Abstract
Bullous pemphigoid (BP) is an autoimmune bullous disease caused by circulating autoantibodies toward the hemidesmosomal antigens BP180 and BP230. Cases of BP have been described following vaccinations against tetanus, poliomyelitis, diphtheria, influenza, pneumococcus, meningococcus, hepatitis B and rabies. The putative mechanism by which COVID-19-vaccines may induce BP has not been clarified. An Italian multicentre study was conducted to collect clinical, histopathological and immunopathological data of patients with BP associated with COVID-19-vaccines. Twenty-one cases were collected, including 9 females and 12 males (M/F = 1.3) with a median age at diagnosis of 82 years. Seventeen patients received the COMIRNATY Pfizer-BioNTech vaccine, two the Moderna mRNA-1273 vaccine, one the ChAdOx1/nCoV-19-AstraZeneca/ Vaxzevria vaccine and one received the first dose with the ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria vaccine and the second dose with the COMIRNATY Pfizer-BioNTech vaccine. Median latency time between the first dose of anti-SARS-CoV-2 vaccine and the onset of cutaneous manifestations was 27 days. Median BPDAI at onset was 42. Eleven out of seventeen patients (65%) had positive titres for anti-BP180 antibodies with a median value of 106.3 U/mL on ELISA; in contrast, only five out of seventeen (29%) were positive for anti-BP230 antibodies, with a median of 35.3 U/mL. In conclusion, in terms of mean age, disease severity at diagnosis and clinical phenotype vaccine-associated BP patients seem to be similar to idiopathic BP with an overall benign course with appropriate treatment. On the other hand, the slight male predominance and the reduced humoral response to BP230 represent peculiar features of this subset of patients.
Keywords: bullous pemphigoid, vaccine, COVID-19, autoantibodies, SARS-CoV-2, triggering factors, BP180, BP230
Introduction
Bullous pemphigoid (BP) is an autoimmune bullous disease caused by circulating autoantibodies toward the hemidesmosomal antigens BP180 and BP230 (1).
Although the majority of cases are considered idiopathic, several trigger factors have been described in literature, such as UV light, radiation, drugs and trauma. Moreover, cases of BP developed following vaccine injection have recently been reported, with a variable latency time, mostly <1 month (2–5). Specifically, multiple vaccinations are reported as trigger for BP, including the ones for influenza (4, 6), pneumococcus (7), meningococcus (2, 8), varicella-zoster (3), rabies (9) and hexavalent (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and Haemophilus influenzae B) (2, 10).
More recently, both new onset and reactivation of BP have been observed after the inoculation of SARS-CoV-2 vaccines (11–14). The putative mechanism by which COVID-19 vaccines may induce BP has not been thoroughly investigated.
Autoimmune mechanisms following SARS-CoV-2 infection may be associated with molecular mimicry (15, 16). On the other hand, vaccination may activate B and T-cell immunity, triggering an autoimmune response in genetically predisposed individuals (17).
The present multicentre study aimed at investigating the demographics, clinical and immunopathological features of SARS-CoV-2 vaccine-associated BP.
Methods
SARS-CoV-2 vaccine-associated BP patients examined between February 1, 2021, and November 15, 2021, were included in the present multicentre study involving six Dermatology Clinics (Milan, Cagliari, Florence, Genoa, Bergamo and Rome). The following eligibility criteria were adopted: (1) age of 18 years or older; (2) recent anti-SARS-CoV2 vaccination (<2 months after either the I or II dose); (3) a Naranjo score of 4 or above concerning the association between BP and SARS-CoV-2 vaccine; (4) absence of newly prescribed medications (in the 3 months preceding BP onset) or dipeptidyl peptidase 4 inhibitors; (5) diagnosis of BP based on typical findings on clinical, histopathological and/or immunopathological [IgG and/or C3 deposits along the dermal-epidermal junction (DEJ) on direct immunofluorescence (DIF) and/or indirect immunofluorescence (IIF) microscopy] examinations. The study was conducted in accordance with the Declaration of Helsinki guidelines and all patients gave written informed consent. The present study is a combined retrospective and prospective study. Clinical data were collected from electronic charts but also directly from patients at baseline or during the follow up visit. Skin manifestations were directly evaluated by a dermatologist. Each patient was examined at least twice (during the period of skin manifestations and after 3 months). Response to treatment was evaluated according to the recommendations from the International Pemphigoid Committee (18). Each participating center was asked to provide the following data: sex; age at onset; SARS-CoV-2 vaccine type; first and second dose date; time from SARS-CoV2 vaccine administration and BP onset; Naranjo score; comorbidities and concomitant medications; clinical scores [Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) and Bullous Pemphigoid Disease Area Index (BPDAI), histopathological and immunopathological features (direct and/or indirect immunofluorescence, ELISA-tests); COVID-19 medications and duration of follow-up.
To identify anti-BP180 and anti-BP230 autoantibodies in patients' serum, commercial ELISA kits (Euroimmun, Padova, Italia) were used, in accordance with the manufacturer's instructions. A cut-off value of >20 U/mL was used for both type of test. As for DIF microscopy the sections stained with fluorescein isothiocyanate-conjugated goat anti-human Ig and C3 (Kallestad Diagnostic, Chaska, MN, USA), were analyzed under a fluorescence microscope. DIF results were recorded by taking into consideration the nature of the immune deposits (IgG, IgA, IgM, C3), the location of the immune deposits and the extent and the pattern of immune complex deposits (granular or linear). IIF was performed on slides containing human epithelial substrate and human salt-split skin as described (19).
Results
Twenty-one cases of SARS-CoV2 vaccine-associated BP were collected, including 9 females and 12 males (M/F = 1.3) with a median age at diagnosis of 82 (IQR: 74–85.5) years (Table 1). Seventeen patients received the COMIRNATY Pfizer-BioNTech vaccine, two the Moderna mRNA-1273 vaccine, one the ChAdOx1/nCoV-19-AstraZeneca/ Vaxzevria vaccine and one received the first dose with the ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria vaccine and the second dose with the COMIRNATY Pfizer-BioNTech vaccine. Median latency time between the first dose of SARS-CoV2 vaccine and the onset of cutaneous manifestations was 27 (IQR: 7–34) days (Table 1). The onset of clinical manifestations occurred in eight patients after the first dose and in 13 after the second dose. Among those with BP appearance between the first and the second dose, median latency time was 6.5 (IQR: 4–7) days from the first dose, whereas among those with BP onset after the second dose, the median latency was 7 (IQR: 4–14.5) days from the second dose [and 32 (IQR: 27–36.5) days from the first one]. Nineteen patients had a Naranjo score ≥6 while two had a Naranjo score of 4. Baseline BPDAI scores were available for all patients. Median BPDAI at onset was 42 (IQR: 18.5–61). Baseline ABSIS scores were available for 16 out of 21 patients. Median ABSIS at onset was 30 (IQR: 15.75–58.5) (Table 1). Laboratory exams were within normal ranges. Eleven out of seventeen patients (64.7%) had positive (>20 U/mL) titres for anti-BP180 antibodies with a median value of 106.3 U/mL on ELISA (IQR: 40–237.5 U/mL); in contrast, only 5 out of 17 (29.4%) were positive for anti-BP230 antibodies, with a median of 35.3 U/mL on ELISA (IQR: 25.9–249.3 U/mL) (Table 2). The clinicopathological picture was typical across our cohort (Figures 1, 2). DIF showed linear IgG and C3 deposits along the DEJ (9 out of 18 cases), isolated linear C3 deposits along the DEJ (6/18), isolated linear IgG deposits along the DEJ (1/18), isolated granular C3 deposits along the DEJ (1/18). DIF turned out negative in one case. IIF performed on salt-split human skin revealed epidermal side binding in all tested cases (13/21) (Table 2).
Table 1.
Demographics and clinical features of reported cases.
| N. | Sex, age (years) | Vaccine | Concomitant medications | Latency from the 1st dose (days) | Naranjo score# | Baseline BPDAI | Baseline ABSIS | Treatment | BPDAI at 3 months | ABSIS at 3 months |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F, 84 | Pfizer | Alendronate | 25 | 6 | 70 | 21 | Topical and systemic CS plus doxycycline | 0 | 0 |
| 2 | M, 83 | Pfizer | Allopurinol, amiodarone, amlodipine, bicalutamide, clonidine, furosemide, insulin, valsartan, warfarin | 32 | 6 | 50 | 18 | Topical and systemic CS plus doxycycline | 0 | 0 |
| 3 | F, 56 | Moderna | none | 7 | 6 | 17 | 4.5 | Topical CS plus doxycycline | 0 | 0 |
| 4 | M, 79 | Pfizer | ASA, amiodarone, atorvastatin, clopidogrel, hydrochlorothiazide, olmesartan, pantoprazole, tamsulosin | 4 | 6 | 23 | 10 | Topical CS plus doxycycline | 0 | 0 |
| 5 | M, 86 | Pfizer | Amiodarone, atorvastatin, clopidrogrel, domperidone, escitalopram, hydrochlorothiazide, levodopa/benserazide | 37 | 6 | 20 | 12 | Topical CS | 0 | 0 |
| 6 | M, 91 | Pfizer | Allopurinol, atorvastatin, furosemide, insulin, nebivolol | 28 | 6 | 80 | 30 | Topical and systemic CS | 0 | 0 |
| 7 | M, 86 | Pfizer | ASA, fenofibrate, isosorbide, ivabradine, pyridostigmine | 36 | 6 | 52 | 20 | Topical and systemic CS plus doxycycline | 0 | 0 |
| 8 | F, 84 | Moderna | Amlodipine, glimepiride, metformin, levothyroxine | 7 | 6 | 40 | 15 | Topical and systemic CS plus doxycycline | 0 | 0 |
| 9 | M, 84 | Pfizer | None | 23 | 6 | 37 | 54 | Systemic CS | 0 | 0 |
| 10 | F, 82 | Pfizer | None | 34 | 6 | 52 | 90 | Systemic CS | 6 | 27 |
| 11 | M, 76 | Pfizer | Candesartan, hydrochlorothiazide | 34 | 6 | 47 | 70 | Systemic CS | NA | NA |
| 12 | M, 78 | Pfizer | none | 4 | 4 | 42 | NA | Topical CS | 0 | NA |
| 13 | F, 90 | Pfizer | Allopurinol, hydrochlorothiazide, losartan | 28 | 4 | 142 | NA | Topical and systemic CS | 25 | NA |
| 14 | M, 90 | Pfizer | Alfuzosin, allopurinol, darbepoetin alfa, furosemide, levothyroxine, pregabalin, warfarin | 64 | 6 | 20 | NA | Systemic CS | 0 | NA |
| 15 | M, 72 | Pfizer | Insulin, telmisartan | 16 | 6 | 80 | NA | Topical and systemic CS plus MTX | 29 | NA |
| 16 | M, 80 | Pfizer | ASA, amlodipine, atenolol, atorvastatin, finasteride, salmeterol/fluticasone, zofenopril | 6 | 6 | 71 | 90 | Topical and systemic CS | 51 | 70 |
| 17 | F, 77 | AstraZeneca | Amlodipine, bisoprolol, furosemide, ramipril, sertraline | 3 | 8 | 42 | 60 | MTX | 0 | 0 |
| 18 | F, 60 | Pfizer | None | 75 | 6 | 10 | 36 | Systemic CS | 0 | 0 |
| 19 | F, 70 | Pfizer | None | 27 | 6 | 15 | 35 | Systemic CS | 1 | 5 |
| 20 | F, 72 | AstraZeneca (1st dose), Pfizer (2nd dose) |
ASA, amlodipine, levothyroxine, perindopril, simvastatin | 7 | 6 | 15 | NA | Systemic CS plus dapsone | 3 | NA |
| 21 | M, 85 | Pfizer | ASA, atenolol, dutasteride, indapamide, perindopril, tamsulosin | 27 | 6 | 15 | 30 | Systemic CS | 41 | 50 |
Naranjo scale interpretation: doubtful (≤0), possible (1-4), probable (5-8), definite (≥9).
CS, corticosteroids; MTX, methotrexate; NA, not available; ABSIS, Autoimmune Bullous Skin Disorder Intensity Score; ASA, acetylsalicylic acid; BPDAI, Bullous Pemphigoid Disease Area Index.
Table 2.
Immunopathological features of reported cases.
| N. | Histopathology§ | DIF | IIF | ELISA IgG anti-BP180 (U/mL) | ELISA IgG anti-BP230 (U/mL) |
|---|---|---|---|---|---|
| 1 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 40 | 8.5 |
| 2 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 492.1 | 425 |
| 3 | + | Neg | IgG along the DEJ. SSS: roof | 136.8 | 73.6 |
| 4 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 237.5 | 0 |
| 5 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 46.9 | 9.7 |
| 6 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 14.9 | 0 |
| 7 | + | Linear IgG/C3 deposits along the DEJ | NA | NA | NA |
| 8 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 247.2 | 5.7 |
| 9 | + | Linear C3 deposits along the DEJ | NA | 0 | 0 |
| 10 | + | Linear IgG/C3 deposits along the DEJ | NA | 0 | 0 |
| 11 | + | Linear C3 deposits along the DEJ | NA | 0 | 0 |
| 12 | + | NA | IgG along the DEJ. SSS: roof | 29.1 | 29.6 |
| 13 | + | Linear IgG deposits along the DEJ | IgG along the DEJ. SSS: roof | 106.3 | 2.9 |
| 14 | + | Linear C3 deposits along the DEJ | neg | 3.3 | 1.3 |
| 15 | + | Linear C3 deposits along the DEJ | neg | 140.4 | 0 |
| 16 | + | Linear IgG/C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | NA | NA |
| 17 | + | Linear C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 52.9 | 22.2 |
| 18 | + | Granular C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 23.7 | 35.3 |
| 19 | + | Linear C3 deposits along the DEJ | IgG along the DEJ. SSS: roof | 5.5 | 1.8 |
| 20 | + | NA | NA | NA | NA |
| 21 | + | NA | NA | NA | NA |
Consistent with bullous pemphigoid, i.e., subepidermal blistering and eosinophil-rich infiltrates.
DIF, direct immunofluorescence; IIF, indirect immunofluorescence; DEJ, dermal-epidermal junction; SSS, salt-split skin; NA, not available.
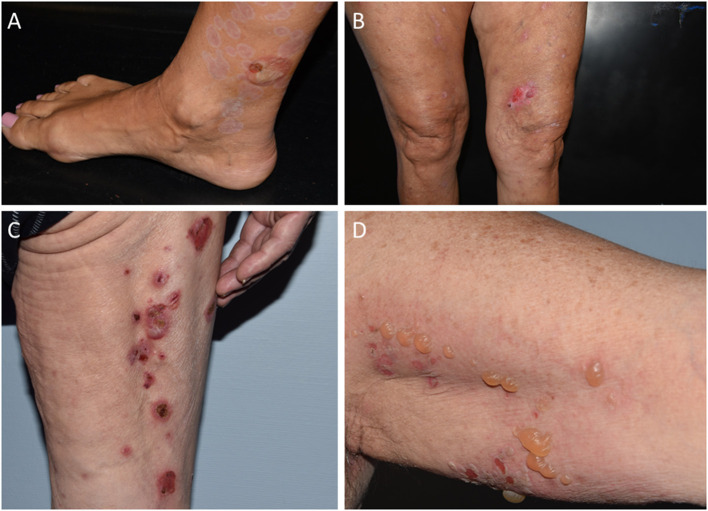
Figure 1.
Clinical spectrum of vaccine-associated BP patients. (A) Acral distribution of active blister associated with older lesions in partial resolution, resulting in mild erythema and hypopigmentation. (B) Sero-hemorrhagic bullous, pruritic eruption on medial surface of left thigh, surrounded by multiple prurigo-like specific lesions. (C) Linear distribution of erythematous blisters, resulting in crusts and erosions. (D) Blisters and erosions with mild erythema located on left axilla.
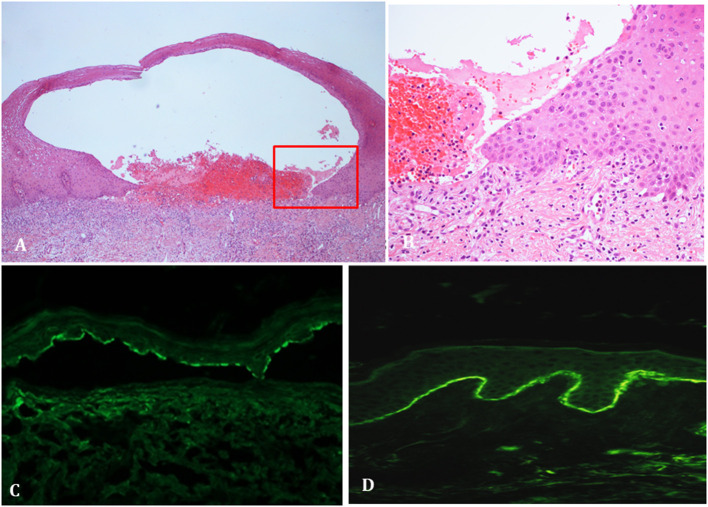
Figure 2.
Histopathological and immunopathological findings of vaccine-associated BP patients. (A) Histopathology showing subepidermal detachment accompanied by inflammatory infiltrates in the dermis (hematoxylin and eosin staining). (B) Close-up view revealing the supepidermal detachment with a dermal inflammatory infiltrate, mainly consisting of lymphocytes and eosinophils (hematoxylin and eosin staining). (C) Salt splin skin in indirect immunofluorescence shows IgG deposits along the dermo-epidermal junction. (D) Direct immunofluorescence shows linear IgG/C3 deposits along the dermo-epidermal junction.
Treatment included systemic corticosteroids (7), topical and systemic corticosteroids (3), topical and systemic corticosteroids plus doxycycline (4), topical corticosteroids plus doxycycline (2), topical and systemic corticosteroids plus methotrexate (1), systemic corticosteroids plus dapsone (1) and topical corticosteroids alone (2), methotrexate alone (1) (Table 1).
At 3 months, 13 patients achieved a complete response, whereas 6 had a partial response and one had stable disease [mean ABSIS percentage change = −80.75% (SD ± 44.25; n = 15); mean BPDAI percentage change = −78.14% (SD ± 60.21; n = 20)] (Table 1).
Discussion
Vaccination has rarely been associated with new-onset dermatoses as well as flaring of pre-existent dermatological disease (11). SARS-CoV-2-vaccine-associated cutaneous eruptions encompass a growing spectrum of clinicopathological varieties, including local injection site reactions, urticarial eruptions, morbilliform eruptions, pernio/chilblain-like lesions, cosmetic filler reactions, herpes zoster and herpes simplex flares, pityriasis rosea-like eruptions (11, 20, 21). Autoimmune bullous skin diseases have also been observed following SARS-CoV-2-vaccination, with approximately 34 individual cases of vaccine-associated BP currently described (12, 14, 17, 22–28) (Supplementary Table 1). According to the registry-based studies by McMahon et al., BP-like eruptions accounted for 20% (12/58) of biopsy-proven SARS-CoV-2-vaccine-associated cutaneous reactions and 1.5% overall (11, 22).
The present multicentre study reports 21 cases of SARS-CoV-2 vaccine-associated BP, representing the largest case series to date.
Median age at onset (81 years) was in line with published observations [82.5 (IQR: 71.25–84.75) years; n = 24/34 with age available] (23–28). Likewise, sex distribution showed a slight male sex preference in both our cohort (M:F = 1.3) and available reports (M:F = 1.2; n = 22 with gender available) (23–28).
Vaccine-induced BP was more frequently associated with the Pfizer vaccine (80.1 vs. 67.6% of available reports), as compared with other mRNA- (Moderna mRNA-1273, 9.5 vs. 29.4% of available reports) or vector-based vaccines (ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria, 9.5 vs. 2.9% of available reports). In line with our data McMahon and coworkers have recently found more BP cases associated with Pfizer vaccine than with Moderna (64 vs. 36%) (21). It is unclear whether this association depends on the greater employment of the Pfizer vaccine or if it underlies a deeper pathogenetic link. In fact, at the time of this study the percentage of Pfizer administration to adult patients was much higher (69.4%) in comparison with Moderna (18.3%), AstraZeneca (10.6%) and Janssen (1.7%) (29). In addition, in the present and all reported studies the sample size is too small to get meaningful result in term of association with a specific vaccine. To assess a possible link further studies with a large sample size standardized by specific vaccine administration should be performed.
Overall, the median latency time between the first dose and onset of cutaneous lesions was 27 days, which is notably higher than that of available reports [median latency time from the first dose to onset: 7 (IQR: 4–22.5) days, n = 17 with timing data available]. However, direct comparison with published cases is hindered by the lack of precise reporting of vaccination timings—especially in the case of vaccines with longer, variable time intervals between doses (e.g., Moderna mRNA-1273 vaccine, ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria). Latency time from last dose was the preferred way of reporting across the literature. In our study, among those with BP appearance between the first and the second dose (n = 8), the median latency time was 6.5 (IQR: 4–7) days after the first dose, in line with available reports [median = 6 (IQR: 3–7.75) days, n = 12]. Similarly, those with BP onset after the second dose (n = 13) had a median latency time of 7 (IQR: 4–14.5) days from the latter, which is in agreement with the literature [median = 7 (2.5–14) days, n = 9]. Speculatively, a latency time shorter than a week (i.e., the minimum time required for antibody production) since the first dose may hint at a role for the stimulation of pre-existent autoimmunity in the pathogenesis of SARS-CoV-2-vaccine-associated BP. Conversely, late onset SARS-CoV-2-vaccine-associated BP may result from a dysregulated primary immune response triggered by the vaccine. Of note, it has been suggested that a one-month latency period from the time of vaccination may be appropriate for anti-basement membrane antibody induction (30).
Clinically, the presentation of SARS-CoV-2-vaccine-associated BP appears to be typical with tense bullae on an erythematous base, various degrees of cutaneous involvement, and an overall benign course with appropriate treatment (only patient n. 21 had stable disease at 3 months). Although many published reports describe a similarly favorable course (17, 24–28), in the study by Tomayko et al., five patients had ongoing disease after a follow-up period ranging from 23 to 105 days (12). Our sample size prevents the possibility to reliably compare different treatments. However, most of the subjects were easily controlled with treatment regimens concepted for milder forms of BP (i.e., topical steroids, low-to-moderate doses of systemic corticosteroids, doxycycline), supporting the assumption that the majority of COVID-19 induced BP cases would be non-severe (17, 24–26). Systemic corticosteroids as well as immunosuppressive adjuvants required to achieve disease control in BP may affect the efficacy of anti-SARS-CoV-2 vaccines. Humoral and cellular immune responses to COVID-19 mRNA vaccines are reduced in patients with immune-mediated inflammatory diseases on background methotrexate (31). Moreover, treatment with mycophenolate mofetil and rituximab also compromise anti-SARS-CoV-2 antibody responses (32). However, according to the updated international recommendations for the management of autoimmune bullous diseases during COVID-19 pandemic, lowering the dosage of immunomodulatory medications before or during the vaccination is not advisable due to the risk of exacerbations (33).
Immunopathological findings also seem to be typical, highlighting linear IgG/C3 deposits along the DEJ on DIF and epidermal side binding on SSS IIF in the vast majority of cases. The serological landscape of SARS-CoV2 vaccine-associated BP is dominated by the presence of anti-BP180 autoantibodies with a frequency (65%) comparable with literature data (34, 35). Of note, positivity for anti-BP230 autoantibodies was infrequent in our cohort with a frequency of reactivity (29%) sharply lower than that previously reported (34, 35). Previous studies, investigating the dynamics of immune response to BP antigens, described that it involves at first extracellular antigens/epitopes (BP180-NC16A domain) followed by intracellular ones (BP230) possibly exposed after tissue damage (36, 37). In the light of these findings, it could be speculated that in vaccine-associated BP, due to very short disease duration, the induction of secondary response to BP230 is not always detectable.
Vaccine-induced BP could stem from vaccine-mediated stimulation of pre-existent, sub-clinical autoreactivity against hemidesmosomal components, as seen in a proportion of pruritic dermatoses of the elderly characterized by IgG-mediated autoimmunity against BP230 (38). However, limited anti-BP230 reactivity across our cohort and published reports would not encourage this interpretation. SARS-CoV-2 vaccine-associated BP may be driven by a specific pathogenetic process in genetically predisposed individuals. Prior to translation, mRNA vaccines could trigger several pro-inflammatory pathways via Toll-like receptor (TLR)-3, TLR7 and TLR8 binding (39). Moreover, through cytokine modulation, novel antigens and adjuvants could promote T-cell-dependent immune responses leading to the production of self-reactive B cells. Indeed, SARS-CoV-2-reactive T cell clones have been reported in the infiltrate of two elderly men with vaccination-induced BP (17). A contributing role of hollow needle-induced tissue disruption during vaccination has also been hypothesized (14, 40). Although no new medications were introduced in our cohort in the 3 months preceding BP onset, the majority of our patients was receiving polypharmacy for various indications. Indeed, drugs potentially linked to drug-induced BP, including antihypertensives, salicylates and diuretics, had been administered for years in some of our cases (Table 1). It is not unconceivable that anti-SARS-CoV-2 vaccines may have created a suitable immune environment to make these individuals more prone to drug-induced BP (41).
In conclusion, SARS-CoV-2-vaccine-associated BP seems to be superimposable to idiopathic BP in terms of median age at onset and clinical presentation. On the other hand, slight male predominance and reduced humoral response to BP230 could represent peculiar features of this subset of patients. A close relationship between vaccination and BP onset is difficult to prove considering the extensive vaccination of the adult population during COVID-19 pandemic. However, the recent immunopathological findings by Gambichler et al. (17) as well as timing reported across our cohort and published cases support the hypothesis of a causal link between SARS-CoV-2 vaccine and BP development. Further research is warranted to better define the nature of SARS-CoV-2-vaccine-associated immune dysregulation leading to BP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Istituto Dermopatico dell'Immacolata (IDI)-IRCCS. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MC, GD, and AM: designed the study. CAM GGe, PV, PS, EC, GGa, AP, EA, LA, RM, MC, EM, AC, SP, BD, and AM: enrolled patients. FM, RM, and GP: carried out the experiment. CAM, CM, GG, MC, GD, and AM: wrote the manuscript. CAM, MC, and GD: contributed to the interpretation of the results. GD and AM: conceived and planned the experiments. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Progetto Ricerca Corrente and Ricerca Finalizzata N 12367807 of the Italian Ministry of Health, Rome, Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Three Italian Centers (IDI-IRCCS; USL Toscana Centro; Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico) participating to this work are members of the European Reference Network for skin diseases.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.841506/full#supplementary-material
References
- 1.Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. (2017) 18:513–28. 10.1007/s40257-017-0264-2 [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente S, Hernández-Martín Á, de Lucas R, González-Enseñat MA, Vicente A, Colmenero I, et al. Postvaccination bullous pemphigoid in infancy: report of three new cases and literature review. Pediatr Dermatol. (2013) 30:741–744. 10.1111/pde.12231 [DOI] [PubMed] [Google Scholar]
- 3.Chacón GR, Sinha AA. Bullous pemphigoid after herpes zoster vaccine administration: association or coincidence? J Drugs Dermatol. (2011) 10:1328-30. [PubMed] [Google Scholar]
- 4.Walmsley N, Hampton P. Bullous pemphigoid triggered by swine flu vaccination: case report and review of vaccine triggered pemphigoid. J Dermatol Case Rep. (2011) 5:74–6. 10.3315/jdcr.2011.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moro F, Fania L, Sinagra JLM, Salemme A, Di Zenzo G. Bullous pemphigoid: trigger and predisposing factors. Biomolecules. (2020) 10:1432. 10.3390/biom10101432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Doval I, Mayo E, Nogueira Fariña J, Cruces MJ. Bullous pemphigoid triggered by influenza vaccination? Ecological study in Galicia, Spain. Br J Dermatol. (2006) 155:820–3. 10.1111/j.1365-2133.2006.07411.x [DOI] [PubMed] [Google Scholar]
- 7.Maki N, Hashimoto T, Yamada T, Ishii N, Tsuruta D, Demitsu T. Case of pemphigoid with immunoglobulin G antibodies to BP180 C-terminal domain and laminin-γ1 (p200) developed after pneumococcal vaccination. J Dermatol. (2021) 48–101–5. 10.1111/1346-8138.15626 [DOI] [PubMed] [Google Scholar]
- 8.Navarro-Navarro I, Jiménez-Gallo D, Valenzuela-Ubiña S, Domínguez-Gomez M, Linares-Barrios M. Infantile bullous pemphigoid following serogroup B meningococcal vaccination. Br J Dermatol. (2021) 184:e53. 10.1111/bjd.19480 [DOI] [PubMed] [Google Scholar]
- 9.Jindal A, Nayak SUK, Shenoi SD, Rao R, Monappa V. Bullous pemphigoid triggered by rabies vaccine. Indian J Dermatol Venereol Leprol. (2020) 86:66–8. 10.4103/ijdvl.IJDVL_666_18 [DOI] [PubMed] [Google Scholar]
- 10.Guerra L, Pedicelli C, Fania L, De Luca N, Condorelli AG, Mazzanti C, et al. Infantile bullous pemphigoid following vaccination. Eur J Dermatol. (2018) 28:708–10. 10.1684/ejd.2018.3383 [DOI] [PubMed] [Google Scholar]
- 11.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. (2021). Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 85, 46-55. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomayko MM, Damsky W, Fathy R, McMahon DE, Turner N, Valentin MN, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. (2021) 148:750–1. 10.1016/j.jaci.2021.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. (2021) 35:e645–7. 10.1111/jdv.17472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt V, Blum R, Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J Eur Acad Dermatol Venereol. (2021) 36:e88–90. 10.1111/jdv.17722 [DOI] [PubMed] [Google Scholar]
- 15.Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: A systematic review. J Am Acad Dermatol. (2021) S0190-9622:00899-9. 10.1016/j.jaad.2021.04.061 [DOI] [PubMed] [Google Scholar]
- 16.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. (2021) 11:617089. 10.3389/fimmu.2020.617089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambichler T, Hamdani N, Budde H, Sieme M, Skrygan M, Scholl L, et al. Bullous pemphigoid after SARS-CoV-2 vaccination: spike protein-directed immunofluorescence confocal microscopy and T cell receptor studies. Br J Dermatol. (2021). 10.1111/bjd.20890 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. (2012) 66:479–85. 10.1016/j.jaad.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gammon WR, Briggaman RA, Inman AO, 3rd, Queen LL, Wheeler CE. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol. (1984) 82:139–44. 10.1111/1523-1747.ep12259692 [DOI] [PubMed] [Google Scholar]
- 20.Català A, Muñoz-Santos C, Galván-Casas C, Roncero Riesco M, Revilla Nebreda D, Solá-Truyols A, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. (2021) 186:142–52. 10.1111/bjd.20639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farinazzo E, Ponis G, Zelin E, Errichetti E, Stinco G, Pinzani C, et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol. (2021). 35:e548–51. 10.1111/jdv.17343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J Am Acad Dermatol. (2022) 86:113–21. 10.1016/j.jaad.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med Clin. (2021) 157:e333–4. 10.1016/j.medcle.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell'Antonia M, Anedda S, Usai F, Atzori L, Ferreli C. Bullous pemphigoid triggered by COVID-19 vaccine: rapid resolution with corticosteroid therapy. Dermatol Ther. (2021) 35:e15208. 10.1111/dth.15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agharbi FZ, Eljazouly M, Basri G, Faik M, Benkirane A, Albouzidi A, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann Dermatol Venereol. (2021) 149:56-7. 10.1016/j.annder.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young J, Mercieca L, Ceci M, Pisani D, Betts A, Boffa MJ. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J Eur Acad Dermatol Venereol. (2022) 36:e13–6. 10.1111/jdv.17676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura K, Kosano M, Sakai Y, Saito N, Takazawa Y, Omodaka T, et al. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J Dermatol. (2021) 48:e606–7. 10.1111/1346-8138.16170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. (2022) 49:34–41. 10.1111/cup.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Italian Ministry of Health . Report of vaccine administration. Available online at: https://www.governo.it/it/cscovid19/report-vaccini/(accessed January 20, 2022).
- 30.Venning VA, Wojnarowska F. Induced bullous pemphigoid. Br J Dermatol. (1995) 132:831–2. 10.1111/j.1365-2133.1995.tb00739.x [DOI] [PubMed] [Google Scholar]
- 31.Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. (2021) 80:1339–44. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzioufas AG, Bakasis AD, Goules AV, Bitzogli K, Cinoku II, Chatzis LG, et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J Autoimmun. (2021) 125:102743. 10.1016/j.jaut.2021.102743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasperkiewicz M, Schmidt E, Amagai M, Fairley JA, Joly P, Murrell DF, et al. Updated international expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. (2021) 35:e412–4. 10.1111/jdv.17207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller JJ, Kittridge AL, Debanne SM, Korman NJ. Evaluation of ELISA testing for BP180 and BP230 as a diagnostic modality for bullous pemphigoid: a clinical experience. Arch Dermatol Res. (2016) 308:269–72. 10.1007/s00403-016-1631-1 [DOI] [PubMed] [Google Scholar]
- 35.Chanprapaph K, Ounsakul V, Pruettivorawongse D, Thadanipon K. Anti-BP180 and anti-BP230 enzyme-linked immunosorbent assays for diagnosis and disease activity tracking of bullous pemphigoid: A prospective cohort study. Asian Pac J Allergy Immunol. (2019) 39:272–8. 10.12932/AP-231118-0446 [DOI] [PubMed] [Google Scholar]
- 36.Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann SC, Lacour JP, et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. (2011) 131: 2271–80. 10.1038/jid.2011.180 [DOI] [PubMed] [Google Scholar]
- 37.Ujiie H, Yoshimoto N, Natsuga K, Muramatsu K, Iwata H, Nishie W, et al. Immune reaction to type XVII collagen induces intramolecular and intermolecular epitope spreading in experimental bullous pemphigoid models. Front Immunol. (2019) 10:1410. 10.3389/fimmu.2019.01410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feliciani C, Caldarola G, Kneisel A, Podstawa E, Pfütze M, Pfützner W et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. (2009) 161:306–12. 10.1111/j.1365-2133.2009.09266.x [DOI] [PubMed] [Google Scholar]
- 39.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases.” Clin Immunol. (2021) 224:108665. 10.1016/j.clim.2021.108665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dănescu S, Chiorean R, Macovei V, Sitaru C, Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: Case report series and a comprehensive review of the published work. J Dermatol. (2016) 43:134–40. 10.1111/1346-8138.13031 [DOI] [PubMed] [Google Scholar]
- 41.Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. (2014) 28:1133–40. 10.1111/jdv.12366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.