Abstract
Background
The aim of this study was to analyze the clinical characteristics of 66 pediatric patients with B.1.617.2 (Delta) variant of coronavirus disease 2019 (COVID-19).
Methods
Sixty-six pediatric patients with B.1.617.2 (Delta) variant of COVID-19 admitted to the hospital from July to August 2021 were classified into mild (n = 41) and moderate groups (n = 25). Clinical characteristics, laboratory data and dynamic trends in different time periods were analyzed retrospectively.
Results
There were no statistically significant differences in age, gender ratios and clinical symptoms between the mild group and the moderate group. All the patients in the moderate group had clusters of onsets, and the incubation period was shorter than that of the mild group. Within 24 hours of admission, the levels of erythrocyte sedimentation rate, cardiac troponin I, D-dimer in the moderate group were higher than that in the mild group (P < 0.05). The titers of immunoglobulin (Ig) G and IgM antibodies gradually increased after disease onset. Thirty-five (53.03%) children were tested positive for antibodies in 4–12 days. IgG increased gradually, while IgM decreased obviously in about 15 days after disease onset. The cycle threshold values of open reading frame 1ab and nucleocapsid protein gene in the severe acute respiratory syndrome coronavirus 2 genomes increased gradually on the 3rd, 6th, 9th, and 12th days after disease onset, compared with those in day 0.
Conclusions
The symptoms of children with B.1.617.2 (Delta) variant of COVID-19 were mild. The description and analysis of the clinical characteristics and laboratory data can help medical staff to evaluate the condition of children with COVID-19 and to accumulate more clinical experience.
Keywords: B.1.617.2 (Delta), Children, Clinical features, Coronavirus disease 2019 (COVID-19)
Introduction
Coronavirus disease 2019 (COVID-19) has spread rapidly worldwide and has become a global public health incident [1, 2]. The B.1.617.2 (Delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in India in December 2020 and became the most reported variant in the country starting in mid-April 2021 [3]. By May 19, 2021, the variant was detected in 43 countries across six continents [4–6]. The B.1.617.2 (Delta) variant has a potentially higher rate of transmission than other variants [7]. There are currently many research papers on COVID-19, but data on the effect of the variant on Chinese pediatric patients are still lacking. This article retrospectively analyzed pediatric patients infected with Delta variant, described their clinical characteristics, laboratory data, and compared various biochemical indicators between the mild group and the moderate group.
Methods
Study design and participants
This single-center retrospective cohort study was conducted at the Second Hospital of Nanjing between July 2021 to August 2021. All 66 pediatric patients with B.1.617.2 (Delta) variant infection were included in the study. The age of the participants ranged from 28 days to 18 years old. The diagnosis of COVID-19 was based on the Guidelines for the Diagnosis and Treatment of Novel Coronavirus Pneumonia (8th trial edition) proposed by the National Health Commission of the People’s Republic of China and the first edition of Children's COVID-19 Diagnosis and Treatment Recommendations of Jiangsu Province [8, 9]. Participants were classified into mild (n = 41) and moderate groups (n = 25). Compared with the mild group, patients in the moderate group had significant clinical features of fever and respiratory symptoms, and most had imaging findings of pneumonia. All confirmed cases of infection were tested for the presence of SARS-CoV-2 in the respiratory tract using real-time polymerase chain reaction (RT-PCR). Patients with viral pneumonia caused by other pathogenic infections, advanced malignant tumors, lung infections caused by autoimmune diseases, and lack of relevant research data were excluded. This study was reviewed and approved by the Medical Ethical Committee of Second Hospital of Nanjing (approval number: 2020-LS-ky003).
Clinical data collection
We collected data on epidemiology including age, gender, underlying diseases, incubation period, cluster onset, and clinical symptoms. Laboratory tests were performed on the first day of hospitalization, including the blood routine variables, such as white blood cell count, lymphocyte count, acute phase reactants including C-reactive protein, procalcitonin, interleukin-6, erythrocyte sedimentation rate (ESR), liver function including alanine aminotransferase and lactate dehydrogenase, blood coagulation function including D-dimer, and myocardial enzymes including cardiac troponin I (cTnI), myokines isoenzyme, type B natriuretic peptide (BNP). Cycle threshold (Ct) values of nucleocapsid protein (N) and open reading frame 1ab (ORF1ab) genes in the SARS-CoV-2 genome tests were taken after disease onset (on day 0, day 3, day 6, day 9, day 12, respectively), while immunoglobulin (Ig) M and IgG antibodies tests were taken after disease onset (on the 1st, 5th, 10th, 15th and 20th days, respectively). All patients underwent X-ray or high-resolution low-dose chest tomography examinations as needed.
Virus test
Nasopharyngeal swabs or oropharyngeal swabs were obtained within 24 hours before admission. The pharyngeal swab was placed in a storage solution (Shengxiang China, × 1001, Sansure Biotech Gene Co. Ltd, China) and then thoroughly shaken. A 2019-nCoV nucleic acid kit (Sansure Biotech Gene Co. Ltd, Hunan, China) was used. We followed the instruction methods to carry out the PCR process. The detection targets of the RT-PCR method were the ORF1ab and N genes in the SARS-CoV-2 genome. The detection process included sample nucleic acid extraction, nucleic acid amplification, product detection and result interpretation. When ORF1ab and N genes were positive, the result was defined as a positive case. Samples of genes of ORF1ab and N with a Ct value ≤ 40 and an S-shaped amplification curve were considered positive.
Anti-human IgG and IgM assays were purchased from Sansure Biotech Gene Co. Ltd, Hunan, China. In all patients, IgG, and IgM antibodies against the SARS-CoV-2 ORF1ab and N protein in serum samples were measured using enzyme-linked immunosorbent assay. The samples with values ≥ 1.0 S/CO were considered positive for SARS-CoV-2 infection.
Statistical analysis
The characteristics of the study participants were summarized using descriptive statistics: number and percentage for categorical variables; mean and standard deviations for continuous variables. Differences in categorical variables were assessed with the χ2 test and Fisher’s exact test (n < 5). The Student’s t test was used to determine if the means of two groups of data differed. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0.
Results
General information
Sixty-six pediatric patients with COVID-19 were not vaccinated, included 30 (45.45%) males and 36 (54.55%) females. The mean age was 10.35 ± 5.86 years, and the average incubation period was 3.25 ± 2.52 days. Fifty-eight (87.8%) cases were clustered; only one child had an underlying disease, which was obesity with hyperuricemia (Table 1).
Table 1.
General information and clinical features of pediatric patients with coronavirus disease 2019
| Variables | Mild group (n = 41) | Moderate group (n = 25) | P |
|---|---|---|---|
| Mean age (y), mean ± SD | 10.99 ± 5.94 | 8.43 ± 5.94 | 0.357 |
| Male, n (%) | 17 (41.5) | 13 (52.0) | 0.281 |
| Cluster onset, n (%) | 33 (80.5) | 25 (100) | 0.018* |
| Preclinical period (d), mean ± SD | 3.73 ± 2.62 | 1.80 ± 1.46 | 0.037* |
| Clinical symptom | |||
| Fever, n (%) | 17 (41.5) | 14 (56.0) | 0.251 |
| Duration of fever, mean ± SD | 2.21 ± 1.10 | 1.93 ± 1.20 | 0.617 |
| Peak heat, mean ± SD | 38.32 ± 0.41 | 38.33 ± 0.56 | 0.949 |
| Cough, n (%) | 16 (39.0) | 15 (60.0) | 0.098 |
| Nasal congestion, n (%) | 17 (41.5) | 6 (24.0) | 0.149 |
| Sore throat, n (%) | 11 (26.8) | 6 (24.0) | 0.799 |
| Fatigue, n (%) | 5 (12.2) | 2 (8.0) | 0.701 |
| Anorexia, n (%) | 4 (9.8) | 2 (8.0) | 0.810 |
| Headache, n (%) | 2 (4.9) | 1 (4.0) | 0.681 |
| Diarrhea, n (%) | 3 (7.3) | 2 (8.0) | 0.633 |
| Vomit, n (%) | 2 (4.9) | 1 (4.0) | 0.681 |
| Decreased smell, n (%) | 2 (4.9) | 1 (4.0) | 0.681 |
| Decreased taste, n (%) | 2 (4.9) | 0 (0) | 0.382 |
| Conjunctivitis, n (%) | 0 (0) | 1 (4.0) | 0.379 |
SD standard deviation. *Compared with mild group, P < 0.05
Clinical features
The 66 pediatric patients included 31 (46.96%) cases of fever (duration of fever was 2.10 ± 1.16 days, peak heat was 38.32 ± 0.47 ℃), 31 cases of cough (46.96%), 23 (34.84%) cases of nasal congestion, 17 (25.75%) cases of sore throat, 7 (10.60%) cases of fatigue, 6 (9.09%) cases of anorexia, 3 (4.54%) cases of headache, 3 (4.54%) cases of decreased sense of smell, 5 (7.57%) cases of diarrhea, 2 (3.03%) cases of decreased sense of taste, 3 (4.54%) cases vomit, and 1 (1.51%) case of conjunctivitis. Patients in the moderate group had clusters of onset, but there were no statistically significant differences of the age, gender ratios and clinical symptoms between the mild group and the moderate group. The preclinical period of the moderate group was significantly shorter than that of the mild group (Table 1, Fig. 1).
Fig. 1.
Clinical symptoms of 66 pediatric patients with coronavirus disease 2019. a Mild group (n = 41); b moderate group (n = 25)
Laboratory data
The levels of ESR, cTnI, D-dimer and IgM antibody in the moderate group were significantly higher than those in the mild group (Table 2). In the unvaccinated 66 pediatric patients, the titers of IgG and IgM antibodies increased gradually after disease onset. On the 5th, 10th, 15th and 20th days, the IgG titers (S/CO) were 1.28 ± 0.60, 3.48 ± 0.71, 7.85 ± 0.92, and 14.56 ± 8.14, respectively; IgM titers (S/CO) were 0.82 ± 0.15, 3.14 ± 0.61, 2.90 ± 1.01, and 1.56 ± 0.63, respectively. Thirty-five (53.03%) children tested positive for antibodies in 4–12 days. IgG increased gradually, whereas IgM decreased obviously in about 15 days (Fig. 2).
Table 2.
Comparison of lab indicators between two groups of children with coronavirus disease 2019 within 24 hours of admission
| Variables | Mild group (n = 41) | Moderate group (n = 25) | P |
|---|---|---|---|
| WBC (× 109/L) | 6.04 ± 2.34 | 5.14 ± 1.37 | 0.149 |
| L (× 109/L) | 2.30 ± 1.37 | 1.82 ± 0.84 | 0.275 |
| PCT (ng/mL) | 0.06 ± 0.02 | 0.07 ± 0.03 | 0.498 |
| CRP (mg/dL) | 4.06 ± 2.37 | 6.08 ± 2.80 | 0.649 |
| ALT (mmol/L) | 17.46 ± 3.00 | 18.99 ± 3.59 | 0.807 |
| IL-6 (pg/mL) | 5.45 ± 3.21 | 9.88 ± 2.42 | 0.397 |
| ESR (mm/h) | 1.00 ± 0.01 | 3.60 ± 0.89 | 0.012* |
| LDH (U/L) | 272.37 ± 80.07 | 299.09 ± 75.01 | 0.776 |
| MB (ng/mL) | 22.09 ± 5.95 | 19.48 ± 4.88 | 0.350 |
| cTNI (pg/mL) | 1.01 ± 0.42 | 1.77 ± 0.34 | 0.047* |
| BNP (pg/mL) | 139.62 ± 28.29 | 204.95 ± 66.78 | 0.401 |
| D-dimer (ng/mL) | 0.26 ± 0.05 | 0.74 ± 0.32 | 0.032* |
| CD3 total T cell count (/µL) | 1608.84 ± 212.77 | 1092.80 ± 140.96 | 0.240 |
| CD8 total T cell count (/µL) | 450.68 ± 252.47 | 411.60 ± 242.19 | 0.283 |
| CD4 total T cell count (/µL) | 801.52 ± 120.06 | 554.00 ± 143.26 | 0.313 |
Values are presented as mean ± standard deviation. WBC white blood cell count, PCT procalcitonin, CRP C-reactive protein, ALT alanine aminotransferase, IL-6 interleukin-6, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenase, MB myokinase isoenzyme, cTnI cardiac troponin I, BNP type B natriuretic peptide. *Compared with a mild group, P < 0.05
Fig. 2.

The dynamic trend of immunoglobulin (Ig) M and IgG antibodies levels in pediatric patients with coronavirus disease 2019 (n = 66)
On day 0 after disease onset, in the mild group, the Ct values of N and ORF1ab genes were higher than those in the moderate group but with no significant differences. The Ct values of ORF1ab and N genes in the SARS-CoV-2 genome increased gradually on the 3rd, 6th, 9th and 12th days after disease onset. On the 9th to 12th days, the Ct values of 51 (77.27%) children were higher than 30. On the 9th to 12th days after disease onset, the Ct values of 51 (77.27%) children were more than 30 (Table 3).
Table 3.
Dynamic trends of nucleic acid cycle threshold values in patients with coronavirus disease 2019
| Variables | Mild group (n = 41) | Moderate group (n = 25) | Total (n = 66) | P |
|---|---|---|---|---|
| N gene (day 0) | 23.57 ± 5.48 | 22.20 ± 6.85 | 22.20 ± 6.30 | 0.484 |
| N gene (day 3) | 26.75 ± 6.00 | 25.58 ± 6.79 | 26.02 ± 6.47 | 0.528 |
| N gene (day 6) | 31.55 ± 5.30 | 29.50 ± 6.27 | 30.29 ± 5.95 | 0.231 |
| N gene (day 9) | 31.93 ± 7.70 | 30.64 ± 5.05 | 31.07 ± 5.04 | 0.443 |
| N gene (day 12) | 35.07 ± 4.42 | 31.78 ± 4.85 | 33.83 ± 5.04 | 0.120 |
| ORF1ab (day 0) | 25.76 ± 6.83 | 25.63 ± 4.99 | 25.69 ± 6.09 | 0.942 |
| ORF1ab (day 3) | 29.45 ± 5.95 | 28.06 ± 6.23 | 28.58 ± 6.10 | 0.428 |
| ORF1ab (day 6) | 33.16 ± 6.79 | 31.91 ± 5.94 | 32.37 ± 5.53 | 0.440 |
| ORF1ab (day 9) | 32.75 ± 5.75 | 32.48 ± 4.97 | 32.56 ± 4.96 | 0.879 |
| ORF1ab (day 12) | 35.64 ± 4.64 | 33.89 ± 5.38 | 34.85 ± 5.06 | 0.471 |
Values are presented as mean ± standard deviation. ORF1ab open reading frame 1ab
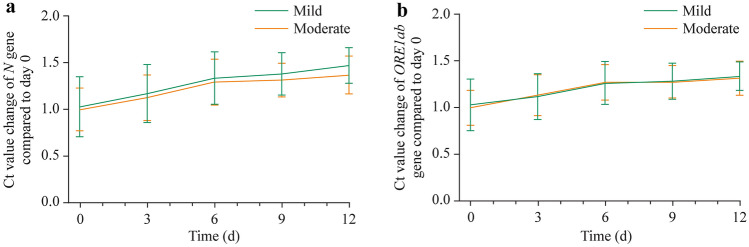
The average Ct values of the N and ORF1ab genes of the two groups on day 0 of the disease onset were assigned as a reference level. Ct values on day 3, day 6, day 9, and day 12 of each patient were compared with the reference levels. On the 3rd day, no significant difference of N and ORF1ab Ct values were found in the mild group, whereas these Ct values increased significantly in the moderator group (P < 0.05). All Ct values measured on the 6th, 9th and 12th days were significantly higher compared to the reference level (P < 0.001) in both mild and moderate groups (Fig. 3).
Fig. 3.
The results of polymerase chain reaction showed variation tendency of N and ORF1ab genes at a different time of both mild and moderate groups. a Ct values of N gene compared to day 0; b Ct values of ORF1ab gene compared to day 0. For both groups of the two genes, 3rd day vs. day 0, no significant statistical difference; 6th, 9th, and 12th days vs. day 0, P < 0.001. Ct cycle threshold, N nucleocapsid protein gene, ORF1ab open reading frame 1ab gene
Discussion
COVID-19 is a new acute respiratory infectious disease, and its worldwide epidemic has produced a major global public health event [10]. According to data from Jiangsu Provincial Center for Disease Control and Prevention, the incidence of COVID-19 among children in the epidemic in Nanjing, 2021 was as high as about 8.0%, and there were no serious cases, critically ill cases, or deaths. In February 2021, China's CDC infection statistics for the whole country indicated the child infection rate was only 2.2%, including one case of death [11]. Novel coronavirus carried stronger transmission power with each mutation. In addition to increasing the possibility of the virus binding to the receptor to gain a greater chance of entering the human body, the Delta variant also brings a higher viral load. Studies have shown that the viral load of Delta variant infection is 1260 times than that of the original strain, which is the reason why the Delta variant strain has gradually evolved into a dominant variant [12].
The present study collected 66 pediatric patients who were diagnosed with COVID-19 [B.1.617.2 (Delta) variant] after July 20, 2021 in Nanjing, China. The epidemic was area clustering. It is suggested that the focus of prevention of children's infection, strengthening the family protection and daily child health care were very important to prevent the spread of epidemic among children.
All pediatric patients were mild or moderate cases, mainly from the mild group with no severe or critically infected patients. The patients showed nonspecific clinical features, such as fever, dry cough, fatigue, and stuffy nose. Some patients presented with a decreased sense of smell and taste as the first symptoms [13], a few patients with conjunctivitis, myalgia, diarrhea, and other symptoms. Compared with mild patients, moderate patients had a higher incidence of fever, shorter incubation period and longer duration. We found there were no severe or critically ill cases. The reasons for this might be as follows: (1) the innate immunity and adaptive immunity decline with age [14]; (2) comorbidity may be risk factors for poor outcome [2]; (3) underdeveloped cellular structure of the lung, which was called angiotensin-converting enzyme 2 to bind the S protein of the SARS-CoV-2 viruses, might play a role in decreased injury to bodies [15, 16]. However, unlike adults with chest pain, shortness of breath, cough, and fever as the main manifestation [17], some children had no clear clinical manifestations at the first diagnosis this year, so suspected asymptomatic children need to be screened repeatedly and diagnosed in combination with epidemiological history.
Several studies have shown that the increase of cTnI is related to the poor prognosis of patients with COVID-19 [18]. Cardiac troponin abnormalities are common in patients with severe novel coronavirus infection, and most of them are myocardial infarction caused by non-ischemic myocardial injury or imbalance of myocardial oxygen supply. Cardiac troponin abnormality has the value of predicting prognosis. In the present study, through the comparison of the clinical test data of the two groups, it was found that the cTnI of moderate patients was significantly higher than that of mild ones, suggesting that cTnI is also an important index to judge the severity in children. D-dimer is also an important index to predict the prognosis of patients. Studies have shown that the value of D-dimer in COVID-19 in critically ill patients is significantly higher than that in non-critically ill patients. It is found that compared with the surviving patients, the level of D-dimer in non-survivors continues to rise [19]. The normal ESR ranges are usually very high during severe inflammation due to infection [20, 21]. ESR also showed differences between the two groups in this study, and the same result had also been verified in adult patients [22]. Different from the results of adult patients, in the present study procalcitonin [23], lymphocyte count, BNP, and CD3 were not found to be different between the two groups. The analysis of the reasons suggested that the condition of these children was relatively mild, and the difference of clinical symptoms was not high, which was consistent with the actual situation.
After infection, no corresponding antibodies were detected in the patients at the initial stage. Thirty-five children tested positive for antibodies in 4–12 days. IgG increased gradually, whereas IgM decreased obviously in about 15 days during the disease. We also have noted a similar trend of specific antibodies in the research of other scholars [24–26]. Owing to the compliance of patients’ discharge and examination, the longest observation time in the present study was 25–31 days. Most of the children showed a steady increase in IgG and a decrease in IgM at discharge. As the virus is cleared, IgM also dropped. The patient will gain a certain degree of immunity within three months after recovery [27].
Lower Ct value and longer viral shedding in B.1.617.2 provided a potential mechanism for increased transmissibility [28]. To explore the dynamic trend of nucleic acid Ct value of COVID-19 in 66 pediatric patients, we compared the Ct values of N and ORF1ab genes at admission. We found that there was no statistical difference between the two groups, which may indicate that the viral load is not related to the severity of the disease. The number showed an upward trend on the 10th-15th day in the course of COVID-19, the Ct value in the vast majority of cases were more than 30, but in the next two weeks, it fluctuated between 30 to 40. We observed that many patients showed clinical asymptomatic or continuous improvement, but the nucleic acid Ct value fluctuated. The reasons are as follows: (1) the influence of sampling depth; (2) the post-negative positive PCR result may be the RNA particles, rather than reinfection [29]. Although most patients become negative in 20–30 days or even longer, the clinical symptoms basically disappear and no further treatment is needed except quarantine. This might suggest that such patients could receive hierarchical management of epidemic prevention measures.
In summary, the epidemic situation has a strong ability to spread among people, and there are fewer cases of COVID-19 infection in children. Most of these cases are mild and moderate infections, whose clinical symptoms are relatively mild. Aggregation in the family is the main way for children to become infected with COVID-19. Early laboratory examination and imaging examination can be nonspecific. It is very important to inquire about the history of epidemiology in detail. Because children are still susceptible to COVID-19 infection, it still needs to be paid close attention to as a special group. According to the epidemiological characteristics of the epidemic in this study, the duration of the disease is short, the number of pediatric patients is small, and there are no severe or critical cases, which may have a certain impact on the statistical results.
Author contributions
HJ and HXC contributed equally to this work. They conceptualized and designed the study, carried out the analyses, and drafted the initial manuscript. FMX, CJ, and CQR designed the data collection instruments, and collected data. LZ and HZL contributed to the important intellectual content during manuscript drafting and revision. GXH contributed to the important intellectual content during manuscript drafting and revision, reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study was supported by the China Postdoctoral Science Foundation (No. 2020M681674, to GXH), and the Nanjing Medical Science and Technique Development Foundation (No. YKK20130, to LZ).
Data availability
All data generated or analyzed during this study are included in this published article. The datasets are also available from the corresponding author on reasonable request.
Declarations
Ethical approval
This work was reviewed and approved by the Medical Ethical Committee of Second Hospital of Nanjing (approval number: 2020-LS-ky003). Written informed consent was obtained from each enrolled patient.
Conflict of interest
Author GXH is a member of the Editorial Board for the World Journal of Pediatrics. The paper was handled by the other Editor and has undergone a rigorous peer-review process. Author GXH was not involved in the journal's review of, or decisions related to, this manuscript. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/6/2022
A Correction to this paper has been published: 10.1007/s12519-022-00557-x
Contributor Information
Zhi-Liang Hu, Email: huzhiliang@njucm.edu.cn.
Xu-Hua Ge, Email: gexuhua@njmu.edu.cn.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam D. What scientists know about new, fast-spreading coronavirus variants. Nature. 2021;594:19–20. doi: 10.1038/d41586-021-01390-4. [DOI] [PubMed] [Google Scholar]
- 4.Reis BY, Barda N, Leshchinsky M, Kepten E, Hernán MA, Lipsitch M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med. 2021;385:2101–2103. doi: 10.1056/NEJMc2114290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M, et al. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms. 2021;9:1542. doi: 10.3390/microorganisms9071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta variant B.1617.2 COVID-19. Clin Pract. 2021;11:778–784. doi: 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China. Diagnosis and treatment protocols of coronavirus disease 2019 (8th trial edition). 2021. http://www.nhc.gov.cn. Accessed 14 May 2021.
- 9.Jiangsu Provincial Expert Group of Pediatrics on the Medical Treatment of Novel Coronavirus Infection and Pneumonia. Expert recommendations on the diagnosis and treatment program for children with novel coronavirus infection in Jiangsu Province (trial version 1). J Nanjing Med Univ (Natural Science). 2020;40:309–14 (in Chinese).
- 10.Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epidemiology Group of New Coronavirus Pneumonia Emergency Response Mechanism of Chinese Center for Disease Control and Prevention. Analysis of epidemiological characteristics of new coronavirus pneumonia. Chin J Epidemiol. 2020;41:145–51 (in Chinese).
- 12.Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021 doi: 10.1038/d41586-021-01986-w. [DOI] [PubMed] [Google Scholar]
- 13.Mak PQ, Chung KS, Wong JS, Shek CC, Kwan MY. Anosmia and ageusia: not an uncommon presentation of COVID-19 infection in children and adolescents. Pediatr Infect Dis J. 2020;39:e199–200. doi: 10.1097/INF.0000000000002718. [DOI] [PubMed] [Google Scholar]
- 14.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang F, Luo XP. Facing the pandemic of 2019 novel coronavirus infections: the pediatric perspectives. Zhonghua Er Ke Za Zhi. 2020;58:81–85. doi: 10.3760/cma.j.issn.0578-1310.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Balla M, Merugu G, Nesheiwat Z, Patel M, Sheikh T, Fatima R, et al. Epidemiological and clinical characteristics of 217 COVID-19 patients in Northwest Ohio, United States. Cureus. 2021;13:e14308. doi: 10.7759/cureus.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panigrahy N, Policarpio J, Ramanathan R. Multisystem inflammatory syndrome in children and SARS-CoV-2: a scoping review. J Pediatr Rehabil Med. 2020;13:301–316. doi: 10.3233/PRM-200794. [DOI] [PubMed] [Google Scholar]
- 21.Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. 2020;148:e196. doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pink I, Raupach D, Fuge J, Vonberg RP, Hoeper MM, Welte T, et al. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection. 2021;49:935–943. doi: 10.1007/s15010-021-01615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mou D, Feng H, Cao R, Weng X, Zhao L, Yang L, et al. Profile of specific antibodies to the SARS-CoV-2. J Med Microbiol. 2021;70:001335. doi: 10.1099/jmm.0.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Xu X, Chang Z, Wang H, Zhong X, Tong X, et al. The dynamic changes of serum IgM and IgG against SARS-CoV-2 in patients with COVID-19. J Med Virol. 2021;93:924–933. doi: 10.1002/jmv.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MPHS, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2021 doi: 10.1093/cid/ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song KH, Kim DM, Lee H, Ham SY, Oh SM, Jeong H, et al. Dynamics of viral load and anti-SARS-CoV-2 antibodies in patients with positive RT-PCR results after recovery from COVID-19. Korean J Intern Med. 2021;36:11–14. doi: 10.3904/kjim.2020.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets are also available from the corresponding author on reasonable request.