CD19-targeting immunotherapies including blinatumomab, a (CD19/CD3) bispecific T-cell engaging antibody, and CD19-chimeric antigen receptor (CAR) T cells have been highly effective for B-cell acute lymphoblastic leukemia (ALL) [1, 2]. However, up to 50% of B-cell ALL patients relapse after CD19-targeted therapies, the majority with CD19-negative disease [3–5]. Given increasing utilization of these newly FDA-approved therapies in standard treatment paradigms, serial evaluations of CD19 surface expression following these therapies will be necessary to monitor for changes in leukemic phenotype.
As a referral center for therapies targeting CD22, an alternate B-cell antigen, we have a unique population of children and young adults with relapse or refractory disease to CD19 targeting [6–8]. Utilizing this cohort, we retrospectively analyzed serial CD19 surface expression to evaluate for dynamic changes in surface expression following immunotherapy.
Patients were defined as CD19 negative, partial (bimodal disease population with at least 5% negative blasts), or positive (>95% CD19 expression) based primarily on disease assessments from the peripheral blood and/or bone marrow by flow cytometry (Supplementary Table S1). CD19 expression was quantified on B-lymphoblasts by the amount of anti-CD19 antibody bound per cell (ABC). “Dim” expression was defined as CD19-positive blasts expressing a CD19 site density between 200 and 2000, a delineation based on the lowest 10% of CD19 expression seen in our prior patients.
This analysis included 56 subjects, 55 with relapsed/refractory ALL, and one with DLBCL (Table S2), the majority of whom were referred for CD22-targeted therapies. Most (47/56, 84%) were relapsed/refractory to CD19-targeted therapies, amongst whom 33 (70%) had previously achieved a complete response (CR) to CD19 targeting. Patients had serial evaluations over a median of 164 days (IQR 69–376 days) as a part of routine assessment during their care at the NIH. Patients were divided by leukemic CD19 expression at referral (not at diagnosis) into CD19-negative, -partial, or -positive subgroups (Table 1). Twenty-three (41%) were CD19 negative, all post one or more CD19-targeted therapies. Eight were CD19 partial; six had received CD19-targeted therapy, the remaining two were immunotherapy naive and had inherent 28% and 81% CD19-expressing partial populations, respectively. Twenty-three (41%) were CD19-positive, 16 had prior CD19-targeted therapy of which 2 were CD19-dim, and both had received blinatumomab.
Table 1.
CD19 expression at the time of first referral to NIH and the first change in CD19 expression from referral.
| Therapy received prior to referral (n = total # who received therapy, # with CR) | CD19 expression at referral (n, %) [median CD19 ABC or %CD19-positive blasts (IQR)] | Interval therapies received (n) | Median days to ↑ CD19 expression (IQR) | First change in CD19 expression (n, %) |
|---|---|---|---|---|
|
| ||||
| CD19-CAR (16, 14) Blinatumomab (3, 2) Both (3, 3a) Other CD19 targetedb (1,0) None (0) |
CD19 negative (23, 41%) [N/A] |
No immunotherapy (1) Stem cell transplant (1) CD22 CAR (15) Inotuzumab (4) Moxetumomab (2) |
NA | Remains CD19 neg (17, 74%) |
| No immunotherapy (2) Stem cell transplant (1) CD22 CAR (2) Inotuzumab (1) Moxetumomab (1) Daratumomab (1) |
133 (351) | Becomes CD19 partial (4, 17%) | ||
| Stem cell transplant (1) CD22 CAR (2) |
416 (39) | Becomes CD19 positive (2, 9%) | ||
| CD19-CAR (1,0) Blinatumomab (5,2) Both (0) None (2) |
CD19 partialc (8, 14%) [68% (59%, 86%)] |
NA | NA | Becomes CD19 neg (0, 0%) |
| No immunotherapy (1) Stem cell transplant (1) CD22 CAR (3) | NA | Remains CD19 partial (4, 50%) | ||
| No immunotherapy (2) CD22 CAR (2) |
53 (66) | Becomes CD19 positive (4, 50%) | ||
| CD19-CAR (5,5) Blinatumomab (9,5) Both (4,2a) None (7) |
CD19 positive (25, 45%) [9004 (4181, 16,240); dimd: 446] |
Stem cell transplant (1) CD22 CAR (1) CD19-CAR (1) Moxetumomab (1) Blinatumomab (1) |
NA | Becomes CD19 nege (2, 8%) |
| No immunotherapy (2) CD19-CAR (1) |
NA | Becomes CD19 partial (3, 12%) | ||
| No immunotherapy (1) | 21 (NA) | CD19 dim → positive (1, 4%) | ||
| No immunotherapy (4) CD22 CAR (14) CD19-CAR (5) CD19/22 CAR (1) Inotuzumab (2) Moxetumomab (1) |
NA | Remains CD19 positivef (19, 76%) | ||
ABC antibody-binding capacity, CR complete remission, IQR interquartile range, CAR chimeric antigen receptor therapy
No response to blinatumomab, complete response to CD19-CAR.
SGN19a.
Partial positivity defined by < 95% antigen positive.
Dim defined as CD19 expression < 2000 sites/cell.
Following interval CD19-targeted therapy.
One patient was CD19-dim and remained CD19-dim.
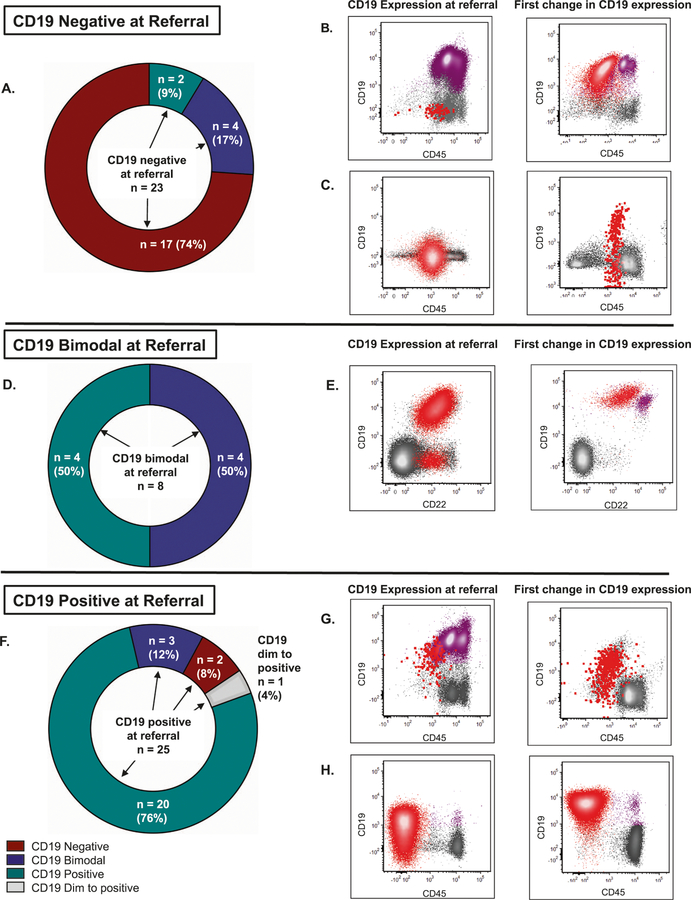
Serial evaluations revealed that 16 (29%) of patients had a change in CD19 expression (Table 1). Most CD19-negative patients remained negative (74%) (Fig. 1a). Two became completely CD19 positive at relapse, one post-CR after CD22 CAR (with CD19-positive isolated CNS relapse) and the other after both CD22 CAR and stem cell transplant (SCT). The latter patient had CD19-positive nonneoplastic B cells (hematogones) concurrently with CD19-negative leukemia; no detectable CD19-positive blasts pretransplant (Fig. 1b). Four of the 23 patients who were CD19 negative became CD19 partial (Fig. 1c).
Fig. 1. Changes in CD19 Expression.
a, d, f Evolution of CD19 expression from referral CD19-negative (N = 23), -partial (N = 8), and -positive (N = 25) subgroups during follow-up at our institution. CD19 expression level at referral is noted in the middle of each graph with arrows pointing to specified changes in expression over time. Two patients elsewhere characterized as CD19-dim expressers (CD19 ABC ≤2000) are included with the CD19-positive patients and one whose expression evolved over time is distinguished (gray color). For the flow cytometry dot plots, referral cases (left-side rows) were chosen as the last case before a change in CD19 expression occurred and subsequent cases (right-side rows) were those immediately following the change. B-ALL blasts are shown in red. Nonneoplastic B cells are shown in purple. Representative flow cytometry dot plots are shown, and additional plots are shown in Fig. S3. b CD19-negative disease and abundant B-cell precursors and mature B cells, which later transition to fully CD19-positive disease with interval CD22 CAR and SCT. Additional flow cytometry plots depicting the disease immunophenotypes at both timepoints can be found in Fig. S3A. c CD19-negative disease which became partially CD19-positive (46% positive expression) with interval CD22 CAR. Additional flow cytometry plots depicting the disease immunophenotypes at both timepoints can be found in Fig. S3B. e CD19-partial/bimodal CD19 expression (87% positive expression), which later became fully CD19-positive with few nonneoplastic, predominantly mature B cells with no interval immunotherapy additional flow cytometry plots depicting the disease immunophenotypes at both timepoints can be found in Fig. S3C. g CD19-positive disease in a background of nonneoplastic B cells, which include abundant B-cell precursors/hematogones and mature B cells. Disease later became CD19-dim/partial positive (85% positive expression) with interval CD22 CAR. Additional flow cytometry plots depicting the disease immunophenotypes at both timepoints can be found in Fig. S3D. h Disease ranging from predominantly CD19-dim to negative (CD19 ABC = 665), which later became fully CD19-positive (CD19 ABC = 3767) with no interval immunotherapy. Few nonneoplastic, predominantly mature B cells are present. Additional flow cytometry plots depicting the disease immunophenotypes at both timepoints can be found in Fig. S3E.
Four (50%) of the CD19-partial patients remained CD19-partial; the remaining four (50%) became CD19-positive, two without interval immunotherapy (Table 1, Fig. 1d, e). Subsequent treatment with CD19-targeted approaches was attempted in three of these four patients who relapsed with CD19 positivity. Two successfully achieved MRD negative remission with CD19-CAR. Another relapsed with CD19-negative disease during treatment with blinatumomab [9]. The last achieved initial remission induction but relapsed with CD19-negative disease post-SCT.
Most (76%) of the patients who were CD19-positive remained so (Table 1, Fig. 1f). Two became CD19-negative after CD19-targeted therapy. Three became CD19 partial, two without any immunotherapy. A small number of CD19-dim/negative blasts, insufficient to definitely identify a subpopulation, were present before this transition in one of these patients (Fig. 1g). Of the two CD19-dim patients, one went from dim postblinatumomab (CD19 ABC = 665) to positive and bright (median CD19 ABC = 3767) without interval immunotherapy and remained so during CD22 CAR and at relapse. At referral, this patient’s disease expressed a spectrum of CD19 from dim to negative, which shifted to express CD19 at a more homogenously bright level (Fig. 1h). The other CD19-dim patient (ABC = 226) remained so during CD22 CAR.
Genomic alterations in CD19 are a major cause of CD19-CAR T-cell resistance [10]. To integrate these changes with the evolution of CD19 surface expression, whole exome sequencing and RNA sequencing were performed in ten patients who had available material. Nine remained in their initial subgroups; six CD19-negative, two CD19-positive, and one CD19-partial (Fig. S1). The remaining case, patient 8, was CD19-dim at referral postblinatumomab and became positive over time as described above. This patient had a relative decrease in CD19 RNA without a detectable CD19 genomic alteration, suggesting that this dim phenotype may be epigenetically or transcriptionally regulated (Tables S3 and S4).
Of the 16 patients with changes in CD19 expression, 7 had a subsequent change, most reverting to their expression at referral (Table S5). Those who transitioned from CD19 negative to positive or vice versa remained so. All four of the patients whose CD19 expression went from negative to partial transitioned back to negative. Of those who transitioned from positive to partial CD19 expression, two of three reverted back to positive.
Sequential antigen targeting strategies may impact response. In our experience with CD22 CAR T cells, prior exposure to Inotuzumab ozogamicin, a CD22-directed-conjugated monoclonal antibody, resulted in lower or partial CD22 expression, thereby predisposing patients to an earlier relapse or preventing complete disease eradication [11, 12]. In this cohort, seven patients were nonresponders to blinatumomab, and were subsequently treated with CD19-CAR. Five responded to CD19-CAR, yet three emerged with CD19-negative disease. While blinatumomab nonresponse did not preclude response to CD19-CAR in this limited cohort, as suggested by Pillai et al., this sequential strategy may be associated with a predisposition toward incomplete disease eradication and increased risk of antigen escape [13]. Further studies are warranted, particularly since sequential CD19 targeting was specifically exclusionary in the global registration study for CD19-targeted CAR T cells in pediatric ALL but is being incorporated in standard practices.
The impact of sequential immunotherapies on nontarget antigens is also relevant. In immunotherapy-naïve patients, CD19 and CD22 expression trends together [14]. To evaluate whether CD19 loss affected CD22 expression, we analyzed CD22 antigen density in a limited dataset of seven patients with CD19-negative disease who had had paired flow reports comparing pre-/post-CD19-targeted therapy (Fig. S2). Six received CD19-CAR and one received blinatumomab. Median CD22 ABC transitioned from 5975 before CD19 loss to 2028 afterwards (p = 0.02), yet all patients remained uniformly CD22-positive. With CD19 loss posttherapy, there is a corresponding decrease in CD22, which may impact response to subsequent therapies.
Differences in the costimulatory domains of CD19-CARs are known to impact CAR T-cell functionality, likely affecting expansion and persistence [15]. Of the 29 patients who relapsed after CD19-CAR, 9 received CARs with CD28 and 20 with 4–1BB costimulatory domain, respectively (Table S6). We investigated whether differences in CAR costimulatory domains was associated with CD19 expression at relapse. Overall, the distribution of relapse CD19 immunophenotype differed between patients receiving 4–1BB- and CD28-containing CARs (p = 0.002). Patients who received 4–1BB-containing CARs had a higher CD19-negative relapse rate compared with those in the CD28-containing CAR group, 85% vs. 22%. Conversely, the CD28-containing CAR group had a higher CD19-positive relapse rate, 67% vs. 15%. 4–1BB-based CARs may exert greater persistence with prolonged selection pressure, predisposing to antigen-negative relapse while the earlier loss of CD28-based CARs may favor antigen-positive relapse.
For the majority of our cohort, once CD19 negativity emerged, leukemia remained CD19 negative. However, the changes in CD19 expression seen in 16/56 (29%) of our patients afford several clinically relevant insights. First, some patients in the CD19-negative and -partial subgroups regained CD19 expression over the follow-up time period. The data on the first change in CD19 expression may indicate that patients who are treated with blinatumomab or who fail to have a CR to CD19-targeted therapies may preferentially develop CD19-partial disease. Second, the observed subsequent changes in CD19 expression suggest that partial CD19 expression may be transient. Taken together, these data suggest that temporary increases in CD19 expression may be amenable to CD19 targeting but associated with a higher likelihood of subsequent CD19 loss, prompting consideration of trials of CD19-targeted therapies as a bridge to SCT.
Despite growing recognition that antigen density may impact treatment response [16] this is amongst one of the first studies to make the clinical distinction of CD19-dim versus fully positive disease. The importance of antigen density in CAR T-cell efficacy remains highly relevant, as evident by CD22-dim disease being sufficient for relapse after CD22 CAR [7] and limiting antitumor efficacy [6]. The antigen expression threshold for therapeutic response may differ by patient and treatment-related factors, and the relevance of antigen-dim disease warrants further study.
Amongst samples analyzed for genomic alterations, which may have been impacted by prior anti-CD19 therapeutic pressure (Table S4); all the prior CD19-CAR patients had received a 4–1BB-based construct and were the only patients who had a frameshift mutation. One explanation for evolution of CD19 surface expression may be the outgrowth of CD19-positive subpopulations once this selective pressure disappears. Two cases examined had preexisting blast subpopulations or cells that explained the evolution of disease CD19 expression (Fig. 1). The relationship of the change in CD19 expression with interval therapies was not straightforward. This indicates that CD19 expression should be periodically monitored even in patients who develop CD19-negative disease.
In the era of targeted immunotherapies, we found that fluctuations in CD19 expression are frequent, nontarget antigens may be impacted, and that a subset of patients with CD19-negative or -partial disease can reexpress CD19, potentially gaining new, albeit transient, susceptibility to CD19-targeting warranting ongoing surveillance for CD19 expression. Additional studies to evaluate expression patterns of CD19 and other targets as they correlate with sequential therapies, as well as genetic underpinnings, will further inform therapeutic decisions.
Supplementary Material
Acknowledgements
We gratefully acknowledge the study participants and their families, referring medical care teams, the faculty and staff of the NIH Clinical Center who provide their expertise in the management of the study participants, and the data managers involved with this work. This work was supported in part by the Intramural Research Program, National Cancer Institute and NIH Clinical Center, National Institutes of Health. This research was made possible through the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the Warren Grant Magnuson Clinical Center.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information The online version of this article (https://doi.org/10.1038/s41375-020-0760-x) contains supplementary material, which is available to authorized users.
References
- 1.Maude SL, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378: 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gokbuget N, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–7. [DOI] [PubMed] [Google Scholar]
- 4.Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92:858–65. [DOI] [PubMed] [Google Scholar]
- 5.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–26. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna S, Highfill SL, Walsh Z, Nguyen SM, Lei H, Shern JF, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res. 2019;25:5329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne AS, Shah NN, Bhojwani D, Silverman LB, Whitlock JA, Stetler-Stevenson M, et al. Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia. Blood. 2017;130:1620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mejstrikova E, Hrusak O, Borowitz MJ, Whitlock JA, Brethon B, Trippett TM, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24:1504–6. [DOI] [PubMed] [Google Scholar]
- 11.Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33:884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates B, Shalabi H, Salem D, Delbrook C, Yuan CM, Stetler-Stevenson M, et al. Sequential CD22 targeting impacts CD22 CAR-T cell response. Blood. 2018;132 Suppl 1:282. [Google Scholar]
- 13.Pillai V, Muralidharan K, Meng W, Bagashev A, Oldridge DA, Rosenthal J, et al. CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv. 2019;3:3539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal J, Naqvi AS, Luo M, Wertheim G, Paessler M, Thomas-Tikhonenko A, et al. Heterogeneity of surface CD19 and CD22 expression in B lymphoblastic leukemia. Am J Hematol. 2018;93:E352–5. [DOI] [PubMed] [Google Scholar]
- 15.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther. 2017;25:2189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.