Abstract
Introduction
There are rare cases of Sjogren’s syndrome presenting with manifestations of encephalitis. There are also rare patients with Sjogren’s presenting with acute thrombotic thrombocytopenic purpura (TTP). There are no cases of both occurring together as the only symptoms of the syndrome. During the COVID-19 pandemic, more cases of autoimmunity are being described given its robust immune response. It is important to keep a wide differential about these varying clinical presentations.
Case Presentation
Our patient is a 19-year-old female with a history of menorrhagia, recent COVID-19 infection, and remote suicidal ideation. She presented with headaches, vomiting, and psychosis. Her labs found platelets of 12,000 and she was soon discovered to have TTP. She was found to have contrast enhancing lesions scattered in her left hemisphere on magnetic resonance imaging as well as seizures. Her workup was negative for infection, but labs revealed a positive antinuclear antibody, elevated anti-Ro antibody (anti-SSA) and anti-La antibody (anti-SSB), and elevated COVID-19 antibodies. She was treated with antiepileptics, pulse dose steroids for 5 days, plasmapheresis, and weekly rituximab for 4 weeks. She had significant clinical improvement.
Conclusion
Sjogren’s syndrome can have varying presentations including TTP with or without encephalitis as a presenting feature. Autoimmunity can also be triggered from COVID-19 infection.
Keywords: Sjogren’s syndrome, encephalitis, COVID-19, thrombotic thrombocytopenic purpura, case report
Case Presentation
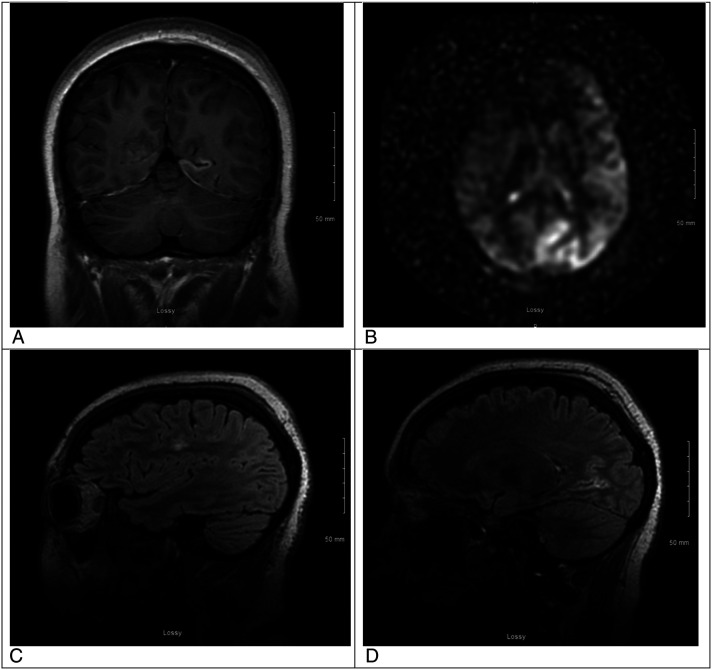
Our patient was a 19-year-old female with a history of menorrhagia, COVID-19 infection, learning disability, and remote suicidal ideation. She presented to an outside facility with 3 days of severe headaches; emesis; and symptoms of psychosis consisting of severe agitation, confusion, and combativeness. She was found to have platelets of 12,000 and was diagnosed with thrombotic thrombocytopenic purpura (TTP) evidenced by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, fevers, and encephalopathy. Lumbar puncture was performed at the outside hospital, and she was transferred to our tertiary care center for emergent plasmapheresis. Neurology was consulted given her progressive encephalopathy. During examination, she was found to have left gaze deviation and right hemiplegia. She was unable to follow commands or open her eyes spontaneously, she did have hyperreflexia in the right upper and lower extremities, and there were no hyperkinetic movements. She underwent emergent magnetic resonance imaging (MRI) of the brain which revealed a contrast enhancing lesion in the left occipital, temporal, and frontal lobes, the vermis folia, and tectal plate colliculi. There was no evidence of diffusion restriction, however there was hyperperfusion on arterial spin labeling in the left hemisphere (Figure 1). Electroencephalography was obtained which showed left hemispheric poly-spike and waves that evolved into several subclinical seizures over a 24-hour monitoring period. She required lacosamide to stop seizure activity and was continued on 200 mg twice daily. Our patient underwent infectious, autoimmune, and hematologic workup including ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) assays (Table 1). In addition to elevated lactate dehydrogenase (LDH), reticulocyte count and bilirubin, schistocytes, and low haptoglobin, she also had a positive antinuclear antibody (ANA), high anti-SSA (anti-Ro) and anti-SSB (anti-La) antibodies, and significantly elevated COVID-19 antibody levels from recent infection given no prior vaccination. At the outside hospital, her symptoms of psychosis were initially thought to be related to her prior history of suicidal ideation but given that she had no prior known psychosis or psychiatric diagnosis, we felt that a primary psychiatric component should be a diagnosis of exclusion. Based on her clinical presentation and further workup, it was determined that this was more likely related to TTP and encephalitis.
Figure 1.
A. This is a magnetic resonance imaging (MRI) brain coronal T1 post contrast view of one of the contrast enhancing lesions in the left hemisphere. B. This is an MRI brain arterial spin labeling sequence (ASL) showing relative hyperperfusion of the left hemisphere, suspicious for seizure activity. C. This is an MRI brain sagittal T2 Flair image without contrast showing an area of edema in the frontal lobe. D. This is an MRI brain sagittal T2 Flair with contrast showing enhancement of a lesion in the left occipital lobe.
Table 1.
This table depicts all of the laboratory testing obtained during the patient's hospital stay.
| Laboratory test | Value | Normal range |
|---|---|---|
| Serum Chemistry | ||
| Sodium | 139 | 136-144 mmol/L |
| Potassium | 3.5 (L) | 3.6-5.1 mmol/L |
| Chloride | 106 | 101-111 mmol/L |
| Carbon dioxide | 27 | 22-32 mmol/L |
| Blood urea nitrogen | 12 | 8-20 mg/dL |
| Glucose | 104 (H) | 65-99 mg/dL |
| Calcium | 8.9 | 8.4-10.2 mg/dL |
| Creatinine | 1.17 | .60-1.30 mg/dL |
| Total protein | 8.3 (H) | 6.1-7.9 g/dL |
| Albumin | 4.2 | 3.5-5.4 g/dL |
| Total bilirubin | 1.7 (H) | .2-1.0 mg/dL |
| Albumin | 4.2 | 3.5-5.4 g/dL |
| Lactate dehydrogenase | 4671 (H) | 313-618 unit/L |
| Alkaline phosphatase | 52 | 38-126 52 unit/L |
| Aspartate aminotransferase | 74 (H) | 15-41 unit/L |
| Alanine transaminase | 34 | 5-35 unit/L |
| Magnesium a | 1.6 (L) | 1.8-2.4 mg/dL |
| Phosphorous a | 4.9 | 3.1-5.6 mg/dL |
| Procalcitonin a | .13 (L) | .50-2.00 ng/dL |
| Creatine kinase a | 222 (H) | 55-170 unit/L |
| Ammonia | <9 (L) | 11-35 mcmol/L |
| Iron | 189 (H) | 28.0-170.0 mcg/dL |
| Total iron binding capacity | 395 (H) | 162-344 mcg/dL |
| Ferritin | 444.00 (H) | 6.00-137.00 ng/mL |
| Transferrin | 265 | 206-381 mg/dL |
| Free T3 a | 1.68 (L) | 2.18-3.98 pg/mL |
| Thyroid stimulating hormone a | .868 | .358-3.740 mclU/mL |
| Free T4 a | 1.16 | .80-2.00 ng/dL |
| Hemoglobin A1C a | 4.1% | ≤5.6% |
| Pregnancy | Negative | N/A |
| Acetaminophen level a | 11.0 (H) | ≤10.0 mcg/mL |
| Salicylate a | <1.0 (L) | 10.0-30.0 mg/dL |
| Ethyl alcohol a | <10 | N/A |
| % Serum ethyl alcohol a | <.010 | N/A |
| Haptoglobin | <8.0 (L) | 30.0-200.0 mg/dL |
| C-reactive protein a | 5.40 | ≤9.00 mg/L |
| Serum C3 (complement) | 75.0 (L) | 90.0-180.0 mg/dL |
| Serum C4 (complement) | 6.6 (L) | 10.0-40.0 mg/dL |
| Cardiac a | ||
| Brain natriuretic peptide | 13.8 | ≤100.0 pg/mL |
| Troponin-I | .014 | .000-.034 ng/mL |
| Hematology | ||
| White blood cells (WBCs) | 6.9 | 4.2-10.2 thou/mcL |
| Red blood cells (RBCs) | 2.85 (L) | 3.50-5.50 x10^6/ mcL |
| Hemoglobin | 8.5 (L) | 12.0-16.0 g/dL |
| Hematocrit | 25.9% (L) | 36.0-48.0% |
| Mean corpuscular volume (MCV) blood | 90.9 | 78.0-98.0 fL |
| Mean corpuscular hemoglobin (MCH) blood | 29.8 | 25.0-35.0 pg |
| MCH concentration | 32.8 | 30.0-36.0 g/dL |
| Red cell distribution width (RDW) | 21.4% (H) | 12.4-16.6% |
| RDW standard deviation | 62.7 (H) | 37.0-54.0 fL |
| Nucleated RBCs | .39 | .00-5.00x10^3/ mcL |
| Platelet count | 6 (L) | 140-400 thou/mcL |
| Absolute neutrophils | 3.1 | 1.8-7.1 thou/mcL |
| Absolute lymphocytes | 3.47 | 1.30-5.94 thou/mcL |
| Absolute reticulocytes | 349.7 (H) | 30.0-90.0 thou/mcL |
| Immature reticulocyte fraction | 40.9% | N/A |
| Segmented neutrophils | 46.1% | N/A |
| Lymphocytes | 51.0% | N/A |
| Monocytes | 1.0% | N/A |
| Eosinophils | 1.9% | N/A |
| Nucleated RBCs | 5.70 (H) | .00-1.00/100 WBC |
| Erythrocyte sedimentation rate | 36 (H) | 0-20 mm/hr |
| Schistocytes | Few | N/A |
| Coagulation | ||
| Fibrinogen level | 244 | 208-475 mg/dL |
| Active partial thrombin time | 30.3 | 23.2-34.1 sec |
| International normalized ratio | 1.11 | .85-1.16 |
| Prothrombin time | 14.1 | 11.7-14.5 sec |
| D-dimer, quantitative a | 4.45 (H) | .00-.44 mcg FEU/mL |
| Russell viper venom | 25.0 (L) | 28.3-43.1 sec |
| Immunology a | ||
| Cardiolipin antibody (Ab), IgA | <2.0 | <20.0, units not specified |
| Cardiolipin Ab, IgG | <2.0 | <20.0, units not specified |
| Cardiolipin Ab, IgM | <2.0 | <20.0, units not specified |
| Anti-cardiolipin IgG | <1.6 | ≤19.9 GPL U/mL |
| Anti-cardiolipin IgM | 1.0 | ≤19.9 MPL U/mL |
| Beta-2-glycoprotein I (GPI) IgG | <1.4 | ≤19.9 unit/mL |
| Phosphatidylserine IgG | <10 | <10 unit/mL |
| Phosphatidylserine IgM | <25 | <25 unit/mL |
| Phosphatidylserine IgA | <20 | <20 unit/mL |
| Beta-2-GPI IgG | <2.0 | <20.0 unit/mL |
| Beta-2-GPI IgM | <2.0 | <20.0 unit/mL |
| Beta-2-GPI IgA | <2.0 | <20.0 unit/mL |
| Antimicrosomal Ab | 95.6 (H) | ≤60 unit/mL |
| Antinuclear Ab (ANA) | Positive (A) | Negative |
| ANA titer | Positive 1:320 (A) | Negative |
| ANA pattern | Speckled | N/A |
| Anti-double stranded DNA Ab | 1 | ≤4 IU/mL |
| Anti-Smith | 0.2 | ≤.9 AI |
| Anti-RNP | <0.2 | ≤.9 AI |
| Anti-SSA | >8.0 (H) | ≤.9 AI |
| Anti-SSB | >8.0 (H) | ≤.9 AI |
| Homocysteine | 7.4 | 4.0-15.5 mcmol/L |
| SARS-CoV-2 total ab | 1310.00 (H) | ≤.99 unit not specified |
| ADAMTS-13 activity | <3% (C) | 68-163% |
| ADAMTS-13 inhibitor | 12.0 (H) | <4 Bethesda units |
| Cerebrospinal fluid studies b | ||
| Glucose | 72 | 40-75 mg/mL |
| Protein | 52.5 (H) | 15-45 mg/100 mL |
| WBCs | 0 | <5/mL |
| RBCS | 0 | <5/mL |
| UA | Unremarkable | |
| Infectious studies | ||
| RPR screen a | Negative | N/A |
| Hepatitis (Hep) A-IgM antibody a | Negative | N/A |
| Hep B surface antigen a | Negative | N/A |
| Hep B core-IgM antibody a | Negative | N/A |
| Hepatitis C antibody | Negative | N/A |
| Human immunodeficiency virus (HIV) antibody/antigen | Negative | N/A |
| Tuberculosis quantiferon gold | Negative | N/A |
| Coronavirus SARS-CoV-2 (COVID-19) PCR test | Negative | N/A |
| Meningitis/encephalitis panel | Negative | N/A |
| Escherichia Coli K1 CSF | ||
| Haemophilus influenzae CSF | ||
| Listeria monocytogenes CSF | ||
| Neisseria meningitidis CSF | ||
| Streptococcus agalactiae CSF | ||
| Streptococcus pneumoniae CSF | ||
| Cytomegalovirus (CMV) CSF | ||
| Enterovirus (EV) CSF | ||
| Herpes-simplex Virus-1 (HSV-1) CSF | ||
| Herpes-simplex Virus-2 (HSV-2) CSF | ||
| Human Herpesvirus-6 (HHV-6) CSF | ||
| Human parechovirus (HPeV) CSF | ||
| Varicella zoster virus (VZV) CSF | ||
| Cryptococcus neoformans/Gatti CSF | ||
| Respiratory pathogen panel | Negative | N/A |
aspecimen collected after first day (24 h) of admission.
bObtained at outside hospital.
Abbreviations: (L): low, (H): high, (A): abnormal, (C): critical.
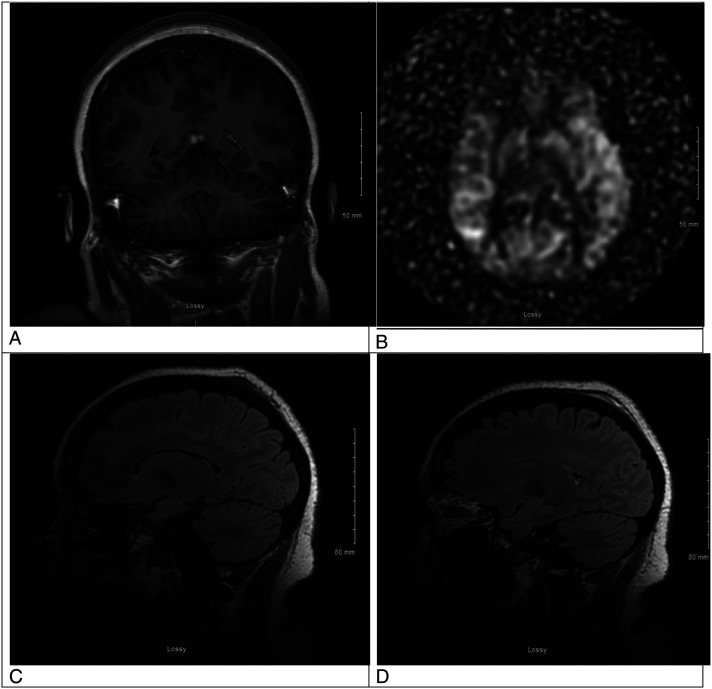
She received eleven sessions of plasmapheresis (PLEX), pulse dosed methylprednisolone for 5 days, and once weekly rituximab 8.3 mg/kg for 4 weeks. Until her infectious workup came back negative, she was treated with empiric antibiotics. After prompt initiation of treatment, our patient had significant improvement of her mental status, neurologic exam, and hematologic labs. Our patient’s repeat MRI 2 weeks after her initial diagnosis was also significantly improved (Figure 2). Specifically, our patient’s gaze deviation and right hemiplegia resolved with prompt administration of lacosamide, more consistent with a Todd’s paralysis phenomenon, whereas her encephalopathy improved over time with each PLEX treatment. Platelets and lactate dehydrogenase (LDH) normalized with continued PLEX and her first dose of rituximab. The patient clinically improved to her neurologic baseline and was able to consent to and read this report.
Figure 2.
A. This is an MRI brain coronal T1 post contrast view showing resolution of the previously contrast enhancing lesion in the left hemisphere. B. This is an MRI brain arterial spin labeling sequence showing resolution of the previous relative hyperperfusion of the left hemisphere. C. This is an MRI brain sagittal T2 Flair image without contrast showing resolution of the previous frontal lobe edema. D. This is an MRI brain sagittal T2 Flair with contrast showing resolution of enhancement the prior left occipital lobe lesion.
Discussion
Sjogren’s syndrome is an autoimmune disease that classically presents with xerostomia and xerophthalmia and is often associated with other autoimmune disorders. 1 Thrombotic thrombocytopenic purpura is a well-studied disease, known to be caused by either a primary or acquired deficiency of ADAMTS-13, a protease that cleaves von Willebrand Factor. This deficiency leads to complications of fever, anemia, thrombocytopenia, renal failure, and neurologic symptoms. 2 There have been reports about acquired TTP in the setting of active COVID-19 infection, including a recent case series.3-5 There are also reports documenting the association of TTP and Sjogren’s syndrome, including a review that recognized diagnoses of the 2 entities at separate times. 3 There are additionally a few cases describing encephalitis as the initial presentation of Sjogren’s syndrome.6,7 This case is a unique presentation of undiagnosed likely Sjogren’s syndrome complicated by a prolonged immune response to COVID-19 outside of the acute infection, with acquired TTP and encephalitis as the initial diagnoses that led to both etiologies’ discovery.
In a patient who presents with severe thrombocytopenia and MAHA, TTP should be high on the differential given its morbidity and mortality if untreated. High suspicion is key to early initiation of treatment. With severe thrombocytopenia and elevated LDH in the setting of severe anemia, schistocytes on peripheral smear, and clinical presentation, treatment should be started prior to confirmatory a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) activity and inhibitor assays. Though reports document a multi-faceted treatment plan, plasmapheresis is the mainstay of TTP treatment acutely. This is often combined with pulse dosed steroids as well as rituximab or cyclophosphamide, depending on the underlying etiology.1,5 Low ADAMTS-13 activity is confirmatory for TTP, and the inhibitor assay distinguishes between primary and acquired TTP, the latter giving a positive result with an elevated titer compared to the negative result of the inhibitor in the primary form. 2
In cases of young females with new onset psychosis, seizures, and altered mentation, the differential must initially be broad. This includes diagnoses such as anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis. In the case of our patient, conducting another lumbar puncture prior to initiating treatment of PLEX was felt to be too risky given her extremely low platelets. There was not enough cerebrospinal fluid (CSF) from her initial tap to send further studies. Additionally, PLEX is the standard of care treatment for both TTP and anti-NMDA receptor encephalitis. Had the patient not improved quickly with PLEX, further investigation was planned, including NMDA testing in the serum and CSF as well as transvaginal ultrasound to evaluate for ovarian teratoma. 8 This same medical decision making was used in testing of other causes of encephalitis, such as anti-myelin oligodendrocyte glycoprotein (anti-MOG) and anti-glial fibrillary acidic protein. In the case of this patient, because her symptomatology improved with PLEX and subsequent normalization of her platelets, it was felt that her encephalopathy was more related to her TTP and these other causes of encephalitis were lower on our differential. Her seizures were also very responsive to a single medication, which is oftentimes not the case for encephalitis from anti-NMDA antibodies.
In our patient, the diagnosis of acquired TTP was presumed and PLEX was initiated immediately. Neurology was involved early in the patient’s course prompting the full infectious and autoimmune workup, including an autoimmune antibody profile that led to the diagnosis of likely Sjogren’s syndrome. Potentially, a robust immune response to COVID-19 triggered the acquired TTP and Sjogren’s associated encephalitis in this patient. It is unknown when exactly our patient was previously infected with COVID-19. The patient’s parents were unable to ascertain when and for how long she previously had respiratory symptoms, however based on the level of her antibodies it was inferred that this robust immune response may have played a part in triggering her underlying autoimmunity. Our patient responded well to her treatment regimen and improved robustly.
While there are specific diagnostic criteria to diagnose Sjogren’s syndrome based on sicca symptoms as well as anti-SSA or anti-SSB antibodies, the positive antinuclear antibody (ANA) in conjunction with positive antibodies increase the likelihood for a patient to develop the physical manifestations of Sjogren’s and later the official diagnosis. 9 There are a few reports in which Sjogren’s syndrome was initially diagnosed as a case of encephalitis and the individual later developed sicca symptoms.6,7 Our patient was significantly encephalopathic from a combination of TTP, encephalitis as evidenced on her images, and intermittent seizures. When her mental status improved, she denied complaints of sicca symptoms however she will need to be monitored closely as an outpatient in the future.
Conclusion
Clinicians should be aware that a robust immune response to illness, like COVID-19, may be present in the setting of TTP and underlying or undiagnosed Sjogren’s syndrome, with encephalitis as a presenting symptom. Though causality cannot be proven, and the highly positive COVID-19 antibodies could have been incidental, we want to present this case to add to the ongoing literature to hopefully assist in challenging cases involving COVID-19, TTP, underlying autoimmunity, and/or encephalitis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Brittany M. Kasturiarachi https://orcid.org/0000-0001-8580-4849
References
- 1.Feltsan T, Stanko P, Mracna J. Sjögren’s syndrome in present. Bratisl lek listy. 2012;113(8):514-516. doi: 10.4149/bll_2012_116. [DOI] [PubMed] [Google Scholar]
- 2.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 3.Tehrani HA, Darnahal M, Vaezi M, Haghighi S. COVID-19 associated thrombotic thrombocytopenic purpura (TTP); a case series and mini-review. Int Immunopharmacol. 2021;93:107397. doi: 10.1016/j.intimp.2021.107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho JF, Shoenfeld Y. Sjögren’s syndrome associated with thrombotic thrombocytopenic purpura: a case-based review. Rheumatol The. 2021;8(1):621-629. doi: 10.1007/s40744-020-00265-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toumeh A, Josh N, Narwal R, Assaly R. Refractory thrombotic thrombocytopenic purpura associated with primary Sjogren syndrome treated with rituximab. Am J The. 2014;21(2):e56-e60. doi: 10.1097/MJT.0b013e3182459aa0. [DOI] [PubMed] [Google Scholar]
- 6.Harboe E, Tjensvoll AB, Maroni S, et al. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and primary Sjogren syndrome: A comparative population-based study. Ann Rheum Dis. 2009;68(10):1541-1546. [DOI] [PubMed] [Google Scholar]
- 7.Iwai K, Amo K, Kuki I, et al. An unusual manifestation of Sjögren syndrome encephalitis. Brain Dev. 2019;41(2):217-220. doi: 10.1016/j.braindev.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Mitra AD, Afifya A. Ovarian teratoma associated Anti N-methyl-D-aspartate receptor encephalitis: A difficult diagnosis with a favorable prognosis. Autops Case Rep. 2018;8(2):e2018019. doi: 10.4322/acr.2018.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis. 2002;61(6):554-558. [DOI] [PMC free article] [PubMed] [Google Scholar]