Abstract
Significance.
Binocular treatment for unilateral amblyopia is an emerging treatment that requires evaluation through a randomized clinical trial.
Purpose.
To compare change in amblyopic eye visual acuity (VA) in children aged 4 to 6 years treated with the dichoptic binocular Dig Rush iPad game plus continued spectacle correction vs. continued spectacle correction alone.
Methods.
Children (mean 5.7 years) were randomly assigned to home treatment for 8 weeks with the iPad game (n=92, prescribed 1 hour/day, 5 days/week or continued spectacle correction alone n=90) in a multi-center randomized clinical trial. Prior to enrollment, children wearing spectacles were required to have at least 16 weeks of wear or no improvement in amblyopic-eye VA (< 0.1 logMAR) for at least 8 weeks. Outcome was change in amblyopic-eye VA from baseline to 4 weeks (primary) and 8 weeks (secondary) assessed by masked examiner.
Results.
182 children with anisometropic (63%), strabismic (16%) (<5Δ near, simultaneous prism and cover test), or combined-mechanism (20%) amblyopia (20/40 to 20/200, mean 20/63) were enrolled. After 4 weeks, mean amblyopic VA improved 1.1 logMAR lines with binocular treatment and 0.6 logMAR lines with spectacles alone (adjusted difference = 0.5 lines; 95.1% CI: 0.1 to 0.9). After 8 weeks, results (binocular treatment: mean amblyopic-eye VA improvement = 1.3 logMAR lines vs. 1.0 logMAR lines with spectacles alone, adjusted difference = 0.3 lines; 98.4% CI: −0.2 to 0.8) were inconclusive because the confidence interval included both zero and the pre-defined difference in mean VA change of 0.75 logMAR lines.
Conclusions.
In 4- to 6-year-old children with amblyopia, binocular Dig Rush treatment resulted in greater improvement in amblyopic-eye VA over 4 weeks but not 8 weeks. Future work is required to determine if modifications to the contrast increment algorithm or other aspects of the game or its implementation could enhance the treatment effect.
Optimum optical correction and patching therapy, are effective treatments for many children with unilateral amblyopia.1 However, the treatment burden can be significant2 with poor adherence,1,3,4 lengthy treatment durations5 and frequent residual amblyopia.1,5,6 Over the past several decades, alternative treatment approaches have been investigated as the understanding of the pathophysiology of amblyopia has grown.7 Dichoptic binocular treatment (hereafter referred to as “binocular treatment”), an approach that may work by a fundamentally different mechanism from patching therapy,8 has shown promise in individuals with anisometropic, strabismic, or combined-mechanism amblyopia.9–12 Binocular therapy for amblyopia is based, in part, on the finding that contrast information from each eye can be combined if the contrast to the amblyopic eye is increased so that it is greater than the contrast presented to the fellow eye, thereby equalizing the input to each eye.13 Based on this finding, a binocular treatment approach for amblyopia was suggested where the fellow eye receives lower contrast stimuli.13 This approach was further supported by the finding of a high positive correlation reported between the magnitude of suppression of the amblyopic eye and the interocular visual acuity difference.14 By appropriate reduction of the contrast to the fellow eye, the input to the two eyes is balanced, reducing suppression and improving the signal from the amblyopic eye.9,14
Binocular therapy has been successful in the lab setting in a small number of adults with amblyopia including some previously treated with patching9, 10 and in several small clinical studies.11, 12, 15, 16 However, results from two recent large multicenter randomized clinical trials using a falling-blocks binocular game played on handheld devices found less improvement in amblyopic-eye visual acuity with binocular treatment than with part-time patching.17, 18 A third randomized clinical trial using the falling-blocks binocular game in children, teens, and adults also found no difference in visual acuity improvement when binocular therapy was compared with a placebo (non-binocular) version of the falling-blocks game.19
Factors that have been proposed to account for the differing results between the laboratory studies and the randomized clinical trials include: subject selection (age, previous therapy, clinical characteristics), the inclusion of a control group and the selection of the type of control, Hawthorne and placebo effects, how the contrast ratio between the two eyes was manipulated, and regression to the mean.7 The three large clinical trials17–19 that controlled for many of these variables suggested that poor adherence with game play was an important factor in the failure to find a treatment effect.
Because no previous large multi-center randomized clinical trial had found binocular treatment to be an effective treatment for amblyopia when compared to patching or a non-binocular game, we designed a proof-of-concept study to compare a newer dichoptic binocular iPad (Apple, Cupertino, CA) game (Dig Rush, not yet commercially available, Ubisoft, Montreal, Canada) with spectacle treatment alone. We speculated that the Dig Rush game would also be more engaging than the falling-blocks game, potentially addressing the previous issues of poor adherence. We conducted two parallel randomized clinical trials using the Dig Rush game for two age cohorts of children. In the older cohort (7 to 12 years old, with the majority having had previous treatment for amblyopia in addition to optical correction), we found no benefit of the binocular Dig Rush compared with continued spectacle wear alone.20
The purpose of the present randomized clinical trial was to compare the effectiveness of treatment with the Dig Rush binocular game plus spectacle wear with continued spectacle wear alone, in children aged 4 to 6 years, when the visual system may be more responsive to treatment than in older children.
METHODS
Fifty-two institution- and community-based clinical sites enrolled participants after obtaining approval from the respective institutional review boards. The study design and protocols adhered to the tenets of the Declaration of Helsinki. Prior to enrollment, a parent or guardian (hereafter referred to as “parent”) of each study participant gave written informed permission, and each participant provided assent as required by the local institutional review board. An independent Data and Safety Monitoring Committee) provided study oversight. The study is listed on www.clinicaltrials.gov, under identifier (NCT02983552) accessed August 18, 2021. The complete study protocol is available on the Pediatric Eye Disease Investigator Group (PEDIG) website (www.pedig.net, accessed August 18, 2021) and parallels the study previously completed in children 7 to 12 years of age.20
To ensure consistency and adherence to the study protocol across participating sites, sites were overseen by a PEDIG protocol monitor and certification of all study personnel was required. Certification included: review and passing an examination of the protocol, a certification phone call, and certification of visual acuity measurement with the ATS-HOTV protocol.21–23 Data entry was done through the PEDIG website where eligibility was confirmed. Study team members participated in monthly phone calls and biannual meetings where the study protocols were reviewed and recruitment updates were provided.
Major eligibility criteria were: age 4 to less than 7 years; amblyopia associated with anisometropia, strabismus, or both; amblyopic-eye visual acuity between 20/40 and 20/200 inclusive; an interocular visual acuity difference of at least 3 logMAR lines; and no more than a 4Δ tropia at near measured by simultaneous prism and cover test. Appendix Table A1, available at http://links.lww.com/OPX/A560 provides all eligibility criteria. To minimize the impact of visual acuity improvement with spectacles alone, prior to enrollment, participants were required to have at least 16 weeks of spectacle wear (if needed) or to demonstrate no improvement in amblyopic-eye visual acuity (<0.1 logMAR improvement) in their current spectacle correction over 2 consecutive visits at least 8 weeks apart.
Randomized Treatment Groups
Participants were randomly assigned via the PEDIG website with equal probability to receive 8 weeks of either binocular treatment with spectacles (if needed) or continued spectacles alone (if needed), subsequently referred to as “binocular treatment” and “control group”, respectively, using a permutated block design stratified by baseline amblyopic-eye visual acuity (20/40 to 20/80 vs. 20/100 to 20/200). Spectacles, if needed as specified in the protocol, were prescribed for both groups for all waking hours. Only one child did not require spectacle correction. Children assigned to the binocular treatment group were loaned an iPad with the Dig Rush application installed. iPads were preset with the auto brightness off and brightness level at 100% with instructions that the settings were not to be modified. The Dig Rush iPad game11 was prescribed for 1 hour/day 5 days/week, allowing the hour to be divided into shorter sessions. Dig Rush is an action-oriented adventure game application with 42 levels that consists of miners digging for gold.11 Participants wore study supplied red-green filter glasses that separate the game elements seen by each eye, with reduced contrast elements seen by the fellow eye, high contrast elements seen by the amblyopic eye, and high contrast background elements seen by both eyes. Both eyes must see their respective game components for successful game play. Contrast to the amblyopic eye remained at 100%, while contrast to the fellow eye started at 20%. Contrast to the fellow eye changed if the game was played ≥ 15 minutes on the preceding day and either increased by 10% of the current contrast with game success or decreased by 5% of the current contrast if game play was not successful.11 Due to a concern by the game developer (Amblyotech, Inc.) that the contrast to the fellow eye could reach 100% with as little as 4 hours and 15 minutes of game play over 17 sessions, the algorithm was changed after 42 participants had been randomized to game play. The new algorithm required at least 30 minutes of game play on the preceding day before a change in contrast could occur. Increasing the time that the contrast to the fellow eye remained below 100% provided more time for potential treatment under the hypothesis that treatment only occurs when fellow eye contrast is below 100%.
Parents recorded the hours of game play and/or spectacle wear each day using study-provided calendars. The iPad tracked game play duration and contrast to the fellow eye. At the end of the study, the iPads were returned to the Jaeb Coordinating Center for retrieval of adherence data.
Study Visits and Testing Procedures
After randomization (subsequently “baseline”), follow-up visits were scheduled at 4 (primary outcome) and 8 weeks (pre-planned secondary outcome) (±1 week). The 4-week outcome was selected based on the encouraging results from an randomized clinical trial with 28 children treated with the Dig Rush game11 and to allow for comparisons with previous PEDIG patching studies.24, 25 The 8-week outcome was selected to provide additional information on the rate of improvement and evaluate treatment adherence over a longer time period. Prior to testing, parents completed questionnaires regarding their child’s symptoms and diplopia while participants completed a separate set of questions about diplopia. Distance visual acuity was measured monocularly with optimal refractive correction (if applicable) and without cycloplegia by a study-certified examiner using the ATS-HOTV protocol.21–23 Ocular alignment was measured at distance and near with the simultaneous prism and cover test and the prism and alternate cover test. Stereoacuity was assessed using the Randot Butterfly and Randot Preschool stereoacuity test (Stereo Optical Co., Chicago IL). A masked examiner measured visual acuity and stereoacuity at 4 and 8 weeks. After the 8-week visit, control group participants were offered 8 weeks of binocular treatment, and a final visit at 16 weeks (±1 week) with masking not mandated for the visual acuity or stereoacuity assessment.
Statistical Analyses
A sample size of 116 participants was selected to have 90% power to detect a treatment group difference at 4 weeks if the true difference in mean visual acuity change was 0.75 logMAR lines, with a 2-sided type I error rate of 5% (0.1% for interim monitoring by the Data and Safety Monitoring Committee and 4.9% for final analysis), assuming a standard deviation (SD) of change in visual acuity of 1.2 logMAR lines based on prior studies,11, 18, 25 and no more than 5% loss to follow-up. A pre-planned sample size re-estimation was conducted using the observed SD of the 4-week outcome from 51 participants with treatment groups combined; given the observed SD of change in visual acuity of 1.6 logMAR lines, the Data and Safety Monitoring Committee recommended increasing sample size to the pre-specified maximum of 182.
The primary outcome measure was change in amblyopic-eye visual acuity from baseline to 4 weeks (21 to <49 days after randomization). A modified intent-to-treat analysis of covariance (ANCOVA), adjusting for baseline visual acuity and only including participants completing the 4-week outcome, was performed to estimate the treatment group difference in mean change in visual acuity at 4 weeks and associated 2-sided 95.1% CI. Alternative approaches to the primary analysis are specified in Appendix Table A2, available at http://links.lww.com/OPX/A560.
Statistical methods for additional analyses are described in the relevant tables and figures. Analyses for secondary outcomes of visual acuity (3 pre-specified analyses, see Table 3 footnote) and stereoacuity (4 pre-specified analyses, see Appendix Tables A3–A4 footer, available at http://links.lww.com/OPX/A560) were adjusted for multiple testing using the Bonferroni method such that the overall type I error rate was 4.9% within the set of secondary visual acuity outcomes and 5% within the set of stereoacuity outcomes. Statistical significance for safety analyses was tested using a 2-sided type I error rate of 1%. Exploratory analyses were conducted in the binocular group for: 1) whether the algorithm for contrast change (≥ 15 vs ≥ 30 minutes of gameplay) made a difference in visual acuity improvement at 4 and 8 weeks, and 2) whether adherence to prescribed treatment as defined by iPad log file data was associated with visual acuity and stereoacuity outcomes. For each participant, the total hours of completed and prescribed game play were calculated from the date the iPad was received until the 4- and 8-week study visits (inclusive), and the percentage of prescribed treatment completed (adherence) was computed as the ratio of completed to prescribed hours of game play for that interval. Contrast presented to the fellow eye was also available from the iPad log files. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). All p-values are 2-sided.
RESULTS
Baseline Characteristics
Between March 2017 and March 2020, 182 participants were randomly assigned to binocular treatment (n=92) or control (continued optical correction, n=90). Baseline characteristics were similar between the groups (Table 1).
Table 1.
Baseline Characteristics for Randomized Participants by Treatment Group.
| Binocular Treatmenta (n=92) | Continued Spectaclesa (n=90) | |||
|---|---|---|---|---|
|
|
||||
| N | % | N | % | |
|
| ||||
| Sex: Female | 50 | 54% | 46 | 51% |
|
| ||||
| Age (Years) | ||||
| 4 to <5 | 12 | 13% | 17 | 19% |
| 5 to <6 | 39 | 42% | 41 | 46% |
| 6 to <7 | 41 | 45% | 32 | 36% |
| Mean (SD) | 5.8 (0.7) | 5.7 (0.7) | ||
|
| ||||
| Race/Ethnicity | ||||
| White | 75 | 82% | 74 | 82% |
| Black/African American | 2 | 2% | 5 | 6% |
| Hispanic | 12 | 13% | 8 | 9% |
| Asian | 1 | 1% | 2 | 2% |
| More than one race | 2 | 2% | 1 | 1% |
|
| ||||
| Prior Amblyopia Treatment b | ||||
| None | 33 | 36% | 32 | 36% |
| Patching | 39 | 42% | 40 | 44% |
| Atropine | 2 | 2% | 2 | 2% |
| Patching & Atropine | 15 | 16% | 15 | 17% |
| Other | 2 | 2% | 0 | 0% |
| Patching & Other | 1 | 1% | 1 | 1% |
|
| ||||
| Prior Binocular Treatment | 0 | 0% | 0 | 0% |
|
| ||||
| Distance Amblyopic-eye VA | ||||
| 20/200 | 1 | 1% | 2 | 2% |
| 20/160 | 2 | 2% | 3 | 3% |
| 20/125 | 7 | 8% | 3 | 3% |
| 20/100 | 3 | 3% | 4 | 4% |
| 20/80 | 9 | 10% | 9 | 10% |
| 20/63 | 24 | 26% | 24 | 27% |
| 20/50 | 27 | 29% | 26 | 29% |
| 20/40 | 19 | 21% | 19 | 21% |
| Mean (SD) LogMAR | 0.48 (0.16) | 0.48 (0.17) | ||
| Snellen equivalent | 20/63 | 20/63 | ||
|
| ||||
| Distance Fellow-eye VA | ||||
| Mean (SD) LogMAR | 0.00 (0.08) | -0.00 (0.09) | ||
| Snellen equivalent | 20/20 | 20/20 | ||
|
| ||||
| Interocular Difference (Lines): Mean (SD) | 4.8 (1.7) | 4.9 (1.7) | ||
|
| ||||
| Stereoacuity: Nil | 27 | 30% | 22 | 27% |
|
| ||||
| Stereoacuity (Seconds of Arc): Median (Range) | 800 (40 to Nil) | 800 (40 to Nil) | ||
|
| ||||
| Amblyopia Cause | ||||
| Strabismus | 15 | 16% | 15 | 17% |
| Anisometropia | 61 | 66% | 54 | 60% |
| Combined mechanism | 16 | 17% | 21 | 23% |
|
| ||||
| Distance SPCT: Maximum angle of deviation (Δ) | ||||
| Orthotropic | 73 | 79% | 76 | 84% |
| 1 to 4 | 15 | 16% | 11 | 12% |
| 5 to 9 | 3 | 3% | 2 | 2% |
| ≥ 10 | 1 | 1% | 1 | 1% |
|
| ||||
| Near SPCT: Maximum angle of deviation (Δ) | ||||
| Orthotropic | 72 | 78% | 75 | 83% |
| 1 to 4 | 20 | 22% | 15 | 17% |
|
| ||||
| Amblyopic-eye Spherical Equivalent (Diopters) | ||||
| Mean (SD) | 4.49 (1.75) | 4.45 (2.76) | ||
|
| ||||
| Fellow-eye Spherical Equivalent (Diopters) | ||||
| Mean (SD) | 2.41 (1.67) | 2.67 (1.99) | ||
|
| ||||
| Spherical Equivalent Anisometropia (Diopters) | ||||
| Mean (SD) | 2.12 (1.52) | 2.25 (1.51) | ||
logMAR = logarithm of minimum angle of resolution; Δ = prism diopters; SD = standard deviation; SPCT = simultaneous prism and cover test; VA = visual acuity.
Two participants in the binocular treatment group and one in the control treatment group were found to be ineligible after enrollment because their visual acuity did not meet stability criteria prior to enrollment.
Other amblyopia treatment included plano (or reduced plus) lens wear, fogging (Bangerter filter, tape, optical), or vision therapy (home or office).
Visit Completion
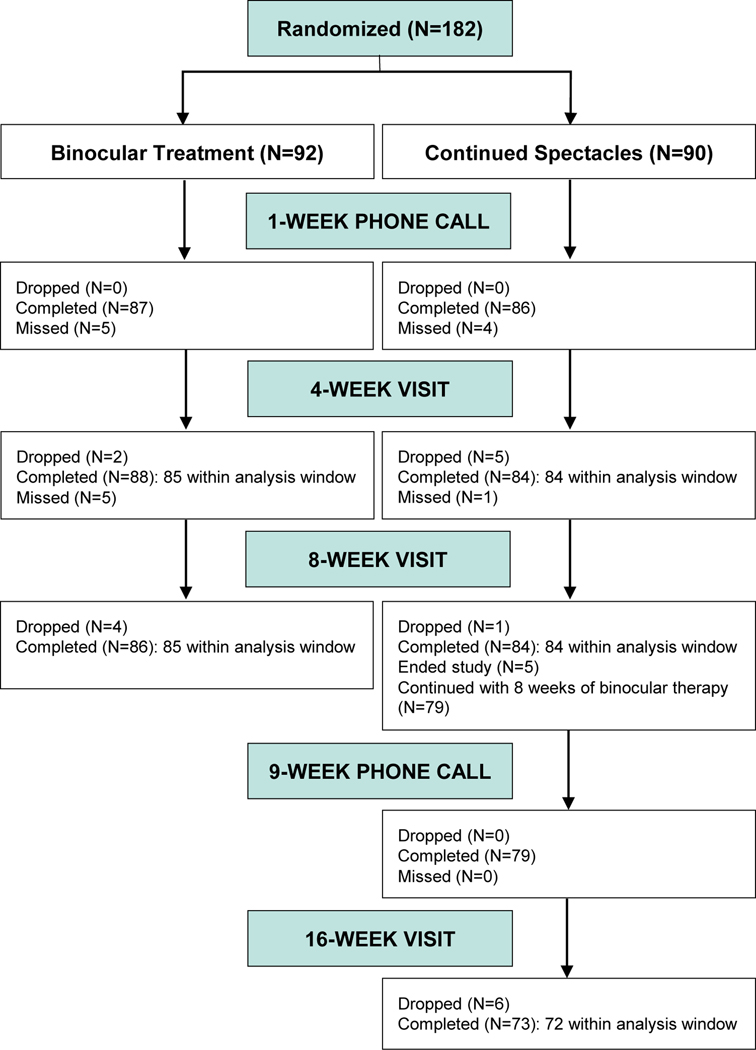
The 4-week (primary outcome) and 8-week (secondary outcome) visits were completed by 85 (92%) in the binocular group and 84 (93%) in the control group (Figure 1). Masking of the visual acuity/stereoacuity testers was maintained at the 4-week visit for 85 (100%) in the binocular group and 83 (99%) in the control group, and at 8 weeks for 83 (98%) in the binocular group and 84 (100%) in the control group.
Figure 1.
Visit completion by treatment group. The 4-week primary outcome visits were classified as being within the analysis window if completed between 21 to <49 days from randomization. The 8-week visits were classified as being within the analysis window if completed between 49 to <105 days from randomization. The 16-week visits were classified as being within the analysis window if completed between 105 to <161 days from randomization.
Adherence
Parent-reported spectacle-wear adherence of >75% for the initial 4 weeks occurred for 66 (78%) participants in the binocular and 74 (95%) in the control group; 69 (78%) participants in the binocular and 78 (95%) in the control group reportedly wore their spectacles >75% throughout the entire 8 weeks. For the binocular group, parent-reported adherence to prescribed game play (1 hour/day, 5 days/week) was >75% for 35 (45%) participants during the initial 4 weeks and 46 (57%) throughout the entire 8 weeks.
Actual game play (adherence) was logged by the iPad. More than 75% of prescribed game play was recorded for 37 (47%) participants during the initial 4 weeks (median=67%, range 3 −145%), similar to parental report, and was >75% for 35 (43%) participants throughout the entire 8 weeks (median=62%, range 2%−122%), slightly lower than the parent’s report. The median hours of game play was 15 of the cumulative 20 hours prescribed (range: 1–28 hours) through 4 weeks and 27 of the cumulative 40 hours prescribed (range: 1–56 hours) through 8 weeks.
Contrast Level of the Binocular Treatment Over the Course of the Study
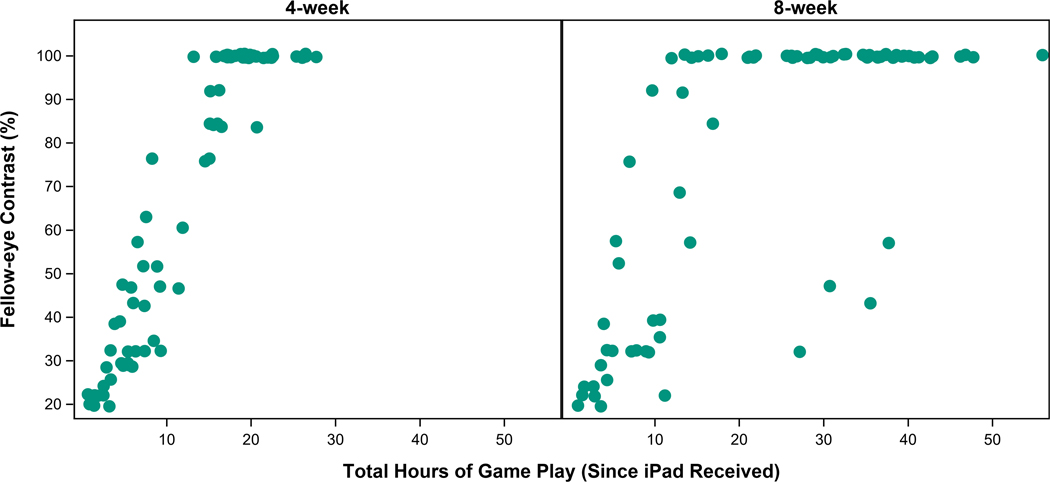
Contrast presented to the fellow eye increased from 20% to 100% for 32 (41%) participants by the 4-week visit and for 50 (62%) by the 8-week visit (Appendix Figure A1, available at http://links.lww.com/OPX/A561).
Amblyopic-eye Visual Acuity
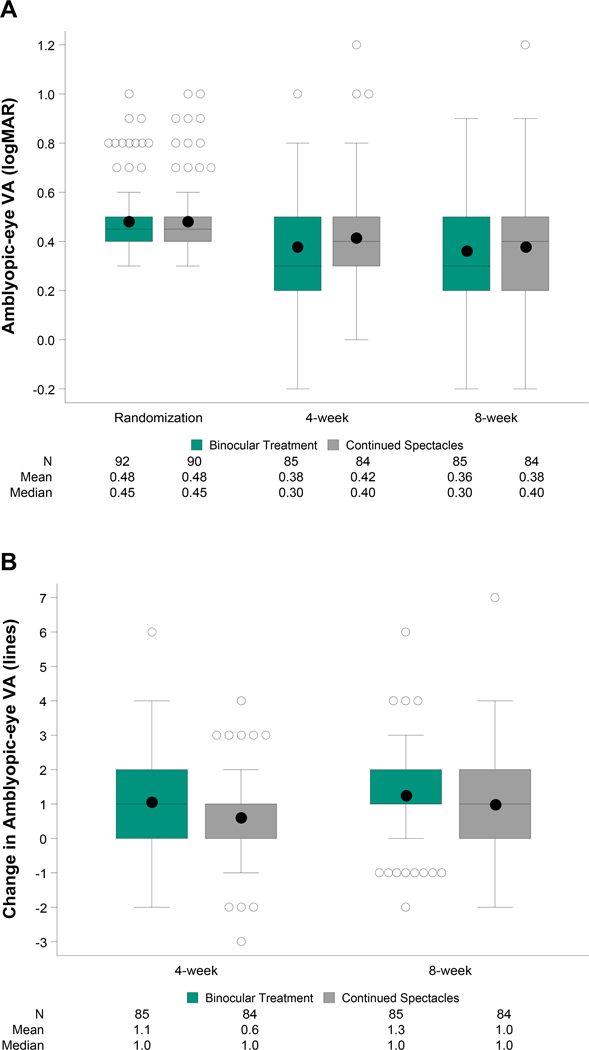
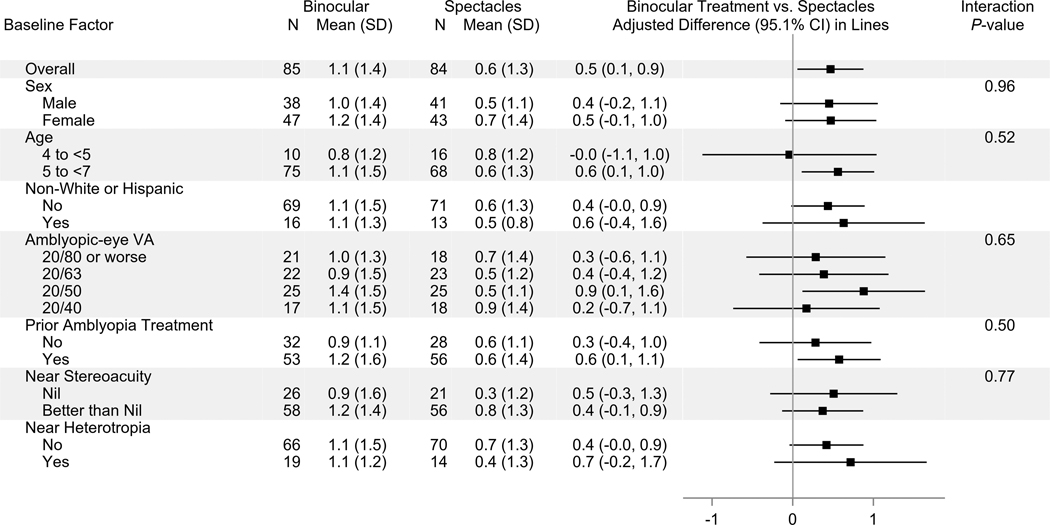
At 4 weeks, mean amblyopic-eye visual acuity improved from baseline by 1.1 logMAR lines in the binocular group and 0.6 logMAR lines in the control group (Table 2, Figures 2A–B). After adjusting for baseline visual acuity, the difference between binocular and control groups was 0.5 logMAR lines (95.1% CI: +0.1 to +0.9, P = .03). Sensitivity analyses yielded similar results (Appendix Table A2, available at http://links.lww.com/OPX/A560). When analyzing the treatment effect according to baseline characteristics (Figure 3), no significant interaction was found.
Table 2.
Distribution of Amblyopic-eye Visual Acuity Outcomes by Treatment Group at Baseline and Outcome Visits.
| Baseline | 4 weeksa | 8 weeksa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | |||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group (N) | 92 | 90 | 85 | 84 | 85 | 84 | ||||||
|
| ||||||||||||
| Amblyopic-eye VA | ||||||||||||
| 20/320 | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% | 1 | 1% |
| 20/250 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| 20/200 | 1 | 1% | 2 | 2% | 1 | 1% | 2 | 2% | 0 | 0% | 0 | 0% |
| 20/160 | 2 | 2% | 3 | 3% | 0 | 0% | 0 | 0% | 1 | 1% | 4 | 5% |
| 20/125 | 7 | 8% | 3 | 3% | 3 | 4% | 4 | 5% | 5 | 6% | 0 | 0% |
| 20/100 | 3 | 3% | 4 | 4% | 5 | 6% | 1 | 1% | 4 | 5% | 1 | 1% |
| 20/80 | 9 | 10% | 9 | 10% | 9 | 11% | 6 | 7% | 8 | 9% | 8 | 10% |
| 20/63 | 24 | 26% | 24 | 27% | 15 | 18% | 20 | 24% | 9 | 11% | 13 | 15% |
| 20/50 | 27 | 29% | 26 | 29% | 9 | 11% | 18 | 21% | 11 | 13% | 18 | 21% |
| 20/40 | 19 | 21% | 19 | 21% | 18 | 21% | 15 | 18% | 19 | 22% | 17 | 20% |
| 20/32 | 0 | 0% | 0 | 0% | 15 | 18% | 10 | 12% | 14 | 16% | 12 | 14% |
| 20/25 | 0 | 0% | 0 | 0% | 6 | 7% | 5 | 6% | 12 | 14% | 7 | 8% |
| 20/20 | 0 | 0% | 0 | 0% | 2 | 2% | 2 | 2% | 1 | 1% | 1 | 1% |
| 20/16 | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 1% |
| 20/12 | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% | 1 | 1% | 1 | 1% |
| Mean (SD) logMAR | 0.48 (0.16) | 0.48 (0.17) | 0.38 (0.22) | 0.42 (0.21) | 0.36 (0.22) | 0.38 (0.22) | ||||||
|
| ||||||||||||
| Mean IOD (SD) Lines | 4.8 (1.7) | 4.9 (1.7) | 3.8 (2.3) | 4.4 (2.2) | 4.0 (2.2) | 4.0 (2.3) | ||||||
|
| ||||||||||||
| Change in amblyopic-eye VA from baseline | ||||||||||||
| ≥ 3 lines better | 12 | 14% | 6 | 7% | 12 | 14% | 9 | 11% | ||||
| 2 lines better | 19 | 22% | 10 | 12% | 23 | 27% | 16 | 19% | ||||
| 1 line better | 22 | 26% | 30 | 36% | 29 | 34% | 33 | 39% | ||||
| 0 line | 23 | 27% | 25 | 30% | 12 | 14% | 13 | 15% | ||||
| 1 line worse | 7 | 8% | 9 | 11% | 8 | 9% | 11 | 13% | ||||
| 2 lines worse | 2 | 2% | 3 | 4% | 1 | 1% | 2 | 2% | ||||
| ≥ 3 lines worse | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% | ||||
| Mean (SD) Linesb | 1.1 (1.4) | 0.6 (1.3) | 1.3 (1.4) | 1.0 (1.4) | ||||||||
| Adjusted mean (95% CI) at 4 weeksc | 1.1 (0.8, 1.4) | 0.6 (0.3, 0.9) | ||||||||||
| Adjusted mean difference (95.1% CI) at 4 weeksc | 0.5 (0.1, 0.9) | |||||||||||
| Adjusted mean (95% CI) at 8 weeksc | 1.3 (1.0, 1.6) | 1.0 (0.7, 1.3) | ||||||||||
| Adjusted mean difference (98.4% CI) at 8 weeksc | 0.3 (−0.2, 0.8) | |||||||||||
|
| ||||||||||||
| Improvement of ≥2 lines from baseline | 31 | 36% | 16 | 19% | 35 | 41% | 25 | 30% | ||||
| Difference (98.4% CI)d | 18% (1%, 34%) | 12% (−6%, 29%) | ||||||||||
|
| ||||||||||||
| Unmasked VA testing | 0 | 0% | 1 | 1% | 2 | 2% | 0 | 0% | ||||
CI = confidence interval; IOD = interocular difference; logMAR = logarithm of minimum angle of resolution; SD = standard deviation; VA = visual acuity.
Limited to follow-up visits completed within the pre-specified analysis windows.
Positive values indicate improvement in amblyopic-eye visual acuity from baseline.
Analysis of covariance model adjusting for baseline amblyopic-eye visual acuity was used to estimate the mean change in amblyopic-eye visual acuity within each treatment group as well as the treatment group difference (positive values favor the binocular treatment group) in the mean change in amblyopic-eye visual acuity from baseline to 4 weeks (P = .03) and to 8 weeks (Bonferroni-adjusted P = .60).
Binomial regression adjusting for baseline amblyopic-eye visual acuity was used to compare the proportion of participants with improvement of ≥2 lines in amblyopic-eye visual acuity from baseline to 4 weeks (Bonferroni-adjusted P = .03) and to 8 weeks (Bonferroni-adjusted P = .34) between the treatment groups. For the 3 exploratory visual acuity outcomes (which included the 8-week treatment group comparison of mean change in amblyopic-eye visual acuity), a Bonferroni adjustment was used to control for multiple testing to preserve the overall type I error rate at 4.9% (2-sided alpha=0.016 per test).
Figure 2.
(A) Amblyopic-eye visual acuity at randomization (baseline) and outcome visits by treatment group. At each time point, the left box represents the binocular treatment group and the right box represents the continued spectacles group. Bottom and top of each box represent the 25th and 75th percentiles; the line in the box is the median and the dot is the mean. Bars above and below extend to the closest observed data point inside 1.5 times the interquartile range and open circles represent statistical outliers. Analyses were limited to 4-week (21 to <49 days after randomization) and 8-week (49 to <105 days after randomization) exams completed within the pre-defined analysis windows.(B) Change in amblyopic-eye visual acuity from baseline to outcome visits by treatment group. At each time point, the left box represents the binocular treatment group and the right box represents the continued spectacles group. Bottom and top of each box represent the 25th and 75th percentiles; the line in the box is the median and the dot is the mean. Bars above and below extend to the closest observed data point inside 1.5 times the interquartile range and open circles represent statistical outliers. Analyses were limited to 4-week (21 to <49 days after randomization) and 8-week (49 to <105 days after randomization) exams completed within the pre-defined analysis windows.
Figure 3.
Treatment group difference in the mean change in amblyopic-eye visual acuity from baseline to 4 weeks according to baseline subgroups. Positive differences favor the binocular treatment group. Subgroup analyses were limited to participants who completed the 4-week outcome visit within the pre-defined analysis window (21 to <49 days after randomization). All subgroup factors were pre-specified. An analysis of covariance was performed to test the 2-way interaction between treatment group and each subgroup factor (baseline age and amblyopic-eye visual acuity were treated as continuous variables), adjusting for baseline amblyopic-eye visual acuity. P-values were not computed for categorical subgroup factors with fewer than 20 participants per treatment group in one or more subgroup levels. Statistical significance of the interaction term was based on a 2-sided alpha of 0.01.
At 8 weeks, after adjusting for baseline visual acuity, the difference in the mean amblyopic-eye visual acuity improvement for the binocular group (1.3 logMAR lines) compared with the control group (1.0 logMAR lines) was 0.3 logMAR lines (98.4% CI: −0.2 to +0.8) (Table 2, Figures 2A–B).
At 4 weeks, amblyopic-eye visual acuity improved ≥2 lines from baseline for 31 (36%) and 16 (19%) for participants in the binocular and control groups, respectively, and at 8 weeks for 35 (41%) and 25 (30%) participants in the binocular and control groups, respectively. The difference in the proportion that improved by ≥ 2 logMAR lines between binocular treatment and controls was 18% (98.4% CI: 1% to +34%) at 4 weeks and 12% (98.4 CI: −6% to +29%) at 8 weeks, favoring the binocular treatment group (Table 2).
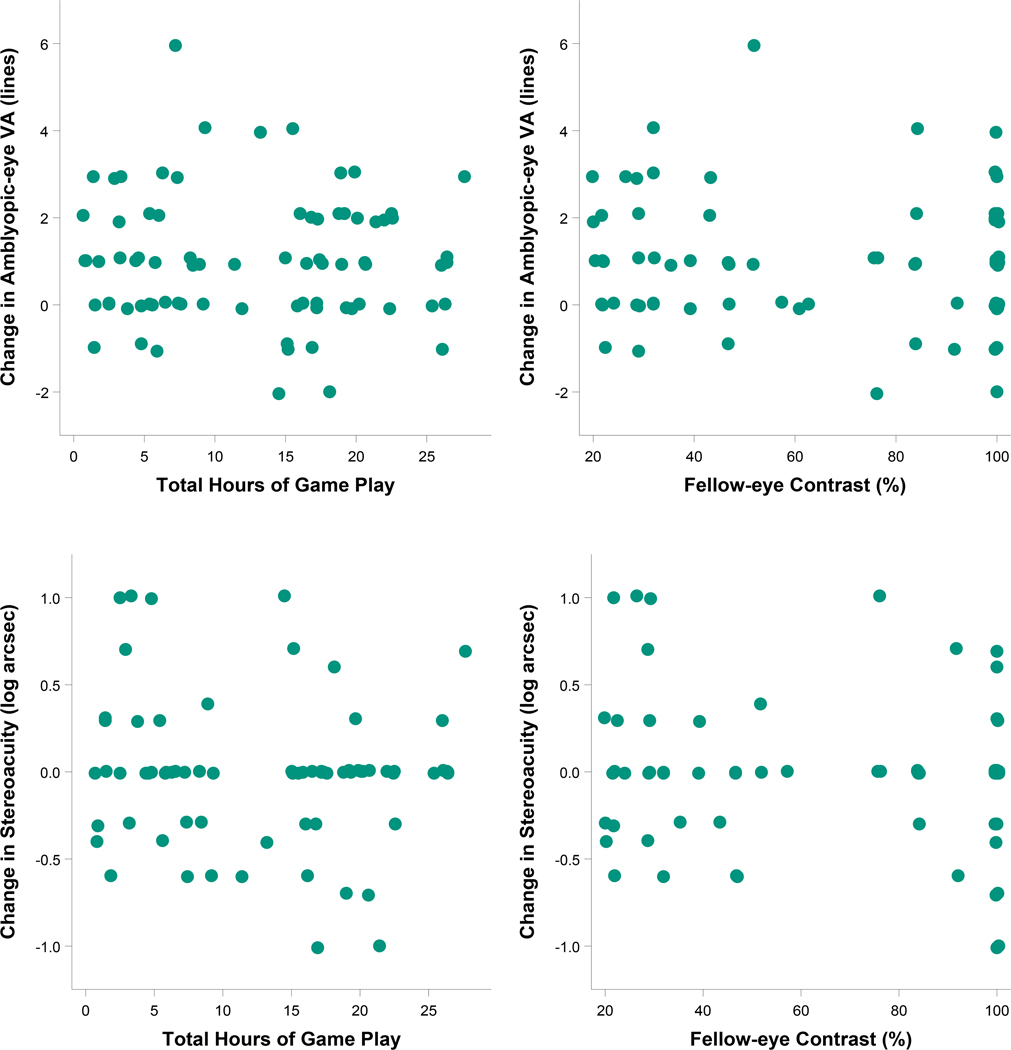
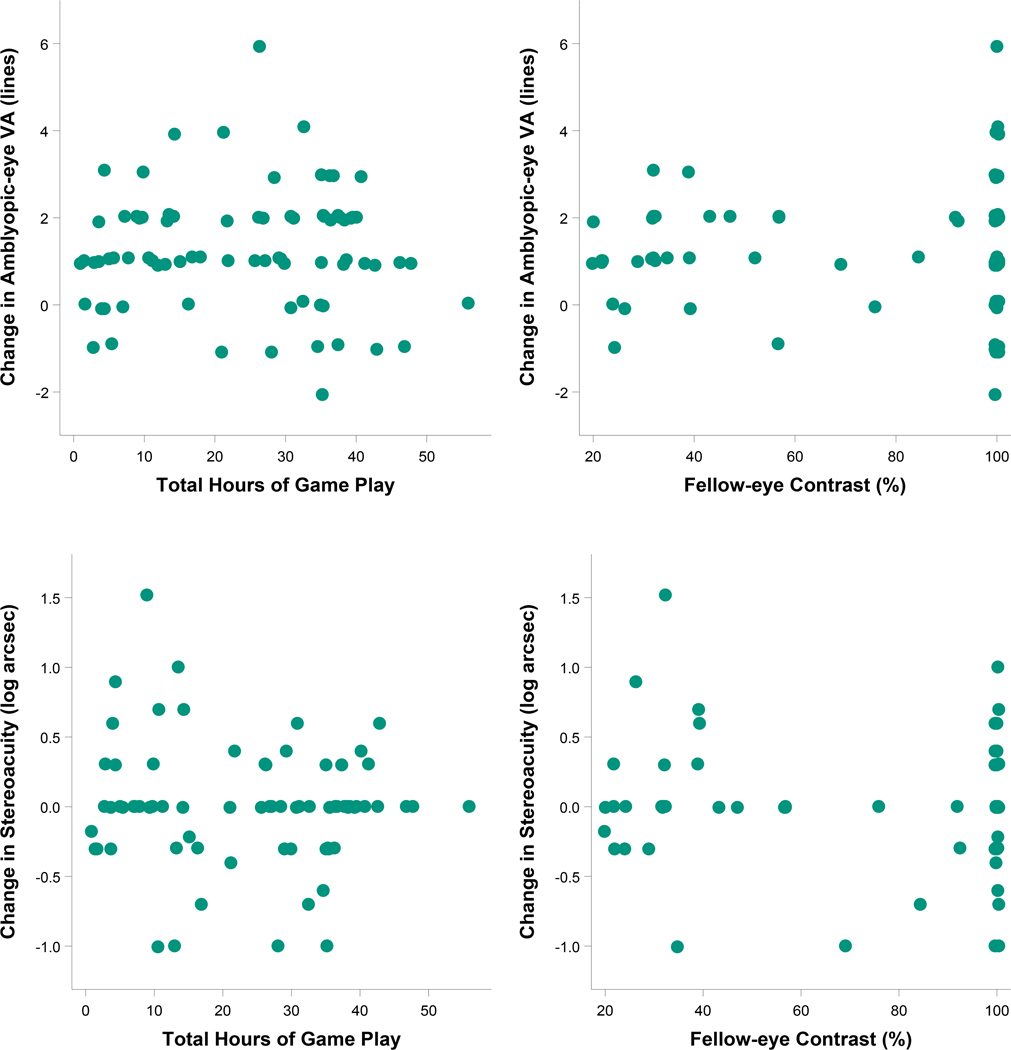
For the binocular group, there was no difference between the algorithm for change in contrast to the fellow eye (≥30 vs. ≥15 minutes of game play) in amblyopic-eye visual acuity improvement at 4 weeks (1.1 vs. 1.1 logMAR lines, difference = 0.02 lines, 95% CI: −0.6 to +0.7) or 8 weeks (1.1 vs. 1.4 logMAR lines, difference = −0.3 lines, 95% CI: −0.9 to +0.4) after adjusting for baseline visual acuity and age. There was no indication of a dose-response relationship between hours of treatment or contrast presented to the fellow eye (iPad® files) and amblyopic-eye visual acuity improvement at 4 or 8 weeks (Figures 4–5, upper panels).
Figure 4.
Relationships of hours played and contrast settings (from the log file) with treatment response for visual acuity and stereoacuity after 4 weeks of binocular therapy (binocular treatment group). Descriptive plots and Pearson correlation coefficients were produced using 4-week data from participants in the binocular treatment group who completed the visit within the pre-defined analysis window (21 to <49 days after randomization). The scatterplots on the top represent the relationships of change in amblyopic-eye visual acuity from baseline (logMAR lines, positive values indicate improvement) with objective measures of (1) total hours of binocular treatment [left column, r (95% CI) = −0.03 (−0.25, 0.20)] and (2) contrast presented to the fellow eye [right column, r (95% CI) = −0.04 (−0.26, 0.18)]. The scatterplots on the bottom represent the relationships of change in stereoacuity from baseline (log seconds of arc, positive values indicate improvement) with objective measures of (1) total hours of binocular treatment [left column, r (95% CI) = −0.10 (−0.33, 0.13)] and (2) contrast presented to the fellow eye [right column, r (95% CI) = −0.13 (−0.35, 0.11)].
Figure 5.
Relationships of hours played and contrast settings (from the log file) with treatment response for visual acuity and stereoacuity after 8 weeks of binocular therapy (binocular treatment group). Descriptive plots and Pearson correlation coefficients were produced using 8-week data from participants in the binocular treatment group who completed the visit within the pre-defined analysis window (49 to <105 days after randomization). The scatterplots on the top represent the relationships of change in amblyopic-eye visual acuity from baseline (logMAR lines, positive values indicate improvement) with objective measures of (1) total hours of binocular treatment [left column, r (95% CI) = −0.002 (−0.22 to 0.22)] and (2) contrast presented to the fellow eye [right column, r (95% CI) = 0.05 (−0.17 to 0.26)]. The scatterplots on the bottom represent the relationships of change in stereoacuity from baseline (log seconds of arc, positive values indicate improvement) with objective measures of (1) total hours of binocular treatment [left column, r (95% CI) = −0.08 (−0.30 to 0.15)] and (2) contrast presented to the fellow eye [right column, r (95% CI) = −0.11 (−0.33 to 0.12)].
Fellow-eye Visual Acuity
After adjusting for baseline visual acuity, mean fellow-eye visual acuity was found to improve similarly for the binocular and control groups at 4 weeks (0.1 vs 0.2 logMAR lines, difference: −0.1 logMAR lines, 99% CI: −0.5 to +0.2) and at 8 weeks (0.3 vs 0.2 logMAR lines, difference: 0.1 logMAR lines, 99% CI: −0.2 to +0.4, Appendix Table A5, available at http://links.lww.com/OPX/A560).
Stereoacuity
Change in stereoacuity from baseline to 4 and 8 weeks did not differ significantly between treatment groups overall, or when limited to participants with no history of strabismus at baseline (median change was 0 seconds of arc for all conditions, Appendix Tables A3–A4, available at http://links.lww.com/OPX/A560). For the binocular group, change in stereoacuity was not associated with either total hours of treatment or contrast presented to the fellow eye (Figures 4–5, lower panels).
Assessment of Potential Adverse Events
There were no differences between treatment groups at 4 or 8 weeks with respect to the proportion of participants with: 1) a new heterotropia at distance or near, 2) worsening of a pre-existing heterotropia by ≥ 10Δ by SPCT, or 3) resolution of heterotropia (Appendix Table A6, available at http://links.lww.com/OPX/A560).
There were only a few cases of diplopia reported in each group. No participants reported diplopia more than once per day at the 4-week visit, and only 2 (1 in each group) reported diplopia frequency of more than once per day at 8 weeks (Appendix Table A7–A8, available at http://links.lww.com/OPX/A560). An increase of 2 or more levels in frequency of headaches (7 (8%) vs 0, P = .002) and eye strain (9 (10%) vs 0, P < .001) was reported by parents significantly more often for the binocular group as compared with the control group, respectively. At 8 weeks the proportion with an increase in frequency remained greater in the binocular treatment group for headaches (5 (6%) vs 0, P = .01) and eye strain (5 (6%) vs 1 (1%), P = .02), but the differences were not statistically significant (Appendix Table A9–A10, available at http://links.lww.com/OPX/A560).
Post 8-Week Phase
At the 8-week visit, 79 (94%) participants in the control group elected to be treated with 8 weeks of binocular therapy (treatment period 8 week to 16 weeks), with 72 (91%) of them completing the 16-week visit (Figure 1). Log file data from this 8-week period indicated that 24 (35%) participants completed >75% of prescribed game play (median=19 hours, range: 1–54 hours), and 40 (59%) participants achieved 100% contrast in the fellow eye. The 16-week mean amblyopic-eye visual acuity was 0.33 logMAR with a mean improvement of 0.6 logMAR lines (95% CI: 0.3 to 0.9) from the 8-week visit. Amblyopic-eye visual acuity improved ≥ 2 lines from 8 to 16 weeks for 18 (25%) participants. The median change in stereoacuity between the 8- and 16-week visits was 0 seconds of arc in the overall cohort and for participants with no history of strabismus at randomization.
No participants reported diplopia more than once per day at 16 weeks. Very few participants had worsening of symptoms.
DISCUSSION
In children 4 to 6 years of age prescribed binocular Dig Rush treatment on an iPad® with continued spectacle correction, amblyopic-eye visual acuity improved more on average after 4 weeks compared with children who were prescribed continued spectacle correction alone. Nevertheless, in the secondary outcome analysis after 8 weeks of binocular treatment, the results were inconclusive. A 0.3 logMAR line point estimate difference and a 98.4% confidence interval including a mean difference of zero is consistent with no treatment group difference, whereas an upper confidence interval limit of 0.8 logMAR lines favoring binocular treatment leaves open the possibility of a small effect of binocular treatment after 8 weeks because the confidence interval included the 0.75D difference of interest.
Comparisons with Previous Studies
Three previous sufficiently powered randomized clinical trials did not find binocular treatment to be effective, when compared with a non-binocular game19 or part-time patching.17, 18 These studies, however, are not directly comparable to the present study because they used a different game, enrolled older participants (including adults in one trial19), used different comparison groups, and had a 6-week19 or 16-week primary outcome.17–19
More comparable to the present study is our parallel randomized clinical trial of children aged 7 to 12 years,20 which only differed from the present study by participant age, and did not find binocular treatment to be effective after 4 weeks or 8 weeks. In general, older children are somewhat less responsive to amblyopia treatment than younger children.26, 27 It is also difficult to separate age from prior treatment. Older children are more likely to have a history of previous treatment and they are more likely to have residual ambyopia that may be less responsive to treatment. Previous amblyopia treatment was reported by a greater proportion of the older cohort participants in the parallel study20 (94%) compared with the present younger cohort (64%), which could have contributed to the different results between studies at 4 weeks.
Another possible explanation for not finding a significant treatment effect in our older-cohort parallel randomized clinical trial20 could have been suboptimal adherence to game play, which has been reported in other binocular treatment trials.17–19 However, our adherence data do not explain the difference in results between the two studies because the median treatment adherence by the iPad® log files at 4 weeks was in fact better in the older cohort20 (80%) than in the younger cohort reported here (67%), and the proportion who completed > 75% of the prescribed game play was higher in the older (58%) than in the younger (47%) cohort. A recent study that looked at barriers to an in-office dichoptic video game treatment in children with amblyopia reported approximately half of the children (the majority less than 7 years old) lost interest in playing the game.28
Some studies of binocular treatment for amblyopia have reported improved stereoacuity in children29, 30 and adults.9, 10, 12, 14 We found no evidence of stereoacuity improvement when using random dot stereotests without monocular cues administered by a masked examiner. One potential reason for not finding stereoacuity improvement, as proposed by Baker et al,13 is that the detection of relative disparities occurs in extrastriate area V2, while binocular summation (targeted by this binocular treatment paradigm) occurs in area VI. It should also be noted that the current, commercially-available random dot stereotests are not designed to evaluate changes in coarse stereopsis. The tests used in this study have only two coarse disparity levels (800 arcseconds on the Preschool® Randot and 2000 arcseconds on the Randot Butterfly stereotest), which made it impossible to detect changes in stereoacuity between nil and 2000 arcseconds and between 2000 and 800 arcseconds.
Why Was the Effect Found at 4 Weeks but Maybe Not at 8 Weeks?
Time Course Differences between Binocular Treatment and Optical Treatment
Although our 8-week results were inconclusive, these results could be consistent with a binocular treatment effect at 4 weeks but not at 8 weeks. It is therefore reasonable to consider why an effect might have been found at 4 weeks only. One possible explanation is the non-linear course of visual acuity improvement observed with binocular treatment, as well as traditional forms of amblyopic treatment, may differ from that seen with optical treatment. Although the time course to achieve maximal optical treatment may vary by age and other factors, it has been reported to be lengthy in some children with anisometropic,31 strabismic, and combined-mechanism amblyopia.32 Similar to optical treatment, atropine penalization also results in a slower improvement in amblyopic visual acuity33, 34 as opposed to patching which has a more rapid treatment effect.24, 25, 33 It appears that visual acuity improvement from binocular treatment may have a similar time course to patching treatment. The mean change in visual acuity with binocular treatment over the first 4 weeks reported here (1.1 logMAR lines) was the same mean improvement found after 5 weeks of 2 hours per day of prescribed patching with 1 hour of near activities (1.1 logMAR lines) in children of a similar age and slightly poorer baseline visual acuity.25 Differences in rates of improvement between binocular treatment and continued glasses alone may have yielded a result that was consistent with a difference at 4 weeks but not at 8 weeks.
Spectacle Wear Adherence
Treatment group differences in spectacle-wear adherence is another potential reason why the 4-week treatment group difference might not be present at 8 weeks. Parent-reported adherence was greater in the spectacle-only group (95%) than in the binocular group (78%) throughout the trial. Poorer spectacle-wear adherence in the binocular treatment group could have reduced the amount of visual acuity improvement related to spectacle use in this group. The better adherence to spectacle wear in the control group coupled with the differences in time course of the two treatments, might have contributed to a treatment difference at 4, but not 8 weeks.
Limitations
This study has some limitations. Prior to enrollment, participants wore appropriate optical correction for 16 weeks or demonstrated less than <1 logMAR line of improved visual acuity at two visits measured 8 weeks apart. The continued mean improvement in amblyopic-eye visual acuity of 1.0 logMAR line with spectacles alone over 8 weeks suggests that these stability criteria were insufficient for the present younger cohort, and that younger children may be more responsive to optical treatment over a longer time period than older children.20 Improvement in amblyopic eye visual acuity with proper optical correction has been reported previously31,32,35,36 and thus it is important to establish stable visual acuity prior to evaluating the effectiveness of amblyopia treatments. Another potential limitation related to our comparison of binocular treatment to continued spectacle wear is that the mean improvement found with game play could be due to the Hawthorne effect, possibly mediated by increased attention from study personnel to those in the binocular treatment group. However, such an effect seems unlikely because the outcome measure (visual acuity) was assessed by masked examiners using a standardized, computerized algorithm. An additional limitation is that it is possible that the amount of binocular treatment received was overestimated. If another person played the game in addition to or instead of the participant, or the participant played the game without the red-green filter glasses, the participant would receive credit for game play but would not be receiving treatment. To avoid these potential pitfalls, clear written and verbal instructions were provided, and the parent was encouraged to supervise the child during game play. As with our parallel study,20 the software ended treatment sessions after approximately one minute of inactivity, so it is unlikely that the duration of treatment was significantly overestimated if the child turned on the game but did not play. The parent was called one week after randomization to review proper game play and adherence and to address any concerns, but the possibility remains that the game could have been played incorrectly and the amount of play was consequently overestimated. However, all children were required to see both elements of the game and demonstrate their ability to play level 3 prior to enrollment, and contrast increments to the fellow eye only occurred with successful game play.
Future Directions
The binocular treatment approach used in this study is based on the premise that amblyopia, at least in part, is due to suppression of the amblyopic eye by the fellow eye. It is theorized that by reducing the contrast to the fellow eye, the signal from the amblyopic eye is less likely to be suppressed and thus visual function will improve. However, similar to the report by Vedamurthy et al,12 who measured and adjusted the interocular lumninace ratios prior to and during game play, we found no dose-response relationship between visual acuity improvement and contrast. For binocular treatment to be most effective, it is unclear whether it is important to individualize the starting level of contrast to the non-amblyopic eye and what rate of contrast adjustment should be used, if at all. It is also unclear whether binocular treatment ceases to be effective after the contrast to the non-amblyopic eye reaches 100%. The initial reports on improved amblyopic-eye visual acuity in adults, using this type of binocular treatment approach, measured the interocular suppression of each participant and individually set the contrast of the fellow eye at the start of treatment.9, 10, 12 However, the one large randomized clinical trial that individually tailored the contrast of the fellow eye at the start of treatment,19 failed to find a significant improvement in amblyopic-eye visual acuity with binocular treatment compared with a non-binocular game. Results from the randomized clinical trials where contrast was not individually tailored have been mixed, with one single-site, small cohort randomized clinical trial,11 and now the present larger-scale study, finding a binocular treatment effect whereas the others have not.17, 18, 20 Further work is needed to determine the importance of individually tailoring the initial contrast to the non-amblyopic eye, how the contrast to the non-amblyopic eye should be incremented, and if treatment continues once contrast reaches 100%. A greater understanding of amblyopic-eye suppression and the best mechanisms to reduce or eliminate suppression’s impact on the amblyopic eye will require additional lab-based studies. These lab-based studies could lead to a more robust binocular treatment approach for amblyopia that may provide an efficacious alternative to the traditional monocular treatment approaches of patching, atropine penalization, and blurring with filters.
CONCLUSIONS
Children 4 to 6 years old with amblyopia who were prescribed home treatment using the binocular Dig Rush game showed greater improvement in amblyopic-eye visual acuity at the 4-week visit compared with that found in children who continued in full-time spectacle wear; adjusted difference = 0.5 lines (95.1% CI: 0.1 to 0.9). Our 4-week results provide proof-of-principle regarding the short-term efficacy of binocular treatment, but our 8-week results were inconclusive adjusted difference = 0.3 lines (98.4% CI: −0.2 to 0.8) because the confidence interval included both zero and the predefined difference in mean visual acuity of 0.75 logMAR lines. This indicates the need for continued investigation of this binocular treatment strategy for amblyopia.
PEDIATRIC EYE DISEASE INVESTIGATOR GROUP.
Clinical Sites
Sites are listed in order by number of participants enrolled (in parenthesis).
Personnel are listed as (I) for Investigator, (C) for Coordinator or (E) for Examiner.
Virginia Beach, VA - Virginia Pediatric Eye Center (19)
Eric Crouch (I); Earl R. Crouch, Jr. (I); Stacy R. Martinson (I); Gaylord G. Ventura (C); Candice Chanel Brown (E); Cynthea M. Carlton (E); Carolina Andrea Fritz (E); Marlene Anne Guillory (E); Iesha Charde ORourke (E)
Boston, MA - Boston Children`s Hospital (11)
Maan S. Alkharashi (I); Linda R. Dagi (I); Gena Heidary (I); David G. Hunter (I); Jason S. Mantagos (I); Aparna Raghuram (I); Deborah K. VanderVeen (I); Carolyn Wu (I); Ryan Chinn (C); Breanne Taylor Beauchamp (E); Kaila Bishop (E); Rachael Calvey (E); Kristyn Magwire (E); Frances M. Pantano-Abele (E); Justyna S. Szczygiel (E); Sarah Whitecross (E); Emily K. Wiecek (E)
Cincinnati, OH - Cincinnati Children`s Hospital (10)
Michael E. Gray (I); Melissa L. Rice (I); Neil Vallabh (C); Jatawna Bush (E); Katherine Castleberry (E); Shemeka Rochelle Forte (E); Amanda R. Johnson (E); Miqua Lynn Stewart (E)
Lubbock, TX - Texas Tech University Health Science Center (10)
Lingkun Kong (I); Misty Rae Sisneros (C); Yvonne Bengoa (E); Connie J. Crossnoe (E)
Kansas City, MO - Children’s Mercy Hospitals and Clinics (7)
Jennifer N. Qayum (I); Justin D. Marsh (I); Amy L. Waters (I); Rebecca J. Dent (C); Lezlie L. Bond (E); Marni M. Harris (E); Lori L. Soske (E); Christina M. Twardowski (E)
Schaumburg, IL - Advanced Vision Center (7)
Ingryd J. Lorenzana (I); Angelyque L. Lorenzana (C); Ashley Francine Fisher (E); Ryan Bracket Mann (E); Danyle Segura (E)
Anaheim, CA - University Eye Center at Ketchum Health (6)
Susan A. Cotter (I); Angela M. Chen (I); Silvia Han (I); Kristine Huang (I); Dashaini V. Retnasothie (I); Sue M. Parker (C); Lucrecia Escobar (E); Catherine L. Heyman (E); Heather D. Mironas (E); Reena A. Patel (E)
Baltimore, MD - Wilmer Institute (6)
Michael X. Repka (I); Courtney Kraus (I); Xiaonong Liu (C); Lora Shirley Bauer (E); Alex Christoff (E)
Columbus, OH - The Ohio State University (5)
Marjean T. Kulp (I); Ann Marie Morrison (I); Maureen D. Plaumann (I); Nancy E. Stevens (C); Michelle J. Buckland (E); Jennifer S. Fogt (E); Steven T. Manning (E); Taylor D. McGann (E); Emmanuel Owusu (E); Erica Rose Shelton (E); Andrew J. Toole (E)
Grand Rapids, MI - Helen DeVos Children`s Hospital Pediatric Ophth. (5)
Brooke E. Geddie (I); Julie A. Conley (I); Samantha K. Rosen (I); Elisabeth T. Wolinski (C); Colette M. Kamaloski (E); Sonia Manuchian (E); Katie L. Patterson (E)
Houston, TX - Texas Children’s Hospital – Department of Ophthalmology (5)
Evelyn A. Paysse (I); Kelsie B. Morrison (I); Irene T. Tung (I); Kimberly G. Yen (I); Gihan Romany (C); Veronica M. Gonzalez (E); Katie D. Malone (E); Christine M. Romero (E)
Portland, OR - OHSU Casey Eye Institute (5)
Allison I. Summers (I); Srianna Narain (C); Grant Andrew Casey (E); Paula K. Rauch (E); Kevin M. Woodruff (E)
Birmingham, AL - UAB School of Optometry (4)
Kristine B. Hopkins (I); Marcela Frazier (I); Tamara S. Oechslin (I); Katherine K. Weise (I); Jenifer Montejo (C); Margaret Kathleen Bailey (E); Candice I. Turner (E)
Memphis, TN - Southern College of Optometry (4)
Marie I. Bodack (I); Randy C. Brafford (C); Marc B. Taub (E)
Rochester, MN - Mayo Clinic (4)
Erick D. Bothun (I); Jonathan M. Holmes (I); Tomohiko Yamada (I); Suzanne M. Wernimont (C); Janet Brinkman (E); Lindsay L. Czaplewski (E); Stacy L. Eastman (E); Julie A. Holmquist (E); Jordan Joseph Huisman (E); Moriah A. Keehn (E); Lindsay D. Klaehn (E); Andrea M. Kramer (E); Laura Lepor (E); Marna L. Levisen (E); Sarah R. Mickow (E); Debbie M. Priebe (E); Laura M. Taylor (E)
Bloomington, IN - Indiana University School Of Optometry (3)
Don W. Lyon (I); Katie S. Connolly (I); Kristy M. Dunlap (C)
Concord, NH - Concord Ophthalmologic Associates (3)
Christie L. Morse (I); Paul J. Rychwalski (I); Melanie L. Christian (E); Caroline C. Fang (E); Jacqueline Kathryn Gavin (E)
Ft. Lauderdale, FL - Nova Southeastern University College of Optometry, The Eye Institute (3)
Michael J. Au (I); Jacqueline Rodena (I); Felicia Jean Timmermann (C); Katherine E. Green (E); Amar Sayani (E); Yin C. Tea (E)
Glendale, AZ - Midwestern University Therapy Institute (3)
Alicia E. Feis (I); Christina A. Esposito (I); Matthew K. Roe (I); Tracy A. Bland (C); Caitlin C. Miller (E); Kelly D. Varney (E)
Lancaster, PA - Conestoga Eye (3)
David I. Silbert (I); Heather Modjesky (C); Dakota W. Hoak (E); Michael A. Raush (E)
Madison, WI - University of Wisconsin, Department of Ophthalmology and Visual Sciences (3)
Yasmin S. Bradfield (I); Angela M. Adler (C); Kristin A. Anderson (E)
Milford, CT - Eye Physicians & Surgeons, PC (3)
Jennifer A. Galvin (I); Taylor Risola (C); Erica O’Brien (E)
Scott Depot, WV - Marshall University (3)
Deborah L. Klimek (I); Ginger Peters (C); Amanda C. Conley (E); Sonya G. Walls (E)
Seattle, WA - Seattle Children’s Hospital (3)
Vivian Manh (I); Lyndsey A. Tews (C/E); Sheila Ganti (C); Bridget Ann Duffy (E); Jennifer Vincent (E)
Spokane, WA - Spokane Eye Clinical Research (3)
Jeffrey D. Colburn (I); Matthew C. Weed (I); Eileen Dittman (C); Felicia C. Korpi (E); Brian G. Skea (E); Dylan C. Waidelich (E)
St. Louis, MO - Saint Louis University Institute (3)
Rafif Ghadban (I); Traci A. Christenson (C); Lisa L. Breeding (E); Emily A. Miyazaki (E)
The Woodlands, TX - Houston Eye Associates (3)
Aaron M. Miller (I); Jorie L. Jackson (C); Angela C. Dillon (E); Carole L. Gray (E); Maria N. Olvera (E)
Baltimore, MD - Greater Baltimore Medical Center (2)
Mary Louise Z. Collins (I); Allison A. Jensen (I); Maureen A. Flanagan (C); Saman Bhatti (E); Cheryl L. McCarus (E); Gail C. Meil (E)
Big Rapids, MI - Michigan College of Optometry at Ferris State Univ (2)
Paula S. McDowell (I); Kerrie Rachelle Currie (C); Emily Jean Aslakson (E); Sarah B. Hinkley (E); Alison M. Jenerou (E)
Gainesville, FL - University of Florida (2)
Swati Agarwal-Sinha (I); Shannon Hampton (C); Kati M. Ostvig (E)
Laramie, WY - Snowy Range Vision Center (2)
Amy E. Aldrich (I); Lauri A. Atencio (C); Ashley Breanne Genoff (E); Samantha D. Lambert (E)
Loma Linda, CA - Loma Linda University Eye Institute (2)
Timothy W. Winter (I); Fatema Q. Esmail (I); Marcia M. Easterly (C); Rosalynn Nguyen-Strongin (E)
Marlton, NJ - Michael F. Gallaway, OD, PC (2)
Michael F. Gallaway (I); Debbie L. Killion (C); Tammy Lynn Thomas (E)
New Haven, CT - Yale Univ. Med. School, Dept. of Ophthal. & Visual Science (2)
Martha A. Howard (I); Margaret B. Therriault (C); Christine Carol Medina (E)
Oklahoma City, OK - Dean A. McGee Eye Institute, University of Oklahoma (2)
R. Michael Siatkowski (I); Janine Collinge (I); Maria E. Lim (I); Tammy Yanovitch (I); Alisha N. Brewer (C); Annette Doughty (C); Shannon Almeida (E); Sonny Icks (E); Laurie Hahn-Parrott (E); Lauren M. Pendarvis (E)
Omaha, NE - Children’s Hospital & Medical Center (2)
Donny W. Suh (I); Whitney R. Brown (I); Rachel M. Smith (I); Carolyn Chamberlain (C); Monica Judy Gomez (E); Jamie R. Lobato (E); Joel O. Rivas (E); Cheryl A. Urzendowski (E)
Poland, OH - Eye Care Associates, Inc. (2)
S. Ayse Erzurum (I); Alysa Christiansen (C); Zainab Dinani (E); Veronica Plessinger (E); Rachel R. Schneider (E)
Pomona, CA - Western University College of Optometry (2)
Ida Chung (I); Elaine C. Ramos (I); Kimberly R. Walker (I); Jennifer Baker (C)
Stratford, CT - Eye Surgery Associates LLC (2)
Jennifer A. Galvin (I); Angelic Garcia (E); Iwona Gorniak (E)
West Des Moines, IA - Wolfe Clinic (2)
Derek P. Bitner (I); Alexis C. Hahn (C); Rhonda J. Countryman (E); Lisa M. Fergus (E); Susan K. Hayes (E)
Boise, ID - St Luke’s Children’s Opthalmology (1)
Katherine A. Lee (I); Daniel R. Brooks (I); Laurie A. Cartwright (C); Kyle J. Perkins (E); Larry W. Plum (E); Bonita R. Schweinler (E)
Buffalo, NY - Ross Eye Institute, University of Buffalo, Med School Dept Ophthalmology (1)
John H. Lillvis (I); Sharon Michalovic (C); Kyle Arnoldi (E); Samantha J. Pape (E)
Chapel Hill, NC - University of North Carolina (1)
Katherine O. Whitfield (I); Elizabeth L. DuBose (C); Sarah Bowes (E)
Durham, NC - Duke University Eye Center (1)
Laura B. Enyedi (I); Nathan L. Cheung (I); Sarah K. Jones (C); Robert J. House (E); Namita Kashyap (E); Rachel N. Loud (E); Courtney E. Wilkins (E)
Houston, TX - University of Houston College of Optometry (1)
Ruth E. Manny (I); Heather A. Anderson (I); Debra C. Currie (I); Karen D. Fern (I)
Iowa City, IA - University of Iowa Hospitals and Clinics (1)
Scott A. Larson (I); Alina V. Dumitrescu (I); Xiaoyan Shan (C); Amy Troll (E)
New York, NY - State University of New York, College of Optometry (1)
Marilyn Vricella (I); Erica L. Schulman-Ellis (I); Monica Joao (C); Rochelle Mozlin (E); Daniella Rutner (E)
Philadelphia, PA - Wills Eye Institute (1)
Kammi B. Gunton (I); Nick R. Bello (C); Lynn H. Trieu (E)
Sartell, MN - PineCone Vision Center (1)
Kevy M. Simmons (I); Abbey Neu (E)
Spokane, WA - Northwest Pediatric Ophthalmology, P.S. (1)
George F. Whitehead (I); SueAnn Marie Stillman (C); Christina N. Nye (E); Caroline J. Shea (E)
St. Louis, MO - St. Louis Children’s Hospital Eye Center (1)
James R. Hoekel (I); Marlo L. Galli (C); Amanda M. Nicklas (E)
Wilmette, IL - Pediatric Eye Associates (1)
Lisa C. Verderber (I); Deborah R. Fishman (I); Roberta A. Forde (C); Adam J. Julian (E)
PEDIG Coordinating Center - Tampa, FL
Raymond T. Kraker, Roy W. Beck, Gillaine Alvarez, Darrell S. Austin, Nicole M. Boyle, Danielle L. Chandler, Patricia L. Connelly, Courtney L. Conner, Trevano W. Dean, Quayleen Donahue, Brooke P. Fimbel, Robert J. Henderson, Amra Hercinovic, James E. Hoepner, Joseph D. Kaplon, Zhuokai Li, B. Michele Melia, Julianne L. Robinson, Jennifer A. Shah, David O. Toro, Rui Wu.
Amblyopia Treatment Study Binocular Gameplay Planning Committee
Jonathan M. Holmes (Co-Chair), Ruth E. Manny (Co-Chair), Eileen E. Birch, Vivian M. Manh, Robert Hess, Vidhya Subramanian, Krista Kelly, Elizabeth L. Lazar, Raymond T. Kraker, David K. Wallace, David A. Leske, Brooke P. Fimbel
PEDIG Executive Committee
Susan A. Cotter (Co-chair), Jonathan M. Holmes (Co-chair), Roy W. Beck, Eileen E. Birch, Angela M. Chen (2017–18), Stephen P. Christiansen (2018-present), Earl R. Crouch (2012–14), Laura B. Enyedi (2014–16), S. Ayse Erzurum (2016-present), Donald F. Everett, Sharon F. Freedman (2016–18), William V. Good (2017–19), Raymond T. Kraker, Katherine A. Lee (2014–16), Richard London (2018-present), Vivian M. Manh (2016–18, 2020- Present), Ruth E. Manny (2017–19), David G. Morrison (2018–19), Stacy L. Pineles (2019-present), Hantamalala Ralay Ranaivo (2019-present), Michael X. Repka, Scott T. Ruark (2018–19), Bonita R. Schweinler (2016–18), Jayne L. Silver (2014–16), Allison I. Summers (2019-present), Lisa C. Verderber (2015–17), David K. Wallace, Katherine K. Weise (2019-present).
Amblyopia Steering Committee
Eileen E. Birch, Nathan L. Cheung (2020), Susan A. Cotter, Rebecca J. Dent (2019–20), Donald F. Everett, Michael E. Gray (2017, 2020-present), Jonathan M. Holmes, Erin C. Jenewein (2018), Srishti Kothari (2019), Raymond T. Kraker, Courtney Kraus (2018, 2020-present), Marjean T. Kulp (2012–17), Sudhi P. Kurup (2019), Sylvia Landa (2016–17), Zhuokai Li (2020-Present), Vivian M. Manh (2020-present), Ruth E. Manny (2016–2020), Stacy R. Martinson (2019–20), B. Michele Melia, Evelyn A. Paysse (2016–20), Michael X. Repka, Dashaini V. Retnasothie (2019), Tawna L. Roberts (2017-present), Veeral S. Shah (2020), Donny W. Suh (2011–17), Allison I. Summers (2017–18), Suzanne M. Wernimont (2017–18)
National Eye Institute - Bethesda, MD
Donald F. Everett
Data and Safety Monitoring Committee
Marie Diener-West (chair), John D. Baker, Barry Davis, Dale L. Phelps, Stephen W. Poff, Richard A. Saunders, Lawrence Tychsen
ACKNOWLEDGMENTS
Research was supported by the National Eye Institute of the National Institutes of Health, under Award Numbers EY011751, EY018810, and EY023198. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The game software was provided at no cost by Amblyotech. Scientific Presentation: 2020 American Academy of Optometry Annual Meeting and at 2020 American Academy of Ophthalmology Annual Meeting.
Zhuokai Li had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical Trials Registration: www.clinicaltrials.gov, under identifier (NCT02983552)
Appendix Table A1.
Study Inclusion and Exclusion Criteria.
| ELIGIBILITY CRITERIA |
| The following criteria must be met for the patient to be enrolled in the study: |
|
|
| 1. Age 4 to <7 years |
|
|
| 2. Amblyopia associated with strabismus, anisometropia, or both (previously treated or untreated) |
| a. Criteria for strabismus: At least one of the following must be met: |
| • Presence of a heterotropia on examination at distance or near fixation (with or without optical correction), must be no more than 4pd by SPCT at near fixation. |
| • Documented history of strabismus which is no longer present |
| b. Criteria for anisometropia: At least one of the following criteria must be met: |
| • ≥1.00 D difference between eyes in spherical equivalent |
| • ≥1.50 D difference in astigmatism between corresponding meridians in the two eyes |
| c. Criteria for combined-mechanism amblyopia: Both of the following criteria must be met: |
| • Criteria for strabismus are met (see above) |
| • ≥1.00 D difference between eyes in spherical equivalent OR ≥1.50 D difference in astigmatism between corresponding meridians in the two eyes |
|
|
| 3. No amblyopia treatment in the past 2 weeks (patching, atropine, Bangerter, vision therapy, binocular treatment) |
|
|
| 4. Requirements for required refractive error correction (based on a cycloplegic refraction (CR) within the last 7 months: |
| • Hypermetropia of 2.50D or more by spherical equivalent (SE) |
| • Myopia of amblyopic eye of 0.50D or more SE |
| • Astigmatism of 1.00D or more |
| • Anisometropia of more than 0.50D SE |
| Note: Subjects with cycloplegic refractive errors that do not fall within the requirements above for spectacle correction may be given spectacles at investigator discretion but must follow the study-specified prescribing guidelines, as detailed below. |
| a. Spectacle prescribing instructions referenced to the CR completed within the last 7 months: |
| •SE must be within 0.50D of fully correcting the anisometropia. |
| •SE must not be under corrected by more than 1.50D SE, and reduction in plus sphere must be symmetric in the two eyes. |
| •Cylinder power in both eyes must be within 0.50D of fully correcting the astigmatism. |
| •Cylinder axis must be within +/− 10 degrees if cylinder power is ≤1.00D, and within +/− 5 degrees if cylinder power is >1.00D. |
| •Myopia must not be under-corrected by more than 0.25D or over corrected by more than 0.50D SE, and any change must be symmetrical in the two eyes. |
| b. Spectacle correction meeting the above criteria must be worn: |
| • 16 weeks OR until VA stability is documented (defined as <0.1 logMAR change by the same testing method measured on 2 consecutive exams at least 8 weeks apart). |
| o Determining visual acuity stability (non-improvement):The first of two measurements may be made 1) in current correction, or 2) in trial frames with or without cycloplegia or 3) without correction (if new correction is prescribed), |
| o The second measurement must be made without cycloplegia in the correct spectacles that have been worn for at least 8 weeks. |
| Note: since this determination is a pre-study procedure, the method of measuring visual acuity is not mandated. |
|
|
| 5. Visual acuity, measured in each eye without cycloplegia incurrent spectacle correction (if applicable) within 7 days prior to randomization using the ATS-HOTV VA protocol for children < 7 years on a study-approved device displaying single surrounded optotypes, as follows: |
| a. VA in the amblyopic eye 20/40 to 20/200 inclusive (ATS-HOTV) |
| b. Best-corrected fellow-eye VA meeting the following criteria: |
| • If age 4, 20/40 or better by ATS-HOTV |
| • If age 5 or 6, 20/32 or better by ATS-HOTV |
| c. IOD ≥ 3 logMAR lines (ATS-HOTV) |
|
|
| 6. Heterotropia with a near deviation of < 5Δ (measured by SPCT) in habitual correction |
|
|
| 7. Subject able to play the Dig Rush game (at least level 3) on the study iPad® under binocular conditions (with red-green glasses). Subject must be able to see both the red “diggers” and blue “gold carts” when contrast is at 20% for the non- amblyopic eye. |
|
|
| 8. Investigator is willing to prescribe computer game play, or continue spectacle wear per protocol. |
|
|
| 9. Parent understands the protocol and is willing to accept randomization. |
|
|
| 10. Parent has phone (or access to phone) and is willing to be contacted by Jaeb Center staff or other study staff. |
| 11. Relocation outside of area of an active PEDIG site for this study within the next 8 weeks is not anticipated. |
|
|
| EXCLUSION CRITERIA |
|
|
| 1. Prism in the spectacle correction at time of enrollment (eligible only if prism is discontinued 2 weeks prior to enrollment). |
| 2. Myopia greater than −6.00D spherical equivalent in either eye. |
| 3. Previous intraocular or refractive surgery. |
| 4. Any treatment for amblyopia (patching, atropine, Bangerter filter, or previous binocular treatment) during the past 2 weeks. Previous amblyopia therapy is allowed regardless of type, but must have been discontinued at least 2 weeks prior to enrollment. |
| 5. Ocular co-morbidity that may reduce VA determined by an ocular examination performed within the past 7 months. |
| 6. (Note: nystagmus per se does not exclude the subject if the above visual acuity criteria are met). |
| 7. No Down syndrome or cerebral palsy |
| 8. No severe developmental delay that would interfere with treatment or evaluation (in the opinion of the investigator). Subjects with mild speech delay or reading and/or learning disabilities are not excluded. |
| 9. Subject has demonstrated previous low compliance with binocular treatment and/or spectacle treatment (as assessed by investigator) |
logMAR = logarithm of minimum angle of resolution; Δ = prism diopters; SD = standard deviation; SPCT = simultaneous prism and cover testATS-HOTV = Amblyopia Treatment Study HOTV; VA = visual acuity; PEDIG = Pediatric Eye Disease Investigator Group
Appendix Table A2.
Primary Efficacy Outcome and Alternative Approaches.
| Analysis Approach | Adjusted Treatment Group Difference in Mean Change in Amblyopic-Eye VA logMAR Lines (95.1% CI)a | P-value |
|---|---|---|
|
| ||
| Primary analysisb | 0.5 (0.1, 0.9) | .03 |
| Performed multiple imputationc to impute VA of participants whose 4-week visit was missing or completed outside of the analysis window (N=7 in binocular group and N=6 in control group) |
0.4 (0.03, 0.8) | .03 |
| Excluded participants who completed the 4-week exam outside of the protocol window (±1 week) (N=5 in binocular group and N=3 in control group) |
0.4 (−0.03, 0.8) | .06 |
| Excluded enrolled participants subsequently found to be ineligible for the study (N=2 in binocular group and N=1 in control group) |
0.5 (0.1, 0.9) | .02 |
| Included cause of amblyopia as an adjustment factor in the model | 0.5 (0.04, 0.9) | .03 |
| Winsorized VA at baseline and 4 weeks at the 10th and 90th percentiles by treatment group | 0.4 (0.03, 0.7) | .03 |
CI = confidence interval; VA = visual acuity.
Adjusted for baseline amblyopic-eye visual acuity. Positive values favor the binocular treatment group.
Modified intent-to-treat analysis limited to participants with a 4-week exam completed within the pre-specified analysis window (21 to <49 days after randomization). No imputation for missing data.
Multiple imputation was performed based on treatment group and amblyopic-eye visual acuity at randomization and the 4- and 8-week visits.
Appendix Table A3.
Distribution of Stereoacuity and Change in Stereoacuity from Baseline by Treatment Group.
| Baseline | 4 weeksa | 8 weeksa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
|||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group(N) | 92 | 90 | 85 | 84 | 85 | 84 | ||||||
|
| ||||||||||||
| Stereoacuity (Seconds of Arc) b | ||||||||||||
| Missing/Not done | 3 | 3% | 8 | 9% | 6 | 7% | 8 | 10% | 5 | 6% | 9 | 11% |
| Nil | 27 | 29% | 22 | 24% | 24 | 28% | 21 | 25% | 24 | 28% | 18 | 21% |
| 2000 | 13 | 14% | 11 | 12% | 8 | 9% | 10 | 12% | 10 | 12% | 6 | 7% |
| 800 | 13 | 14% | 10 | 11% | 13 | 15% | 7 | 8% | 10 | 12% | 15 | 18% |
| 400 | 11 | 12% | 19 | 21% | 10 | 12% | 15 | 18% | 9 | 11% | 12 | 14% |
| 200 | 13 | 14% | 12 | 13% | 9 | 11% | 9 | 11% | 12 | 14% | 9 | 11% |
| 100 | 9 | 10% | 5 | 6% | 10 | 12% | 6 | 7% | 10 | 12% | 7 | 8% |
| 60 | 1 | 1% | 2 | 2% | 3 | 4% | 5 | 6% | 2 | 2% | 3 | 4% |
| 40 | 2 | 2% | 1 | 1% | 2 | 2% | 3 | 4% | 3 | 4% | 5 | 6% |
| Median (Range) | 800 (40 to Nil) | 800 (40 to Nil) | 800 (40 to Nil) | 400–800 (40 to Nil) | 800 (40 to Nil) | 800 (40 to Nil) | ||||||
|
| ||||||||||||
| Change in Level of Stereoacuity from Baseline c | ||||||||||||
| > 2 Levels Worse | 11 | 14% | 8 | 11% | 7 | 9% | 3 | 4% | ||||
| Within 1 Level | 56 | 72% | 57 | 78% | 61 | 78% | 54 | 75% | ||||
| > 2 Levels Better | 11 | 14% | 8 | 11% | 10 | 13% | 15 | 21% | ||||
Limited to follow-up visits completed within the pre-specified analysis windows.
Results of the Randot Butterfly stereoacuity test were analyzed as 2000 seconds of arc (if correct response) or nil (if incorrect response) in the presence of an incorrect response on the 800 seconds of arc level of the Randot Preschool stereoacuity test.
The exact Wilcoxon rank-sum test was used to compare the change in stereoacuity from baseline to the 4-week visit (Bonferroni-adjusted P > .99) and to the 8-week visit (Bonferroni-adjusted P > .99) between the treatment groups.
Appendix Table A4.
Distribution of Stereoacuity Scores and Change in Stereoacuity Scores from Baseline by Treatment Group (Limited to Participants without Strabismus).
| Baseline | 4 weeksa | S weeksa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
|||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group (N) | 61 | 54 | 58 | 51 | 5l | 51 | ||||||
|
| ||||||||||||
| Stereoacuity (Seconds of Arc) b | ||||||||||||
| Missing/Not done | 0 | 0% | 2 | 4% | 4 | 7% | 4 | 8% | 4 | 7% | 5 | 10% |
| Nil | 15 | 25% | 11 | 20% | 11 | 19% | 8 | 16% | 11 | 19% | 8 | 16% |
| 2000 | 9 | 15% | 6 | 11% | 5 | 9% | 7 | 14% | 7 | 12% | 2 | 4% |
| 800 | 5 | 8% | 5 | 9% | 8 | 14% | 4 | 8% | 5 | 9% | 8 | 16% |
| 400 | 10 | 16% | 12 | 22% | 8 | 14% | 10 | 20% | 5 | 9% | 8 | 16% |
| 200 | 10 | 16% | 12 | 22% | 9 | 16% | 9 | 18% | 11 | 19% | 8 | 16% |
| 100 | 9 | 15% | 3 | 6% | 10 | 1l% | 4 | 8% | 9 | 16% | 6 | 12% |
| 60 | 1 | 2% | 2 | 4% | 3 | 5% | 4 | 8% | 2 | 4% | 2 | 4% |
| 40 | 2 | 3% | 1 | 2% | 0 | 0% | 1 | 2% | 3 | 5% | 4 | 8% |
| Median (Range) | 400 (40 to Nil) | 400 (40 to Nil) | 400 (60 to Nil) | 400 (40 to Nil) | 400 (40 to Nil) | 400 (40 to Nil) | ||||||
|
| ||||||||||||
| Change in Level of Stereoacuity from Baseline c | ||||||||||||
| ≥ 2 Levels Worse | 8 | 15% | 6 | 13% | 5 | 9% | 3 | 7% | ||||
| Within 1 Level | 37 | 69% | 34 | l6% | 40 | l5% | 30 | 6l% | ||||
| ≥ 2 Levels Better | 9 | 1l% | 5 | 11% | 8 | 15% | 12 | 27% | ||||
Limited to follow-up visits completed within the pre-specified analysis windows.
Results of the Randot Butterfly stereoacuity test were analyzed as 2000 seconds of arc (if correct response) or nil (if incorrect response) in the presence of an incorrect response on the 800 seconds of arc level of the Randot Preschool stereoacuity test.
The exact Wilcoxon rank-sum test was used to compare the change in stereoacuity from baseline to the 4-week visit (Bonferroni-adjusted P > .99) and to the 8-week visit (Bonferroni-adjusted P > .99) between treatment groups for participants without strabismus.
Appendix Table A5.
Distribution of Change in Fellow-eye Visual Acuity from Baseline to Follow-up Visits by Treatment Group.
| 4 weeks | 8 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | |||||
|
|
||||||||
| N | % | N | % | N | % | N | % | |
|
| ||||||||
| Per Group (N) | 88 | 84 | 86 | 84 | ||||
| Change in fellow-eye VA from baseline | ||||||||
| ≥ 3 logMAR lines better | 0 | 0% | 2 | 2% | 1 | 1% | 1 | 1% |
| 2 logMAR lines better | 2 | 2% | 2 | 2% | 7 | 8% | 5 | 6% |
| 1 logMAR line better | 20 | 23% | 23 | 27% | 25 | 29% | 21 | 25% |
| 0 logMAR line | 50 | 57% | 44 | 52% | 40 | 47% | 40 | 48% |
| 1 logMAR line worse | 14 | 16% | 8 | 10% | 12 | 14% | 17 | 20% |
| 2 logMAR lines worse | 2 | 2% | 4 | 5% | 1 | 1% | 0 | 0% |
| ≥ 3 logMAR lines worse | 0 | 0 | 1 | 1% | 0 | 0% | 0 | 0% |
| Mean (SD) Linesa | 0.1 (0.8) | 0.2 (1.0) | 0.3 (0.9) | 0.2 (0.9) | ||||
| Adjusted mean (95% CI) Linesb | 0.1 (−0.1, 0.2) | 0.2 (0.02, 0.4) | 0.3 (0.1, 0.5) | 0.2 (0.04, 0.4) | ||||
| Adjusted mean difference (99% CI) Linesb | -0.1 (−0.5, 0.2) | 0.1 (−0.2, 0.4) | ||||||
|
| ||||||||
| Worsening of ≥ 2 lines from baseline | 2 | 2% | 5 | 6% | 1 | 1% | 0 | 0% |
| Difference (99% CI)c | -4% (−15%, 6%) | 1% (−6%, 9%) | ||||||
CI = confidence interval; logMAR = logarithm of minimum angle of resolution; SD = standard deviation; VA = visual acuity.
Positive values indicate improvement in fellow-eye visual acuity from baseline.
Analysis of covariance model adjusting for baseline fellow-eye visual acuity was used to estimate the mean change in fellow-eye visual acuity within each treatment group as well as the treatment group difference (positive values favor the binocular treatment group) in the mean change in fellow-eye visual acuity from baseline to 4 weeks (P = .26) and to 8 weeks (P = .41).
Barnard’s exact test was used to compare the proportion of participants with worsening of ≥ 2 lines in fellow-eye visual acuity from baseline to 4 weeks (P = .25) and to 8 weeks (P = .52) between treatment groups.
Appendix Table A6.
Change in Ocular Deviation from Baseline by Treatment Group.
| 4 weeks | 8 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | |||||
|
|
||||||||
| N with Change/ N Eligible | % | N with Change/ N Eligible | % | N with Change/ N Eligible | % | N with Change/ N Eligible | % | |
|
| ||||||||
| New strabismusa | 7/88 | 8% | 7/84 | 8% | 6/86 | 7% | 7/84 | 8% |
| Increased magnitude of strabismus by ≥10Δb | 0/20 | 0% | 2/17 | 12% | 0/20 | 0% | 2/17 | 12% |
| Strabismus at baseline no longer presentc | 6/20 | 30% | 6/17 | 35% | 6/20 | 30% | 4/17 | 24% |
Barnard’s exact test was used to compare the proportion of participants with new strabismus at 4 weeks (P = .98) and at 8 weeks (P = .81) between the treatment groups.
Barnard’s exact test was used to compare the proportion of participants with strabismus magnitude that increased by ≥ 10Δ at 4 weeks (P = .15) and at 8 weeks (P = .15) between the treatment groups.
Barnard’s exact test was used to compare the proportion of participants with a strabismus at baseline that was no longer present at 4 weeks (P = .77) or at 8 weeks (P = .71) between the treatment groups.
Appendix Table A7.
Distribution of Participant-reported Frequency of Diplopia and Change in Diplopia Frequency from Baseline by Treatment Group.
| Baseline | 4 weeks | 8 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Participant-reporteda | Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group(N) | 91 | 90 | 87 | 84 | 86 | 84 | ||||||
|
| ||||||||||||
| Diplopia frequency b | ||||||||||||
| Never | 87 | 96% | 86 | 96% | 78 | 90% | 79 | 94% | 78 | 91% | 78 | 93% |
| Less than once a week | 2 | 2% | 0 | 0% | 2 | 2% | 2 | 2% | 4 | 5% | 4 | 5% |
| Once a week | 0 | 0% | 3 | 3% | 4 | 5% | 0 | 0% | 1 | 1% | 1 | 1% |
| Once a day | 1 | 1% | 1 | 1% | 3 | 3% | 3 | 4% | 2 | 2% | 0 | 0% |
| Up to 10 times a day | 1 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| More than 10 times a day | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| All the time | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% |
|
| ||||||||||||
| Change in diplopia frequency from baseline c | ||||||||||||
| Increased frequency (> 2 levels) | 6 | 7% | 2 | 2% | 2 | 2% | 1 | 1% | ||||
| Similar frequency (within 1 level) | 79 | 92% | 80 | 95% | 82 | 96% | 80 | 95% | ||||
| Reduced frequency (> 2 levels) | 1 | 1% | 2 | 2% | 1 | 1% | 3 | 4% | ||||
Three participants in the binocular treatment group (one at each visit) and one in the control treatment group at the 4-week visit had monocular diplopia.
The exact Wilcoxon rank sum test was used to compare diplopia frequency at 4 weeks (P = .36) and 8 weeks (P = .65) between the treatment groups.
The exact Wilcoxon rank sum test was used to compare the change in diplopia frequency from baseline to 4 weeks (P = .36) and to 8 weeks (P = .60) between the treatment groups.
Appendix Table A8.
Distribution of Parent-reported Frequency of the Child’s Diplopia and Change in Diplopia Frequency from Baseline By Treatment Group.
| Baseline | 4 weeks | 8 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Parent-reporteda | Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
Binocular Treatment |
Continued Spectacles |
||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group(N) | 91 | 90 | 87 | 84 | 86 | 84 | ||||||
|
| ||||||||||||
| Diplopia frequency b | ||||||||||||
| Never | 87 | 96% | 86 | 96% | 78 | 90% | 79 | 94% | 78 | 91% | 78 | 93% |
| Less than once a week | 2 | 2% | 0 | 0% | 2 | 2% | 2 | 2% | 4 | 5% | 4 | 5% |
| Once a week | 0 | 0% | 3 | 3% | 4 | 5% | 0 | 0% | 1 | 1% | 1 | 1% |
| Once a day | 1 | 1% | 1 | 1% | 3 | 3% | 3 | 4% | 2 | 2% | 0 | 0% |
| Up to 10 times a day | 1 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| More than 10 times a day | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| All the time | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% |
|
| ||||||||||||
| Change in diplopia frequency from baseline c | ||||||||||||
| Increased frequency (> 2 levels) | 6 | 7% | 2 | 2% | 2 | 2% | 1 | 1% | ||||
| Similar frequency (within 1 level) | 79 | 92% | 80 | 95% | 82 | 96% | 80 | 95% | ||||
| Reduced frequency (> 2 levels) | 1 | 1% | 2 | 2% | 1 | 1% | 3 | 4% | ||||
The parental assessment may be missing if the parent/guardian was not available at the visit.
The exact Wilcoxon rank sum test was used to compare diplopia frequency at 4 weeks (P > .99) and at 8 weeks (P = .50) between the treatment groups.
The exact Wilcoxon rank sum test was used to compare the change in diplopia frequency from baseline to 4 weeks (P = .49) and to 8 weeks (P = .25) between the treatment groups.
Appendix Table A9.
Distribution of Symptom Survey Frequency Scores for Individual Questionnaire Items at Baseline and Follow- up Visits by Treatment Group.
| Baseline | 4 weeksa | 8 weeksa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | |||||||
|
|
||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Per Group (N) | 92 | 90 | 88 | 83b | 86 | 84 | ||||||
|
| ||||||||||||
| Blurred Vision | ||||||||||||
| Never | 62 | 67% | 61 | 68% | 72 | 82% | 71 | 86% | 74 | 86% | 62 | 74% |
| Almost never | 11 | 12% | 10 | 11% | 10 | 11% | 6 | 7% | 5 | 6% | 17 | 20% |
| Sometimes | 17 | 18% | 12 | 13% | 5 | 6% | 4 | 5% | 7 | 8% | 5 | 6% |
| Often | 2 | 2% | 5 | 6% | 1 | 1% | 2 | 2% | 0 | 0% | 0 | 0% |
| Almost always | 0 | 0% | 2 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||||||
| Eyestrain | ||||||||||||
| Never | 57 | 62% | 47 | 52% | 53 | 60% | 58 | 70% | 58 | 67% | 58 | 69% |
| Almost never | 19 | 21% | 18 | 20% | 15 | 17% | 19 | 23% | 16 | 19% | 20 | 24% |
| Sometimes | 12 | 13% | 19 | 21% | 17 | 19% | 6 | 7% | 12 | 14% | 6 | 7% |
| Often | 4 | 4% | 6 | 7% | 3 | 3% | 0 | 0% | 0 | 0% | 0 | 0% |
| Almost always | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||||||
| Headache | ||||||||||||
| Never | 59 | 64% | 47 | 52% | 60 | 68% | 68 | 82% | 62 | 72% | 65 | 77% |
| Almost never | 19 | 21% | 27 | 30% | 14 | 16% | 13 | 16% | 13 | 15% | 17 | 20% |
| Sometimes | 12 | 13% | 15 | 17% | 9 | 10% | 1 | 1% | 10 | 12% | 2 | 2% |
| Often | 2 | 2% | 0 | 0% | 5 | 6% | 1 | 1% | 1 | 1% | 0 | 0% |
| Almost always | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||||||
| Looking over spectacles c | ||||||||||||
| Never | 30 | 33% | 34 | 38% | 44 | 50% | 40 | 48% | 38 | 44% | 40 | 48% |
| Almost never | 23 | 25% | 21 | 23% | 17 | 19% | 19 | 23% | 16 | 19% | 17 | 20% |
| Sometimes | 25 | 27% | 24 | 27% | 19 | 22% | 17 | 20% | 26 | 30% | 23 | 27% |
| Often | 11 | 12% | 9 | 10% | 6 | 7% | 6 | 7% | 5 | 6% | 2 | 2% |
| Almost always | 3 | 3% | 2 | 2% | 2 | 2% | 1 | 1% | 1 | 1% | 2 | 2% |
|
| ||||||||||||
| Taking off spectacles c | ||||||||||||
| Never | 28 | 30% | 39 | 43% | 43 | 49% | 46 | 55% | 45 | 52% | 44 | 52% |
| Almost never | 34 | 37% | 27 | 30% | 26 | 30% | 24 | 29% | 28 | 33% | 30 | 36% |
| Sometimes | 24 | 26% | 19 | 21% | 15 | 17% | 11 | 13% | 8 | 9% | 7 | 8% |
| Often | 5 | 5% | 4 | 4% | 4 | 5% | 1 | 1% | 5 | 6% | 3 | 4% |
| Almost always | 1 | 1% | 1 | 1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
The exact Wilcoxon rank sum test was used to compare symptom frequency at 4 weeks (P = .59 for blurred vision; P = .06 for eyestrain; P = .02 for headache; P = .98 for looking over spectacles; P = .30 for taking off spectacles) and at 8 weeks (P = .08 for blurred vision; P = .63 for eyestrain; P = .27 for headache; P = .52 for looking over spectacles; P = .83 for taking off spectacles) between the treatment groups.
One participant in the control treatment group did not have a completed Symptom Survey at the 4-week visit.
There were no responses that spectacles were not worn.
Appendix Table A10.
Distribution of Change in Symptom Survey Frequency Scores from Baseline for Individual Questionnaire Items at Follow-up Visits by Treatment Group.
| 4 weeksa | 8 weeksa | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Binocular Treatment | Continued Spectacles | Binocular Treatment | Continued Spectacles | |||||
|
|
||||||||
| N | % | N | % | N | % | N | % | |
|
| ||||||||
| Per Group (N) | 88 | 83b | 86 | 84 | ||||
|
| ||||||||
| Blurred Vision | ||||||||
| Increased frequency (≥ 2 levels) | 4 | 5% | 0 | 0% | 4 | 5% | 2 | 2% |
| Similar frequency (within 1 level) | 73 | 83% | 72 | 87% | 67 | 78% | 71 | 85% |
| Reduced frequency (≥ 2 levels) | 11 | 13% | 11 | 13% | 15 | 17% | 11 | 13% |
|
| ||||||||
| Eyestrain | ||||||||
| Increased frequency (≥ 2 levels) | 9 | 10% | 0 | 0% | 5 | 6% | 1 | 1% |
| Similar frequency (within 1 level) | 73 | 83% | 73 | 88% | 75 | 87% | 72 | 86% |
| Reduced frequency (≥ 2 levels) | 6 | 7% | 10 | 12% | 6 | 7% | 11 | 13% |
|
| ||||||||
| Headache | ||||||||
| Increased frequency (≥ 2 levels) | 7 | 8% | 0 | 0% | 5 | 6% | 0 | 0% |
| Similar frequency (within 1 level) | 76 | 86% | 72 | 87% | 76 | 88% | 77 | 92% |
| Reduced frequency (≥ 2 levels) | 5 | 6% | 11 | 13% | 5 | 6% | 7 | 8% |
|
| ||||||||
| Looking over spectacles c | ||||||||
| Increased frequency (≥ 2 levels) | 4 | 5% | 2 | 2% | 6 | 7% | 3 | 4% |
| Similar frequency (within 1 level) | 72 | 82% | 73 | 88% | 70 | 81% | 76 | 90% |
| Reduced frequency (≥ 2 levels) | 12 | 14% | 8 | 10% | 10 | 12% | 5 | 6% |
|
| ||||||||
| Taking off spectacles c | ||||||||
| Increased frequency (≥ 2 levels) | 1 | 1% | 0 | 0% | 0 | 0% | 2 | 2% |
| Similar frequency (within 1 level) | 78 | 89% | 80 | 96% | 76 | 88% | 77 | 92% |
| Reduced frequency (≥ 2 levels) | 9 | 10% | 3 | 4% | 10 | 12% | 5 | 6% |
The exact Wilcoxon rank sum test was used to compare the change in symptom frequency from baseline to 4 weeks (P = .35 for blurred vision; P < .001 for eyestrain; P = .002 for headache; P = .43 for looking over spectacles; P = .97 for taking off spectacles) and to 8 weeks (P = .97 for blurred vision; P = .02 for eyestrain; P = .01 for headache; P = .74 for looking over spectacles; P = .65 for taking off spectacles) between the treatment groups.
One participant in the control treatment group did not have a completed Symptom Survey at the 4-week visit.
There were no responses that spectacles were not worn.
Appendix Figure A1,
available at http://links.lww.com/OPX/A561: Relationship between total hours of binocular game play and contrast presented to the fellow eye after 4 and 8 weeks of treatment (binocular treatment group).
REFERENCES
- 1.Papageorgiou E, Asproudis I, Maconachie G, et al. The Treatment of Amblyopia: Current Practice and Emerging Trends. Graefes Arch Clin Exp Ophthalmol 2019;257:1061–78. [DOI] [PubMed] [Google Scholar]
- 2.Carlton J, Kaltenthaler E. Amblyopia and Quality of Life: A Systematic Review. Eye (Lond) 2011;25:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart CE, Moseley MJ, Stephens DA, et al. Treatment Dose-Response in Amblyopia Therapy: The Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 2004;45:3048–54. [DOI] [PubMed] [Google Scholar]
- 4.Awan M, Proudlock FA, Gottlob I. A Randomized Controlled Trial of Unilateral Strabismic and Mixed Amblyopia Using Occlusion Dose Monitors to Record Compliance. Invest Ophthalmol Vis Sci 2005;46:1435–9. [DOI] [PubMed] [Google Scholar]
- 5.Hiscox F, Strong N, Thompson JR, et al. Occlusion for Amblyopia: A Comprehensive Survey of Outcome. Eye 1992;6:300–4. [DOI] [PubMed] [Google Scholar]
- 6.Repka MX. Amblyopia Outcomes through Clinical Trials and Practice Measurement: Room for Improvement: The LXXVII Edward Jackson Memorial Lecture. Am J Ophthalmol 2020;219:A1–a26. [DOI] [PubMed] [Google Scholar]
- 7.Levi DM. Rethinking Amblyopia 2020. Vision Res 2020;176:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, He Z, Mao Y, et al. Patching and Suppression in Amblyopia: One Mechanism or Two? Front Neurosci 2019;13:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess RF, Mansouri B, Thompson B. A New Binocular Approach to the Treatment of Amblyopia in Adults Well Beyond the Critical Period of Visual Development. Restor Neurol Neurosci 2010;28:793–802. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Thompson B, Deng D, et al. Dichoptic Training Enables the Adult Amblyopic Brain to Learn. Curr Biol 2013;23:R308–R9. [DOI] [PubMed] [Google Scholar]
- 11.Kelly KR, Jost RM, Dao L, et al. Binocular Ipad Game Vs Patching for Treatment of Amblyopia in Children: A Randomized Clinical Trial. JAMA Ophthalmology 2016;134:1402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedamurthy I, Nahum M, Huang SJ, et al. A Dichoptic Custom-Made Action Video Game as a Treatment for Adult Amblyopia. Vision Res 2015;114:173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DH, Meese TS, Mansouri B, Hess RF. Binocular Summation of Contrast Remains Intact in Strabismic Amblyopia. Invest Ophthalmol Vis Sci 2007;48:5332–8. [DOI] [PubMed] [Google Scholar]
- 14.Hess RF, Thompson B. Amblyopia and the Binocular Approach to Its Therapy. Vision Res 2015;114:4–16. [DOI] [PubMed] [Google Scholar]
- 15.Tsirlin I, Colpa L, Goltz HC, Wong AM. Behavioral Training as New Treatment for Adult Amblyopia: A Meta-Analysis and Systematic Review. Invest Ophthalmol Vis Sci 2015;56:4061–75. [DOI] [PubMed] [Google Scholar]
- 16.Birch EE, Li SL, Jost RM, et al. Binocular Ipad Treatment for Amblyopia in Preschool Children. J AAPOS 2015;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manh VM, Holmes JM, Lazar EL, et al. A Randomized Trial of a Binocular Ipad Game versus Part-Time Patching in Children Aged 13 to 16 Years with Amblyopia. Am J Ophthalmol 2018;186:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes JM, Manh VM, Lazar EL, et al. Effect of a Binocular Ipad Game vs. Part-Time Patching in Children Aged 5 to 12 Years with Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol 2016;134:1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao TY, Guo CX, Babu RJ, et al. Effectiveness of a Binocular Video Game Vs Placebo Video Game for Improving Visual Functions in Older Children, Teenagers, and Adults with Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol 2018;136:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pediatric Eye Disease Investigator Group. A Randomized Trial of Binocular Dig Rush Game Treatment for Amblyopia in Children Aged 7 to 12 Years. Ophthalmology 2019;126:456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes JM, Beck RW, Repka MX, et al. The Amblyopia Treatment Study Visual Acuity Testing Protocol. Arch Ophthalmol 2001;119:1345–53. [DOI] [PubMed] [Google Scholar]
- 22.Moke PS, Turpin AH, Beck RW, et al. Computerized Method of Visual Acuity Testing: Adaptation of the Amblyopia Treatment Study Visual Acuity Testing Protocol. Am J Ophthalmol 2001;132:903–9. [DOI] [PubMed] [Google Scholar]
- 23.Drover JR, Felius J, Cheng CS, et al. Normative Pediatric Visual Acuity Using Single Surrounded Hotv Optotypes on the Electronic Visual Acuity Tester Following the Amblyopia Treatment Study Protocol. J AAPOS 2008;12:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pediatric Eye Disease Investigator Group. The Course of Moderate Amblyopia Treated with Patching in Children: Experience of the Amblyopia Treatment Study. Am J Ophthalmol 2003;136:620–9. [DOI] [PubMed] [Google Scholar]
- 25.Pediatric Eye Disease Investigator Group. A Randomized Trial to Evaluate 2 Hours of Daily Patching for Strabismic and Anisometropic Amblyopia in Children. Ophthalmology 2006;113:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pediatric Eye Disease Investigator Group. Randomized Trial of Treatment of Amblyopia in Children Aged 7 to 17 Years. Arch Ophthalmol 2005;123:437–47. [DOI] [PubMed] [Google Scholar]
- 27.Holmes JM, Lazar EL, Melia BM, et al. Effect of Age on Response to Amblyopia Treatment in Children. Arch Ophthalmol 2011;129:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadhum A, Tan ETC, Levi DM, et al. Barriers to Successful Dichoptic Treatment for Amblyopia in Young Children. Graefes Arch Clin Exp Ophthalmol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly KR, Jost RM, Wang YZ, et al. Improved Binocular Outcomes Following Binocular Treatment for Childhood Amblyopia. Invest Ophthalmol Vis Sci 2018;59:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jost RM, Kelly KR, Hunter JS, et al. A Randomized Clinical Trial of Contrast Increment Protocols for Binocular Amblyopia Treatment. J AAPOS 2020;24:282.e1-.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pediatric Eye Disease Investigator Group, Cotter SA, Edwards AR, et al. Treatment of Anisometropic Amblyopia in Children with Refractive Correction. Ophthalmology 2006;113:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pediatric Eye Disease Investigator Group, Cotter SA, Foster NC, et al. Optical Treatment of Strabismic and Combined Strabismic-Anisometropic Amblyopia. Ophthalmology 2012;119:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pediatric Eye Disease Investigator Group. A Randomized Trial of Atropine Vs Patching for Treatment of Moderate Amblyopia in Children. Arch Ophthalmol 2002;120:268–78. [DOI] [PubMed] [Google Scholar]
- 34.Pediatric Eye Disease Investigator Group. The Course of Moderate Amblyopia Treated with Atropine in Children: Experience of the Amblyopia Treatment Study. Am J Ophthalmol 2003;136:630–9. [DOI] [PubMed] [Google Scholar]
- 35.Stewart CE, Moseley MJ, Fielder AR, Stephens DA. Refractive Adaptation in Amblyopia: Quantification of Effect and Implications for Practice. Br J Ophthalmol 2004;88:1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao TY, Anstice N, Babu RJ, et al. Optical Treatment of Amblyopia in Older Children and Adults Is Essential Prior to Enrolment in a Clinical Trial. Ophthalmic Physiol Opt 2018;38:129–43. [DOI] [PubMed] [Google Scholar]