Abstract
BACKGROUND
Surgical resection after neoadjuvant treatment is the main driver for improved survival in locally advanced pancreatic cancer (LAPC). However, the diagnostic performance of computed tomography (CT) imaging to evaluate the residual tumour burden at restaging after neoadjuvant therapy is low due to the difficulty in distinguishing neoplastic tissue from fibrous scar or inflammation. In this context, radiomics has gained popularity over conventional imaging as a complementary clinical tool capable of providing additional, unprecedented information regarding the intratumor heterogeneity and the residual neoplastic tissue, potentially serving in the therapeutic decision-making process.
AIM
To assess the capability of radiomic features to predict surgical resection in LAPC treated with neoadjuvant chemotherapy and radiotherapy.
METHODS
Patients with LAPC treated with intensive chemotherapy followed by ablative radiation therapy were retrospectively reviewed. One thousand six hundred and fifty-five radiomic features were extracted from planning CT inside the gross tumour volume. Both extracted features and clinical data contribute to create and validate the predictive model of resectability status. Patients were repeatedly divided into training and validation sets. The discriminating performance of each model, obtained applying a LASSO regression analysis, was assessed with the area under the receiver operating characteristic curve (AUC). The validated model was applied to the entire dataset to obtain the most significant features.
RESULTS
Seventy-one patients were included in the analysis. Median age was 65 years and 57.8% of patients were male. All patients underwent induction chemotherapy followed by ablative radiotherapy, and 19 (26.8%) ultimately received surgical resection. After the first step of variable selections, a predictive model of resectability was developed with a median AUC for training and validation sets of 0.862 (95%CI: 0.792-0.921) and 0.853 (95%CI: 0.706-0.960), respectively. The validated model was applied to the entire dataset and 4 features were selected to build the model with predictive performance as measured using AUC of 0.944 (95%CI: 0.892-0.996).
CONCLUSION
The present radiomic model could help predict resectability in LAPC after neoadjuvant chemotherapy and radiotherapy, potentially integrating clinical and morphological parameters in predicting surgical resection.
Keywords: Computed tomography, Radiomics, Predictive model, Resectability, Locally advanced pancreatic cancer, Radiation oncology
Core Tip: The present study proposes a computed tomography (CT)-based radiomics model to predict resectability in locally advanced pancreatic cancer (LAPC) treated with intensive chemotherapy followed by ablative radiation therapy. The model was built, tested, and validated in a homogeneous cohort of LAPC patients, using clinical data and radiomic features extracted from the simulation-CT, and showed a reliable performance to predict surgical resection. If further confirmed, the results of this study may allow integrating radiomic information into the pool of clinical and morphological parameters to consider when a LAPC patient is candidate for surgical exploration after neoadjuvant therapy.
INTRODUCTION
Pancreatic cancer (PC) is an aggressive disease, with increasing incidence and mortality rates, and a 5-year survival of less than 10%[1,2]. At the time of diagnosis, roughly one-third of patients present with locally advanced PC (LAPC), typically due to extensive involvement of peripancreatic vessels (e.g., celiac axis, superior mesenteric artery and vein, portal vein, common hepatic artery), that precludes surgical resection[3]. Nowadays, multiagent chemotherapy regimens, including gemcitabine plus nanoparticle albumin-bound (nab)-paclitaxel and 5-fluorouracil, leucovorin, irinotecan, plus oxaliplatin (FOLFIRINOX), represent the standard of care for LAPC, able to significantly improve survival compared to mono-chemotherapy schedules[4-6]. In parallel, the integration of dose-escalated radiation therapy (RT) approaches to intensive chemotherapy regimens have suggested the possibility to maximize the benefits of the oncological treatment[7,8]. Technological innovations, such as the use of stereotactic techniques, advanced organ motion management solutions, and accurate image guidance before treatment delivery, have allowed to safely deliver ablative doses to LAPC while sparing the surrounding critical organs at risk (OARs)[9,10]. In addition, different series reported that a multimodal approach, including systemic induction therapy followed by (chemo-) radiotherapy, might represent an effective therapeutic option for LAPC, potentially improving the oncological outcome and the probability of surgical resection, at the price of acceptable postoperative complication rates[11-14]. Thus, there is a strong need to determine the resectability of LAPC for treatment decision-making.
After neoadjuvant treatment, the therapeutic decision whether to perform surgical exploration is typically based on a multidisciplinary and multiparametric evaluation that includes patients- and tumour-specific features. Cross-sectional imaging at restaging is essential to rule out disease progression that would contraindicate surgery and drive the surgical strategy if radical resectability is considered feasible. Indeed, several studies have reported that computed tomography (CT) misestimates the resectability of LAPC after neoadjuvant treatment[15-17]. After RT, CT cannot discriminate between post-therapy fibrosis, locoregional oedema, inflammatory changes, and viable tumour, thus underestimating the histological response[18-20].
Radiomics is gaining more and more popularity for the possibility of decoding crucial information underneath medical imaging. Unlike conventional CT image analysis, radiomics can use imaging data for the high-throughput extraction of large numbers of quantitative features, able to offer additional information related to tumour phenotype, its heterogeneity, and microenvironment, as well as post-treatment changes[21]. Additionally, this information can be used to create accurate, reliable, and efficient predictive models throughout the application of machine learning algorithms[22]. To date, only a few studies have investigated the application of the radiomics framework to CT imaging to obtain potential biomarkers predictive and prognostic for treatment response and survival in PC treated with chemotherapy and radiotherapy[23-27]. More recently, the possibility of using CT radiomic biomarkers as predictors of resectability in oesophageal cancer[28] and thymic malignancies[29] has been investigated.
This study aimed to explore, for the first time, whether a CT-based radiomic model could assess LAPC patients' resectability after neoadjuvant treatment, including induction chemotherapy followed by ablative RT.
MATERIALS AND METHODS
Study design
Clinical, radiological, laboratory, surgical, and pathology data of LAPC patients receiving intensive induction chemotherapy followed by ablative RT from January 2017 to April 2020 were prospectively collected and retrospectively evaluated. The Institutional Review Board (IRB) approved the prospective collection of patient data (PAD-R n.1101 CESC). The National Comprehensive Cancer Network (NCCN) classification was used to define LAPC[30]. As described in the original published study[14], inclusion criteria for Risk Adapted Ablative Radiation Therapy (RAdAR) were: Histologically-proven pancreatic ductal adenocarcinoma, ECOG performance status < 2, at least 3 mo of chemotherapy (with gemcitabine/nab-paclitaxel or FOLFIRINOX), biochemical response, and absence of disease progression at restaging CT-scan after induction chemotherapy.
RT protocol and surgery
Details of the radiation treatment have previously been reported[14]. Briefly, the RAdAR approach consisted of anatomy- and simultaneous integrated boost (SIB)-based dose prescription strategy. If anatomically and dosimetrically feasible, the first treatment choice was stereotactic ablative RT (SAbR). However, in the following cases, the (hypo-) fractionated ablative radiotherapy (HART) schedule was adopted: Tumour 6 cm in greatest dimension, nodal spread of disease that could not be included in the SAbR target volume, tumour adhesion/infiltration of the stomach or duodenum, and/or impossibility to achieve SAbR planning objectives (e.g., non-respect of OARs dose constraints).
Following induction chemotherapy, RT was delivered with SAbR, administering 30 Gy in 5 fractions to the tumour volume (PTVt) and 50 Gy SIB to the vascular involvement, or with HART prescribing 50.4 Gy in 28 fractions to the PTVt, with a vascular SIB of 78.4 Gy. Thus, an ablative biologically effective dose (BED10 = 100 Gy) within the tumour was prescribed for both SAbR and HART. The RAdAR was delivered using RapidArc® Technology (Varian Medical Systems, Palo Alto, CA, United States) or TomoTherapy® System (Accuray, Sunnyvale, CA, United States). Daily on-line volumetric image-guided radiotherapy (cone beam or megavoltage CT) was performed before each treatment fraction. After restaging, a multidisciplinary team re-evaluated the patient and, in the absence of tumour progression, re-considered surgery if radical resection was deemed feasible; if not, patients were candidate for follow-up.
Image acquisitions and tumour segmentation
For simulation CT, patients were immobilized in the supine position with arms over the head. After a scan without contrast, a tri-phase contrast-enhanced simulation CT scan was carried out, including an arterial, a pancreatic parenchymal, and a portal venous phase, using a 64-row scanner (Brilliance 64; Philips, Eindhoven, The Netherlands). A minimum 3-h fasting period was required for all patients before simulation CT. For contrast-enhanced imaging, a weight-based amount of iodinated nonionic contrast agent was used, with an automatic power injector at a flow rate of 2–3.5 mL/s. A bolus-tracking technique at standardized time was used for contrast-enhanced phases. CT images were acquired with a 64 mm × 0.625–1.25 mm collimation, with a reconstruction thickness of 2 mm.
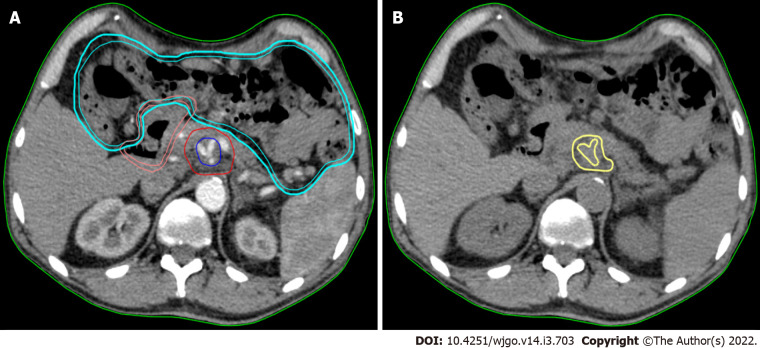
Texture analysis was performed using contrast-free simulation CT imaging. Tumour segmentation was performed using a software for medical image processing (MIM software; Mim Software Inc., United States). The volume of interest (VOI) corresponded to the gross tumour volume (GTV) and was defined by a radiation oncologist with experience in PC and validated by a second radiation oncologist (Figure 1). The segmentation excluded vessels, biliary stent, calcifications, fiducial markers, or any other potential source of artifact from the GTV (VOI) (Figure 1).
Figure 1.
Texture analysis performed using contrast-free simulation computed tomography imaging. A: Target volumes delineation in a stereotactic ablative radiation therapy case. The high-dose planning target volume (blue) encompasses the tumour-vessel interface (TVI, encasement of celiac axis) inside the tumour planning target volume (PTVt, red). The prescription dose is 30 Gy and 50 Gy in 5 fractions, with simultaneous integrated boost, to PTVt and TVI, respectively. The following organ at risk are shown: duodenum (pink) and bowel (cyan); B: The gross tumour volume (GTV) of the pancreatic lesion without vessels (yellow) is shown in the same axial computed tomography (CT)-simulation image without contrast. This is the final CT used for analysis.
Radiomic feature extraction
Radiomic features were extracted from the VOI using PyRadiomics v3.0[31]. Firstly, the VOI was resampled with an isotropic voxel of 2 mm × 2 mm × 2 mm, and HU were binned considering a width of 5 HU. The extracted features are defined according to Imaging Biomarker Standardized Initiative (IBSI)[32] and include first order statistics, shaped based both 2D and 3D, Gray Level Co-occurrence Matrix, Gray Level Run Length Matrix, Gray Level Size Zone Matrix, Neighbouring Gray Tone Difference Matrix, and Grey Level Dependence Matrix, as well as filtered features (logarithm exponential, gradient, LBP3D, and wavelet). A total of 1655 radiomic features were considered.
Statistical analysis
Statistical analysis of clinical (tumour location and size, cancer antigen 19-9 (CA19-9) value at diagnosis and after chemotherapy, clinical stage, chemotherapy regimen and radiation approach) and radiomic data was implemented in R (v3.6.3). For the analysis, patients surgically explored (e.g. exploratory laparotomy after RT) but not resected were integrated into the non-resected group. The complete workflow of statistical analysis included a first step of variable selection and a training/validation step to find the model that better predicted the outcome. Subsequently the validated model was applied to the whole dataset.
Multivariate analysis was performed firstly on the training set that included 70% of the whole database. Clinical data and radiomic features were tested for their capability to predict surgical resection using Wilcoxon rank-sum test. Correlated features were identified using Pearson correlation considering a threshold of 0.9 and were removed for further analysis. P values of the remaining variables were corrected for multiple comparisons considering Bonferroni correction (P corrected < 0.01). Multivariate LASSO regression analysis was performed to select relevant variables and build the model. The optimal lambda parameter was chosen as an average of the regularization parameter obtained from 50 times repetition of the 5-fold cross-validation process.
The model was then tested on the validation dataset, and corresponding area under the receiver operating characteristic curve (AUC) and their confidence intervals were computed. To improve the statistical significance and robustness of our analysis, all the steps were repeated 100 times. More precisely, among these repeated analyses, the solution that presents the median AUC was chosen as representative, and corresponding confidence intervals were computed throughout a bootstrap. The already tested model was re-trained on the whole dataset to obtain robust selected variables, and AUC was computed. To assess the predictive capability of our model, this was finally applied to the entire cohort to predict the surgical resection status and compute the OS for predicted surgery vs no predicted surgery patients. The log-rank test was used to assess statistical significance for survival curves.
RESULTS
Study population
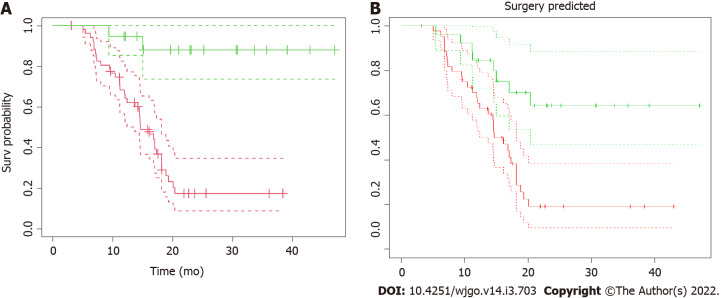
Seventy-one LAPC patients were included in the analysis. Baseline characteristics are outlined in Table 1. The median age was 65 years [interquartile range (IQR) 57-69], and 57.8% of patients were male. The median period of induction chemotherapy was 6 mo (IQR 6-6 mo). SAbR was used in 59 (83.1%) patients and HART in the remaining 12 (16.9%). All patients completed the prescribed treatment. Thirty-two patients (45.1%) underwent exploratory laparotomy after RT, and 19 (26.8%) patients ultimately received surgical resection, with a resection/exploration ratio of 59.4%. Postoperative 90-d mortality was nil. The median follow-up for the analysis was 15.0 mo (IQR 11.2-20.2 mo). Overall survival (OS) curves and their confidence intervals, estimated by Kaplan–Meier method as a function of surgical resection (resected vs non-resected patients; P < 0.001), are shown in Figure 2A.
Table 1.
Baseline characteristics and treatment details
|
Characteristic
|
Value
|
| No. of patients | 71 |
| Age, yr, median (IQR) | 64.8 (57.1-69.6) |
| Sex, male, n (%) | 41 (57.8) |
| ECOG, 0, n (%) | 61 (85.9) |
| Primary tumour location, n (%) | |
| Head | 44 (62.0) |
| Body | 25 (35.2) |
| Neck | 2 (2.8) |
| Tumour size (mm), median (IQR) | 40 (30-45) |
| Biliary stent, present, n (%) | 25 (35.2) |
| CA19-9 (U/mL) at diagnosis | 951 (± 1134) |
| CA19-9 (U/mL) after chemotherapy (before RAdAR) | 106 (± 160) |
| Clinical T stage1, n (%) | |
| T2 | 6 (8.5) |
| T3 | 11 (15.5) |
| T4 | 54 (76.0) |
| Clinical N stage1, n (%) | |
| N0 | 34 (47.9) |
| N1 | 37 (52.1) |
| Pre-RAdAR chemotherapy regimen, n (%) | |
| FOLFIRINOX | 30 (42.3) |
| Gemcitabine + nab-paclitaxel | 41 (57.7) |
| RAdAR approach, n (%) | |
| SAbR | 59 (83.2) |
| HART | 12 (16.9) |
| Delivery technique, n (%) | |
| RapidArc® Technology | 55 (77.5) |
| TomoTherapy® System | 16 (22.5) |
| Resected patients2, n (%) | 19 (26.8) |
| R status3, n (%) | |
| R0 | 12 (63.2) |
| R1 | 7 (36.8) |
Per the AJCC staging system, eighth edition.
Exploratory laparotomy in 32 patients (45.1%), resection/exploration ratio 59.4%.
Among resected patients, n = 19.
CA: Celiac artery; CHA: Common hepatic artery; FOLFIRINOX: Fluorouracil, leucovorin, oxaliplatin; HART: Hypofractionated ablative radiotherapy; IQR: Interquartile range; PV: Portal vein; SMA: Superior mesenteric artery; SAbR: Stereotactic ablative radiation therapy; SMV: Superior mesenteric vein.
Figure 2.
Kaplan–Meier overall survival. A: Kaplan–Meier overall survival (OS) as a function of surgical resection. Patients were stratified according to resectability status and OS curves from radiotherapy for resected (green line) vs non-resected (red line) and their confidence intervals (dotted lines) were plotted. The median OS from radiation therapy for resected patients has not been reached, compared to 14.6 mo (CI: 12.4-18.1 mo) of non-resected patients (P < 0.001); B: Kaplan–Meier OS as a function of surgical resection as predicted by the model. The “predicted” OS curves from radiotherapy for resected (green line) vs non-resected (red line) patients and their confidence intervals (dotted lines) showed here were obtained applying the model to the dataset to predict the resectability status. The two curves are significantly different (P < 0.001).
Prediction model for resectability
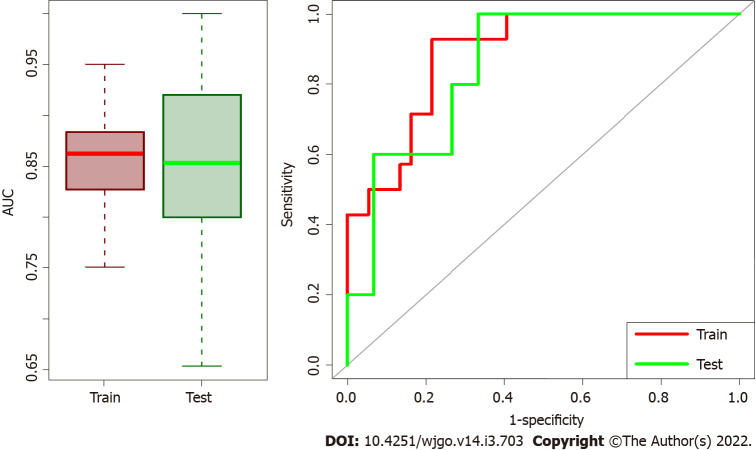
Median AUC for training and validation sets were 0.862 (95%CI: 0.792-0.921) and 0.853 (95%CI: 0.706-0.960), respectively. Box plots in Figure 3 summarized the AUC distributions for both datasets for all the 100 repetitions and the ROC curve of the median solution both for train and validation test. Among the 100 repetitions of the training process, the clinical variables were rarely selected from LASSO. On average, 98% of the selected variables were radiomic features, indicating a higher predictive power of radiomic data with respect to clinical data.
Figure 3.
Box plots of area under the receiver operating characteristic curve distributions and receiver operating characteristic curves for test/train datasets. The first part of training model and validation was repeated 100 times and Box plots of area under the receiver operating characteristic (ROC) curve (AUC) distributions for test/train datasets are represented on the left side. Median AUC both for train and validation sets are higher than 0.85 meaning that the model has high performance in both datasets. On the right the ROC curves of the median solution among the repetitions both for train and validation test are shown.
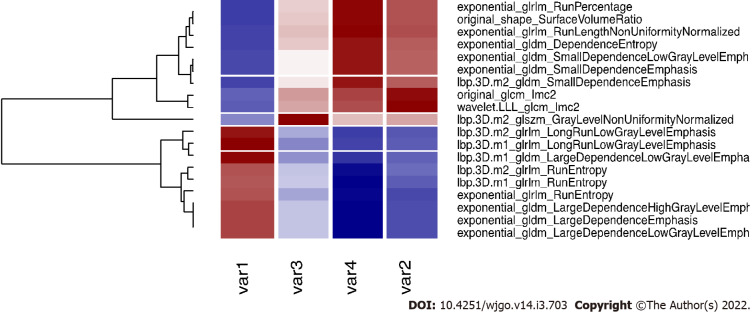
Applying the validated model on the entire dataset, 4 features were selected from the LASSO regression. Figure 4 depicts the LASSO variable selection process and the AUC as a function of the lambda parameter. Four variables survived to LASSO regression in correspondence with the best lambda value (dotted line in Figure 4). Lambda was chosen as the value at one standard deviation after the value that maximises AUC. The selected features, the P value from the Wilcoxon test, and their adjusted P-value for Bonferroni correction are reported in Table 2. In addition, the slope of LASSO regression coefficients for each variable is shown. Starting from the selected variables, the correlation matrix between these features and all other features with at least one Pearson correlation higher than 0.9 are reported in Figure 5, as a reference for external validation.
Figure 4.
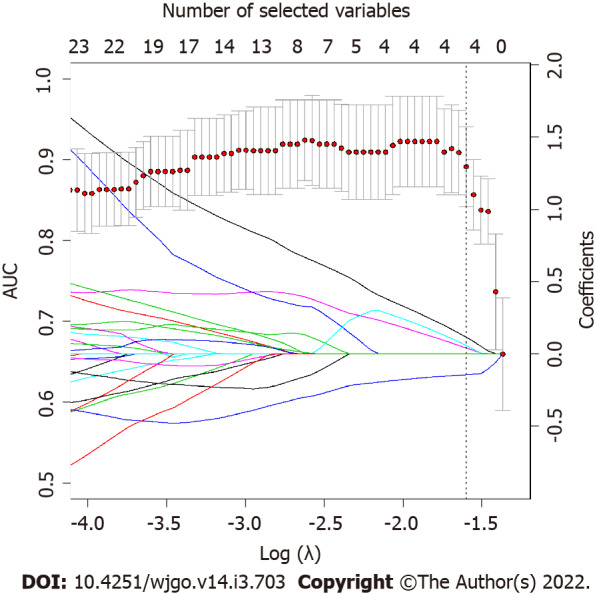
LASSO regression coefficients and area under the receiver operating characteristic curve as function of lambda parameter. LASSO regression coefficients for each variable (represented by continuous lines, each line is a variable that are imposed to reach zero by LASSO regression as lambda increases) and area under the receiver operating characteristic curve (AUC) as function of Log(λ) are superimposed. Dotted line represents the best lambda parameters that corresponds to the value at one standard deviation after the lambda that maximize AUC. As it is possible to notice, the best lambda value corresponds to four variables selected by LASSO.
Table 2.
Selected features, P value from Wilcoxon test, adjust P value for Bonferroni correction, and LASSO regression coefficients (for each variable)
|
Variable name
|
P
value Wilcoxon
|
P
value corrected
|
LASSO slope
|
| lbp.3D.m1_glrlm_LongRunLowGrayLevelEmphasis | 1.01E-09 | 4.82E-07 | -0.146 |
| wavelet.LLL_glcm_Imc2 | 1.17E-06 | 5.61E-04 | 0.039 |
| lbp.3D.m2_glszm_GrayLevelNonUniformityNormalized | 4.01E-06 | 1.92E-03 | 0.113 |
| exponential_glrlm_RunLengthNonUniformityNormalized | 4.02E-06 | 1.92E-03 | 0.056 |
Figure 5.
Correlation plot of the selected variables and other significant features. The four selected variables (named var1, var2, var3 and var4 for simplicity, please refers to the end of this caption for the correspondence with variable names), are correlated (r > 0.9) with other variables that are rejected for further analysis. Here we report the correlated variables as reference for further studies that could find significant features correlated with our variables. Var1: Lbp.3D.m1_glrlm_LongRunLowGrayLevelEmphasis; Var2: Wavelet.LLL_glcm_Imc2; Var3: Lbp.3D.m2_glszm_GrayLevelNonUniformityNormalized; Var4: Exponential_glrlm_RunLengthNonUniformityNormalized.
AUC obtained for the model applied on the entire dataset is 0.944 (95%CI: 0.892-0.996) (Figure 6). OS curves and their confidence intervals, estimated by Kaplan–Meier method as a function of surgical resection (resected vs non-resected patients) as predicted by the model, are shown in Figure 2B (P < 0.001).
Figure 6.
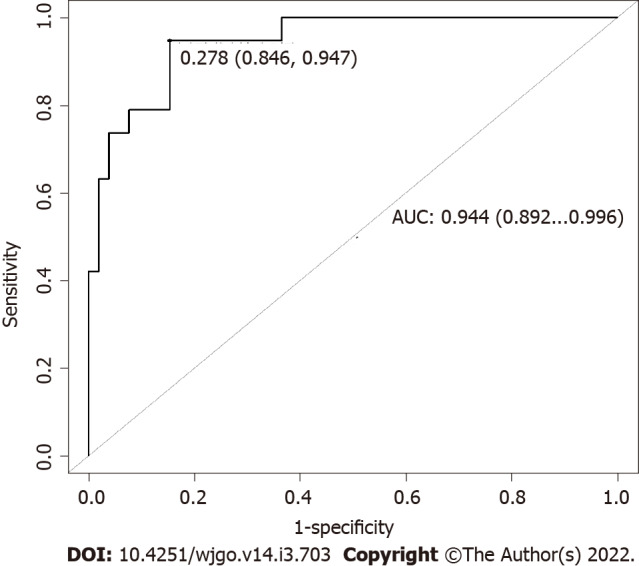
Area under the receiver operating characteristic curve obtained for the model applied on the entire dataset. To test the performance of our model in the entire dataset, area under the receiver operating characteristic curve obtained applying the model to the entire dataset was computed and represented here. AUC: Area under the receiver operating characteristic curve.
DISCUSSION
To the best of our knowledge, the present study is the first to propose a CT-based radiomics model to predict resectability in LAPC treated with intensive chemotherapy followed by ablative RT. The model was built, tested, and validated in a homogeneous cohort of LAPC patients, using clinical data and radiomic features extracted from the simulation-CT, and showed a reliable performance to predict surgical resection.
Surgical resection after neoadjuvant treatment is the main driver for improved survival in LAPC[11]. However, the diagnostic performance of CT imaging to evaluate the residual tumour burden at restaging after neoadjuvant therapy is low, due to the difficulty in distinguishing neoplastic tissue from fibrous scar or inflammation[33]. In this context, radiomics has gained popularity over conventional imaging as a complementary clinical tool capable of providing additional, unprecedented information regarding the intratumor heterogeneity and the residual neoplastic tissue, potentially serving in the therapeutic decision-making process.
In the present study, we identified four potential CT-based radiomic features (lbp.3D.m1_glrlm_LRLGE; wavelet.LLL_glcm_Imc2; lbp.3D.m2_glszm_GLLN; exponential_glrlm_RLNN) to build a CT radiomic model, which could be useful in predicting resectability in LAPC patients undergoing neoadjuvant therapy. Notably, the AUC obtained for the model was 0.94 if applied to the entire dataset. Radiomics has already been used in other contexts of surgical oncology, predicting resectability in oesophageal[28] and thymic cancers[29].
An important consideration of the current study is that clinical data was not significant to separate resected from not-resected patients and did not contribute to the building of the predictive model. This means that radiomic represents a fundamental tool to decode information that cannot be obtained from the direct observation of CT images or other clinical data alone[34]. Notably, the “real” OS curves (Figure 2A) and “predicted” OS curves obtained applying the validated model to the entire database without considering the information of resectability status (Figure 2B) are comparable. This is an indirect validation of the model that can independently predict before RT whether the patient can be a candidate for surgical resection, leading to comparable OS curves. This finding is particularly relevant from a clinical point of view. Indeed, although recent retrospective series have suggested that RT can complement induction chemotherapy and improve LAPC resectability[11-13], the role of (chemo-) RT after systemic therapy in LAPC is still controversial[35].
The potential to predict surgical resection of LAPC, especially in patients still considered unresectable after induction chemotherapy, could drive to a more aggressive effort of conversion to surgery by the means of RT. For this purpose, the use of simulation CT for features' extraction appears particularly appropriate to add homogeneity, repeatability, robustness, and simplicity to the model under evaluation. The use of free-contrast planning CT scans was previously reported as a basis of the analysis to derive the textural features related to survival in LAPC treated with SAbR. Analysing data of 100 patients, the authors found a significant association between a clinical-radiomic signature and survival of LAPC in both training and validation sets (P = 0.01 and 0.05 and concordance index 0.73 and 0.75, respectively)[25].
The analysis of the present study has some significant strengths. First, a three-step machine learning method was implemented. More precisely, the model was trained on a training subset of patients finding a robust AUC (median value: 0.86); subsequently, this model was validated and confirmed on a smaller subset (30% of the total patients), confirming high AUC values (median value: 0.85). Ultimately, it was re-applied on the whole database to extract the more significant features that contribute to the prediction, obtaining a high AUC value (0.94). Second, the statistical significance of all the steps gives robust and reliable predictions. Indeed, radiomic analysis and machine learning implementation are prone to lead to several errors and unreproducible results[36]. To give strengths and add reproducibility to the analysis, all results were cross-validated or obtained throughout multiple repetitions and corrected for multiple testing.
In the analysis, all the features correlated with the most significant ones were rejected to maintain only those variables that explained more variance. All the significant features correlated with at least one of the selected features are shown in Figure 5. The names of correlated variables are provided, allowing other centres to reproduce the presented results. Further studies could find significant variables different to those reported in our study, but correlated with them, indicating they have the same underlying radiomic information. Lastly, the possibility to extract valuable information on planning CT without additional diagnostic methodologies adds simplicity and the possibility to apply this in the clinical practice.
A further frontier of the application of radiomics, not evaluated in the present study, is to analyse the change in radiomic features during or after treatment (the so-called delta radiomics)[37,38]. The application of delta radiomic might further improve the ability of radiomics to provide predictive models for oncological outcomes and instruct clinical decisions. Furthermore, another potential application of radiomics in oncology is to realize prognostic and predictive models of the tumour pathological response (TRG) to neoadjuvant treatment[39-41]. This information could lead to better identification of patients who may or may not benefit from preoperative approach, in order to allow for more effective treatment personalization.
This study had several limitations. First, the design was observational, with a retrospective analysis and a relatively small sample size. Second, the results may have been biased by the patient selection process since indications to chemotherapy, radiotherapy, and surgery were defined on a case-by-case basis by the Institutional board. The lack of external validation represents a third limit. An external multi-institutional validation may have been preferable. Finally, due to the limited number of events in the resected group, we were unable to perform an analysis on the impact of resection status (R0 vs R1) on survival.
CONCLUSION
The present CT-radiomic model demonstrated a reliable performance to predict resectability in LAPC treated with induction chemotherapy followed by ablative RT. Radiomic information may complement clinical and morphological parameters in predicting surgical resectability. If further confirmed, the results of this study may allow integrating radiomic information into the pool of clinical and biochemical data to consider when a LAPC patient is candidate for surgical exploration after neoadjuvant therapy.
ARTICLE HIGHLIGHTS
Research background
Radiomics is emerging as a promising tool in oncology, potentially improving, through the development of predictive and prognostic models, the therapeutic decision-making process. To date, however, few data are available regarding the use of radiomics in pancreatic cancer (PC). Since computed tomography (CT) misestimate the resectability of locally advanced PC (LAPC) after neoadjuvant treatment, the role of radiomics could be decisive to integrate traditional morphological parameters in predicting surgical resection.
Research motivation
To explore the potential role of CT-radiomic features to integrate clinical and morphological data to predict surgical resection in LAPC treated with neoadjuvant chemotherapy and radiotherapy.
Research objectives
To create and validate a predictive model to predict LAPC resectability, throughout the application of machine learning algorithms to planning CT-radiomic features.
Research methods
A total of 1655 radiomic features were extracted from planning CT inside the gross tumour volume. Resectability status predictive model was build starting from these radiomic features and clinical data. A first step of variable selection and a training/validation step to find the model that better predicted the outcome was adopted. Subsequently, the validated model was applied to the whole dataset. The discriminating performance of each model was assessed with the area under the receiver operating characteristic curve (AUC).
Research results
Seventy-one LAPC patients were included in the analysis. After neoadjuvant chemotherapy and radiotherapy, 19 (26.8%) patients underwent surgical resection. The training and validation steps resulted in a predictive model of resectability with a median AUC of 0.862 (95%CI: 0.792-0.921) and 0.853 (95%CI: 0.706-0.960), respectively. This model applied to the entire dataset allowed to select 4 radiomic features that predict the respectability status with an AUC of 0.944 (95%CI: 0.892-0.996). No clinical data contributed to the predictive model.
Research conclusions
The present radiomic model could help predict resectability in LAPC treated with neoadjuvant therapy, suggesting a promising role in the context of a complex long-course downstaging and a challenging indication to surgery.
Research perspectives
The analysis of the change of radiomic features during or after treatment (delta radiomics) and the correlation with tumour response (e.g., tumour regression grade) represent another intriguing application of radiomics that needs further exploration.
Footnotes
Institutional review board statement: The Institutional Review Board (IRB) approved the prospective collection of patient data, No. PAD-R n.1101 CESC.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: We have no financial relationships to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: AIRO; ESTRO.
Peer-review started: May 17, 2021
First decision: July 14, 2021
Article in press: February 11, 2022
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: He YQ, Kamiyama T S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Gabriella Rossi, Department of Radiation Oncology, University of Verona Hospital Trust, Verona 37126, Italy.
Luisa Altabella, Department of Medical Physics, University of Verona Hospital Trust, Verona 37126, Italy.
Nicola Simoni, Department of Radiation Oncology, University of Verona Hospital Trust, Verona 37126, Italy. nicolasimoni81@gmail.com.
Giulio Benetti, Department of Medical Physics, University of Verona Hospital Trust, Verona 37126, Italy.
Roberto Rossi, Department of Radiation Oncology, University of Verona Hospital Trust, Verona 37126, Italy.
Martina Venezia, Department of Radiation Oncology, University of Verona Hospital Trust, Verona 37126, Italy.
Salvatore Paiella, Department of General and Pancreatic Surgery, Pancreas Institute, University of Verona Hospital Trust, Verona 37126, Italy.
Giuseppe Malleo, Department of General and Pancreatic Surgery, Pancreas Institute, University of Verona Hospital Trust, Verona 37126, Italy.
Roberto Salvia, Department of General and Pancreatic Surgery, Pancreas Institute, University of Verona Hospital Trust, Verona 37126, Italy.
Stefania Guariglia, Department of Medical Physics, University of Verona Hospital Trust, Verona 37126, Italy.
Claudio Bassi, Department of General and Pancreatic Surgery, Pancreas Institute, University of Verona Hospital Trust, Verona 37126, Italy.
Carlo Cavedon, Department of Medical Physics, University of Verona Hospital Trust, Verona 37126, Italy.
Renzo Mazzarotto, Department of Radiation Oncology, University of Verona Hospital Trust, Verona 37126, Italy.
Data sharing statement
No additional data are available.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 4.Maggino L, Malleo G, Marchegiani G, Viviani E, Nessi C, Ciprani D, Esposito A, Landoni L, Casetti L, Tuveri M, Paiella S, Casciani F, Sereni E, Binco A, Bonamini D, Secchettin E, Auriemma A, Merz V, Simionato F, Zecchetto C, D'Onofrio M, Melisi D, Bassi C, Salvia R. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019;154:932–942. doi: 10.1001/jamasurg.2019.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, El-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, Moorcraft SY, Conroy T, Hohla F, Allen P, Taieb J, Hong TS, Shridhar R, Chau I, van Eijck CH, Koerkamp BG. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reni M, Zanon S, Balzano G, Nobile S, Pircher CC, Chiaravalli M, Passoni P, Arcidiacono PG, Nicoletti R, Crippa S, Slim N, Doglioni C, Falconi M, Gianni L. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol. 2017;28:2786–2792. doi: 10.1093/annonc/mdx495. [DOI] [PubMed] [Google Scholar]
- 7.Chung SY, Chang JS, Lee BM, Kim KH, Lee KJ, Seong J. Dose escalation in locally advanced pancreatic cancer patients receiving chemoradiotherapy. Radiother Oncol. 2017;123:438–445. doi: 10.1016/j.radonc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan S, Chadha AS, Suh Y, Chen HC, Rao A, Das P, Minsky BD, Mahmood U, Delclos ME, Sawakuchi GO, Beddar S, Katz MH, Fleming JB, Javle MM, Varadhachary GR, Wolff RA, Crane CH. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toesca DAS, Ahmed F, Kashyap M, Baclay JRM, von Eyben R, Pollom EL, Koong AC, Chang DT. Intensified systemic therapy and stereotactic ablative radiotherapy dose for patients with unresectable pancreatic adenocarcinoma. Radiother Oncol. 2020;152:63–69. doi: 10.1016/j.radonc.2020.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Reyngold M, O'Reilly EM, Varghese AM, Fiasconaro M, Zinovoy M, Romesser PB, Wu A, Hajj C, Cuaron JJ, Tuli R, Hilal L, Khalil D, Park W, Yorke ED, Zhang Z, Yu KH, Crane CH. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 2021;7:735–738. doi: 10.1001/jamaoncol.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, Fishman EK, Hruban RH, Yu J, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg. 2019;270:340–347. doi: 10.1097/SLA.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, Ferrone CR, Parikh AR, Weekes CD, Nipp RD, Kwak EL, Allen JN, Corcoran RB, Ting DT, Faris JE, Zhu AX, Goyal L, Berger DL, Qadan M, Lillemoe KD, Talele N, Jain RK, DeLaney TF, Duda DG, Boucher Y, Fernández-Del Castillo C, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simoni N, Micera R, Paiella S, Guariglia S, Zivelonghi E, Malleo G, Rossi G, Addari L, Giuliani T, Pollini T, Cavedon C, Salvia R, Milella M, Bassi C, Mazzarotto R. Hypofractionated Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost and Simultaneous Integrated Protection in Pancreatic Ductal Adenocarcinoma. Clin Oncol (R Coll Radiol) 2021;33:e31–e38. doi: 10.1016/j.clon.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G, Simoni N, Paiella S, Rossi R, Venezia M, Micera R, Malleo G, Salvia R, Giuliani T, Di Gioia A, Auriemma A, Milella M, Guariglia S, Cavedon C, Bassi C, Mazzarotto R. Risk Adapted Ablative Radiotherapy After Intensive Chemotherapy for Locally Advanced Pancreatic Cancer. Front Oncol. 2021;11:662205. doi: 10.3389/fonc.2021.662205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang JK, Byun JH, Kang JH, Son JH, Kim JH, Lee SS, Kim HJ, Yoo C, Kim KP, Hong SM, Seo DW, Kim SC, Lee MG. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur Radiol. 2021;31:813–823. doi: 10.1007/s00330-020-07188-8. [DOI] [PubMed] [Google Scholar]
- 16.Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, Lucidarme O, Bachet JB. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol. 2017;27:3104–3116. doi: 10.1007/s00330-016-4632-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim YE, Park MS, Hong HS, Kang CM, Choi JY, Lim JS, Lee WJ, Kim MJ, Kim KW. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology. 2009;250:758–765. doi: 10.1148/radiol.2502080501. [DOI] [PubMed] [Google Scholar]
- 19.Cassinotto C, Cortade J, Belleannée G, Lapuyade B, Terrebonne E, Vendrely V, Laurent C, Sa-Cunha A. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol. 2013;82:589–593. doi: 10.1016/j.ejrad.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Cassinotto C, Mouries A, Lafourcade JP, Terrebonne E, Belleannée G, Blanc JF, Lapuyade B, Vendrely V, Laurent C, Chiche L, Wagner T, Sa-Cunha A, Gaye D, Trillaud H, Laurent F, Montaudon M. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2014;273:108–116. doi: 10.1148/radiol.14132914. [DOI] [PubMed] [Google Scholar]
- 21.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 22.Giraud P, Giraud P, Gasnier A, El Ayachy R, Kreps S, Foy JP, Durdux C, Huguet F, Burgun A, Bibault JE. Radiomics and Machine Learning for Radiotherapy in Head and Neck Cancers. Front Oncol. 2019;9:174. doi: 10.3389/fonc.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciaravino V, Cardobi N, DE Robertis R, Capelli P, Melisi D, Simionato F, Marchegiani G, Salvia R, D'Onofrio M. CT Texture Analysis of Ductal Adenocarcinoma Downstaged After Chemotherapy. Anticancer Res. 2018;38:4889–4895. doi: 10.21873/anticanres.12803. [DOI] [PubMed] [Google Scholar]
- 24.Borhani AA, Dewan R, Furlan A, Seiser N, Zureikat AH, Singhi AD, Boone B, Bahary N, Hogg ME, Lotze M, Zeh HJ III, Tublin ME. Assessment of Response to Neoadjuvant Therapy Using CT Texture Analysis in Patients With Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. AJR Am J Roentgenol. 2020;214:362–369. doi: 10.2214/AJR.19.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozzi L, Comito T, Fogliata A, Franzese C, Franceschini D, Bonifacio C, Tozzi A, Di Brina L, Clerici E, Tomatis S, Reggiori G, Lobefalo F, Stravato A, Mancosu P, Zerbi A, Sollini M, Kirienko M, Chiti A, Scorsetti M. Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS One. 2019;14:e0210758. doi: 10.1371/journal.pone.0210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandrasegaran K, Lin Y, Asare-Sawiri M, Taiyini T, Tann M. CT texture analysis of pancreatic cancer. Eur Radiol. 2019;29:1067–1073. doi: 10.1007/s00330-018-5662-1. [DOI] [PubMed] [Google Scholar]
- 27.Parr E, Du Q, Zhang C, Lin C, Kamal A, McAlister J, Liang X, Bavitz K, Rux G, Hollingsworth M, Baine M, Zheng D. Radiomics-Based Outcome Prediction for Pancreatic Cancer Following Stereotactic Body Radiotherapy. Cancers (Basel) 2020;12 doi: 10.3390/cancers12041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou J, Li R, Zeng R, Wu CQ, Chen Y, Chen TW, Zhang XM, Wu L, Jiang Y, Yang JQ, Cao JM, Tang S, Tang MJ, Hu J. CT radiomic features for predicting resectability of oesophageal squamous cell carcinoma as given by feature analysis: a case control study. Cancer Imaging. 2019;19:66. doi: 10.1186/s40644-019-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista Araujo-Filho JA, Mayoral M, Zheng J, Tan KS, Gibbs P, Shepherd AF, Rimner A, Simone CB 2nd, Riely G, Huang J, Ginsberg MS. CT Radiomic Features for Predicting Resectability and TNM Staging in Thymic Epithelial Tumors. Ann Thorac Surg. 2021 doi: 10.1016/j.athoracsur.2021.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma Version 2. 2015. NCCN Guidelines. (2015). [cited 10 April 2021]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf .
- 31.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, El Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Götz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegård A, Maier-Hein KH, Morin O, Müller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers RJHM, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Löck S. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology. 2020;295:328–338. doi: 10.1148/radiol.2020191145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassinotto C, Sa-Cunha A, Trillaud H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn Interv Imaging. 2016;97:1225–1232. doi: 10.1016/j.diii.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, Schernberg A, Paragios N, Deutsch E, Ferté C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. 2017;28:1191–1206. doi: 10.1093/annonc/mdx034. [DOI] [PubMed] [Google Scholar]
- 35.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouché O, Shannon J, André T, Mineur L, Chibaudel B, Bonnetain F, Louvet C LAP07 Trial Group. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, Bellomi M. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp. 2018;2:36. doi: 10.1186/s41747-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasief H, Zheng C, Schott D, Hall W, Tsai S, Erickson B, Allen Li X. A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol. 2019;3:25. doi: 10.1038/s41698-019-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusumano D, Boldrini L, Yadav P, Casà C, Lee SL, Romano A, Piras A, Chiloiro G, Placidi L, Catucci F, Votta C, Mattiucci GC, Indovina L, Gambacorta MA, Bassetti M, Valentini V. Delta Radiomics Analysis for Local Control Prediction in Pancreatic Cancer Patients Treated Using Magnetic Resonance Guided Radiotherapy. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li ZY, Wang XD, Li M, Liu XJ, Ye Z, Song B, Yuan F, Yuan Y, Xia CC, Zhang X, Li Q. Multi-modal radiomics model to predict treatment response to neoadjuvant chemotherapy for locally advanced rectal cancer. World J Gastroenterol. 2020;26:2388–2402. doi: 10.3748/wjg.v26.i19.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Z, Frazer M, Zhang GG, Latifi K, Moros EG, Feygelman V, Felder S, Sanchez J, Dessureault S, Imanirad I, Kim RD, Harrison LB, Hoffe SE, Frakes JM. CT-based radiomic features to predict pathological response in rectal cancer: A retrospective cohort study. J Med Imaging Radiat Oncol. 2020;64:444–449. doi: 10.1111/1754-9485.13044. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, He B, Zhuang X, Gao X, Wang D, Li M, Lin Z, Luo R. CT-based radiomic signatures for prediction of pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J Radiat Res. 2019;60:538–545. doi: 10.1093/jrr/rrz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.