Abstract
Cholangiocarcinomas are a heterogeneous group of highly aggressive cancers that may arise anywhere within the biliary tree. There is a wide geographical variation with regards to its incidence, and risk-factor associations which may include liver fluke infection, primary sclerosing cholangitis, and hepatolithiasis amongst others. These tumours are classified into intrahepatic, perihilar and distal based on their anatomical location. Morphologically, intrahepatic cholangiocarcinomas are further sub-classified into small and large duct variants. Perihilar and distal cholangiocarcinomas are usually mucin-producing tubular adenocarcinomas. Cholangiocarcinomas develop through a multistep carcinogenesis and are preceded by dysplastic and in situ lesions. While clinical characteristics and management of these tumours have been extensively elucidated in literature, their ultra-structure and tumour biology remain relatively unknown. This review focuses on the current knowledge of pathological characteristics, molecular alterations of cholangiocarcinoma, and its precursor lesions (including biliary intraepithelial neoplasia, intraductal papillary neoplasms of the bile duct, intraductal tubulopapillary neoplasms and mucinous cystic neoplasm).
Keywords: Cholangiocarcinoma, Classification, Pathology, Molecular features, Precursors lesions, Treatment
Core Tip: Cholangiocarcinoma is a heterogeneous and aggressive epithelial malignancy of the biliary system. The majority of cholangiocarcinomas are diagnosed at an advanced stage when choice of treatment is limited. Cholangiocarcinoma is classified into intrahepatic, perihilar, and distal bile duct cancer, according to the anatomical location. This review focuses on the current knowledge of histopathological features, molecular alterations and clinical characteristics of cholangiocarcinoma and its precursor lesions (including biliary intraepithelial neoplasia, intraductal papillary neoplasms of the bile duct, intraductal tubulopapillary neoplasms and hepatobiliary mucinous cystic neoplasm. Recently, actionable genetic alterations, mainly IDH1 mutations and FGFR2 fusions have been described, in cholangiocarcinoma.
INTRODUCTION
Cholangiocarcinoma, although rare, is the second most common primary hepatic cancer after hepatocellular carcinoma (HCC). It accounts for approximately 15% of cases and represents 3% of all gastrointestinal malignancies[1-3] These are a diverse group of highly fatal cancers that arise along the biliary tree[4]. Majority of cholangiocarcinoma are diagnosed in older individuals, with a peak incidence in the 7th decade. It afflicts both genders almost equally (slight male preponderance)[5]. Cholangiocarcinomas encompasse three distinct anatomical categories based on the site of biliary tract involvement, namely intrahepatic (IHCC), perihilar (PHCC) and distal cholangiocarcinoma (DCC). Each of these categories differ in their risk factors, clinical presentations, epidemiological features, morphologic and molecular characteristics[6,7]. Approximately 6%-10% are intrahepatic, 30% are distal and a majority (60%) are PHCC[8]. IHCC are tumours located proximal to the second-order bile ducts within the liver and thus arise from segmental or smaller intrahepatic biliary channels[9]. PHCC are localized to an area between the second-order bile ducts and the insertion of the cystic duct into the common bile duct[10]. DCC include tumours between the origin of the cystic duct and ampulla of Vater[11].
EPIDEMIOLOGY
Due to a wide geographical variation in the risk factors (both environmental and genetic), incidence and mortality rates of cholangiocarcinoma vary across regions[12]. The highest incidences of cholangiocarcinoma are reported in the northeast provinces of Thailand where the liver fluke Opisthorchis viverrini is endemic[13,14]. Age-standardized incidence rates in this region is an alarming 85/100000 population. Lowest incidences of this tumour are in Israel and Costa Rica (0.3/100000 population), while in the United States it is 1.6/100000 population[1,5]. Studies in the last few decades have reported increasing incidence of IHCC and a stable or decreasing incidence of PHCC and/or DCC in many European countries (Italy, Germany, England and Wales), United States, Australia and Japan[5,6,12]. The incidence of IHCC, PHCC and DCC has remained stable in France, and decreasing incidence of IHCC have been reported in Denmark[12].
RISK FACTORS
Most cases (70%) of cholangiocarcinoma are sporadic, occurring without any probable or known risk factors. Table 1 Lists all known risk factors for cholangiocarcinoma. Parasitic infections like Opisthorchis viverrini and Clonorchis sinensis (liver flukes) induce chronic bile duct inflammation, and periductal scarring which increase the risk of biliary tract malignancy[14]. In the Western world, primary sclerosing cholangitis (PSC) remains the most prevalent risk factor[4]. PSC induces chronic inflammation, biliary epithelial proliferation, and production of endogenous bile mutagens leading to biliary tumorigenesis[5]. Malignant transformation in epithelial lining of biliary cysts can occur as there is reflux of pancreatic enzymes, bile stasis and increased bile acid concentration[5]. Increased risk is also reported in Caroli disease and hepatolithiasis where there is bile stasis, chronic inflammation, bacterial infection, and recurrent cholangitis[12]. In cirrhotic patients, an increased risk of cholangiocarcinoma is observed due to the presence of amplified cell proliferation, release of inflammatory cytokines and scarring[5]. Apart from the presence of cirrhosis, hepatitis B (HBV) and hepatitis C (HCV) viruses have a direct carcinogenic effect on hepatic progenitor cells resulting in an increased risk of cholangiocarcinoma in these patients. Obesity increases the risk of cancer by affecting the levels of leptin, adiponectin and proinflammatory cytokines[15]. Non-alcoholic fatty liver disease may promote cholangiocarcinoma development directly by induction of hepatic inflammation or, indirectly, by resulting in cirrhosis.
Table 1.
Definite and probable risk factors for cholangiocarcinoma
|
Definite risk factors
|
| Primary sclerosing cholangitis |
| Liver fluke infection (Opisthorchis viverrine, Clonorchis sinensis) |
| Hepatolithiasis |
| Biliary malformation (choledochal cysts, Caroli’s disease, congenital hepatic fibrosis) |
| Thorotrast |
| Probable risk factors |
| Alcohol |
| Hepatitis B |
| Hepatitis C |
| Cirrhosis |
| Toxins (dioxin, polyvinyl chloride) |
| Biliary–enteric drainage procedures |
| Inflammatory bowel disease |
| Asbestos |
| Non-alcoholic fatty liver disease |
| Metabolic syndrome, type 2 diabetes, obesity |
| Smoking |
| Chronic pancreatitis |
BILIARY TRACT ANATOMY
The biliary tract extends from the canals of Hering to the common bile duct and are broadly subdivided into extrahepatic and intrahepatic segments[16,17]. It is a complex structure showing wide variation in anatomy and histology[18]. The intrahepatic bile ducts (ducts proximal to the right and left duct confluence) are further subclassified into large and small intrahepatic bile ducts. Large intrahepatic bile ducts (> 300 μm in diameter) are also referred to as the ‘perihilar’ bile ducts and consist of the segment, right and left hepatic bile ducts. These ducts are lined by tall columnar epithelium with basally placed hyperchromatic-isomorphic nuclei, mucin filled cytoplasm and a fibro-collagenous duct wall[18]. The intrahepatic biliary tree begins at the level of canals of Hering which are lined partly by biliary epithelium and hepatocytes[19]. The canals of Hering continue into a channel, termed the ductule (< 20 μm in diameter). These are entirely lined by cholangiocytes and continue as the interlobular bile ducts (20-100 μm in diameter). The interlobular bile ducts are lined by cuboidal cells resting on a basement membrane. Multiple Interlobular bile ducts fuse to form septal (> 100 μm in diameter), area and segmental bile ducts. The septal ducts are lined by tall columnar cells with fibro-collagenous duct walls[18].
Embryologically, small intrahepatic bile ducts evolve from hepatoblasts through a process of ductal plate formation. During the first weeks of gestation, the ductal plate develops as a cylindrical, double-layered sleeve of cholangiocytes with a slit-like lumen surrounding a portal vein branch. Remodelling and partial involution of these cylindrical ductal plates give rise to bile ducts. Large ducts are formed from the caudal portion of the hepatic diverticulum[18]. The exact process of fusion of these ducts has not been entirely elucidated, but they appear in continuity throughout development. Peribiliary glands are physiologically distributed within the fibromuscular walls of extrahepatic bile ducts and large intrahepatic bile ducts[20].
GROWTH PATTERNS
Macroscopic pattern
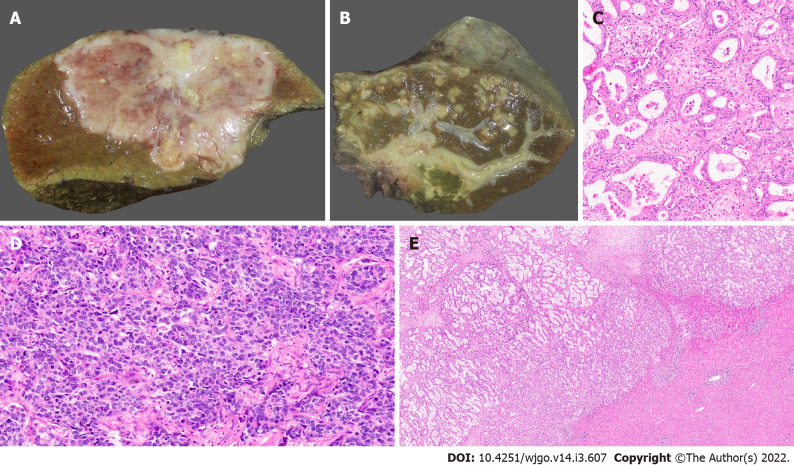
Based on their macroscopic growth patterns, IHCC are classified into mass forming, periductal infiltrating, and intraductal growth types[21,22]. Mass forming lesions are the predominant type, accounting for 60-80% of IHCC[23-26]. These are firm, solid tumours with a white or greyish cut-surface, with well-defined borders within the hepatic parenchyma (Figure 1A). Intrahepatic metastases or coalescing lesions may be observed. Mucin may also be identified along the cut-surface. These tumours are thought to arise in small intrahepatic bile ducts and are commonly characterized by central necrosis or scaring. The periductal infiltrating type accounts for 15%-35% of IHCC and extends along the portal tracts presenting as bile duct strictures with luminal narrowing. The intraductal growth variant of IHCC is characterized by a papillary or polypoid lesion within a dilated bile duct and most often represent a malignant progression of intraductal papillary mucinous neoplasm (IPNB)[26]. They are the least common variant and account for 8-29% of cases. IHCC can have mixed growth patterns, for example, mass forming and periductal infiltrating[27]. Rarer undefined patterns have also been reported[22].
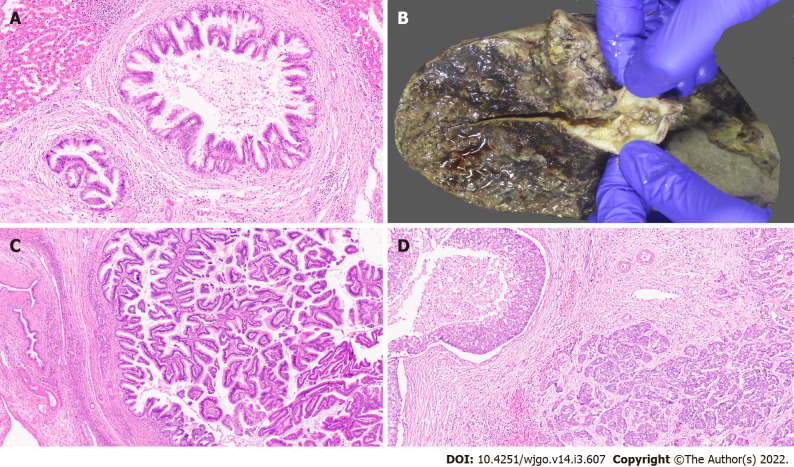
Figure 1.
Gross features and morphology of cholangiocarcinoma. A: Mass forming intrahepatic cholangiocarcinoma (IHCC); B: Extrahepatic cholangiocarcinoma with periductal infiltrating growth (arrow) and markedly greenish liver; C: Well differentiated cholangiocarcinoma [hematoxylin and eosin (H&E, × 25)]; D: Poorly differentiated cholangiocarcinoma (H&E, × 25); E: Large duct variant of IHCC (H&E, × 8).
Macroscopically, PHCC and DCC have similar growth patterns; they present as flat or poorly defined nodular sclerosing tumours with thickening of the duct wall, often with diffuse infiltration into adjacent structures (approximately 80%) (Figure 1B) and, less frequently, as intraductal papillary tumours[26,28]. American Joint Committee on Cancer (AJCC)/ Union for International Cancer Control (UICC) and College of American Pathologists (CAP) recognize only the mass forming and periductal infiltrating types (or mixed types); they do not recognize intraductal or undefined growth patterns. This adds to the uncertainty in classifying these tumours[29].
Microscopic pattern
The histological classification of cholangiocarcinoma is highlighted in Table 2.
Table 2.
Histological classification of cholangiocarcinoma
|
Based on histological differentiation
|
| Well (> 95% of tumour composed of glands) |
| Moderately (50%-95% of tumour composed of glands) |
| Poor (5%-49% of tumour composed of glands) |
| Undifferentiated type (< 5% of tumor composed of glands) |
| Based on glandular features |
| Conventional type (bile duct type) |
| Small bile duct type (intrahepatic) |
| Large bile duct type |
| Cholangiocellular (intrahepatic) |
| Uncommon variants |
| Ductal plate malformation type (intrahepatic) |
| Lymphoepithelioma type |
| Clear cell type |
| Squamous/adenosquamous type |
| Mucinous carcinoma |
| Sarcomatoid |
| Signet ring carcinoma |
| Neuroendocrine |
| HCC-like |
HCC: Hepatocellular carcinoma.
IHCC
Traditionally, IHCC are sub-classified into two broad sub-groups: well, moderately (Figure 1), poorly differentiated tubular/acinar adenocarcinoma or the uncommon morphological variants[21,30]. These tumours are lined by cuboidal to columnar epithelial cells, resembling biliary lining epithelial cells and demonstrate stromal desmoplasia and variable inflammation[26]. Tumour cells may show mucin production into the lumen of tubular structures on their apical aspect, and within the cell cytoplasm (Figure 1C). Poorly differentiated tumours demonstrate solid, cord-like, or cribriform growth patterns with variable cytological and nuclear pleomorphisms (Figure 1D). Variable necrosis has also been demonstrated. The cancer may show compression of hepatic parenchyma and no evidence of fibrous capsule. The neoplastic cells display invasion between hepatocytes and appear to infiltrate the sinusoids[31-33]. IHCC demonstrate a prominent desmoplastic microenvironment characterized by a dense collagenized stroma and abundance of cancer-associated fibroblasts and, to a lesser extent, tumour-associated histiocytes with a varying number of innate immune cells.
Large and small duct variants of IHCC
The large duct variant of IHCC (LD-IHCC) arises from intrahepatic bile ducts or its associated peribiliary glands. The neoplastic cells lining the malignant acini are cuboidal to tall columnar containing cytoplasmic mucin and usually form large acini within open luminal spaces associated with abundant desmoplastic stroma (Figure 1E)[34,35]. Sites of hepatic parenchymal infiltration show variable tumour histology, some resemble the small duct type or bile ductular type.
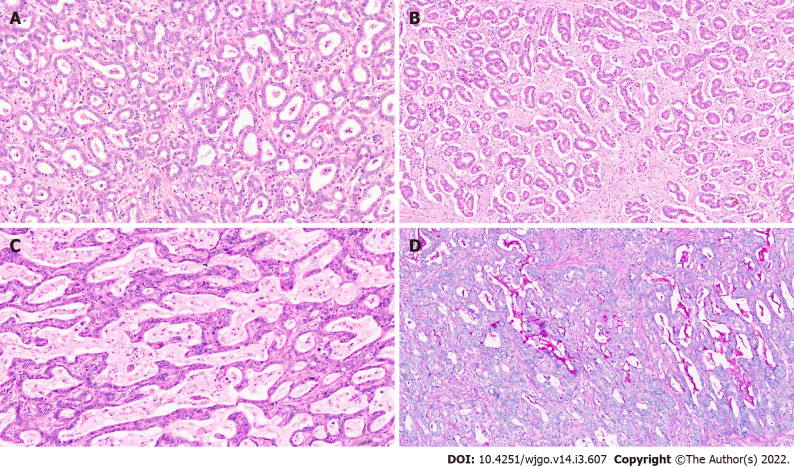
Small bile duct type of IHCC (SD-IHCC) arise from progenitor cells and mature hepatocytes and resemble -cholangiolar cells. These tumours show small monotonous or anastomosing glands which are lined with cuboidal cells. The cells have uniform nuclei with scant to moderate eosinophilic or amphophilic cytoplasm and no mucin production (Figure 2A, 2B). This classification into large and small duct types also has clinicopathologic, immunohistochemical, and molecular importance[22,26]. SD-IHCC are associated with chronic liver disease/cirrhosis (especially viral hepatitis), whereas LD-IHCC are linked to chronic biliary disease, precursor lesions, and hepatolithiasis. SD-IHCC nearly always has a mass forming macroscopic growth pattern and often with a central scar, whereas LD-IHCC have variable macroscopic growth patterns with mucin production (Figure 2C, 2D), poorer differentiation, perineural invasion, and lymph node metastases[26].
Figure 2.
Intrahepatic cholangiocarcinoma. A: Small duct variant of Intrahepatic cholangiocarcinoma (IHCC) (SD-IHCC) with closely packed small glands [hematoxylin and eosin (H&E, × 15)]; B: SD-IHCC with desmoplastic stroma (H&E, × 15); C: Large duct variant of IHCC (LD-IHCC) with mucin production (H&E, × 20); D: LD-IHCC with mucin [Periodic acid Schiff after diastase, × 15].
Ductal plate malformation type of IHCC
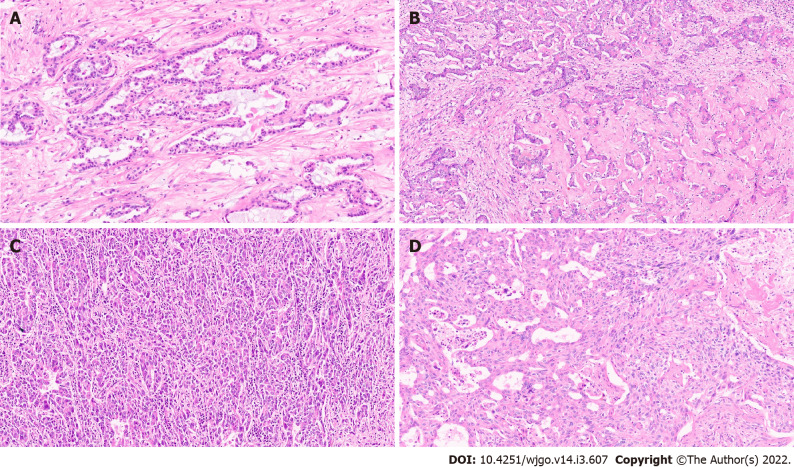
This subtype was first reported in 2012. The tumour was noted to mimic ductal plate malformations (DPM)[36]. DPM are developmental anomalies resulting from a lack of ductal plate remodelling during bile duct morphogenesis. Common examples of DPM include fibrocystic diseases such as Caroli’s disease, congenital hepatic fibrosis, and von Meyenburg complex. Majority of patients with DPM type of IHCC are males aged over 60 years at diagnosis. Sixty percent of cases are associated with chronic liver disease and the remaining show mild steatosis and/or portal inflammation. The tumours are usually solitary with whitish solid cut surface. Microscopically, the tumours are arranged in small nodules with desmoplastic stroma. The malignant acini show irregularly dilated lumen lined with a single layer of cuboidal or low columnar neoplastic cells with mild nuclear isomorphism and irregular protrusions and bulges (Figure 3A). The neoplastic cells are frequently positive for CK19, epithelial cell adhesion molecule (EpCAM), and epithelial membrane antigen (EMA). Neural cell adhesion molecule (NCAM) may also be variably expressed. These tumours show low proliferation with Ki67 index (< 10%) and p53 is scarcely expressed.
Figure 3.
Intrahepatic cholangiocarcinoma. A: Intrahepatic cholangiocarcinoma (IHCC) with ductal plate malformation phenotype [hematoxylin and eosin (H&E, × 20)]; B: Cholangiocellular or bile ductular type of IHCC (H&E, × 20); C: IHCC lymphoepithelioma subtype (H&E, × 20); D: IHCC with sarcomatoid areas (H&E, × 20).
Cholangiocellular or bile ductular type of IHCC
This is a distinct biliary derived tumour and grossly, this subtype shows mass forming growth pattern[37]. Brightfield microscopy of these tumours show homogeneous morphology with well to moderately differentiated tumour cells forming small tubules, acini or cord-like structures with a slit-like lumen along with arborization resembling proliferating reactive bile ductules (Figure 3B)[38-39]. The neoplastic cells are small in size (small compared to conventional IHCC cells). The non-neoplastic hepatocytes are extensively replaced by tumour cells in the hepatic lobules. Marked collagenisation is also noted around the tumour cells. Immunopositivity for NCAM and EpCAM are observed in the tumour cells. DPM pattern has also been described in cholangiocellular IHCC[40].
Lymphoepithelioma-like cholangiocarcinoma
These are rare tumours resembling undifferentiated nasopharyngeal cancers. They have clusters of large cells with vesicular nuclei and prominent lymphoid cell inflammatory infiltrates. These cancers have been reported in various organs including salivary glands, stomach, lung, and the liver, where they present with hepatocellular or biliary features. The latter is labelled as lymphoepithelioma like cholangiocarcinoma (LLCC)[41,42]. These tumours are often associated with Epstein-Barr virus (EBV). 70% of the cases are EBV positive based on Epstein-Barr encoding region (EBER) in situ hybridization. This is contrary to what is observed in conventional biliary tract cancers, which are not associated with EBV infection[43].
Hsu et al[45] reported the first case of LLCC in 1996[44]. Compared to conventional cholangiocarcinoma, LLCC present at a younger age and have a female preponderance (female-to-male ratio > 3:1). Histologically, these tumours are composed of acini, clusters and cords of neoplastic cells associated with prominent lymphoplasmacytic infiltration (Figure 3C)[41]. The intimate relationship between the cancer cells and numerous lymphoid cells can make a pathologic diagnosis challenging. Pathologic tools which help confirm the diagnosis of LLCC in the midst of dense lymphoid tissue include a low threshold for cytokeratin immunohistochemistry and EBER in situ hybridization[45].
Clear cell cholangiocarcinoma
These are exceedingly rare liver tumours and are recognized as a special variant of IHCC[46,47]. Diagnostic difficulties may occur in differentiating this carcinoma from other types of clear cell cancers (clear cell HCCs and metastatic clear cell cancers from the kidney, ovary, thyroid, or gastrointestinal tract)[47]. Patients are usually in the 5th or 6th decade of life and there is no gender predilection. Predisposing factors for this tumour are as yet unknown, and there is no report on its relation with hepatitis. The prognosis is relatively better than conventional cholangiocarcinoma. The mechanism for the clear cell change has been speculated to involve glycogen, mucin, or lipid[48]. CD56 immunostaining is useful in diagnosis, as it is frequently expressed in clear cell cholangiocarcinoma, and scarcely in clear cell renal cell carcinoma or lung tumours.
Sarcomatoid cholangiocarcinoma
Intrahepatic sarcomatoid cholangiocarcinoma is an extremely rare tumour accounting for less than 1% of hepatobiliary system malignancies[49]. Light microscopy reveals a relatively well-delineated tumour characterized by spindle to epithelioid cancer cells having variable nuclear pleomorphism with hyperchromatic nuclei and inconspicuous-to-prominent nucleoli (Figure 3D). The tumour cells are interspersed with stromal tissue. Occasionally, cancer cells with mucin are observed. Inflammatory cell infiltration was present in the abundant stroma[49]. Pleomorphic giant cells have also been reported in the tumour[50]. The sarcomatoid subtype is an independent predictor of tumour recurrence, and has a poorer overall survival among IHCC sub-types[50].
Other rare subtypes
Other rare histological subtypes include squamous and adenosquamous carcinoma, mucinous carcinoma, signet ring cell carcinoma, undifferentiated, HCC-like, and mucoepidermoid carcinoma.
PHCC AND DCC
PHCC and DCC are histologically similar to LD-IHCC. The conventional types show well to moderately differentiated acinar structures (Figure 4A). Rarely, tumours with poor differentiation with cells arranged in solid sheets or cords may also be seen. Associated sclerotic desmoplastic stroma is usually identified. The nodular-sclerosing type demonstrates marked cancerous thickening of the affected bile ducts. Micropapillary or flat adenocarcinoma are observed on the luminal surface. Perineural invasion is common (Figure 4B), and lymphovascular invasion is variably observed. Mucin-producing epithelial cells lining the large bile duct and/or the hepatic progenitor cells are the purported cells of origin for DCC and PHCC[51]. Extrahepatic cholangiocarcinoma may also demonstrate intraductal growth with papillary, tubular or superficial spreading patterns. Adenosquamous carcinoma (Figure 4C) displaying mixed, isolated, or adjoining keratinizing squamous and tubular components have rarely been reported. Keratinizing squamous cell carcinoma, mucinous carcinoma, signet ring cell carcinoma, clear cell carcinoma, hepatoid and neuroendocrine tumours (Figure 4D) have also been reported.
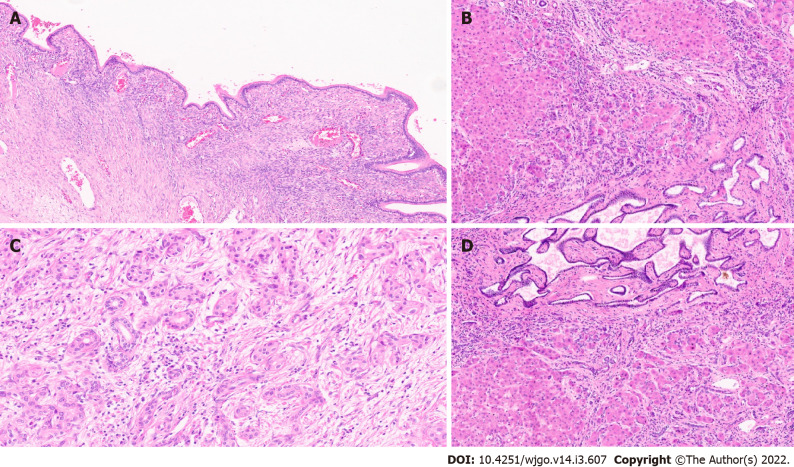
Figure 4.
Extrahepatic cholangiocarcinoma. A: Extrahepatic cholangiocarcinoma (EHCC) with large mucin producing malignant glands and abundant desmoplastic stroma [haematoxylin and eosin (H&E, × 8)]; B: EHCC with perineural invasion (H&E, × 20); C: EHCC adenosquamous subtype (H&E, × 15); D: Well differentiated neuroendocrine tumour of the bile duct (H&E, × 20).
IMMUNOHISTOCHEMICAL FEATURES
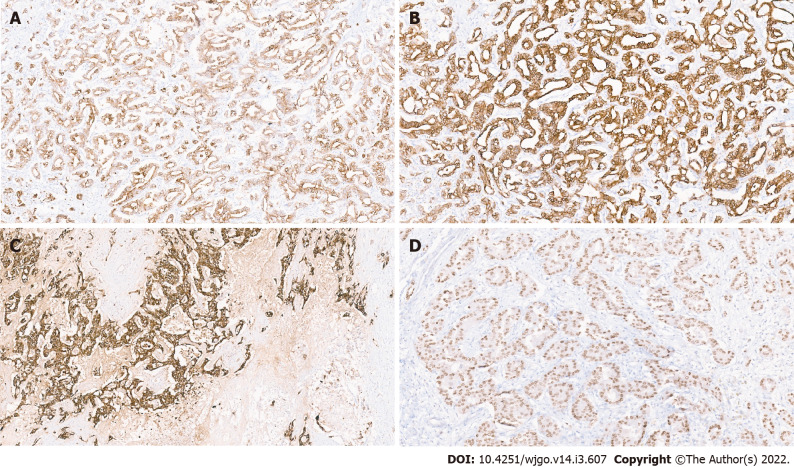
Cholangiocarcinomas (intrahepatic and extrahepatic) show immunopositivity for CK7, CK19, and EMA (Figure 5A, B and C)[29]. Positivity for Hepatocyte nuclear factor-1β (HNF-1β) (Figure 5D) and C-reactive protein have also been observed[52]. They are usually negative for CDX2 and SAT-B2, however some cases of IHCC may show focal mild positivity for CDX2 and SAT-B2. CK20 immunostain is typically negative or focally positive. These immunostains can help exclude a diagnosis of metastatic colorectal adenocarcinoma, which are typically strong positive for CK20, CDX2, and SAT-B2 but negative for CK7 and CK19. Differentiating cholangiocarcinomas from metastatic pancreatic ductal adenocarcinoma and upper gastrointestinal tract cancers using immunostains is difficult, as these tumours are also positive for CK7 and CK19. Several studies have shown that the LD-IHCC and SD-IHCC have distinct immunohistochemical characteristics[53-56]. LD-IHCC are positive for S100P and TFF1, whereas SD-IHCC tend to be positive for NCAM(CD56) and N-cadherin. NCAM and EMA are often negative or weakly positive for tumour cell cytoplasm in HCC-like IHCC, however strong expression for stem cell makers, including TROP2, EpCAM and Nestin have been reported[57] Fernández Moro et al[58] proposed a comprehensive immunohistochemical panel including CK19, CK20, MUC2, MUC5AC, CA19–9, mCEA, CA125 and SMAD4 to aid in the differentiation of metastatic and pancreatobiliary adenocarcinomas.
Figure 5.
Immunohistochemistry in cholangiocarcinoma. A: Positive CK7 immunostaining; B: Positive CK19 immunostaining; C: Positive epithelial membrane antigen immunostaining; D: Hepatocyte nuclear factor-1 β nuclear immunostaining in a small duct variant of intrahepatic cholangiocarcinoma.
PRECURSORS LESIONS OF CHOLANGIOCARCINOMA
Carcinogenesis of cholangiocarcinoma is a multistep process beginning with transformed biliary epithelial cells or from stem/progenitor cells. Table 3 describes precursor lesions of both intrahepatic and extrahepatic cholangiocarcinoma.
Table 3.
Clinicopathologic, immunohistochemical, and molecular characteristics
|
|
Large duct type
|
Small duct type
|
| Location | Proximal to hepatic hilum | Peripheral |
| Risk factors | PSC, Liver fluke infection, Hepatolithiasis | Chronic liver disease, viral hepatitis |
| Gross features | Periductal infiltrating, Mixed pattern | Mass forming |
| Precursor lesion | BilIN, IPNB, ITPN | Unknown |
| Pathology | Large, widely spaced glands, Columnar with mucin production, desmoplastic stroma | Small tubules, fused or anastomosing glands, cuboidal to low columnar, central scarring, minimal to no mucin |
| Perinerual invasion | Common | Rare |
| Lymphovascular invasion/lymph node metastases | Common | Rare |
| Tumour border | Infiltrative | Expansile or pushing, rarelyinfiltrative |
| Immunohistochemical features | S100P and TFF1 | CD56, N-cadherin, CRP |
| Molecular alterations | KRAS and GNAS mutationsCOX2 upregulations | IDH1/IDH2 and BRAF mutations, FGFR2 fusion |
BilIN: Biliary intraepithelial neoplasia; CRP: C-reactive protein; IPNB: Intraductal papillary neoplasm of the bile duct; ITPN: Intraductal tubulopapillary neoplasms; PSC: Primary sclerosing cholangitis.
Biliary epithelial neoplasia
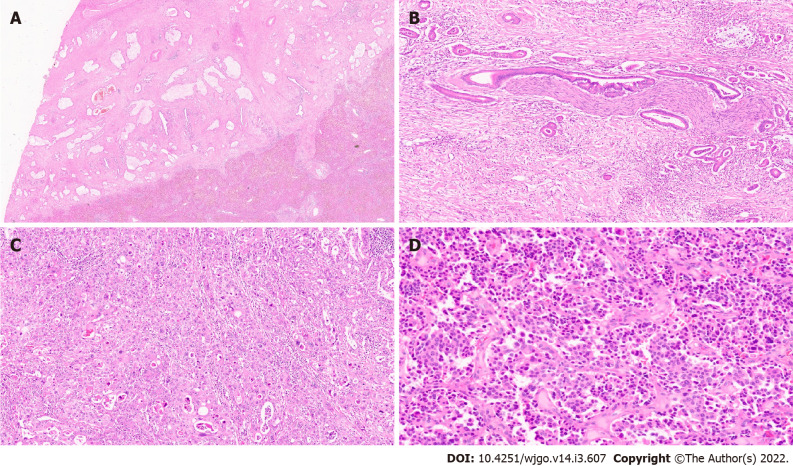
Biliary intraepithelial neoplasia (BilIN) represents the most frequent precursor lesion of invasive adenocarcinoma in the biliary tract[59]. BilIN are non-invasive microscopic flat, micropapillary (papillary projection with fibrovascular stalk) or pseudopapillary (papillary projection without fibrovascular stalk) lesions with dysplasia. BilIN do not produce clinical symptoms and are not detectable on imaging studies[60]. The 2019 WHO classification histologically stratifies BilIN into a two-tiered classification based on the tumour grade (high vs low) and intraepithelial extent of cellular and nuclear atypia (Figure 6A). This replaces an earlier classification which was three-tiered (BilIN-1, BilIN-2, and BilIN-3). BilIN-1 and BilIN-2 categories from the previous classification are now classified as low-grade and the former BilIN-3 is now classified as high-grade[61]. Furthermore, high grade BilIN is considered as carcinoma in situ.
Figure 6.
Precursor lesions of cholangiocarcinoma. A: Biliary intraepithelial neoplasia with low grade dysplasia [haematoxylin and eosin (H&E, × 15)]; B: Intraductal papillary neoplasm of the bile duct (IPNB) (arrow); C: IPNB pancreaticobiliary subtype (H&E, × 10); D: Intraductal tubulopapillary neoplasms of the bile duct with invasive carcinoma (H&E, × 10).
The presence of BilIN have been associated with hepatolithiasis, PSC and choledochal cysts. It has also been observed in the mucosa adjacent to invasive adenocarcinoma. They have also been detected in cirrhotic livers from nonbiliary diseases (e.g., alcoholic liver disease and hepatitis C)[61,62]. Multicentricity is common in BilIN. Macroscopically, BilIN may manifest as fine granularity, thickened velvety mucosa, or effacement of underlying tissue layers. However, it often appears grossly normal. Low grade BilIN are usually flat lesions with high N:C ratio, hyperchromatic stratified nuclei, and nucleoli. High grade lesions have papillary projections with loss of polarity, marked nuclear atypia and frequent mitosis[61]. BilIN may further be sub-classified as the classic type demonstrating columnar/cuboidal cells with eosinophilic cytoplasm and round nuclei, and the intestinal type characterized by columnar cells with elongated and hyperchromatic, pseudostratified nuclei along with occasional goblet-type cytoplasmic mucin resembling intestinal adenoma[60]. The classic type shows CK7 immunopositivity whereas intestinal type shows immunopositivity for any of the intestinal immunomarkers (CK20, CDX2, or MUC2)[63]. Distinguishing low-grade dysplasia from reactive atypia can occasionally be difficult. The presence of intraepithelial neutrophils which are observed in reactive changes help solve this conundrum. The term ‘indefinite for dysplasia’ has been proposed in cases where sufficient doubt precludes a definitive classification.
Intraductal papillary neoplasms of the bile duct
Intraductal papillary neoplasia of the bile duct (IPNB) is a unique macroscopic premalignant neoplasm that may arise within intra- or extrahepatic bile ducts[64]. IPNB is typically diagnosed in middle-aged or elderly adults and has a slight male predominance[65]. IPNB is a rare disease entity with a prevalence of 4% to 15% among bile duct tumours, and higher incidence is noted in south-east Asian countries[65,66]. Risk factors include hepatolithiasis, liver fluke infections, PSC and congenital biliary tract disease. These tumours may be single or multiple and can present clinically as large duct obstruction with recurrent abdominal pain, cholangitis and cholestatic hepatic dysfunction[22]. Macroscopically, IPNBs present as visible polypoid, papillary, greyish white or brownish, soft tissue growths within a dilated bile duct lumen (Figure 6B). Some patients may present with mucus hypersecretion. Like intraductal papillary mucinous neoplasm (IPMN) of the pancreas, IPNB is histologically classified into four types based on their histological and immunohistochemical features: pancreaticobiliary (Figure 6C), intestinal, gastric and oncocytic types[67]. Pancreaticobiliary and intestinal subtypes are the most common types, although its frequency varies across geographical regions. Mixed subtypes are observed frequently in these neoplasms, and hence their classification is based on the most prevalent subtype. High-grade dysplasia is often extensive and invasive carcinomas are identified in approximately half the cases.
Carcinomas that arise from these lesions are usually the pancreatobiliary-type cholangiocarcinomas with tubular growth pattern, although other rare variants including neuroendocrine and mucinous tumours have been reported. Recently, a panel of Japanese and Korean biliary pathologists proposed a consensus classification for IPNB. These lesions are grouped into types 1 and 2, supplementing the traditional two-tiered grading system (low-grade and high-grade dysplasia[68]. Type 1 IPNB is characterized by regular structures, whereas type 2 show irregular structures. Foci of complicated lesions, such as cribriform or solid structures, are frequently observed in type 2. Pancreatobiliary type shows MUC1 immunostaining, while MUC2 is observed in the intestinal type[69]. MUC5AC is positive in all four types.
Intraductal tubulopapillary neoplasms of the bile duct
This is a recently identified distinct intraductal neoplasm with a predominantly tubular growth pattern. It occurs in the large intrahepatic and extrahepatic bile ducts and is often associated with invasive adenocarcinoma at the time of diagnosis (Figure 6D)[21,22]. ITPNs are rare premalignant lesions characterised by polypoid or solid tumours inside a dilated bile duct[70]. The mean age at presentation is 60 years, with no gender predilection. Purely intraductal tumours appear to have favourable outcomes, but metastases are known to occur in the presence of invasive carcinoma[21]. The tumour shows high cellularity with back-to-back tubular glands and solid sheets with minimal papillary architecture. The cells are cuboidal to columnar with mild to moderate cytological atypia[71]. Despite being associated with invasive carcinoma, overall, ITPNs have a better prognosis than IPNBs. This may be due to an earlier diagnosis resulting from a large in situ intraductal component, or could possibly be due to the inherent differences in the molecular background[72].
Hepatobiliary mucinous cystic neoplasm
Hepatobiliary mucinous cystic neoplasm (HMCN) are lesions characterized by neoplastic mucinous and/or nonmucinous biliary epithelium surrounded by ovarian-type mesenchymal stroma (Figure 7A)[73]. This is a rare tumour representing less than 5% of all cystic neoplasms of the liver and is diagnosed almost exclusively in women in their fourth or fifth decade of life[73,74]. HMCN display no communication with bile ducts and were previously included in the biliary cystadenoma/adenocarcinoma type[21]. Grossly, they are multilocular neoplasms ranging in size from 5 to 29 cm and show a cyst-in-cyst appearance on pre-operative imaging[75]. HMCNs present either as low- or intermediate-grade dysplasia or malignant features with high-grade dysplasia[75]. The benign and borderline categories are however, more common. Their ovarian-type stroma is positive for estrogen and progesterone receptor, inhibin-α and FOXL2[73]. Non-invasive HMCNs have an excellent prognosis, especially when resected completely[76].
Figure 7.
Other precursor lesions. A: Hepatobiliary mucinous cystic neoplasm with mucinous lining epithelial and ovarian stroma [haematoxylin and eosin (H&E, × 10)]; B: Von Mayenberg complex (H&E, × 20); C: Bile duct adenoma (H&E, × 20).
Other precursors to lesions
Premalignant lesions of IHCC, particularly those of the mass forming type remain relatively undefined. Hepatic adenofibroma is a benign tumour similar to biliary micro hamartoma with abundant fibrotic stroma and glandular cystic dilatation. They have potential for malignant transformation[77]. Biliary hamartoma, also known as the von Meyenburg complex are histopathological lesions composed of irregular small bile ducts or dilated ductular structures, frequently containing bile, with a fibrous stroma (Figure 7B). The epithelial lining cells are flattened or cuboidal, monomorphic, and lack mitoses. Biliary hamartomas are typically found adjacent to a portal area and may be multiple. Biliary hamartomas are generally regarded as benign. Few reports of cholangiocarcinoma arising from biliary hamartomas raise the question of its potential role as being a precursor lesion[78,79]. Bile duct adenomas (BDA) and atypical epithelial lesions of small bile ducts have occasionally been reported as candidate preinvasive lesions of peripheral IHCC[26]. BDAs are usually solitary and subcapsular (nearly 90% of the cases), and over 90% are less than 1cm in size. They are composed of small, normal looking bile ducts (Figure 7C). In small biopsies differentiation between a well-differentiated IHCC and BDA may be difficult. Both p53 and p16 immunohistochemistry can be helpful to distinguish these two lesions. P53 shows a strong and diffuse expression in malignant lesions while p16 is constantly expressed in bile duct adenoma.
MOLECULAR PATHOMECHANISMS
The knowledge of molecular pathology of cholangiocarcinoma has markedly evolved over the past decade. With the advent of high-throughput gene sequencing technologies, multiple new genetic and epigenetic alterations in cholangiocarcinoma have been uncovered[80]. In-depth sequencing has also highlighted the molecular complexity and heterogeneity of these tumours. A better understanding of the underlying pathomechanisms of cholangiocarcinogenesis will help to improve the description of the tumour and its subtypes. Moreover, it will also pave the way for personalized treatment for these rare primary liver cancers[17]. It is important for future studies to search for distinct subgroups within the subtypes on a morphomolecular basis.
Molecular characteristics of intrahepatic cholangiocarcinoma
Table 3 summarizes the clinicopathologic, immunohistochemical, and molecular characteristics of IHCC[53]. Mutation analysis of both LD-IHCC and SD-IHCC reveal KRAS as the most frequently mutated oncogene in LD-IHCC[54]. Large-duct type also show a high mutation frequency of tumour suppressor genes (e.g., p53). SD-IHCC show higher frequency mutations of IDH1 and IDH2[81,82]. IDH1 and IDH2 are relevant in carcinogenesis due to their involvement in cell metabolism[83]. Nakamura described FGFR2 fusion genes in SD-IHCC[81]. With a prevalence of 14%–23% in IHCC, FGFR2 rearrangement is the most common type of FGFR aberration[84]. Lowery et al[85] performed targeted next-generation sequencing assay and reported alterations in ARID1A, BAP1, and TP53, along with IDH1, and FGFR2 gene fusions. They also reported a tendency toward mutual exclusivity between multiple genes including TP53:IDH1, IDH1:KRAS, TP53:BAP1, and IDH1:FGFR2. FGFR2 rearrangements seem to occur more frequently in younger patients and possibly confer a better prognosis[86]. Rarely NTRK fusions have also been reported[86].
Jang et al[87] investigated the molecular landscape of IHCC in both histologically unremarkable livers and in those with chronic liver disease (CLD). They employed a high throughput mass spectrometry-based platform and compared the mutation profiles of 43 IHCC with histologically unremarkable livers and 38 with CLD[87] The most commonly mutated gene was KRAS followed by MLH1, NRAS, GNAS and EGFR. The frequency of BRAF, APC, PIK3CA, CDKN2A, PTEN, and TP53 mutations was < 5%. Overall mutation rates of biliary cancer with CLD were lower than that of cancers in a histologically unremarkable liver. Sia et al[88] classified IHCC into two unique subclasses: inflammation and proliferation, each with distinct features, activated genes, and clinical outcomes. The inflammation class demonstrated activated inflammatory signalling pathways, with overexpression of cytokines, and STAT3 activation, while proliferation class was characterized by the activation of RAS, MAPK and MET oncogenic signalling pathways, mutations in KRAS and BRAF as well as expression of genes that were previously associated with worse outcome in patients with HCC. Kim et al[89] classified IHCC into two classes, those primarily driven by either somatic mutations (M class) or by DNA copy number alterations (C class). Compared to M class IHCC with a relative deficit of copy number alterations, C class IHCC harbour recurrent focal copy number alterations including deletions involving CDKN2A, ROBO1, ROBO2, RUNX3, and SMAD4.
DNA mismatch repair (MMR) deficiency leading to microsatellite instability (MSI) have been demonstrated as a distinct pathway for carcinogenesis[90]. MSI is clinically relevant, since these cancers are responsive to immune checkpoint inhibitor therapy[91]. Although MSI most commonly occurs in colorectal and endometrial cancers, a wide variety of other cancers, including biliary cancer exhibit MSI. Goeppert et al[91] analysed the mononucleotide MSI marker panel consisting of BAT25, BAT26, and CAT25 in 159 IHCC and detected high-level of MSI (MSI-H) in 2 cases. Patients affected by MSI-H cholangiocarcinoma were younger and showed atypical histomorphology along with a longer overall survival and high tumour stage. Correlation analysis of MSI status with tumour-infiltrating immune cells, MHC I, and PD-L1 expression in the same cholangiocarcinoma cohort showed increased numbers of CD8, FOXP3, CD20 positive cells and moderate or high MHC I expression levels in MSI-H IHCC[90]. Overall, the frequency of MSI-H based on various studies is 10%[90]. Very recently Zhou et al[92] evaluated the role of Brahma-related gene 1 (Brg1) in IHCC and demonstrated that a high Brg1 expression in hepatic progenitor cells (HPC) promoted HPC expansion, liver cirrhosis, and IHCC development in response to chronic biliary injury.
Intratumoural heterogeneity in intrahepatic cholangiocarcinoma
Dong et al[93] performed multiregional whole-exome sequencing to investigate intratumoural heterogeneity (ITH) and its impact on IHCC progression. They demonstrated many factors, such as parallel evolution and chromosome instability may participate and promote the branch diversity of IHCC. In primary and recurrent metastatic tumours, they found evidence of polyclonal metastatic seeding, indicating that symbiotic communities of multiple clones existed and were maintained during metastasis.
Molecular alterations of extrahepatic cholangiocarcinoma
These lesions show similar molecular profiles as LD-IHCC and have the presence of KRAS mutation. As demonstrated in a recent study, KRAS, TP53, ARID1A, and SMAD4 are the most prevalent mutations[94]. Mutations in IDH1/2 and BAP1 and FGFR2-fusions reported in SD-IHCC have not been identified in these tumours. Four distinct transcriptome-based molecular classes of EHCC were identified. Metabolic class showed a hepatocyte-like phenotype with activation of the transcription factor HNF4A and enrichment in gene signatures related to bile acid metabolism. The proliferation class was characterized by enrichment of MYC targets, ERBB2 mutations/ amplifications and activation of mTOR signalling and was more common in patients with DCC. The mesenchymal class was defined by signatures of epithelial mesenchymal transition, aberrant TGFβ signalling and poor overall survival and immune class showed lymphocyte infiltration of the tumour, overexpression of PD-1/PD-L1 and molecular features associated with a better response to immune checkpoint inhibitors. Kim et al[95] investigated and found MSI-H in 1 of 18 EHCC (6%). Overall, the reported frequency of MSI-H in carcinomas of the large bile ducts is estimated to be 5%[90].
Epigenetics of cholangiocarcinoma
Epigenetics are heritable elements that regulate gene expression without modifying the nucleotide sequence of the DNA. They play an important role in cholangiocarcinogenesis[96]. A multitude of alterations of key epigenetic players have been observed in cholangiocarcinoma: DNA methylation, histone modifications, chromatin remodelling and noncoding RNAs (ncRNAs). In tumours, the aberrant DNA methylation occur at the 50 methylcytosine (5-mc) in CpG rich area in the promotor sequence of tumour suppressor genes resulting in gene inactivation[80]. A study investigating 489 cases identified four clusters (cluster 1 to cluster 4) of cholangiocarcinoma based on their DNA methylation pattern with different clinical outcomes[97]. Cluster 1 and 4 were clearly distinguished by their highly distinctive patterns of genome-wide DNA hypermethylation, targeting either promoter CpG islands or promoter CpG shores. Further analysis demonstrated that Cluster 1 cholangiocarcinoma were fluke-positive with increased mutation rates (mutation signature 1 enrichment, and increased point-mutation subclonality), while Cluster 4 were fluke-negative and by comparison relatively clonal. Cluster 1 showed downregulation of the DNA demethylation enzyme TET1 and upregulation of the histone methyltransferase EZH2. Hypermethylation of CpG sites was also observed in cluster 4 with enrichment of FGFR translocations and IDH1/2 and BAP1 mutations.
Several histone deacetylase (HDAC) enzymes are overexpressed in cholangiocarcinoma[98,99]. Overexpression of HDAC6 is associated with shortening and/or loss of ciliary appendages, an important feature of malignant transformation of cholangiocytes[100] HDAC1 was found to be overexpressed in IHCC cells in vitro as a result of elevated SPRR2A, a gene involved in maintenance of epithelial barriers and wound repair, resulting in deacetylation of p53[101]. Evidence also suggests that a variety of HDAC inhibitors, such as valproic acid (VPA) and vorinostat can in vitro and in vivo inhibit the growth of cholangiocarcinoma individually or in combination with chemotherapeutic agents[99]. ncRNAs are the newly defined players in cholangiocarcinogenesis, being able to act as tumour suppressor genes or oncogenes. Therefore, representing potentially valuable tools in diagnosis and targets for treatment[102].
Molecular alterations of precursor lesions
Genomic alterations accumulate in precursor lesions during the multistep biliary carcinogenesis. An increased expression of p21, p53, cyclin D1 along with a decreased expression of Dcp4 is observed in a histological progression of BilIN[103]. Expression of EZH2 shows a stepwise increase from low grade to high grade to invasive cancer[104]. Molecular alterations of KRAS have also been reported in BilIN[105]. Molecular analysis of IPNBs reveal KRAS mutations, over-expressions of TP53 and losses of p16 in low-grade dysplasia. A loss of SMAD4 is noted in late phases of tumour development[106]. Another study investigated the genetic landscape of biliary papillary neoplasms by whole exome sequencing. Mutations in either APC or CTNNB1 were detected in 4 of 7 cases. Somatic mutations were also identified in KRAS, BRAF, CDC27, KMT2C, KMT2D, and MSH3, MSH6, PMS1[107]. Genetic alterations reported in ITPN are CDKN2A/p16 and TP53[108]. Very recently, Gross et al[109] performed whole exome sequencing of ITPN and demonstrated a high genetic diversity with recurrent copy number variants (CNVs) (loss of chromosome 1p36 and others), and only a few recurrent somatic mutations in TG, SLIT2, FGFR2, and HMCN1. They also identified cell cycle, chromatin remodelling, and DNA damage/repair as key signalling pathways in these neoplasms. In HMCN, there is activation of hedgehog and wnt pathways; and downregulation of T-helper 1 and 2 pathways[73].
Role of molecular pathology in diagnosis and management
Molecular characterisation of cholangiocarcinoma is now being considered a way to differentiate benign and cancerous biliary strictures. It will potentially help clinicians decide optimal treatment plan. Recently, a study evaluated a 28-gene next-generation sequencing panel (genes that are commonly mutated, amplified, and/or deleted in malignant biliary neoplasms) named BiliSeq using endoscopic retrograde cholangiopancreatography-obtained biliary specimens from patients with bile duct strictures[110]. Combining BiliSeq with pathological evaluation of biliary tissue improved the detection of malignant biliary strictures and allowed for the identification of potentially targetable molecular alterations, thus guiding treatment decisions.
LIQUID BIOPSY IN CHOLANGIOCARCINOMA
The term liquid biopsies comprise a diverse group of methodologies centring around the detection and analysis of tumour cells or tumour cell products obtained from blood or other body fluids[111]. Different types of liquid biopsies include circulating tumour cells (CTCs), cell free nucleic acids (cfDNA, mRNA, non-coding RNA such as micro-RNA or long non-coding RNA), “tumour-educated platelets” (TEPs) and vesicles such as exosomes[112]. The clinical application of liquid biopsies includes early detection of cancer or tumour recurrence, individual risk-assessment and treatment monitoring. Few studies have evaluated role of liquid biopsies in cholangiocarcinoma. Yang et al[113] showed that CTCs were associated with more-aggressive tumour characteristics and were independently associated with a poorer survival in patients with cholangiocarcinoma. Wintachai et al[114] investigated the diagnostic and prognostic values of plasma cfDNA levels from 62 cholangiocarcinoma patients, 33 benign biliary disease patients and 30 normal controls. They demonstrated a superior diagnostic efficacy of cfDNA in detecting cholangiocarcinoma than CEA and CA19-9. Most commonly identified genetic alterations were in ARID1A (30%), PBRM1 (30%), mTOR (30%), and FGFR3 (30%). The current role of liquid biopsies in cholangiocarcinoma remain limited and further research is required to appreciate its full potential[111].
CLINICAL FEATURES AND MANAGEMENT
Clinically and management-wise cholangiocarcinomas can be classified according to anatomical location of lesion along the biliary tract. IHCC arise proximal to second order of biliary tree and hence are harder to diagnose before they become symptomatic. Symptoms usually arise from the size and pressure on vascular and biliary structures. Compression on the biliary system leads to jaundice. Occasionally, IHCC can co-exist with HCC in a cirrhotic liver and are usually incidentally diagnosed in liver transplant (LT) recipients. In a non-cirrhotic liver, standard of care remains anatomical liver resection with an aim to achieve microscopically negative (R0) resection margins. Radiologically, hypervascular IHCC associated with high microvascular density, arterial vessel density, and cholangiocellular or bile ductular subtype on pathology have a more favourable outcome. These tumours are less aggressive in nature than those with hypovascular features[57]. The roles of adjuvant and neoadjuvant chemo-radiotherapy have not been entirely defined. However, gemcitabine-based adjuvant chemotherapy has shown to improve the overall and disease-free survival. Nonetheless, overall survival remains dismal due to a delay in the diagnosis[115]. Early results from targeted therapies including inhibitors of IDH or fibroblast growth factor receptor (FGFR) in IHCC have been promising[116]. Emerging clinical data from immune checkpoint inhibitors therapy suggest modest efficacy in cholangiocarcinoma[116]. Role of NTRK, BRAF and MEK inhibitors are also being investigated in cholangiocarcinoma[117].
Due to their anatomical location, PHCC and DCC usually present earlier than IHCC with symptoms of vascular or biliary compression. The most common presentation is in the form of obstructive jaundice. PHCC also known as Klatskin’s tumour have been variously classified based on the anatomical location, extent of tumour involvement and resectability[118-121]. Bismuth’s classification is based on the anatomical location of the tumour is the most commonly used classification[121]. Standard of care is again R0 resection followed by adjuvant therapy with Gemcitabine based on stage of the lesion[122]. Newer modalities in terms of focussed radiation therapy has shown some promise[123]. LT has been successfully performed in selected cases of unresectable cholangiocarcinoma. Several series have shown good 5-year overall survival in highly selected patients who have undergone neo-adjuvant therapy as a part of specially designed algorithms (e.g., Mayo protocol)[124]. DCC behave akin to periampullary carcinoma and the treatment is mainly surgical in the form of a pancreaticoduodenectomy (Whipple’s procedure)[125]. Surgical resection is the standard therapy for IPNBs confined to the liver. IPNB-associated invasive adenocarcinoma has demonstrated a better prognosis than conventional IHCC[126]. Complete surgical resection is also the treatment of choice for ITPNs.
Surgical resection and lymphadenectomy
Treatment of choice as mentioned above, is surgical resection for these tumours. Proximal and distal extent of the lesion, along with the degree of vascular involvement combined with the quality and volume of the liver are crucial factors in the surgical management algorithm in cholangiocarcinoma[127]. Arterial and portal vein local resections are indicated when R0 resection can be potentially achieved. Anatomical resection of the liver is sufficient in IHCC, however for PHCC & DCC an extensive locoregional lymphadenectomy is indicated. Lymphadenectomy for these tumours have shown to improve survival, and furthermore allow accurate staging, prognostication and institution of adjuvant therapy. Although data on the extent of lymphadenectomy is not sufficient and conclusive, the involvement of para-aortic lymph nodes is unequivocally a bad prognostic indicator and lymphadenectomy should not be extended to the same[128,129].
CONCLUSION
Cholangiocarcinomas are a heterogeneous group of cancers arising from the biliary tree demonstrating marked geographical variation due to regional differences in risk factors. Traditionally considered as a single disease, extensive genomic and epigenomic characterization in the last decade have uncovered various molecular alterations associated with specific subtypes of cholangiocarcinoma. Mutated genes may be specifically targeted for therapeutic intervention, a few of which include inhibitors of IDH and FGFR in intrahepatic cancers and trastuzumab in HER2-positive extrahepatic cancers. Despite recent advances in our understanding of biliary cancer, many important questions remain for the prevention and treatment of this lethal disease. Currently, there is no international consensus on the histological classification of cholangiocarcinoma, and there remains a need for standardization of nomenclature and diagnostic criteria of these tumours.
Footnotes
Conflict-of-interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 25, 2021
First decision: December 4, 2021
Article in press: February 23, 2022
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu J, Rompianesi G, Shi YJ S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Mukul Vij, Department of Pathology, Dr Rela Institute and Medical center, Chennai 600044, Tamil Nadu, India. mukul.vij.path@gmail.com.
Yogesh Puri, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Ashwin Rammohan, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Gowripriya G, Department of Pathology, Dr Rela Institute and Medical center, Chennai 600044, Tamil Nadu, India.
Rajesh Rajalingam, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Ilankumaran Kaliamoorthy, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
Mohamed Rela, Institute of Liver Disease and Transplantation, Dr Rela Institute and Medical Center, Chennai 600044, Tamil Nadu, India.
References
- 1.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6:956–969. doi: 10.1016/S2468-1253(21)00171-0. [DOI] [PubMed] [Google Scholar]
- 5.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 6.Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5:61. doi: 10.21037/cco.2016.10.09. [DOI] [PubMed] [Google Scholar]
- 7.Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, Fiel MI, Schwartz M, Thung SN. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31:49–60. doi: 10.1055/s-0031-1272839. [DOI] [PubMed] [Google Scholar]
- 8.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging. 2014;14:14. doi: 10.1186/1470-7330-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, Carpino G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira IS, Kilcoyne A, Everett JM, Mino-Kenudson M, Harisinghani MG, Ganesan K. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdom Radiol (NY) 2017;42:1637–1649. doi: 10.1007/s00261-017-1094-7. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 14.Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:301–308. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, Therneau TM, Olson JE, Chaiteerakij R, Roberts LR. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64:785–796. doi: 10.1002/hep.28529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM, Alvaro D. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9:231–240. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 17.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–635. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 18.Zen Y, Hübscher SG, Nakanuma Y. Bile duct diseases. In: Burt AD, Ferrell LD, Hubscher SG, editors. MacSween’s Pathology of the Liver. 7th edition. Philadelphia: Elsevier, 2018: 516-593. [Google Scholar]
- 19.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 21.Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419–427. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lendvai G, Szekerczés T, Illyés I, Dóra R, Kontsek E, Gógl A, Kiss A, Werling K, Kovalszky I, Schaff Z, Borka K. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol Oncol Res. 2020;26:3–15. doi: 10.1007/s12253-018-0491-8. [DOI] [PubMed] [Google Scholar]
- 23.Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, Geschwind JF, Pawlik TM. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750.e4. doi: 10.1016/j.jamcollsurg.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Vyas M, Deshpande V. How do I distinguish cholangiocarcinoma from metastatic carcinoma and why does it matter? Diagnostic Histopathology. 2021 [Google Scholar]
- 25.Nakanuma Y, Klimstra DS, Komuta M, Zen Y. Intrahepatic Cholangiocarcinoma. In: WHO Classification of Tumours Editorial Board, Editor. Digestive system tumours. WHO classification of tumours series. 5th ed. Lyon: International Agency for Research on Cancer, 2019: 254-259. [Google Scholar]
- 26.Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract Res Clin Gastroenterol. 2015;29:277–293. doi: 10.1016/j.bpg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 28.Nakanuma Y, Sato Y, Ojima H, Kanai Y, Aishima S, Yamamoto M, Ariizumi S, Furukawa T, Hayashi H, Unno M, Ohta T Hepatolithiasis Subdivision of Intractable Hepatobiliary Diseases Study Group of Japan (Chairman, Hirohito Tsubouchi) Clinicopathological characterization of so-called "cholangiocarcinoma with intraductal papillary growth" with respect to "intraductal papillary neoplasm of bile duct (IPNB)". Int J Clin Exp Pathol. 2014;7:3112–3122. [PMC free article] [PubMed] [Google Scholar]
- 29.Krasinskas AM. Cholangiocarcinoma. Surg Pathol Clin. 2018;11:403–429. doi: 10.1016/j.path.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B, et al. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carnoiro F, Hruba RH, Theise ND, editors. WHO classification of tumors of the digestive system. Lyon: IARC Press, 2010: 217-224. [Google Scholar]
- 31.Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen Y, Kosaka K, Harada K. Pathology of peripheral intrahepatic cholangiocarcinoma with reference to tumorigenesis. Hepatol Res. 2008;38:325–334. doi: 10.1111/j.1872-034X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 32.Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, Sawada S, Minato H, Matsui O, Nakanuma Y. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: 'bile ductular carcinoma'. Histopathology. 2007;51:390–400. doi: 10.1111/j.1365-2559.2007.02735.x. [DOI] [PubMed] [Google Scholar]
- 33.Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:22–34. doi: 10.21037/hbsn.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigel CS, Drill E, Zhou Y, Basturk O, Askan G, Pak LM, Vakiani E, Wang T, Boerner T, Do RKG, Simpson AL, Jarnagin W, Klimstra DS. Intrahepatic Cholangiocarcinomas Have Histologically and Immunophenotypically Distinct Small and Large Duct Patterns. Am J Surg Pathol. 2018;42:1334–1345. doi: 10.1097/PAS.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akita M, Fujikura K, Ajiki T, Fukumoto T, Otani K, Azuma T, Itoh T, Ku Y, Zen Y. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol. 2017;30:986–997. doi: 10.1038/modpathol.2017.22. [DOI] [PubMed] [Google Scholar]
- 36.Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Ariizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant "ductal plate malformation" pattern: a new subtype. Am J Surg Pathol. 2012;36:1629–1635. doi: 10.1097/PAS.0b013e31826e0249. [DOI] [PubMed] [Google Scholar]
- 37.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, Montal R, Torrens L, Martinez-Quetglas I, Fiel MI, Hao K, Villanueva A, Thung SN, Schwartz ME, Llovet JM. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Vogel A, Saborowski A. Cholangiocellular Carcinoma. Digestion. 2017;95:181–185. doi: 10.1159/000454763. [DOI] [PubMed] [Google Scholar]
- 39.Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M, Sato Y, Nakanuma Y. Cholangiolocellular Carcinoma With "Ductal Plate Malformation" Pattern May Be Characterized by ARID1A Genetic Alterations. Am J Surg Pathol. 2019;43:352–360. doi: 10.1097/PAS.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 41.Huang YH, Zhang CZ, Huang QS, Yeong J, Wang F, Yang X, He YF, Zhang XL, Zhang H, Chen SL, Zheng YL, Deng R, Lin CS, Yang MM, Li Y, Jiang C, Kin-Wah Lee T, Ma S, Zeng MS, Yun JP. Clinicopathologic features, tumor immune microenvironment and genomic landscape of Epstein-Barr virus-associated intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:838–849. doi: 10.1016/j.jhep.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Lin A, Alpert L, Hart J, Chapman C, Pillai AA. Lymphoepithelioma-Like Carcinomas: A Rare Variant of Cholangiocarcinoma. Hepatology. 2020;72:353–355. doi: 10.1002/hep.31102. [DOI] [PubMed] [Google Scholar]
- 43.Labgaa I, Stueck A, Ward SC. Lymphoepithelioma-Like Carcinoma in Liver. Am J Pathol. 2017;187:1438–1444. doi: 10.1016/j.ajpath.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Hsu HC, Chen CC, Huang GT, Lee PH. Clonal Epstein-Barr virus associated cholangiocarcinoma with lymphoepithelioma-like component. Hum Pathol. 1996;27:848–850. doi: 10.1016/s0046-8177(96)90460-8. [DOI] [PubMed] [Google Scholar]
- 45.Mostyka M, Birch MM, Samstein B, Pittman ME. Hidden Carcinoma: Pitfalls in the Diagnosis of Lymphoepithelioma-Like Cholangiocarcinoma. Int J Surg Pathol. 2020;28:872–878. doi: 10.1177/1066896920921560. [DOI] [PubMed] [Google Scholar]
- 46.Haas S, Gütgemann I, Wolff M, Fischer HP. Intrahepatic clear cell cholangiocarcinoma: immunohistochemical aspects in a very rare type of cholangiocarcinoma. Am J Surg Pathol. 2007;31:902–906. doi: 10.1097/PAS.0b013e31802c0c8a. [DOI] [PubMed] [Google Scholar]
- 47.Tihan T, Blumgart L, Klimstra DS. Clear cell papillary carcinoma of the liver: an unusual variant of peripheral cholangiocarcinoma. Hum Pathol. 1998;29:196–200. doi: 10.1016/s0046-8177(98)90235-0. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T, Abe T, Oshita A, Yonehara S, Katamura Y, Matsumoto N, Kobayashi T, Nakahara M, Ohdan H, Noriyuki T. Intrahepatic cholangiocarcinoma with clear cell type following laparoscopic curative surgery. Surg Case Rep. 2020;6:264. doi: 10.1186/s40792-020-01041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim DK, Kim BR, Jeong JS, Baek YH. Analysis of intrahepatic sarcomatoid cholangiocarcinoma: Experience from 11 cases within 17 years. World J Gastroenterol. 2019;25:608–621. doi: 10.3748/wjg.v25.i5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang T, Kong J, Yang X, Shen S, Zhang M, Wang W. Clinical features of sarcomatoid change in patients with intrahepatic cholangiocarcinoma and prognosis after surgical liver resection: A Propensity Score Matching analysis. J Surg Oncol. 2020;121:524–537. doi: 10.1002/jso.25815. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Ming JL, Ren XY, Qiu L, Zhou LJ, Yang SD, Fang XM. Sarcomatoid intrahepatic cholangiocarcinoma mimicking liver abscess: A case report. World J Clin Cases. 2020;8:208–216. doi: 10.12998/wjcc.v8.i1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patil PA, Taddei T, Jain D, Zhang X. HNF-1β is a More Sensitive and Specific Marker Than C-Reactive Protein for Identifying Biliary Differentiation in Primary Hepatic Carcinomas. Arch Pathol Lab Med. 2021 doi: 10.5858/arpa.2020-0725-OA. [DOI] [PubMed] [Google Scholar]
- 53.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40:1021–1030. doi: 10.1097/PAS.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 55.Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 56.Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type vs peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94–100. doi: 10.1002/jhbp.154. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen Canh H, Takahashi K, Yamamura M, Li Z, Sato Y, Yoshimura K, Kozaka K, Tanaka M, Nakanuma Y, Harada K. Diversity in cell differentiation, histology, phenotype and vasculature of mass-forming intrahepatic cholangiocarcinomas. Histopathology. 2021 doi: 10.1111/his.14417. [DOI] [PubMed] [Google Scholar]
- 58.Fernández Moro C, Fernandez-Woodbridge A, Alistair D'souza M, Zhang Q, Bozoky B, Vasan SK, Catalano P, Heuchel R, Shtembari S, Del Chiaro M, Danielsson O, Björnstedt M, Löhr JM, Isaksson B, Verbeke C, Bozóky B. Correction: Immunohistochemical Typing of Adenocarcinomas of the Pancreatobiliary System Improves Diagnosis and Prognostic Stratification. PLoS One. 2017;12:e0171283. doi: 10.1371/journal.pone.0171283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato Y, Sasaki M, Harada K, Aishima S, Fukusato T, Ojima H, Kanai Y, Kage M, Nakanuma Y, Tsubouchi H Hepatolithiasis Subdivision of Intractable Hepatobiliary Diseases Study Group of Japan (Chairman, Hirohito Tsubouchi) Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J Gastroenterol. 2014;49:64–72. doi: 10.1007/s00535-013-0810-5. [DOI] [PubMed] [Google Scholar]
- 60.Ainechi S, Lee H. Updates on Precancerous Lesions of the Biliary Tract: Biliary Precancerous Lesion. Arch Pathol Lab Med. 2016;140:1285–1289. doi: 10.5858/arpa.2015-0396-RS. [DOI] [PubMed] [Google Scholar]
- 61.Busturk O, Aishima S, Esposito I. Bilairy intraepithelial neoplasia. In: WHO Classification of Tumours Editorial Board, editor. Digestive system tumours. WHO classification of tumours series. 5th edition. Lyon: International Agency for Research on Cancer, 2019: 273-275. [Google Scholar]
- 62.Wu TT, Levy M, Correa AM, Rosen CB, Abraham SC. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer. 2009;115:4564–4575. doi: 10.1002/cncr.24471. [DOI] [PubMed] [Google Scholar]
- 63.Zen Y, Quaglia A, Heaton N, Rela M, Portmann B. Two distinct pathways of carcinogenesis in primary sclerosing cholangitis. Histopathology. 2011;59:1100–1110. doi: 10.1111/j.1365-2559.2011.04048.x. [DOI] [PubMed] [Google Scholar]
- 64.Nakanuma Y, Uesaka K, Okamura Y, Terada T, Fukumura Y, Kakuda Y, Sugino T, Sato Y, Taek JK, Park YN. Reappraisal of pathological features of intraductal papillary neoplasm of bile duct with respect to the type 1 and 2 subclassifications. Hum Pathol. 2021;111:21–35. doi: 10.1016/j.humpath.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, Fukumura Y, Isayama H, Terada T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J Clin Med. 2020;9 doi: 10.3390/jcm9123991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakanuma Y, Busturk O, Esposito I, Klimstra DS, Komuta M, Zen Y. Intraductal Papillary Neoplasm of Bile Duct. In: WHO Classification of Tumours Editorial Board, editor. Digestive system tumours. WHO classification of tumours series. 5th edition. Lyon: International Agency for Research on Cancer, 2019: 279-282. [Google Scholar]
- 67.Aoki Y, Mizuma M, Hata T, Aoki T, Omori Y, Ono Y, Mizukami Y, Unno M, Furukawa T. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. 2020;251:38–48. doi: 10.1002/path.5398. [DOI] [PubMed] [Google Scholar]
- 68.Nakanuma Y, Jang KT, Fukushima N, Furukawa T, Hong SM, Kim H, Lee KB, Zen Y, Jang JY, Kubota K. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci. 2018;25:181–187. doi: 10.1002/jhbp.532. [DOI] [PubMed] [Google Scholar]
- 69.Nakanuma Y, Uesaka K, Miyayama S, Yamaguchi H, Ohtsuka M. Intraductal neoplasms of the bile duct. A new challenge to biliary tract tumor pathology. Histol Histopathol. 2017;32:1001–1015. doi: 10.14670/HH-11-892. [DOI] [PubMed] [Google Scholar]
- 70.Akita M, Hong SM, Sung YN, Kim MJ, Ajiki T, Fukumoto T, Itoh T, Zen Y. Biliary intraductal tubule-forming neoplasm: a whole exome sequencing study of MUC5AC-positive and -negative cases. Histopathology. 2020;76:1005–1012. doi: 10.1111/his.14103. [DOI] [PubMed] [Google Scholar]
- 71.Katabi N, Torres J, Klimstra DS. Intraductal tubular neoplasms of the bile ducts. Am J Surg Pathol. 2012;36:1647–1655. doi: 10.1097/PAS.0b013e3182684d4f. [DOI] [PubMed] [Google Scholar]
- 72.Schlitter AM, Jang KT, Klöppel G, Saka B, Hong SM, Choi H, Offerhaus GJ, Hruban RH, Zen Y, Konukiewitz B, Regel I, Allgäuer M, Balci S, Basturk O, Reid MD, Esposito I, Adsay V. Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Mod Pathol. 2015;28:1249–1264. doi: 10.1038/modpathol.2015.61. [DOI] [PubMed] [Google Scholar]
- 73.Van Treeck BJ, Lotfalla M, Czeczok TW, Mounajjed T, Moreira RK, Allende DS, Reid MD, Naini BV, Westerhoff M, Adsay NV, Kerr SE, Rizvi SH, Smoot RL, Liu Y, Davila J, Graham RP. Molecular and Immunohistochemical Analysis of Mucinous Cystic Neoplasm of the Liver. Am J Clin Pathol. 2020;154:837–847. doi: 10.1093/ajcp/aqaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079–1089. doi: 10.1038/modpathol.2011.71. [DOI] [PubMed] [Google Scholar]
- 75.Busturk O, Nakanuma Y, Aishima A, Esposito I. Mucinous cystic neoplasms of the liver and biliary systtem. In: WHO Classification of Tumours Editorial Board, editor. Digestive system tumours. WHO classification of tumours series. 5th editon. Lyon: International Agency for Research on Cancer, 2019: 250-253. [Google Scholar]
- 76.Nakajima T, Sugano I, Matsuzaki O, Nagao K, Kondo Y, Miyazaki M, Wada K. Biliary cystadenocarcinoma of the liver. A clinicopathologic and histochemical evaluation of nine cases. Cancer. 1992;69:2426–2432. doi: 10.1002/1097-0142(19920515)69:10<2426::aid-cncr2820691007>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 77.Thai E, Dalla Valle R, Evaristi F, Silini EM. A case of biliary adenofibroma with malignant transformation. Pathol Res Pract. 2016;212:468–470. doi: 10.1016/j.prp.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 78.Jain D, Sarode VR, Abdul-Karim FW, Homer R, Robert ME. Evidence for the neoplastic transformation of Von-Meyenburg complexes. Am J Surg Pathol. 2000;24:1131–1139. doi: 10.1097/00000478-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Song JS, Lee YJ, Kim KW, Huh J, Jang SJ, Yu E. Cholangiocarcinoma arising in von Meyenburg complexes: report of four cases. Pathol Int. 2008;58:503–512. doi: 10.1111/j.1440-1827.2008.02264.x. [DOI] [PubMed] [Google Scholar]
- 80.Kongpetch S, Jusakul A, Ong CK, Lim WK, Rozen SG, Tan P, Teh BT. Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways. Best Pract Res Clin Gastroenterol. 2015;29:233–244. doi: 10.1016/j.bpg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 82.Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Personeni N, Lleo A, Pressiani T, Colapietro F, Openshaw MR, Stavraka C, Pouptsis A, Pinato DJ, Rimassa L. Biliary Tract Cancers: Molecular Heterogeneity and New Treatment Options. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, Kemeny NE, O'Reilly EM, El-Dika I, Jarnagin WR, Harding JJ, D'Angelica MI, Cercek A, Hechtman JF, Solit DB, Schultz N, Hyman DM, Klimstra DS, Saltz LB, Abou-Alfa GK. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedica F, Grassini G. Pathology and molecular pathology of cholangiocarcinoma. Hepatoma Res. 2021;7:71. [Google Scholar]
- 87.Jang S, Chun SM, Hong SM, Sung CO, Park H, Kang HJ, Kim KP, Lee YJ, Yu E. High throughput molecular profiling reveals differential mutation patterns in intrahepatic cholangiocarcinomas arising in chronic advanced liver diseases. Mod Pathol. 2014;27:731–739. doi: 10.1038/modpathol.2013.194. [DOI] [PubMed] [Google Scholar]
- 88.Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C, Cornella H, Klotzle B, Fan JB, Cotsoglou C, Thung SN, Fuster J, Waxman S, Garcia-Valdecasas JC, Bruix J, Schwartz ME, Beroukhim R, Mazzaferro V, Llovet JM. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim YH, Hong EK, Kong SY, Han SS, Kim SH, Rhee JK, Hwang SK, Park SJ, Kim TM. Two classes of intrahepatic cholangiocarcinoma defined by relative abundance of mutations and copy number alterations. Oncotarget. 2016;7:23825–23836. doi: 10.18632/oncotarget.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva VW, Askan G, Daniel TD, Lowery M, Klimstra DS, Abou-Alfa GK, Shia J. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5:62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 91.Goeppert B, Roessler S, Renner M, Singer S, Mehrabi A, Vogel MN, Pathil A, Czink E, Köhler B, Springfeld C, Pfeiffenberger J, Rupp C, Weiss KH, Schirmacher P, von Knebel Doeberitz M, Kloor M. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer. 2019;120:109–114. doi: 10.1038/s41416-018-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y, Chen Y, Zhang X, Xu Q, Wu Z, Cao X, Shao M, Shu Y, Lv T, Lu C, Xie M, Wen T, Yang J, Shi Y, Bu H. Brahma-Related Gene 1 Inhibition Prevents Liver Fibrosis and Cholangiocarcinoma by Attenuating Progenitor Expansion. Hepatology. 2021;74:797–815. doi: 10.1002/hep.31780. [DOI] [PubMed] [Google Scholar]
- 93.Dong LQ, Shi Y, Ma LJ, Yang LX, Wang XY, Zhang S, Wang ZC, Duan M, Zhang Z, Liu LZ, Zheng BH, Ding ZB, Ke AW, Gao DM, Yuan K, Zhou J, Fan J, Xi R, Gao Q. Spatial and temporal clonal evolution of intrahepatic cholangiocarcinoma. J Hepatol. 2018;69:89–98. doi: 10.1016/j.jhep.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 94.Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabró R, Pinyol R, Torres-Martin M, Bassaganyas L, Moeini A, Peix J, Cabellos L, Maeda M, Villacorta-Martin C, Tabrizian P, Rodriguez-Carunchio L, Castellano G, Sempoux C, Minguez B, Pawlik TM, Labgaa I, Roberts LR, Sole M, Fiel MI, Thung S, Fuster J, Roayaie S, Villanueva A, Schwartz M, Llovet JM. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73:315–327. doi: 10.1016/j.jhep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim SG, Chan AO, Wu TT, Issa JP, Hamilton SR, Rashid A. Epigenetic and genetic alterations in duodenal carcinomas are distinct from biliary and ampullary carcinomas. Gastroenterology. 2003;124:1300–1310. doi: 10.1016/s0016-5085(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 96.Rodrigues PM, Olaizola P, Paiva NA, Olaizola I, Agirre-Lizaso A, Landa A, Bujanda L, Perugorria MJ, Banales JM. Pathogenesis of Cholangiocarcinoma. Annu Rev Pathol. 2021;16:433–463. doi: 10.1146/annurev-pathol-030220-020455. [DOI] [PubMed] [Google Scholar]
- 97.Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7:1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morine Y, Shimada M, Iwahashi S, Utsunomiya T, Imura S, Ikemoto T, Mori H, Hanaoka J, Miyake H. Role of histone deacetylase expression in intrahepatic cholangiocarcinoma. Surgery. 2012;151:412–419. doi: 10.1016/j.surg.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 99.O'Rourke CJ, Munoz-Garrido P, Aguayo EL, Andersen JB. Epigenome dysregulation in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1423–1434. doi: 10.1016/j.bbadis.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 100.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizuguchi Y, Specht S, Lunz JG 3rd, Isse K, Corbitt N, Takizawa T, Demetris AJ. SPRR2A enhances p53 deacetylation through HDAC1 and down regulates p21 promoter activity. BMC Mol Biol. 2012;13:20. doi: 10.1186/1471-2199-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Esparza-Baquer A, Labiano I, Bujanda L, Perugorria MJ, Banales JM. MicroRNAs in cholangiopathies: Potential diagnostic and therapeutic tools. Clin Res Hepatol Gastroenterol. 2016;40:15–27. doi: 10.1016/j.clinre.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts. World J Hepatol. 2009;1:35–42. doi: 10.4254/wjh.v1.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175–183. doi: 10.1002/path.2345. [DOI] [PubMed] [Google Scholar]
- 105.Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119:1669–1674. doi: 10.1002/cncr.27955. [DOI] [PubMed] [Google Scholar]
- 106.Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B, Zen Y, Schuster T, Höfler H, Perren A, Klöppel G, Esposito I. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2014;27:73–86. doi: 10.1038/modpathol.2013.112. [DOI] [PubMed] [Google Scholar]
- 107.Fujikura K, Akita M, Ajiki T, Fukumoto T, Itoh T, Zen Y. Recurrent Mutations in APC and CTNNB1 and Activated Wnt/β-catenin Signaling in Intraductal Papillary Neoplasms of the Bile Duct: A Whole Exome Sequencing Study. Am J Surg Pathol. 2018;42:1674–1685. doi: 10.1097/PAS.0000000000001155. [DOI] [PubMed] [Google Scholar]
- 108.Kuan LL, Dennison AR, Garcea G. Intraductal Tubulopapillary Neoplasm of the Pancreas and Bile Duct: A Review. Pancreas. 2020;49:498–502. doi: 10.1097/MPA.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 109.Gross C, Engleitner T, Lange S, Weber J, Jesinghaus M, Konukiewitz B, Muckenhuber A, Steiger K, Pfarr N, Goeppert B, Keller G, Weichert W, Adsay NV, Klöppel G, Rad R, Esposito I, Schlitter AM. Whole Exome Sequencing of Biliary Tubulopapillary Neoplasms Reveals Common Mutations in Chromatin Remodeling Genes. Cancers (Basel) 2021;13 doi: 10.3390/cancers13112742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singhi AD, Nikiforova MN, Chennat J, Papachristou GI, Khalid A, Rabinovitz M, Das R, Sarkaria S, Ayasso MS, Wald AI, Monaco SE, Nalesnik M, Ohori NP, Geller D, Tsung A, Zureikat AH, Zeh H, Marsh JW, Hogg M, Lee K, Bartlett DL, Pingpank JF, Humar A, Bahary N, Dasyam AK, Brand R, Fasanella KE, McGrath K, Slivka A. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. 2020;69:52–61. doi: 10.1136/gutjnl-2018-317817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poulet G, Massias J, Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63:449–455. doi: 10.1159/000499337. [DOI] [PubMed] [Google Scholar]
- 112.Rompianesi G, Di Martino M, Gordon-Weeks A, Montalti R, Troisi R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J Gastrointest Oncol. 2021;13:332–350. doi: 10.4251/wjgo.v13.i5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang JD, Campion MB, Liu MC, Chaiteerakij R, Giama NH, Ahmed Mohammed H, Zhang X, Hu C, Campion VL, Jen J, Venkatesh SK, Halling KC, Kipp BR, Roberts LR. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148–158. doi: 10.1002/hep.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wintachai P, Lim JQ, Techasen A, Lert-Itthiporn W, Kongpetch S, Loilome W, Chindaprasirt J, Titapun A, Namwat N, Khuntikeo N, Jusakul A. Diagnostic and Prognostic Value of Circulating Cell-Free DNA for Cholangiocarcinoma. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11060999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, Invernizzi P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9 doi: 10.3390/cells9030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.KLATSKIN G. ADENOCARCINOMA OF THE HEPATIC DUCT AT ITS BIFURCATION WITHIN THE PORTA HEPATIS. AN UNUAL TUMOR WITH DISTINCTIVE CLINICAL AND PATHOLOGICAL FEATURES. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 119.Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 120.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC cancer staging manual: Springer New York, 2010. [Google Scholar]
- 121.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3:18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ren B, Guo Q, Yang Y, Liu L, Wei S, Chen W, Tian Y. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy vs no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat Oncol. 2020;15:15. doi: 10.1186/s13014-020-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125–1130. doi: 10.1097/TP.0000000000003212. [DOI] [PubMed] [Google Scholar]
- 125.Kim BH, Kim K, Chie EK, Kwon J, Jang JY, Kim SW, Oh DY, Bang YJ. Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution. Cancer Res Treat. 2017;49:473–483. doi: 10.4143/crt.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Covelli C, Capelli P, Furlanetto A, Guido M. Benign biliary neoplasms and biliary tumor precursors. Pathologica. 2021;113:147–157. doi: 10.32074/1591-951X-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sano T, Shimizu Y, Senda Y, Kinoshita T, Nimura Y. Assessing resectability in cholangiocarcinoma. Hepat Oncol. 2014;1:39–51. doi: 10.2217/hep.13.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aoba T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, Nagino M. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 2013;257:718–725. doi: 10.1097/SLA.0b013e3182822277. [DOI] [PubMed] [Google Scholar]
- 129.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]