Abstract
Background
Rates of shared decision making (SDM) are relatively low in early stage prostate cancer decisions, as patients’ values are not well integrated into a preference-sensitive treatment decision. The study objectives were to develop a SDM training video, measure usability and satisfaction, and determine the effect of the intervention on preparing patients to participate in clinical appointments.
Methods
A randomized controlled trial was conducted to compare a plain-language decision aid (DA) to the DA plus a patient SDM training video. Patients with early stage prostate cancer completed survey measures at baseline and after reviewing the intervention materials. Survey items assessed patients’ knowledge, beliefs related to SDM, and perceived readiness/intention to participate in their upcoming clinical appointment.
Results
Of those randomized to the DA + SDM video group, most participants (91%) watched the video and 93% would recommend the video to others. Participants in the DA + SDM video group, compared to the DA-only group, reported an increased desire to participate in the decision (mean = 3.65 v. 3.39, P < 0.001), less decision urgency (mean = 2.82 v. 3.39, P < 0.001), and improved self-efficacy for communicating with physicians (mean = 4.69 v. 4.50, P = 0.05). These participants also reported increased intentions to seek a referral from a radiation oncologist (73% v. 51%, P = 0.004), to take notes (mean = 3.23 v. 2.86, P = 0.004), and to record their upcoming appointments (mean = 1.79 v. 1.43, P = 0.008).
Conclusions
A novel SDM training video was accepted by patients and changed several measures associated with SDM. This may be a scalable, cost-effective way to prepare patients with early stage prostate cancer to participate in their clinical appointments.
Highlights.
This study describes the development of a novel shared decision making training video, which was found to be well received and highly used by patients.
The shared decision making video combined with a decision aid was more effective at improving several measures of shared decision making outcomes (e.g., increased desire to participate, self-efficacy) compared to receipt of the decision aid alone.
Videos may be an ideal low-resource format to deliver shared decision making training to patients facing preference-sensitive decisions.
Keywords: communication skills, decision aid, patient engagement, prostate cancer, shared decision making
Introduction
With improvements in the early detection of prostate cancer among men, a growing number of patients are faced with the difficult decision of active surveillance, radiation, or surgery. 1 The best treatment for each patient depends on both his medical factors (e.g., cancer severity and medical comorbidities) and also on how he personally values the risks (e.g., erectile dysfunction) and benefits (e.g., chance of curing the cancer) of each choice 2 ; therefore, this is a clinical situation that can be considered a preference-sensitive decision. The process of shared decision making (SDM) offers a framework for patients and physicians to identify the best treatment option based on both patients’ medical factors and personal values. 3 To be able to fully participate in SDM, patients must 1) be knowledgeable about their medical condition and treatment choices, 2) feel empowered to engage in decision making processes, and 3) have the skills required to participate in the actual clinical appointment.
Unfortunately, baseline rates of SDM in early stage prostate cancer are relatively low, and physicians frequently do not incorporate patients’ values into their recommendations.4–6 One reason for these low rates of SDM may be the information asymmetry between patients and physicians, such that patients do not feel qualified to participate during clinical appointments. 3 Traditional decision aids have focused on addressing this issue by increasing patients’ knowledge about their medical conditions and treatment options. However, even with decision aids designed to decrease information asymmetry, patients with early stage prostate cancer still often do not receive treatments that are aligned with their preferences.6,7 One reason for this may be that most decision aids focus on increasing patients’ knowledge about their medical conditions and treatment options but do not necessarily empower them to engage in decision making. Men with early stage prostate cancer face particularly high barriers to participating in clinical appointments, given that they are generally older in age and may need to talk about sensitive topics such as impotence and incontinence. 8 Many interventions have attempted to improve patient participation in clinical appointments by teaching physicians better communication skills, with good success across multiple domains. 9 However, effective SDM “takes two to tango,” 3 and improving communication skills in physicians is only part of the equation. Physicians cannot fully engage in SDM if patients are not prepared to engage with them.
Combining a decision aid (DA) with a patient SDM training video may be a new approach to improve SDM by teaching patients more specifically about the SDM process, including how to participate actively in clinical appointments. Despite their promise, there have been relatively few intervention studies on how to improve patient communication skills 10 and even fewer that have looked at the effect of combining a DA with a communication skills intervention. Currently, the effects of communication skills interventions have been mixed, although it does seem that patients are overall more participatory during clinical appointments.10–14 Notably, many patient communication skills interventions suffer from 3 broad limitations: 1) lack of conceptual or theoretical framework, 2) outcome measures that do not match their interventions, and 3) an exclusive focus on question asking, which is only 1 form of patient participation. 15 Finally, many studies have provided patients with communication coaching through one-on-one trainings, which are relatively resource intensive and not easily scalable.16,17
In this study, we conducted a randomized trial to compare the effect of a DA alone v. the DA plus a SDM training video on patients’ knowledge, beliefs about the SDM process, and perceived readiness to participate in their upcoming clinical appointments. Our study addressed previous studies’ limitations by incorporating a theoretical basis for the SDM video training, examining multiple aspects of SDM beyond question asking, and matching our outcome measures to the content that was being taught within the video. This article reports 1) the development of a novel SDM training video, 2) patients’ evaluation of the training video, and 3) the extent to which a DA paired with a SDM training video (relative to the DA alone) changed patients’ knowledge of prostate cancer, beliefs about the SDM process, and perceived readiness to participate in their upcoming clinical appointments. Specifically, we hypothesized that compared with patients who only received the DA, patients who received the DA plus the SDM training video would report 1) equivalent knowledge about their medical condition and treatment options since the SDM video was not designed to affect knowledge, 2) feel empowered to engage in decision making processes (e.g., desire to participate in the decision), and 3) feel more prepared to participate in upcoming clinical appointments (e.g., greater self-efficacy for communicating with physician).
Methods
Materials
Decision aid
The DA was based on the existing booklet “Making the Choice: Deciding What to Do about Early Stage Prostate Cancer,” which was designed to empower men with early stage prostate cancer to engage in treatment decision making. 18 The DA uses plain language and simple graphics to be accessible to men with eighth-grade literacy levels. It was previously shown to improve patient knowledge about their treatment options compared to a standard decision aid and increased patients’ initial interest in active surveillance but ultimately did not change patient treatment choice or participation in the decision making process. 18 We made modifications based on medical updates and feedback we elicited since the original publication.
Patients who received the SDM training video received a slightly modified DA that summarized the important material in the video. First, there was a summary page of the communication skills and participatory behaviors emphasized in the video, with pictures of a patient modeling the behavior. Second, the “questions you may wish to ask your doctor” page listed more questions about preferences and the decision making process rather than purely about medical knowledge. Finally, there was more space for “notes.” Otherwise, the DAs were identical.
SDM training video
The SDM training video incorporates several theoretical models of communication and human behavior, including Bandura’s (1977) model of self-efficacy, 19 the Comskill model of physician communication skills training, 20 and previously established barriers to and facilitators of SDM. 8 The intervention primarily focused on increasing patients’ self-efficacy through 3 separate methods outlined by Bandura 19 : 1) “vicarious experience” by actors modeling communication skills, 2) “verbal persuasion” of the importance of SDM by verbal suggestion and encouragement, and 3) “performance accomplishments” by asking patients to visualize using SDM skills. A video was chosen as the method for demonstrating adaptive patient communication in a clinical visit because visual modeling has been shown to be an effective way to increase self-efficacy regarding new behaviors. 19
To develop the content of the SDM training video, we assembled a team of clinicians and health communication experts to review audio-recorded clinical appointments from another study 6 in which patients received their prostate cancer diagnoses and discussed treatment options with their urologists. The team first identified the typical appointment flow as follows: introduction, discussion of biopsy experience and results, discussion of treatment options, and making a treatment decision or deciding the next steps. 21 Then, using an iterative process, the team identified categories of “shared decision making problems” that often occurred within each of these sections of the appointment. In consultation with communication and SDM experts, the team identified strategies that patients could use to overcome each of these communication problems (Table 1). An initial video script was created using the identified problems and solutions as a template. The video script was then reviewed by clinician stakeholders (i.e., urologists, radiation oncologists, medical oncologists, and primary care providers) and experts in the field of medical decision making, decision aids, and patient-centered care. The tool was also influenced by the results of a previous focus group and semistructured interviews that were conducted with men who had a history of prostate cancer during the development of the first patient decision aid. In the focus group and interviews, men read the DVD script and listened to an audio recording with readers portraying the patient, doctor, and narrator and gave their reactions to it. The focus group and interviews were conducted in a semistructured format to identify specific content that was useful, confusing, helpful, upsetting, and/or valuable and to suggest improvements. Revisions to the script were made based on feedback from the focus group, interviews, and our experts prior to filming.
Table 1.
Barriers to Shared Decision Making Identified in Previously Recorded Appointments and Strategies to Overcome the Barriers That Were Demonstrated within the SDM Skills Training Video
| Timing within Appointment | Barrier to Shared Decision Making | Strategies to Overcome Barrier |
|---|---|---|
| Diagnosis delivery/risk classification | There’s no time to process bad news. | It’s hard to give bad news and doctors may be trying to “get past” a hard part of the job. Ask for time to process the bad news. |
| Information may be given too quickly with extensive use of numbers. | It’s hard to take it all in—ask doctors to slow down. Numbers can be confusing—ask doctors to clarify or explain percentages. | |
| Discussion of treatment options: active surveillance | Doctors may quickly reject active surveillance. | Clarify why surveillance is inappropriate. |
| Discussion of treatment options: active treatments | Doctors are biased toward their own treatment option. | Realize that doctors understand their own treatment best and are biased. |
| Doctors spend most of their time talking about their treatment and may only be able to provide specific statistics for that treatment. | Make sure to get a referral to other specialists (radiation oncologist or urologist). Consider talking to your primary care doctor before making a decision. | |
| Recommendations | Doctors may not know the “full story” and may make recommendations that do not incorporate patients’ preferences. | Let your doctor know your preferences. |
| Making a decision | Doctors may pressure patients to make a decision that day. Doctors offer to schedule surgery with the option of “cancelling” if patients decide they do not want surgery. | Tell your doctor that you want to take time to think about your options before making a decision. |
| Wrap-up | Doctors often ask “any final questions?” without really pausing or allowing time for questions. | Make sure you get all of your questions answered before you leave. |
| Ending may be rushed and patients may be unsure of next steps. | Clarify the “next steps” with your doctor. |
At the end of this process, the video was designed to address elements of the decision making process that are optimal for SDM, including the 1) importance of participating in the treatment decision and communicating one’s personal preferences and values, 2) acceptability of taking time to make the decision, 3) utility of getting a second opinion, and 4) feeling confident and prepared to participate in the clinical appointment (e.g., bring recorder, list of questions, communication self-efficacy).
The final video was 26 minutes long and portrayed a hypothetical clinical appointment in which a patient met with a urologist to receive his prostate biopsy results, learned that he had a diagnosis of localized prostate cancer, and discussed his treatment options. The videos portrayed clinical interactions between a patient and physician (played by professional actors) to demonstrate areas where there are often communication problems and where SDM could be beneficial. The video then modeled 1 or more solutions. Throughout the video, the narrator emphasized other aspects of how to participate in SDM, such as reassuring patients they could take time for their decision, that their preferences mattered, and encouraging them to take notes during their appointments.
There were two versions of the final video: 1 primarily featuring a black patient actor and 1 primarily featuring a white patient actor (although both videos included both patient actors). The urologist in both videos was white. Patients who self-identified as white or black received the version that more predominantly featured the patient actor that matched their own race to increase their likelihood of identifying with the individual performing the desired behavior, which has been shown to more effectively improve self-efficacy.19,22,23 Patients who identified as a race other than black or white (e.g., Asian) were randomized between the 2 video versions. Due to limited resources and the patient population served at the site of the study, the study team decided to limit the patient actor races to white and black since these were the primary races in the anticipated study population, while acknowledging that unfortunately this means that some individuals (e.g., Hispanic or Asian) would not see themselves reflected racially in the video.
See Appendix A for a copy of the video script.
Study Design
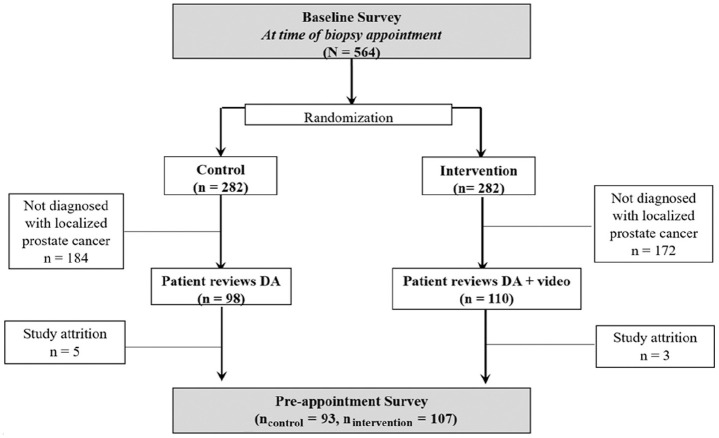
The study took place from November 2010 through April 2014 at 2 clinics within a university-affiliated hospital in the midwestern United States. We recruited male patients at the time of their prostate biopsy appointment to test for cancer (primarily due to elevated prostate-specific antigen [PSA] levels on screening tests). Participants completed an initial baseline survey and were then randomized to 1 of 2 groups: DA only v. the DA plus the SDM training video. Randomization at biopsy ensured that men had the opportunity to review the intervention materials (i.e., decision aid ± video) before they discussed their diagnoses and treatment options with their physicians (which typically occurred 1–2 weeks after the biopsy). Only men diagnosed with low- or intermediate-risk prostate cancers (Gleason score of 6 or 7 and PSA <20) were eligible to continue in the study, as these are the cancers for which patient preferences were the most salient at the time of the study and the clinical condition for which the decision aid was designed. 2 Participants completed a preappointment survey after reviewing the intervention materials but prior to their appointment with their physician to discuss biopsy results. Figure 1 shows the CONSORT diagram for who was included in the study sample for analyses.
Figure 1.
CONSORT diagram for study sample. Data were collected from all participants for both the baseline and preappointment surveys. However, we only included participants who were diagnosed with localized prostate cancer in these analyses, as they met predefined eligibility criteria to continue participation in the full study.
Measures
Table 2 indicates in which survey(s) each measure was assessed.
Table 2.
Measures Included within Each Survey
| Survey Measure | Baseline Survey | Preappointment Survey |
|---|---|---|
| Demographics | X | |
| Use and evaluation of study materials (DA ± video) | X | |
| Prostate cancer knowledge | X | X |
| Desire to participate in decision | X | X |
| Decision urgency | X | X |
| Intention to seek other opinions | X | |
| Self-efficacy for communication with doctor | X | X |
| Intention to engage in SDM-related behaviors | X | X |
SDM, shared decision making.
Demographic and medical information
Participants self-reported their age, race, ethnicity, education, and marital status on the baseline survey. Each participant’s PSA and Gleason score (measure of their cancer severity) were recorded from their medical charts.
Use and evaluation of the decision aid and video
Participants completed 7 survey questions to assess their use and evaluation of the materials they received. See Table 3 for the list of questions and response options.
Table 3.
Use and Evaluation of Decision Aid and Shared Decision Making Video: Survey Questions and Response Options
| Survey Question | Response Anchors |
|---|---|
| Only participants who received the video were asked the following questions: | |
| Did you watch the video? | 1 = not at all, 2 = some, 3 = I did not get a chance to watch it |
| Would you recommend this video to other men who are making decisions about prostate cancer treatment? | 1 = probably not, 4 = definitely |
| All participants were asked the following questions: | |
| About how much time did you spend looking at or reading the decision aid booklet? | 1 = less than 30 minutes, 2 = 30–60 minutes, 3 = 1–2 hours, 4 = more than 2 hours, 5 = I did not get a chance to look at it |
| Would you recommend this decision aid booklet to other men who are making decisions about prostate cancer treatment? | 1 = probably not, 4 = definitely |
| I felt that the amount of information in the decision aid booklet (decision aid materials) was ____. a | 1 = too little, 5 = too much |
| I felt that the information in the decision aid booklet (decision aid materials) was balanced and not slanted towards any one treatment. a | 1 = strongly disagree, 5 = strongly agree |
| How trustworthy was the decision aid booklet (decision aid materials)? a | 0 = not at all trustworthy, 11 = completely trustworthy |
For participants who received the decision aid (DA) only, these questions used the phrase “decision aid booklet.” For participants who received both the DA and the training video, the questions used the phrase “decision aid materials.”
Prostate cancer knowledge
We assessed participants’ knowledge of prostate cancer and their treatment options using 13 questions primarily adapted from previous studies.24–26 For example, patients answered the following question: “For most men with early stage prostate cancer, how much would waiting 4 weeks to make a treatment decision affect their chances of survival?” (1 = a lot, 2 = somewhat, 3 = a little or not at all, 4 = I am not sure); participants were given credit if they answered “a little or not at all.” We calculated the percentage of questions that were answered correctly. Questions and answers are listed in Appendix B.
Desire to participate in the decision
Patients’ preferred decision role was assessed with the Control Preferences Scale, in which higher numbers indicate a preference for a more active decision making role. 27 Specifically, response options for preferred decision role were as follows: 1 = My doctor(s) will make/made the decision with little input from me; 2 = My doctor(s) will make/made the decision but will seriously consider my opinion, 3 = My doctor(s) and I will make/made the decision together, 4 = I will make/made the decision after seriously considering my doctor(s) opinion, or 5 = I will make/made the decision with little input from my doctors.
Decision urgency
Participants were asked to respond to 2 items to assess the sense of urgency they had related to making a treatment decision. They were asked, “If you find out you have early stage prostate cancer, how important would it be to make a decision quickly?” Response options ranged from 1 = not at all important to 5 = extremely important. They were also asked how much they agreed with the following statement (1 = strongly disagree to 5 = strongly agree): “It is important to make a decision in the first week after a diagnosis of early stage prostate cancer.” Higher scores for both items reflected greater decision urgency.
Intentions to seek other opinions
Participants were asked, “If you find out you have early stage prostate cancer, will you plan to talk to more than one urologist?” (0 = no, 1 = yes, 2 = not sure). They were asked the same question regarding whether they planned to talk to “a radiation oncologist” and “your primary care doctor” (0 = no, 1 = yes, 2 = not sure).
Self-efficacy regarding communication with the doctor
Participants answered 3 items adapted from the “communication with physician” subscale of the Chronic Disease Self-Efficacy Scale. 28 Participants responded to “How confident are you that you could” 1) “Ask your doctor things about your treatment that concern you?” 2) “Tell your doctor that you do not understand something he/she said?” and 3) “Tell your doctor that you disagree with something that he/she is saying?” (1 = not at all confident, 3 = somewhat confident, 5 = extremely confident). A mean score was computed with a higher score indicating greater self-efficacy for communicating with the physician.
Intention to engage in SDM-related behaviors
Participants answered questions designed to assess their intentions to engage in several behaviors associated with SDM during their upcoming appointments. Questions were based on patient behaviors that were emphasized in the SDM training video. Specifically, participants were asked how likely they were to do the following participatory behaviors: (1) “Take notes when talking with your doctor,” (2) “Bring a tape recorder when talking with your doctor,” (3) “Bring in a list of questions for your doctor,” and (4) “Bring someone to the doctor’s visit with you” (1 = not at all likely, 5 = very likely).
Statistical Analyses
To test the impact of the SDM video, we conducted 1-way analyses of covariance (ANCOVAs) using IBM SPSS Version 26 (SPSS, Inc.) for continuous outcomes and χ2 tests for categorical outcomes. In all analyses for which we had data from both the baseline and preappointment surveys, we included participants’ baseline values as a covariate after ensuring that these baseline values did not differ across conditions.
Funding
All funding agreements ensured independence of authors to design and conduct the study, analyze data, and publish study findings.
Results
Overall, 564 patients were recruited into the study, and 208 participants were diagnosed with early stage prostate cancer and remained in the study. The remainder of participants were either diagnosed with later-stage prostate cancer or did not have cancer; data from those patients are not included in this study. On average, participants were 61.58 years old (SD = 7.69, range = 43–84). Most of the sample had at least a bachelor’s degree (64%) and were primarily white (88%). Basic demographics and clinical characteristics did not differ between the DA-only and the DA plus SDM video groups (Table 4).
Table 4.
Demographic and Clinical Characteristics of Participants by Study Arm. a
| DA Only (n = 98) | DA plus SDM Video (n = 110) | Total Sample (N = 208) | Group Comparison | |
|---|---|---|---|---|
| Age, mean (SD), y | 61.23 (8.0) | 61.89 (7.5) | 61.58 (7.7) | t(206) = .63; P = 0.54 |
| Ethnicity | ||||
| Hispanic | 2 (2.0) | 4 (3.6) | 6 (2.9) | χ2(1) = .47; P = 0.49 |
| Middle Eastern | 1 (1.0) | 2 (1.8) | 3 (1.4) | χ2(1) = .23; P = 0.63 |
| Race | χ2(4) = 3.77; P = 0.44 | |||
| White or Caucasian | 85 (86.7) | 98 (89.1) | 183 (88) | |
| Black or African American | 10 (10.2) | 7 (6.4) | 17 (8.2) | |
| American Indian or Alaskan Native | 0 (0.0) | 1 (0.9) | 1 (.5) | |
| Pacific Islander or Native Hawaiian | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Asian | 1 (1.0) | 4 (3.6) | 5 (2.4) | |
| Other | 2 (2.0) | 0 (0.0) | 2 (1.0) | |
| Education | t(206) = .50; P = 0.32 | |||
| Less than high school | 2 (2.0) | 2 (1.8) | 4 (1.9) | |
| High school or GED | 9 (9.2) | 10 (9.1) | 19 (9.1) | |
| Trade school | 2 (2.0) | 1 (0.9) | 3 (1.4) | |
| Some college/associate’s degree | 24 (24.5) | 24 (21.9) | 48 (23.1) | |
| College degree | 61 (62.3) | 73 (66.4) | 134 (64.4) | |
| Marital status | χ2(3) 1.83; P = 0.61 | |||
| Married/partner | 78 (79.6) | 94 (85.5) | 172 (82.7) | |
| Divorced/separated | 9 (9.1) | 11 (10.0) | 20 (9.7) | |
| Widowed | 3 (3.1) | 3 (2.7) | 6 (2.9) | |
| Never married | 7 (7.1) | 2 (1.8) | 9 (4.3) | |
| Missing | 1 (1.0) | 0 (0.0) | 1 (0.5) | |
| Gleason score | χ2(1) = 1.01; P = 0.31 | |||
| Gleason 6 | 45 (45.9) | 44 (40.0) | 89 (42.8) | |
| Gleason 7 | 50 (51.0) | 65 (59.1) | 115 (55.3) | |
| Missing | 3 (3.1) | 1 (0.9) | 4 (1.9) | |
| PSA (ng/mL), mean (SD) | 6.03 (2.6) | 6.41 (2.8) | 6.23 (2.7) | t(202) = .80; P = 0.33 |
| Missing | 2 (2.0) | 2 (2.0) | 4 (1.9) |
PSA, prostate-specific antigen.
Values are presented as number (%) unless otherwise indicated.
Use and Evaluation of Study Materials
Most participants reported using the study materials (i.e., DA ± video) and would recommend them to other men with prostate cancer. Overall, 76% of participants reported that they spent 30 minutes or more reading the DA (a reasonable amount of time to fully review the contents) and 92% would “probably” or “definitely” recommend it. Neither of these assessments differed across condition (for time spent reviewing, F(1, 198) = .03, P = 0.70, and for recommending the DA, F(1, 198) = 2.31, P = 0.10). Of the participants who received the SDM training video, 91% of participants reported that they watched the entire video, and 93% of those would “probably” or “definitely” recommend it.
Participants felt that the amount of information they received from the materials they received (either DA only or DA plus video) was appropriate in quantity, with mean ratings not significantly different from the midpoint (3) of the response scale (1 = too little and 5 = too much), t(199) = .59, P = 0.56. Participants’ evaluation did not differ by condition (F(1, 198) = 1.14, P = 0.29). Ninety-five percent of participants “agreed” or “strongly agreed” that the information was balanced. Participants’ evaluation did not differ by condition (F(1, 198) = 1.47, P = 0.23). Finally, participants believed that the information was trustworthy, with a mean trustworthiness score of 9.36 (SD = 1.65) (0 = completely untrustworthy and 11 = completely trustworthy). Participants in the DA plus SDM video group rated the materials they received as more trustworthy than participants in the DA-only group (mean [SD]: DA + video, 9.63 [1.5] v. DA only, 9.04 [1.74]; F(1, 195) = 6.39, P = 0. 012), although average trustworthiness ratings were high across both conditions.
Differences in Key Study Outcomes
Table 5 presents the analyses assessing differences between the DA only v. DA plus SDM video on key SDM outcomes. As expected, prostate cancer knowledge did not differ across conditions. Participants who received the DA plus SDM training video reported a significantly greater preference for taking a more active role in decision making, even though participants in both groups were significantly above the midpoint of the scale (for DA-only group: t(92) = 6.53, P < 0.001; for DA + SDM video group: t(106) = 12.86, P < 0.001). This indicates a preference for patient-led decision making regardless of condition. Participants who received the DA plus SDM video reported significantly less decision urgency and more frequently planned to seek a referral to a radiation oncologist. However, participants’ plan to seek a second opinion from another urologist or speak to a primary care physician did not differ across condition. Participants who received the DA plus SDM training video reported significantly higher communication self-efficacy and reported that they were more likely to take notes and bring a tape recorder to their appointment. Participants’ reported likelihood of bringing a list of questions or bringing someone with them to the appointment did not differ by condition.
Table 5.
Differences in Key Outcomes by Study Arm a
| Outcome | DA Only | DA plus SDM Video | One-Way ANCOVA or χ2 |
|---|---|---|---|
| Prostate cancer knowledge, % correct answers, mean (SD) | 78.66 (19.11) | 82.89 (15.86) | F(1, 197) = 1.19, P = 0.28 |
| n | 93 | 107 | |
| Desire to participate in decision, b mean (SD) | 3.39 (.58) | 3.65 (.52) | F(1, 190) = 8.66, P = 0.004 |
| n | 90 | 103 | |
| Decision urgency, b mean (SD) | |||
| Make decision quickly | 3.30 (1.26) | 2.82 (1.21) | F(1, 192) = 6.00, P < 0.001 |
| Make decision first week of diagnosis | 2.34 (1.24) | 1.73 (1.02) | F(1, 192) = 20.29, P < 0.001 |
| n | 90 | 105 | |
| Plan to seek other opinion, % yes | |||
| Radiation oncologist | 51 | 73 | χ 2 (2) = 11.24, P = 0.004 |
| Second urologist | 42 | 46 | χ2(2) = 1.49, P = 0.48 |
| Primary care physician | 81 | 72 | χ2(2) = 2.29, P = 0.32 |
| n | 93 | 107 | |
| Communication self-efficacy, b mean (SD) | 4.50 (0.57) | 4.69 (0.43) | F(1, 190) = 3.91, P = 0.05 |
| n | 90 | 103 | |
| SDM-related behavioral intentions, b mean (SD) | |||
| Take notes | 2.86 (1.43) | 3.23 (1.39) | F = 8.33, P = 0.004 |
| Bring tape recorder | 1.43 (0.95) | 1.79 (1.20) | F = 7.30, P = 0.008 |
| Bring list of questions | 3.87 (1.14) | 3.90 (1.06) | F = 1.47, P = 0.23 |
| Bring someone to doctor’s appointment | 3.69 (1.45) | 3.83 (1.46) | F = 1.39, P = 0.24 |
| n | 90 | 103 | |
ANCOVA, analysis of covariance; DA, decision aid; SDM, shared decision making.
For all measures except plan to seek other opinions, participants’ baseline responses to the same item were included as a covariate. Significant differences are in bold text.
Items measured on a 1 to 5 Likert scale, where higher numbers indicate a higher desire to participate, more decision urgency, higher communication self-efficacy, and higher likelihood of participating in SDM-related behaviors.
Discussion
In this study, we developed a novel SDM training video for patients with early stage prostate cancer, evaluated its usability and acceptability, and conducted a randomized trial to evaluate the effect of the training video plus an educational DA v. the educational DA alone on a number of outcome measures associated with SDM. Both the DA and SDM training video were well received and highly used by patients. As expected, the SDM training video did not affect patients’ knowledge of their condition or treatment options because both groups received the DA, which aligns with the intention of the DA to improve factual knowledge. 29 Rather, the training video improved several measures of outcomes associated with effective SDM: an increased desire to participate in the decision, less decision urgency, improved physician communication self-efficacy, and increased intentions to seek a referral from a radiation oncologist, take notes, and record their upcoming appointments.
While DAs have been shown to improve patient knowledge, 29 studies have found that patients with early stage prostate cancer do not receive treatment concordant with their preferences,6,7 suggesting that SDM among patients with prostate cancer could be improved. Video may be an effective medium to provide patients with SDM training, including elements of communication skills training. In our study, we found that using a SDM training video that had trained actors who modeled SDM and effective communication with a physician improved both participants’ desire to engage in SDM and related behaviors (e.g., intention to take notes) and their self-efficacy for communicating with their physician. Prior studies attempting to improve patients’ communication self-efficacy have relied on “high-intensity” one-to-one coaching sessions between patients and professional communication coaches.10,16,17,30 Although several of these interventions showed promise for improving SDM, the utility of these interventions is limited by their resource intensiveness. Other approaches that focus on encouraging patients to ask questions can improve physician provision of information, which is an important aspect of the SDM process. 31 These approaches do not, however, address some of the more nuanced aspects and challenges of engaging in a full SDM process during clinical appointments. Our study suggests that videos may be an ideal low-resource format to deliver this more nuanced training to patients facing preference-sensitive decisions. Distribution of the materials could be easily incorporated into the clinic workflow; for example, the staff member rooming the patient for their biopsy could provide the patient with a packet of information that contained both the decision aid and the training video, as well as a letter explaining that all patients who undergo biopsies receive this information to help prepare them in case their biopsy comes back positive for early stage prostate cancer. Because clinicians often provide biopsy results and discuss treatment options in the same visit, this is likely best timing for distribution of the materials. For patients who do not end up getting diagnosed with early stage prostate cancer, the video would still be beneficial because the SDM skills taught in the video can be applied across multiple health care settings.
There are some limitations to our study. First, our participant group lacked diversity. Most men were white and highly educated, which was a reflection of the patient population at the study site. Replication of our study with a more diverse sample would be important to evaluate for external validity, especially given that prior studies have found race differences in treatment preference after viewing decision aids for prostate cancer. 32 Unfortunately, we were underpowered to reliably test for interactions between patient characteristics (e.g., race or income) and the effectiveness of the intervention within our data set, but future research should be attuned to this possibility and more actively recruit a diverse patient population. Second, it is not clear whether the increases in self-efficacy and intentions we observed will translate into increases in empowerment during clinical appointments. Self-efficacy expectations are a significant determinant of behavior, 19 but clinical appointments are complex, and other factors may affect whether or not patients participate in clinical appointments. Future analyses that examine actual patient participation in appointments will help elucidate whether the statistically significant increase in self-efficacy and SDM intentions associated with the video intervention translated into a practically significant difference in patient participation in SDM. Third, we intentionally chose to evaluate the effect of the addition of a SDM training video to a DA to isolate the effects of a SDM training video from the effects of knowledge provision, and the DA was slightly modified for the SDM training video group to reinforce material in the video. As a result, we did not have a “true control” in which no intervention was provided. We made this choice given the extensive evidence that decision aids improve decisional outcomes compared to no decision aid. 33 We feel that the benefits gained from our approach outweigh the cons but recognize that there are implications for the interpretation of our results. Fourth, due to logistical constraints and our anticipated patient population, we only developed 2 versions of the video: one that primarily featured an African American patient and one that primarily featured a white patient. As a result, some patients may not have seen themselves racially represented in the training video. Future research could examine whether it would be equally efficacious to use a single video that included patients from more racial groups and/or multiracial individuals. This would also decrease the resources needed to produce the training video and simplify the video distribution process. Fifth, although we chose our outcome measures intentionally to reflect the targets of our intervention, we acknowledge that all outcome measures have inherent limitations, and there are aspects of SDM that we did not address in our choice of measures. Last, the American Urology Association updated treatment guidelines in 2017, 34 after this study was conducted, to distinguish recommendations for low-risk (recommend active surveillance) v. intermediate-risk prostate cancer (recommend surgery or radiotherapy). However, they still recommend that counseling of patients should incorporate SDM principles as there are often multiple clinically accepted options. Therefore, we believe these findings continue to provide guidance in best practices for engaging patients with clinically localized prostate cancer (both low and intermediate risk).
Conclusion
Patient communication skills training interventions show promise for increasing patients’ participation in SDM, but previous interventions were limited in the scalability of their interventions. We developed and tested a novel, theory-based SDM training video as an alternative to one-on-one intensive communication training interventions. We found that a SDM training video plus a DA, compared to a DA alone, improved several measures associated with patient participation in SDM. Specifically, it 1) increased patients’ intentions to record their appointments and take notes, 2) increased patients’ sense of self-efficacy regarding patient–physician communication, 3) decreased decision urgency, and 4) increased patients’ desire to participate in the decision. The SDM video may be a cost-effective, scalable approach to teach patients with early stage prostate cancer how to more fully participate in the SDM process, although additional research is needed to determine whether these changes will translate into differences in patient behavior during clinical appointments and final decision making process.
Supplemental Material
Supplemental material, sj-docx-1-mdm-10.1177_0272989X211028563 for Preparing Patients with Early Stage Prostate Cancer to Participate in Clinical Appointments Using a Shared Decision Making Training Video by Karen Scherr, Rebecca K. Delaney, Peter Ubel, Valerie C. Kahn, Daniel Hamstra, John T. Wei and Angela Fagerlin in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X211028563 for Preparing Patients with Early Stage Prostate Cancer to Participate in Clinical Appointments Using a Shared Decision Making Training Video by Karen Scherr, Rebecca K. Delaney, Peter Ubel, Valerie C. Kahn, Daniel Hamstra, John T. Wei and Angela Fagerlin in Medical Decision Making
Footnotes
KS and RKD share first authorship.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The financial support for this study was provided entirely by a grant from the National Institutes of Health, National Cancer Institute [P50 CA101451-10] to Drs. Fagerlin and Ubel and Dr. Delaney’s research effort was supported in part by National Institutes of Health under Ruth L. Kirschstein National Research Service Award [T32HL007576] from the National Heart, Lung, and Blood Institute. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
first-authorship.
ORCID iDs: Karen Scherr  https://orcid.org/0000-0002-1972-6472
https://orcid.org/0000-0002-1972-6472
Rebecca K. Delaney  https://orcid.org/0000-0002-1589-1374
https://orcid.org/0000-0002-1589-1374
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making website at http://journals.sagepub.com/home/mdm.
Contributor Information
Karen Scherr, Department of Family Medicine and Community Health, Duke University, Durham, NC, USA.
Rebecca K. Delaney, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA.
Peter Ubel, Stanford School of Public Policy, Duke University, Durham, NC, USA.
Valerie C. Kahn, Center for Bioethics and Social Sciences in Medicine, University of Michigan, Ann Arbor, MI, USA
Daniel Hamstra, Department of Radiation Oncology, Oakland University William Beaumont School of Medicine, Auburn Hills, MI, USA.
John T. Wei, Department of Urology, University of Michigan, Ann Arbor, MI, USA
Angela Fagerlin, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA; Salt Lake City VA Center for Informatics Decision Enhancement and Surveillance (IDEAS), Salt Lake City, UT, USA.
References
- 1. Sidana A, Hernandez DJ, Feng Z, et al. Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31. [DOI] [PubMed] [Google Scholar]
- 3. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–92. [DOI] [PubMed] [Google Scholar]
- 4. Pieterse AH, Henselmans I, de Haes HCJM, Koning CCE, Geijsen ED, Smets EMA. Shared decision making: Pprostate cancer patients’ appraisal of treatment alternatives and oncologists’ eliciting and responding behavior, an explorative study. Patient Educ Couns. 2011;85(3):e251–e9. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman KE, Niu J, Shen Y, et al. Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med. 2014;174(9):1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scherr KA, Fagerlin A, Hofer T, et al. Physician recommendations trump patient preferences in prostate cancer treatment decisions. Med Decis Making. 2017;37(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Violette PD, Agoritsas T, Alexander P, et al. Decision aids for localized prostate cancer treatment choice: systematic review and meta-analysis. CA Cancer J Clin. 2015;65(3):239–51. [DOI] [PubMed] [Google Scholar]
- 8. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. [DOI] [PubMed] [Google Scholar]
- 9. Post DM, Cegala, Donald J., Miser, William F. The other half of the whole: teaching patients to communicate with physicians. Fam Med. 2002;34(5):344–52. [PubMed] [Google Scholar]
- 10. Cegala DJ, Eisenberg D. Enhancing cancer patients’ participation in medical consultations. In: Kissane D, Bultz B, Butow P, Finlay I, eds. Handbook of Communication in Oncology and Palliative Care. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 11. Cegala DJ. Patient communication skills training: a review with implications for cancer patients. Patient Educ Couns. 2003;50(1):91–4. [DOI] [PubMed] [Google Scholar]
- 12. Cegala DJ, McClure L, Marinelli TM, Post DM. The effects of communication skills training on patients’ participation during medical interviews. Patient Educ Couns. 2000;41(2):209–22. [DOI] [PubMed] [Google Scholar]
- 13. Katz ML, Fisher JL, Fleming K, Paskett ED. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012;21(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilkes MS, Day FC, Srinivasan M, et al. Pairing physician education with patient activation to improve shared decisions in prostate cancer screening: a cluster randomized controlled trial. Ann Fam Med. 2013;11(4):324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parker PA, Davison BJ, Tishelman C, Brundage MD. What do we know about facilitating patient communication in the cancer care setting? Psychooncology. 2005;14(10):848–58. [DOI] [PubMed] [Google Scholar]
- 16. Brown R, Butow PN, Boyer MJ, Tattersall MH. Promoting patient participation in the cancer consultation: evaluation of a prompt sheet and coaching in question-asking. Br J Cancer. 1999;80(1–2):242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mishel MH, Germino BB, Lin L, et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial. Patient Educ Couns. 2009;77(3):349–59. [DOI] [PubMed] [Google Scholar]
- 18. Holmes-Rovner M, Stableford S, Fagerlin A, et al. Evidence-based patient choice: a prostate cancer decision aid in plain language. BMC Med Inform Decis Making. 2005;5(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 20. Brown R, Bylund CL. Theoretical models of communication skills training. In: Kissane D, Bultz B, Butow P, Finlay I, eds. Handbook of Communication in Oncology and Palliative Care. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 21. Henry SG, Czarnecki D, Kahn VC, et al. Patient-physician communication about early stage prostate cancer: analysis of overall visit structure. Health Expect. 2015;18(5):1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelman HC. Processes of opinion change. Public Opin Q. 1961;25(1):57–78. [Google Scholar]
- 23. Feick L, Higie RA. The effects of preference heterogeneity and source characteristics on ad processing and judgements about endorsers. J Advertising. 1992;21(2):9–24. [Google Scholar]
- 24. Lee CN, Dominik R, Levin CA, et al. Development of instruments to measure the quality of breast cancer treatment decisions. Health Expect. 2010;13(3):258–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sepucha K. Spinal stenosis decision quality instrument. Available from: https://mghdecisionsciences.org/wp-content/uploads/2018/06/ss_dqi-user-manual.pdf
- 27. Degner L, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 28. Lorig K, Sterwart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: SAGE; 1996. [Google Scholar]
- 29. Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379–90. [DOI] [PubMed] [Google Scholar]
- 30. Mishel MH, Belyea M, Germino BB, et al. Helping patients with localized prostate carcinoma manage uncertainty and treatment side effects. Cancer. 2002;94(6):1854–66. [DOI] [PubMed] [Google Scholar]
- 31. Shepherd HL, Barratt A, Trevena LJ, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross-over trial. Patient Educ Couns. 2011;84(3):379–85. [DOI] [PubMed] [Google Scholar]
- 32. Langford AT, Scherer LD, Ubel PA, Holmes-Rovner M, Scherr KA, Fagerlin A. Racial differences in veterans’ response to a standard vs. patient-centered decision aid for prostate cancer: implications for decision making in African American and white men [published online June 3, 2020]. Patient Educ Couns. [DOI] [PubMed] [Google Scholar]
- 33. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: Risk stratification, shared decision making, and care options. J Urol. 2018;199(3):683–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mdm-10.1177_0272989X211028563 for Preparing Patients with Early Stage Prostate Cancer to Participate in Clinical Appointments Using a Shared Decision Making Training Video by Karen Scherr, Rebecca K. Delaney, Peter Ubel, Valerie C. Kahn, Daniel Hamstra, John T. Wei and Angela Fagerlin in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X211028563 for Preparing Patients with Early Stage Prostate Cancer to Participate in Clinical Appointments Using a Shared Decision Making Training Video by Karen Scherr, Rebecca K. Delaney, Peter Ubel, Valerie C. Kahn, Daniel Hamstra, John T. Wei and Angela Fagerlin in Medical Decision Making