic induction (pat1-114 or pat1-as1 strains), cells were treated as described previously (Hyppa and Smith, 2009; Guerra-Moreno et al., 2012). At each meiotic timepoint, 500 µl of culture was collected for microscopic assay.
Fluorescence microscopy
Cells induced for meiosis were suspended in EMM2-N with Hoechst 33342 (5 μg/ml) to stain chromatin. The cell suspension was spread on a poly-L-lysine-coated slide before observation in one of three types of microscope: (1) a SIM with 100×/1.4 NA STED White Objective (Leica Microsystems) and VT-iSIM system (Visitech International); (2) a DeltaVision Elite microscope (DeltaVision) with 100×/1.4 UPlanSApo lens (GE Healthcare); or (3) an Evos FL Auto 2 inverted microscope (Evos, Thermo Fisher Scientific) with a 60×/1.42 PlanApo N Lens. Image data were processed using Micro Evolution Deconvolution Software running in FIJI (SIM) or SoftWoRX (DeltaVision). Images are maximum intensity projections of sections with a step size of 0.2 μm to cover the whole cell. For cells treated with 1,6-hexanediol, cells were suspended in EMM2-N with Hoechst 33342 (5 μg/ml) and 1,6-hexanediol (10% w/v), and observed in a microscope within 5 min after the addition of 1,6-hexanediol. Experiments using two different sources of 1,6-hexanediol (Sigma-Aldrich and Hampton Research) showed similar results. For live-cell time-lapse analysis, the meiotic-induced cells were mounted on an agarose pad (Escorcia et al., 2019). The slide was observed with a DeltaVision microscope and the data processed as above.
Quantification of fluorescence microscopy images
For quantification of LinE morphology, we used images taken by SIM. The image analysis pipeline was developed in MATLAB_R2019b and is available from GitHub at https://github.com/FredHutch/Smith_Lab_LinEs. After importing the z-stacks of the GFP signal, the global background was determined from the maximum projection image, and the GFP signal was segmented by thresholding with a value equal to 3× the mean background intensity. The resulting three-dimensional volume was then subjected to skeletonization, and the endpoints of each resulting skeletonized GFP element were identified. Then, the longest path between any endpoint combination was measured by geodesic distance. For each cell of each genotype analyzed, the longest distance for each element (in voxels, the three-dimensional analog of pixel) and the number of GFP elements per cell were gathered. Based on images of maximum projection and images of skeleton projection, elements 6 voxels or longer were defined as (long) linear structures, whereas elements with 5 or fewer voxels were defined as (short) dotty structures (Fig. S4, Table S1).
For quantification of nuclear GFP intensity after treatment with 1,6-hexanediol, images were taken by DeltaVision. Wild-type h90 strain (GP50) was used as a negative control (i.e. lacking a GFP fusion protein). The nuclear region was identified by Hoechst 33342 staining. Nuclear GFP was quantified using ImageJ (National Institutes of Health), and the nuclear region threshold was adjusted using the Li method (Li and Lee, 1993; Li and Tam, 1998; Sezgin and Sankur, 2004).
For quantification of LinE structure formation and disassembly, images were taken using an Evos microscope. For each timepoint of each sample, at least 250 cells with clear nuclear staining from at least ten individual fields were examined. Three biological repeats were performed for each condition.
Supplementary Material
Acknowledgements
We thank Daqiao Ding for sending us unpublished micrographs of live S. pombe zygotic meiotic cells expressing LinE-GFP proteins in the linear configuration; Yoshinori Watanabe for a strain with the rec8+-mCherry-Tspo5≪natr allele; Kami Ahmad for use of his Evos FL Auto 2 inverted microscope; Julien Dubrulle for image quantification; and Sue Amundsen, Randy Hyppa, Mai-Chi Nguyen and especially Cristina Martín-Castellanos for helpful comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.-C.C., G.R.S.; Methodology: Y.-C.C.; Software: Y.-C.C.; Validation: Y.-C.C.; Formal analysis: Y.-C.C.; Investigation: Y.-C.C., G.R.S.; Resources: Y.-C.C., G.R.S.; Data curation: Y.-C.C.; Writing - original draft: Y.-C.C.; Writing - review & editing: G.R.S.; Visualization: Y.-C.C.; Supervision: G.R.S.; Project administration: G.R.S.; Funding acquisition: G.R.S.
Funding
This research was supported by the National Institutes of Health (R35 GM118120 to G.R.S. and P30 CA015704 to the Fred Hutchinson Cancer Research Center). Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259061.
References
- Bähler, J., Wyler, T., Loidl, J. and Kohli, J. (1993). Unusual nuclear structures in meiotic prophase of fission yeast: a cytological analysis. J. Cell Biol. 121, 241-256. 10.1083/jcb.121.2.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat, F., Manova, K., Yuen, J. P., Jasin, M. and Keeney, S. (2000). Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6, 989-998. 10.1016/S1097-2765(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Boddy, M. N., Gaillard, P.-H. L., Mcdonald, W. H., Shanahan, P., Yates, 3rd, J. R. and Russell, P. (2001). Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107, 537-548. 10.1016/S0092-8674(01)00536-0 [DOI] [PubMed] [Google Scholar]
- Bowring, F. J., Yeadon, P. J., Stainer, R. G. and Catcheside, D. E. (2006). Chromosome pairing and meiotic recombination in Neurospora crassa spo11 mutants. Curr. Genet. 50, 115-123. 10.1007/s00294-006-0066-1 [DOI] [PubMed] [Google Scholar]
- Brown, S. D., Audoynaud, C. and Lorenz, A. (2020). Intragenic meiotic recombination in Schizosaccharomyces pombe is sensitive to environmental temperature changes. Chromosome Res. 28, 195-207. 10.1007/s10577-020-09632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes, M. D., Farah, J. A. and Smith, G. R. (2000). Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5, 883-888. 10.1016/S1097-2765(00)80328-7 [DOI] [PubMed] [Google Scholar]
- Colaiácovo, M. P., MacQueen, A. J., Martinez-Perez, E., McDonald, K., Adamo, A., La Volpe, A. and Villeneuve, A. M. (2003). Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5, 463-474. [DOI] [PubMed] [Google Scholar]
- Cromie, G. A., Hyppa, R. W., Cam, H. P., Farah, J. A., Grewal, S. I. and Smith, G. R. (2007). A discrete class of intergenic DNA dictates meiotic DNA break hotspots in fission yeast. PLoS Genet. 3, e141. 10.1371/journal.pgen.0030141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L., Rozalén, A. E., Moreno, S., Smith, G. R. and Martín-Castellanos, C. (2008). Rec25 and Rec27, novel linear-element components, link cohesin to meiotic DNA breakage and recombination. Curr. Biol., 18, 849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., McDonald, K., Moulder, G., Barstead, R., Dresser, M. and Villeneuve, A. M. (1998). Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94, 387-398. [DOI] [PubMed] [Google Scholar]
- Ding, D.-Q., Yamamoto, A., Haraguchi, T. and Hiraoka, Y. (2004). Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev. Cell 6, 329-341. 10.1016/S1534-5807(04)00059-0 [DOI] [PubMed] [Google Scholar]
- Ding, D.-Q., Matsuda, A., Okamasa, K., Nagahama, Y., Haraguchi, T. and Hiraoka, Y. (2016). Meiotic cohesin-based chromosome structure is essential for homologous chromosome pairing in Schizosaccharomyces pombe. Chromosoma 125, 205-214. 10.1007/s00412-015-0551-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, D.-Q., Matsuda, A., Okamasa, K. and Hiraoka, Y. (2021). Linear elements are stable structures along the chromosome axis in fission yeast meiosis. Chromosoma 130, 149-162. 10.1007/s00412-021-00757-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser, M. E. and Giroux, C. N. (1988). Meiotic chromosome behavior in spread preparations of yeast. J. Cell Biol. 106, 567-573. 10.1083/jcb.106.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel, R. (1977). Frequency of mating-type switching in homothallic fission yeast. Nature 266, 172-174. 10.1038/266172a0 [DOI] [PubMed] [Google Scholar]
- Egel-Mitani, M., Olson, L. W. and Egel, R. (1982). Meiosis in Aspergillus nidulans: another example for lacking synaptonemal complexes in the absence of crossover interference. Hereditas 97, 179-187. 10.1111/j.1601-5223.1982.tb00761.x [DOI] [PubMed] [Google Scholar]
- Ellermeier, C. and Smith, G. R. (2005). Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U.S.A. 102, 10952-10957. 10.1073/pnas.0504805102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorcia, W. and Forsburg, S. L. (2018). Random spore analysis in fission yeast. Methods Mol. Biol. 1721, 189-195. 10.1007/978-1-4939-7546-4_17 [DOI] [PubMed] [Google Scholar]
- Escorcia, W., Shen, K. F., Yuan, J. P. and Forsburg, S. L. (2019). Examination of mitotic and meiotic fission yeast nuclear dynamics by fluorescence live-cell microscopy. J. Vis. Exp. 148, e59822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorcia, W., Tripathi, V. P., Yuan, J.-P. and Forsburg, S. L. (2021). A visual atlas of meiotic protein dynamics in living fission yeast. Open Biol. 11, 200357. 10.1098/rsob.200357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estreicher, A., Lorenz, A. and Loidl, J. (2012). Mug20, a novel protein associated with linear elements in fission yeast meiosis. Curr. Genet. 58, 119-127. 10.1007/s00294-012-0369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, K. R., Gutiérez-Velasco, S., Martín-Castellanos, C. and Smith, G. R. (2013). Protein determinants of meiotic DNA break hot spots. Mol. Cell 49, 983-996. 10.1016/j.molcel.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, K. R., Sasaki, M., Milman, N., Keeney, S. and Smith, G. R. (2014). Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 24, 1650-1664. 10.1101/gr.172122.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, K. R., Hyppa, R. W., Cromie, G. A. and Smith, G. R. (2018). Physical basis for long-distance communication along meiotic chromosomes. 115, E9333-E9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Moreno, A., Alves-Rodrigues, I., Hidalgo, E. and Ayté, J. (2012). Chemical genetic induction of meiosis in Schizosaccharomyces pombe. Cell Cycle 11, 1621-1625. 10.4161/cc.20051 [DOI] [PubMed] [Google Scholar]
- Gutz, H. (1971). Site specific induction of gene conversion in Schizosaccharomyces pombe. Genetics 69, 317-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung, F., Wurz-Wildersinn, R., Fuchs, J., Schubert, I., Suer, S. and Puchta, H. (2007). The catalytically active tyrosine residues of both SPO11-1 and SPO11-2 are required for meiotic double-strand break induction in Arabidopsis. Plant Cell 19, 3090-3099. 10.1105/tpc.107.054817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyppa, R. W. and Smith, G. R. (2009). Using Schizosaccharomyces pombe meiosis to analyze DNA recombination intermediates. Methods Mol. Biol. 557, 235-252. 10.1007/978-1-59745-527-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyppa, R. W. and Smith, G. R. (2010). Crossover invariance determined by partner choice for meiotic DNA break repair. Cell 142, 243-255. 10.1016/j.cell.2010.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyppa, R. W., Fowler, K. R., Cipak, L., Gregan, J. and Smith, G. R. (2014). DNA intermediates of meiotic recombination in synchronous S. pombe at optimal temperature. Nucleic Acids Res. 42, 359-369. 10.1093/nar/gkt861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino, Y. and Yamamoto, M. (1985). Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U.S.A. 82, 2447-2451. 10.1073/pnas.82.8.2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper, J., Evans, R., Pritzel, A., Green , T., Figurnov , M., Ronneberger , O., Tunyasuvunakool , K., Bates, R., Žídek, A., Potapenko, A.et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583-589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyazono, R., Oda, A., Yamada, T. and Ohta, K. (2019). Conserved HORMA domain-containing protein Hop1 stabilizes interaction between proteins of meiotic DNA break hotspots and chromosome axis. Nucleic Acids Res. 47, 10166-10180. 10.1093/nar/gkz754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, S. (2001). Mechanism and control of meiotic recombination initiation. In Current Topics in Developmental Biology, Vol. 52, pp. 1-53. Academic Press. [DOI] [PubMed] [Google Scholar]
- Keeney, S., Giroux, C. N. and Kleckner, N. (1997). Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88, 375-384. 10.1016/S0092-8674(00)81876-0 [DOI] [PubMed] [Google Scholar]
- Lange, J., Yamada, S., Tischfield, S. E., Pan, J., Kim, S., Zhu, X., Socci, N. D., Jasin, M. and Keeney, S. (2016). The landscape of mouse meiotic double-strand break formation, processing, and repair. Cell 167, 695-708.e16. 10.1016/j.cell.2016.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. H. and Lee, C. K. (1993). Minimum cross entropy thresholding. Pattern Recogn. 26, 617-625. 10.1016/0031-3203(93)90115-D [DOI] [Google Scholar]
- Li, Y. F. and Smith, G. R. (1997). The Schizosaccharomyces pombe rec16 gene product regulates multiple meiotic events. Genetics 146, 57-67. 10.1093/genetics/146.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. and Tam, P. K. S. (1998). An iterative algorithm for minimum cross entropy thresholding. Pattern Recognit. Lett. 19, 771-776. 10.1016/S0167-8655(98)00057-9 [DOI] [Google Scholar]
- Loidl, J. (2006). S. pombe linear elements: the modest cousins of synaptonemal complexes. Chromosoma, 115, 260-271. [DOI] [PubMed] [Google Scholar]
- Lorenz, A., Wells, J. L., Pryce, D. W., Novatchkova, M., Eisenhaber, F., McFarlane, R. J. and Loidl, J. (2004). S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J. Cell Sci., 117, 3343-3351. [DOI] [PubMed] [Google Scholar]
- Lorenz, A., Estreicher, A., Kohli, J. and Loidl, J. (2006). Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma 115, 330-340. 10.1007/s00412-006-0053-9 [DOI] [PubMed] [Google Scholar]
- Ma, L., Fowler, K. R., Martín-Castellanos, C. and Smith, G. R. (2017). Functional organization of protein determinants of meiotic DNA break hotspots. Sci. Rep. 7, 1393. 10.1038/s41598-017-00742-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub, M., Paiano, J., Bruno, M., Wu, W., Pathuri, S., Zhang, X., Ralls, S., Cheng, X., Nussenzweig, A. and MacFarlan, T. S. (2020). Dual histone methyl reader ZCWPW1 facilitates repair of meiotic double strand breaks in male mice. eLife 9, e53360. 10.7554/eLife.53360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, A. L. and Amon, A. (2004). Meiosis: cell-cycle controls shuffle and deal. Nat. Rev. Mol. Cell Biol. 5, 983-997. 10.1038/nrm1526 [DOI] [PubMed] [Google Scholar]
- Martín-Castellanos, C., Blanco, M., Rozalén, A. E., Pérez-Hidalgo, L., García, A. I., Conde, F., Mata, J., Ellermeier, C., Davis, L., San-Segundo, P.et al. (2005). A large-scale screen in S. pombe identifies seven novel genes required for critical meiotic events. Curr. Biol., 15, 2056-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S. and Hayashi-Hagihara, A. (1998). mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev., 12, 2932-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi, T., Ito, M., Kugou, K., Yamada, S., Furuichi, M., Oda, A., Yamada, T., Hirota, K., Masai, H. and Ohta, K. (2012). A central coupler for recombination initiation linking chromosome architecture to S phase checkpoint. Mol. Cell 47, 722-733. 10.1016/j.molcel.2012.06.023 [DOI] [PubMed] [Google Scholar]
- Molnar, M., Doll, E., Yamamoto, A., Hiraoka, Y. and Kohli, J. (2003). Linear element formation and their role in meiotic sister chromatid cohesion and chromosome pairing. J. Cell Sci. 116, 1719-1731. 10.1242/jcs.00387 [DOI] [PubMed] [Google Scholar]
- Nambiar, M., Chuang, Y.-C. and Smith, G. R. (2019). Distributing meiotic crossovers for optimal fertility and evolution. DNA Repair (Amst) 81, 102648. 10.1016/j.dnarep.2019.102648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, L. W., Edén, U., Egel-Mitani, M. and Egel, R. (1978). Asynaptic meiosis in fission yeast? Hereditas 89, 189-199. 10.1111/j.1601-5223.1978.tb01275.x [DOI] [Google Scholar]
- Pan, J., Sasaki, M., Kniewel, R., Murakami, H., Blitzblau, H. G., Tischfield, S. E., Zhu, X., Neale, M. J., Jasin, M., Socci, N. D.et al. (2011). A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719-731. 10.1016/j.cell.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki, M., Siomos, M. F. and Nasmyth, K. (2003). Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423-440. 10.1016/S0092-8674(03)00083-7 [DOI] [PubMed] [Google Scholar]
- Rog, O., Kohler, S. and Dernburg, A. F. (2017). The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. eLife 6, e21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno, T. and Watanabe, Y. (2015). Phosphorylation of cohesin Rec11/SA3 by casein kinase 1 promotes homologous recombination by assembling the meiotic chromosome axis. eLife 32, 220-230. [DOI] [PubMed] [Google Scholar]
- Sato, M., Dhut, S. and Toda, T. (2005). New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22, 583-591. 10.1002/yea.1233 [DOI] [PubMed] [Google Scholar]
- Sezgin, M. and Sankur, B. (2004). Survey over image thresholding techniques and quantitative performance evaluation. J. Electron. Imaging 13, 146-168. 10.1117/1.1631315 [DOI] [Google Scholar]
- Smith, G. R. (2009). Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. Methods Mol. Biol. 557, 65-76. 10.1007/978-1-59745-527-5_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. R., Boddy, M. N., Shanahan, P. and Russell, P. (2003). Fission yeast Mus81.Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics, 165, 2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirek, M., Estreicher, A., Csaszar, E., Wells, J., McFarlane, R. J., Watts, F. Z. and Loidl, J. (2010). SUMOylation is required for normal development of linear elements and wild-type meiotic recombination in Schizosaccharomyces pombe. Chromosoma 119, 59-72. 10.1007/s00412-009-0241-5 [DOI] [PubMed] [Google Scholar]
- Wells, D., Bitoun, E., Moralli, D., Zhang, G., Hinch, A., Jankowska, J., Donnelly, P., Green, C. and Myers, S. R. (2020). ZCWPW1 is recruited to recombination hotspots by PRDM9 and is essential for meiotic double strand break repair. eLife 9, e53392. 10.7554/eLife.53392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintrebert, M., Nguyen, M.-C. and Smith, G. R. (2021). Activation of meiotic recombination by nuclear import of the DNA break hotspot-determining complex. J. Cell Sci. 134, jcs253518. 10.1242/jcs.253518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M. (1996). The molecular control mechanisms of meiosis in fission yeast. Trends Biochem. Sci. 21, 18-22. 10.1016/S0968-0004(06)80022-8 [DOI] [PubMed] [Google Scholar]
- Young, J. A., Schreckhise, R. W., Steiner, W. W. and Smith, G. R. (2002). Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell 9, 253-263. 10.1016/S1097-2765(02)00452-5 [DOI] [PubMed] [Google Scholar]
- Zahn-Zabal, M. and Kohli, J. (1996). The distance-dependence of the fission yeast ade6-M26 marker effect in two-factor crosses. Curr. Genet. 29, 530-536. 10.1007/BF02426957 [DOI] [PubMed] [Google Scholar]
- Zickler, D. and Kleckner, N. (1999). Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33, 603-754. 10.1146/annurev.genet.33.1.603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.