Abstract
Aim
Capric acid (also known as decanoic acid or C10) is one of the fatty acids in the medium‐chain triglycerides (MCTs) commonly found in dietary fats. Although dietary treatment with MCTs is recently of great interest for the potential therapeutic effects on neuropsychiatric disorders, the effects of oral administration of C10 on behavior remain to be examined. This study investigated acute and chronic effects of oral administration of C10 on locomotor activity and anxiety‐like and depression‐related behaviors in adult male C57BL/6J mice.
Methods
To explore the acute effects of C10 administration, mice were subjected to a series of behavioral tests in the following order: light/dark transition, open field, elevated plus maze, Porsolt forced swim, and tail suspension tests, 30 minutes after oral gavage of either vehicle or C10 solution (30 mmol/kg dose in Experiment 1; 0.1, 0.3, 1.0, 3.0 mmol/kg doses in Experiment 2). Next, to examine chronic effects of C10, mice repeatedly administered with either vehicle or C10 solution (0.3, 3.0 mmol/kg doses per day, for 21 days, in Experiment 3) were subjected to behavioral tests without oral administration immediately before each test.
Results
The mice administrated with the high dose of C10 (30 mmol/kg) showed lower body weights, shorter distance traveled, and more anxiety‐like behavior than vehicle‐treated mice, and the results reached study‐wide statistical significance. The C10 administration at a lower dose of 0.3 mmol/kg had no significant effects on body weights and induced nominally significantly longer distance traveled than vehicle administration. Repeated administration of C10 at a dose of 3.0 mmol/kg for more than 21 days caused lower body weights and decreased depression‐related behavior, although the behavioral differences did not reach study‐wide significance.
Conclusions
Although these results suggest dose‐dependent effects of oral administration of capric acid on locomotor activity and anxiety‐like and depression‐related behaviors, further study will be needed to replicate the findings and explore the underlying brain mechanisms.
Keywords: anxiety, capric acid, decanoic acid, depression, medium‐chain fatty acid, mice
Repeated oral administration of the medium‐chain fatty acid, capric acid, decreased depression‐related behavior in C57BL/6J mice. This study suggests that capric acid exerts an antidepressant effect.
1. INTRODUCTION
A ketogenic diet (KD), a high‐fat and low‐carbohydrate diet, was introduced as an effective treatment of pharmacoresistant epilepsy, 1 and it has been used worldwide over the past two decades following a resurgence of interest in its efficacy for epilepsy.2, 3, 4 The medium‐chain triglyceride (MCT) KD was developed to make the classic KD more palatable, allowing less restriction of other foods. 5 The MCTs have attracted great interest due to the importance of their direct or indirect effects on seizure control of epilepsy and energy metabolism. 6 Recently, KD/MCT diet has also been studied as a potential therapeutic treatment for other neuropsychiatric disorders, such as Alzheimer's disease, Parkinson's disease, autism spectrum disorder, and depression, although the efficacies and mechanisms underlying the diet actions remain elusive.4, 7
The MCTs are abundant in coconut oil, palm oil, and fats. MCTs produce more ketones per kilocalorie of energy than long‐chain triglycerides, thus being more ketogenic. 3 Rodent studies have reported that chronic administration of a KD or a diet containing a ketone ester reduces anxiety‐like and depression‐related behaviors and improves cognitive functions.8, 9 Acute injection of fatty acids, including one of the medium‐chain fatty acids, lauric acid (dodecanoic acid; C12), is suggested to contribute to a decrease in anxiety‐like behavior. 10 Chronic administration of coconut oil or MCT diet has also been reported to decrease anxiety‐like behaviors in rats.11, 12 These findings suggest that acute and chronic administrations of MCTs have anxiolytic and antidepressant effects. MCTs are composed of fatty acid esters of glycerol with 6‐12 carbons arranged in a straight chain, such as caprylic acid (also known as octanoic acid; C8), capric acid (decanoic acid; C10), and lauric acid. However, the effects of each medium‐chain fatty acid on behavior remain to be determined.
In this study, we investigated the acute and chronic effects of oral administration of capric acid (C10) at different doses on locomotor activity, anxiety‐like behavior, and depression‐related behavior in adult male C57BL/6J mice. In Experiment 1, to examine acute behavioral effects of C10, mice were subjected to a battery of behavioral tests in the following order: the light/dark transition test, open field test, elevated plus maze test, Porsolt forced swim test, and tail suspension test, 30 minutes after oral administration of C10 at the dose of 30 mmol/kg, which is reported to have anticonvulsant effects. 13 In Experiment 2, acute behavioral effects of C10 were further explored when C10 was administered at the lower doses (0.1‐3.0 mmol/kg). Finally, in Experiment 3, to examine behavioral effects of repeated administration of C10 at the lower doses (0.3 and 3.0 mmol/kg), mice were administered with C10 once a day for 3 weeks and were subjected to behavioral tests without C10 administration immediately before the tests. Fatty acids are rapidly metabolized to ketone bodies, such as beta‐hydroxybutyrate (BHB), which have been suggested to regulate anxiety‐like and depression‐related behaviors.14, 15 Thus, plasma BHB levels were measured from blood samples collected 30 minutes after oral gavage of C10 and were also analyzed approximately 24 hours after the last oral administration of C10 in Experiment 3 to examine the involvement of possible changes in ketone levels after C10 administration in the regulation of behaviors.
2. MATERIALS AND METHODS
2.1. Animals
Male C57BL/6J mice at 7 weeks old were purchased from Charles River Laboratories Japan, Inc. After arrival to our animal facility, mice were group‐housed (four per cage) in plastic cages (250 × 182 × 139 mm) with paper chips for bedding (Paper Clean; Japan SLC, Inc, Shizuoka, Japan). Rooms were maintained under a 12 hours light/dark cycle (lights on at 7:00 AM) at 23 ± 2°C. Animals had free access to food (CRF‐1; Oriental Yeast Co., Ltd., Tokyo, Japan) and filtered water throughout the experiments. All experimental procedures started 2 weeks or more after the arrival. All the procedures were approved by the Institutional Animal Care and Use Committee of Fujita Health University.
2.2. Oral administration
Capric acid (C10; C10H20O2, FW 172.26 g/mol; decanoic acid‐natural, >98%, food‐grade: Sigma‐Aldrich Co, St Louis, MO, USA) was dissolved in vehicle (0.5% methylcellulose 400 centipoise; Sigma‐Aldrich Co, St Louis, MO, USA). All mice were weighed immediately before oral administration. In Experiment 1, 24 mice were divided into the vehicle‐ and C10‐treated groups (n = 12, each group). Mice of the C10‐treated group were orally administered with C10 (30 mmol/10 ml in the vehicle) at the dose of 30 mmol/kg body weight. Mice of the vehicle‐treated group were orally administered with the vehicle at a volume of 10 ml/kg. Mice were subjected to behavioral tests 30 minutes after administration on each test day as described below (see Figure 1A). The pretreatment time (30 minutes) was determined according to the previous study reporting a maximal anticonvulsant efficacy of C10 in the pilot experiments. 13 In Experiment 2, 108 mice were divided into five groups: vehicle‐ and C10 (0.1, 0.3, 1.0, and 3.0 mmol/kg)‐treated groups (n = 21‐22, each group). Mice were subjected to a series of behavioral tests approximately 30 minutes after oral administration in the same manner as Experiment 1 (see Figure 2A). In Experiment 3, 48 mice were divided into three groups: vehicle‐ and C10 (0.3 and 3.0 mmol/kg)‐treated groups (n = 16, each group). Animals were orally administered once a day with either vehicle or 0.3 mmol/kg of C10 or 3.0 mmol/kg of C10 for 21 days and were subjected to behavioral tests after the 21 days treatment (see Figure 3A). In Experiment 3, mice were administered with vehicle or C10 solution after the behavioral test on each test day.
FIGURE 1.
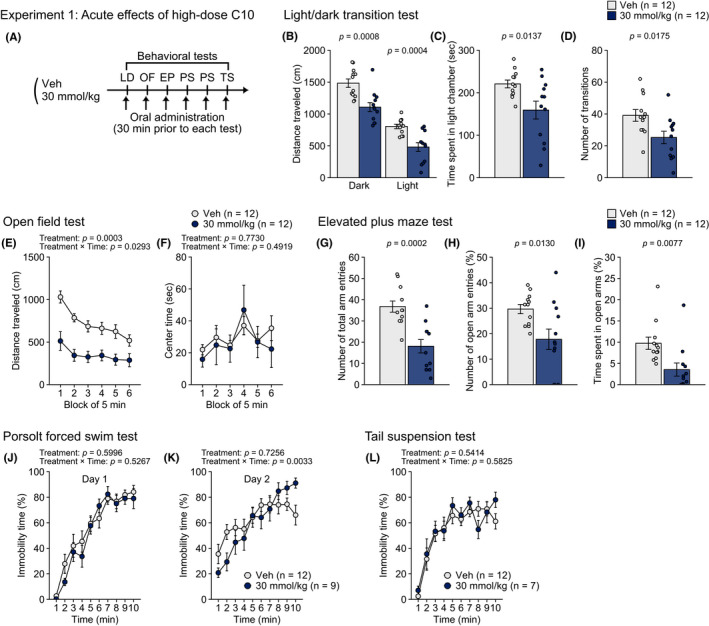
Acute effects of oral administration of CA10 at a high dose on behaviors in male C57BL/6J mice in Experiment 1. (A) Schematic diagram of experimental procedures in Experiment 1. (B–D) Light/dark transition test: (B) distance traveled (cm) in the light (right) and dark (left) chambers, (C) time spent in the light chamber (sec), and (D) number of transitions. (E–F) Open field test: (E) distance traveled (cm) and (F) center time (sec) during six blocks of 5 min. (G–I) Elevated plus maze test: (G) number of total arm entries, (H) percentage of open arm entries (%), and (I) percentage of time spent in open arms (%). (J–K) Porsolt forced swim test: percentage of immobility time (%) for ten 1 min blocks of the test session on test day 1 (J) and on test day 2 (K). (L) Percentage of immobility time (%) in the tail suspension test. Values are means ± SEM. Statistical analysis was performed by unpaired t‐test or two‐way repeated measures ANOVA, and the P values for main effects and interactions are shown
FIGURE 2.
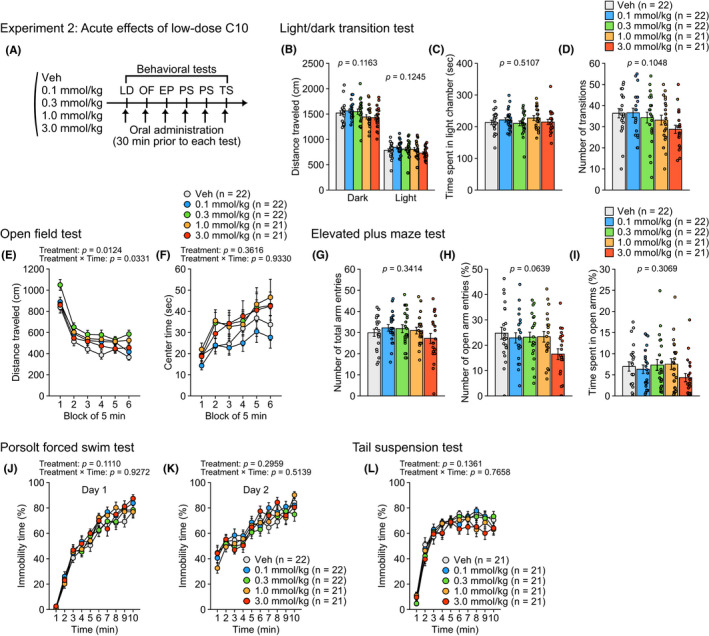
Acute effects of oral administration of CA10 at low doses on behaviors in male C57BL/6J mice in Experiment 2. (A) Schematic diagram of experimental procedures in Experiment 2. (B–D) Light/dark transition test: (B) distance traveled (cm) in the light (right) and dark (left) chambers, (C) time spent in the light chamber (sec), and (D) number of transitions. (E–F) Open field test: (E) distance traveled (cm) and (F) center time (sec) during six blocks of 5 min. (G–I) Elevated plus maze test: (G) number of total arm entries, (H) percentage of open arm entries (%), and (I) percentage of time spent in open arms (%). (J–K) Porsolt forced swim test: percentage of immobility time (%) for ten 1 min blocks of the test session on test day 1 (J) and on test day 2 (K). (L) Percentage of immobility time (%) in the tail suspension test. Values are means ± SEM. Statistical analysis was performed by one‐way ANOVA or two‐way repeated measures ANOVA, and the P values for main effects and interactions are shown
FIGURE 3.
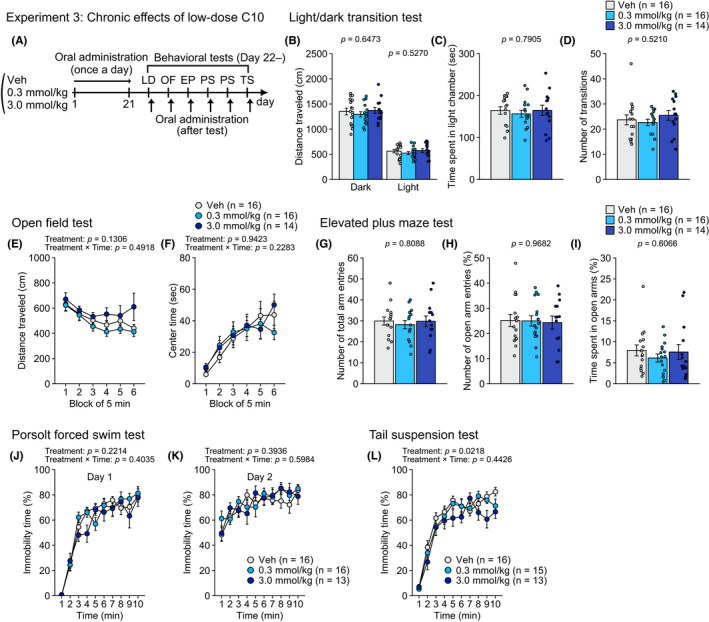
Chronic effects of repeated oral administration of CA10 on behaviors in male C57BL/6J mice in Experiment 3. (A) Schematic diagram of experimental procedures in Experiment 3. (B–D) Light/dark transition test: (B) distance traveled (cm) in the light (right) and dark (left) chambers, (C) time spent in the light chamber (sec), and (D) number of transitions. (E–F) Open field test: (E) distance traveled (cm) and (F) center time (sec) during six blocks of 5 min. (G–I) Elevated plus maze test: (G) number of total arm entries, (H) percentage of open arm entries (%), and (I) percentage of time spent in open arms (%). (J–K) Porsolt forced swim test: percentage of immobility time (%) for ten 1 min blocks of the test session on test day 1 (J) and on test day 2 (K). (L) Percentage of immobility time (%) in the tail suspension test. Values are means ± SEM. Statistical analysis was performed by one‐way ANOVA or two‐way repeated measures ANOVA, and the P values for main effects and interactions are shown
2.3. Behavioral test
Mice were subjected to a battery of behavioral tests performed in the following order: light/dark transition test (LD), open field test (OF), elevated plus maze test (EP), Porsolt forced swim test (PS; one trial per day, for two consecutive days), and tail suspension test (TS) with at least 1 day between tests, as previously described.16, 17 After each test, apparatuses were cleaned with hypochlorous acid water to prevent bias based on olfactory cues. The tests were performed between 9:00 AM and 5:00 PM In Experiment 1, three C10‐treated mice were excluded from the analysis of the forced swim test due to death after the test, and two C10‐treated mice were further excluded from the analysis of the tail suspension test because of death before the test (n = 1) and due to a technical problem of the test (n = 1; one mouse climbed the tail; see 18 ). In Experiment 2, in the tail suspension test, the behavioral data of two mice were lost due to a technical problem related to the limitation of data storage of a computer with a behavior analyzing system (Veh, n = 1; 0.1 mmol/kg, n = 1), and data of one mouse (0.3 mmol/kg, n = 1) was also excluded from the analysis because of falling off the apparatus. In Experiment 3, two mice treated with C10 at 3.0 mmol/kg dose died during the 21 days treatment. Additionally, one mouse treated with C10 at 3.0 mmol/kg dose was excluded from the analysis of the forced swim test due to death after the test, and one mouse treated with C10 at 0.3 mmol/kg dose was excluded from the analysis of the tail suspension test because it climbed the tail during the test.
2.3.1. Light/dark transition test
The light/dark transition test, originally developed by Crawley and colleagues, 19 was performed to assess anxiety‐like behavior as previously described. 16 The apparatus consisted of a cage (21 × 42 × 25 cm) divided into two sections of equal size by a partition with a door (O’Hara & Co., Tokyo, Japan). One chamber consisted of white plastic walls and was brightly illuminated (390 lux) by lights attached above the ceiling of the chamber. The other chamber had black plastic walls and was dark (2 lux). Both chambers had a white plastic floor. Mice were placed into the dark chamber and allowed to move freely between the two chambers for 10 minutes with the door open. The distance traveled (cm), total number of transitions, latency to first enter the light chamber (sec), and time spent in the light chamber (sec) were recorded automatically using the ImageLD program (see “Image analysis”).
2.3.2. Open field test
The open field test was performed to assess locomotor activity and anxiety‐like behavior 20 in the open field apparatus with the VersaMax Animal Activity Monitoring System (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH, USA), in which the center area was illuminated to 100 lux by lights attached above the ceiling. The center area was defined as a 20 cm × 20 cm area. Each mouse was placed in one corner of an open field. The total distance traveled (cm), vertical activity (measured by counting the number of photobeam interruptions), time spent in the center area (sec), and stereotypic counts (beam‐break counts for stereotyped behaviors), were automatically recorded using the activity monitoring system for the entire 30 minutes period after mice were placed in the apparatus. The behavioral data were analyzed in 5 minutes blocks.
2.3.3. Elevated plus maze test
The elevated plus maze test, widely used to assess anxiety‐like behavior, 21 was performed as previously described. 22 The apparatus consisted of two open arms (25 × 5 cm) and two enclosed arms of the same size with 15 cm‐high transparent walls and a central square (5 × 5 cm) connecting the arms (O’Hara & Co., Tokyo, Japan). The floors of arms and central square were made of white plastic plates and were elevated to a height of 55 cm above the floor. To prevent mice from falling off the open arms, those arms were surrounded by a raised ledge (3 mm thick and 3 mm high, transparent plastic). Arms of the same type were located opposite one another. The illumination level of the central area was 100 lux. Each mouse was placed in the central square of the maze facing one of the closed arms. The number of arm entries, distance traveled (cm), percentage of entries into open arms, and percentage of time spent in open arms were measured during a 10 minutes test period. Data acquisition and analysis were performed automatically using the ImageEP program.
2.3.4. Porsolt forced swim test
The Porsolt forced swim test, originally developed by Porsolt and colleagues, 23 was performed to assess depression‐related behavior. A Plexiglas cylinder (20 cm height × 10 cm diameter, O’Hara & Co., Tokyo, Japan), filled with water (approximately 21‐23°C) up to a height of 8 cm, was placed into a test chamber with a video camera mounted on the ceiling (49 cm height × 44 cm length × 32 cm width, inside dimensions; O'Hara & Co., Tokyo, Japan). Each mouse was placed into the cylinder for 10 minutes per day for two consecutive days. Images were captured at two frames per sec through the video camera. The amount of area (pixels) that the mouse moved was calculated for each pair of successive frames. When the amount of area was below a certain threshold, mouse behavior was judged as "immobile." When the amount of area equaled or exceeded the threshold, the mouse was considered "mobile." The optimal threshold was determined by adjusting it based on the amount of immobility measured by human observation. Immobility lasting for less than 2 seconds. was not included in the analysis. Data acquisition and analysis for the percentage of immobility time and distance traveled (cm) were automatically performed by the ImagePS program.
2.3.5. Tail suspension test
The tail suspension test, which was developed by Steru et al, 24 was used to evaluate depression‐related behavior, as previously described.16, 17 Mice were suspended 30 cm above the floor in a visually isolated area by adhesive tape placed approximately 1 cm from the tip of the tail in a white plastic chamber with a video camera mounted on the sidewall (44 cm height × 49 cm length × 32 cm width, inside dimensions; O'Hara & Co., Tokyo, Japan). The behavior was recorded for 10 minutes. Images were captured at two frames per second through the video camera. The immobility of each mouse was judged according to a certain threshold using ImagePS program.
2.3.6. Image analysis
Image analysis programs (ImageLD/EP/PS) were used to analyze mouse behaviors automatically in the light/dark transition, elevated plus maze, Porsolt forced swim, and tail suspension tests. These programs, based on the public domain ImageJ program (developed by Wayne Rasband at the National Institute of Mental Health, Bethesda), were developed and modified for each test by Tsuyoshi Miyakawa. The ImageLD program can be freely downloaded from the “Mouse Phenotype Database” (http://www.mouse‐phenotype.org/).
2.4. Measurement of plasma BHB levels
Blood was taken from the facial vein or submandibular vein using Goldenrod Animal Lancet (MEDIpoint, Inc, NY, USA) 30 minutes after oral injection of vehicle or C10 (0.3, 3.0, or 30 mmol/kg) in mice, which were experimentally naive before the oral gavage (Veh, n = 5; 0.3 mmol/kg, n = 6; 3.0 mmol/kg, n = 6; 30 mmol/kg, n = 6). Additionally, trunk blood was collected from three groups of mice used in Experiment 3 (Veh, n = 16; 0.3 mmol/kg, n = 16; 3.0 mmol/kg, n = 13) approximately 24 hours after the tail suspension test followed by the last oral administration (1 day after the 28 days treatment of C10). Blood samples were placed in tubes containing sodium heparin (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) and centrifuged at 3000 g for 10 minutes at 4°C. Supernatants were collected and stored at −80°C until measurement. Plasma BHB levels were determined using the Wako Autokit 3‐HB (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) according to the manufacturer's instructions.
2.5. Statistical analysis
Statistical analyses were performed using SAS University Edition (SAS Institute, Cary, NC, USA). Behavioral data were analyzed using unpaired t‐test, one‐way ANOVA with treatment with vehicle and C10 as a between‐subjects variable, and two‐way repeated‐measures ANOVA with treatment as a between‐subjects variable and time as a within‐subject variable. In addition, the percentage of body weight gain (= 100 × (body weight at each treatment day/body weight at the first treatment day) was analyzed by two‐way repeated‐measures ANOVA with treatment as a between‐subjects variable and with time as a within‐subject variable. Statistical analysis for plasma BHB levels was performed by one‐way ANOVA with treatment as a between‐subjects variable. We defined “study‐wide significance” as statistical significance that survived the Benjamini–Hochberg false discovery rate (FDR) correction for controlling for multiple hypothesis testing based on the number of behavioral measures of the test battery.25, 26 “Nominal significance” was defined as having achieved statistical significance without the FDR correction (uncorrected P < 0.05). The ANOVA results in Experiments 1‐3 were summarized in Table S1‐S3. Post hoc multiple comparisons were further performed using Fisher's LSD with Bonferroni correction for multiple comparisons. All of the P‐values are presented as unadjusted values. Values in graphs are expressed as the mean ± SEM.
3. RESULTS
3.1. Acute effects of oral high‐dose C10 administration on behavior
In Experiment 1 (Figure 1A), in the light/dark transition test, the mice treated with C10 at the dose of 30 mmol/kg traveled significantly shorter distances in the light and dark chambers (Figure 1B), spent less time in the light chamber (Figure 1C) and showed fewer transitions between the chambers (Figure 1D), than vehicle‐treated mice (all, P < 0.05; Table S1). Similar findings were obtained in the open field and elevated plus maze tests: a shorter distance traveled (Figure 1E), smaller number of vertical counts (Figure S1B), and fewer stereotypic counts (Figure S1C) in the open field test were observed in C10‐treated mice than in vehicle‐treated mice (all, P < 0.05; Table S1), although the group difference was not found in the center time, which has been used as an index of anxiety‐like behavior (Figure 1F). In the elevated plus maze test, shorter distance traveled (Figure S1D), fewer number of arm entries (Figure 1G), and lower percentages of open arm entries and open arm time (Figure 1H,I) were also found in C10‐treated mice (all, P < 0.05; Table S1). There was no significant effect of treatment on the latency to enter the light chamber (Figure S1A). In the Porsolt forced swim test, there was a significant treatment × time interaction on immobility on test day 2 (Table S1), and post hoc analysis revealed that C10‐treated mice exhibited lower immobility during the second minute of the test period and then showed more immobility during the tenth minute compared to vehicle‐treated mice (Figure 1K; P < 0.05, for each of the respective time points). There were no significant main effects of treatment and no significant treatment × time interactions on immobility on day 1 in the forced swim test (Figure 1J), distance traveled on day 1 (Figure S1E) and day 2 (Figure S1F), and immobility in the tail suspension test (Figure 1L). These observations indicate that administration of C10 at the 30 mmol/kg dose causes deceased locomotor activity and increased anxiety‐like behavior.
3.2. Acute effects of oral low‐dose C10 administration on behavior
In Experiment 2 (Figure 2A), there was a nominally significant main effect of treatment and a nominally significant treatment × time interaction on distance traveled in the open field test (Figure 2E; Table S2). In the open field test, post hoc analysis revealed that mice administered with C10 at doses of 0.1‐1.0 mmol/kg traveled longer distances than vehicle‐treated mice during the first, fourth, or sixth blocks of 5 minutes (0.1 mmol/kg > Veh during the fourth block, P = 0.0229; 0.3 mmol/kg > Veh during the first, fourth, and sixth blocks, P = 0.0002, P = 0.0008, and P = 0.0001, respectively; 1.0 mmol/kg > Veh during the fourth and sixth blocks, P = 0.0094 and P = 0.0052, respectively). There were no significant main effects of treatment or treatment × time interactions on the other behavioral measures (Figure 2B‐D, Figure 2F‐l, and Figure S2A‐F; Table S2).
3.3. Chronic effects of repeated oral administration of low‐dose C10 on behavior
In Experiment 3 (Figure 3A), there was a nominally significant main effect of treatment on immobility in the tail suspension test (Figure 3L; Table S3). Post hoc analysis revealed that mice administered with C10 at the dose of 3.0 mmol/kg spent less time in immobility than vehicle‐treated mice (P = 0.0065). There were no significant main effects of treatment or treatment × time interactions on the other behavioral measures (Figure 3B‐K and Figure S3A–F; Table S3).
3.4. Effects of oral C10 administration on body weights
In Experiments 1‐3, the body weights of each mouse were measured immediately before oral administration of C10 solution or vehicle. A significant main effect of treatment and a significant treatment × time interaction on body weights were found in Experiment 1 (Figure S4A: treatment effect, F 1,17 = 52.63, P < 0.0001; time effect, F 5,85 = 16.07, P < 0.0001; treatment × time interaction, F 5,85 = 12.74, P < 0.0001). Body weights of mice administered with C10 at the dose of 30 mmol/kg were significantly lower than vehicle‐administered mice from one day after the first administration day to the day of the tail suspension test (Figure S4A). In Experiment 2, there was no significant main effect of treatment and no significant treatment × time interaction on body weights (Figure S4B: treatment effect, F 4,100 = 0.47, P = 0.7594; time effect, F 5,500 = 7.44, P < 0.0001; treatment × time interaction, F 20,500 = 0.59, P = 0.9208). In Experiment 3, a significant treatment × time interaction on body weights was found (Figure S4C: treatment effect, F 2,41 = 2.56, P = 0.0893; time effect, F 26,1066 = 8.64, P < 0.0001; treatment × time interaction, F 52,1066 = 2.38, P < 0.0001). There were significant simple main effects of treatment on body weights on days 12‐15 and 18‐20 and on test days of the first and second forced swim tests and tail suspension test (all, P < 0.05): body weights of mice administered with C10 at the dose of 3.0 mmol/kg were significantly lower than mice administered with vehicle and C10 at dose of 0.3 mmol/kg (on all the days, 3.0 mmol/kg < Veh, all P < 0.05; on days 18 and 19 and the last 3 days of behavioral tests, 3.0 mmol/kg < 0.3 mmol/kg, all P < 0.05).
3.5. Acute and chronic effects of oral C10 administration on plasma BHB levels
There was a significant effect of treatment on BHB levels in plasma collected 30 minutes after oral administration of C10 (Figure S5A; F 3,19 = 9.22, P = 0.0006). Plasma BHB levels were higher in mice treated with 30 mmol/kg dose of C10 than the other groups treated with vehicle, 0.3 mmol/kg C10, and 3.0 mmol/kg C10 (all, P = 0.001), and no significant differences in BHB levels were found between the three groups except for 30 mmol/kg dose group. There was no significant effect of treatment on plasma BHB levels in groups of mice repeatedly administered vehicle, 0.3 mmol/kg C10, or 3.0 mmol/kg C10 (Figure S5B; F 2,42 = 0.53, P = 0.5937).
4. DISCUSSION
The present study showed that acute administration of capric acid (C10) at the high dose of 30 mmol/kg decreased distance traveled and increased anxiety‐like behaviors in the open field, light/dark transition, and elevated plus maze tests. In contrast, acute CA10 administration at the lower doses of 0.1‐1.0 mmol/kg increased distance traveled in the open field test. These results suggest dose‐dependent effects of C10 on locomotor activity and anxiety‐like behavior in adult male C57BL/6J mice. This study also showed that repeated administration of C10 at the dose of 3.0 mmol/kg slightly decreased immobility in the tail suspension test, while it had no significant effects on locomotor activity and anxiety‐like behavior, suggesting that chronic treatment with C10 may potentially have an antidepressant effect. However, none of the chronic effects of C10 reached the study‐wide statistical significance, and further study will be needed to confirm the findings of antidepressant effect. It has been recently reported that chronic treatment with a KD and MCT diet induced decreased anxiety‐ and depression‐like behaviors in rodents.8, 9, 11, 12, 27 Our study suggests that C10 is one of the fatty acids partially contributing to the antidepressant‐like effects.
Ketogenic diet has been used to induce decreased seizure frequency in epileptic patients.2, 3 A rodent study revealed that C10 showed anticonvulsant properties by increasing seizure thresholds when it was orally administered at the doses of 10 and 30 mmol/kg but not at 1 and 3 mmol/kg doses, 13 which implied that C10 could suppress excessive synchronization of neuronal activity in the brain. A high‐dose administration of anticonvulsant drugs, such as valproic acid that is a branched short‐chain fatty acid, is reported to decrease locomotor activity28, 29 and increase immobility in the forced swim test.30, 31 Similarly, C10 administration at the high dose of 30 mmol/kg reduced the distance traveled in some behavioral tests in Experiment 1 of the present study. Thus, the C10‐inhibitory properties of brain neuronal activity might be related to the hypolocomotor activity in C10‐treated mice. Given that the median toxic dose (TD50) value of C10 for an impaired motor function was estimated to be 101.4 mmol/kg and C10 administration at 1‐50 mmol/kg dose had no significant effects on the grip strength in mice, 13 it seems unlikely that the decreased distance traveled after C10 administration at the 30 mmol/kg dose is due to reductions in motor function and muscle strength.
The open field test has been widely used for assessing anxiety‐like behavior, locomotor activity, and exploratory behavior and for screening anxiolytic drugs. 20 Some researchers have interpreted high activity as an index of low emotionality or an indicator of high exploratory behavior. 32 In this study, in contrast to the sedative effects of acute high‐dose C10, a lower‐dose (0.1‐1.0 mmol/kg) administration of C10 slightly increased distance traveled in the open field test. The increased locomotor activity might reflect attenuation of anxiety and/or disinhibition of exploratory behavior suppressed by exposure to a novel environment. It should be noted that the results did not reach study‐wide statistical significance. Additionally, such behavioral effects of C10 were not found in the light/dark transition test and elevated plus maze test. Further examinations will be required to assess anxiety‐like behavior under various test situations in C10‐treated mice for evaluating its potential anxiolytic effects.
Any significant behavioral effects of acute low‐dose C10 administration were not observed in the forced swim and tail suspension tests, widely used for screening antidepressant drugs.23, 24, 33 Although the mice administered with C10 at the high dose of 30 mmol/kg also exhibited no significant differences in immobility from vehicle‐treated mice on the first trial of the forced swim test and in the tail suspension test, they showed lower immobility during the early test period and then higher immobility during the last test period in the second trial of the forced swim test than vehicle‐treated mice. One interpretation is that the reduced immobility suggests the antidepressant effect of high‐dose C10 administration, and the increased immobility during the last test period might be due to the hypolocomotor activity, fatigue, or possibly adaptive response without the energy expenditure required in swimming, 34 in high‐dose C10‐treated mice.
Repeated C10 administration for more than 3 weeks at relatively low doses of 0.3 and 3.0 mmol/kg, without administration immediately before behavioral tests, had no significant effects on locomotor activity and anxiety‐like behavior. Considering the findings of the acute behavioral effects of C10, these results suggest that low‐dose C10 administration has no long‐lasting and no cumulative effects of repeated exposure to C10 on locomotor activity and anxiety‐like behavior. Interestingly, there were no behavioral effects of repeated C10 administration in the forced swim test, seemingly consistent with the findings of previous studies in rats treated with MCT for 2 weeks 12 and in mice treated with KD for 3 months, 35 while some studies have reported a decreased immobility after 7 days treatment with KD 27 and prenatal exposure to KD. 9 Oral gavage administration used in this study could be more stressful than ad libitum dietary intake. Such an administration method might have masked some behavioral effects of C10. However, our results showing decreased immobility in mice repeatedly administered with C10 in the tail suspension test suggest that chronic exposure to C10 may have a certain type of antidepressant effect, although effects of MCTs including C10 may not be detectable in the forced swim test.
Unfortunately, in the present study, oral C10 administration at 30 mmol/kg dose led to death in four mice. In addition, the mice administered with C10 at the highest dose showed marked decreases in body weights 1 day after administration. These results suggest that there could be some toxic effects of high‐dose C10. Such adverse effects on body weights and behaviors might be partially due to increased plasma BHB levels or a ketosis state induced by high‐dose C10 administration. In people with epilepsy, the most commonly reported adverse effects of KD/MCT diet treatment were vomiting, constipation, and diarrhea in addition to gastrointestinal disturbances and body weight loss. 36 We did not examine the occurrence of vomiting, diarrhea, and gastrointestinal disturbances in C10‐treated mice, and thus it is important to analyze the digestive issues or appetite loss in a future study to understand the potential adverse effects of C10 in mice. On the other hand, mice acutely exposed to C10 at 0.1‐3.0 mmol/kg dose appeared normal and showed no significant changes in their body weights. Repeated exposure to C10 at 3.0 mmol/kg dose induced a significant but slight decrease in body weights after 2 weeks administration, seemingly consistent with the findings of some previous studies of chronic treatment with KD or MCT diet.8, 37, 38, 39 Since all mice used in our study had free access to a standard diet without any dietary restriction, the present findings suggest that supplementary administration of C10 with a standard diet is sufficient to reduce body weights. In this study, however, we did not measure food intake in each group of mice, and thus we cannot exclude the possibility that the decreased body weights of C10‐treated groups might be due to changes of food intake.
The action mechanisms of anxiolytic‐ and antidepressant‐like effects of KD and MCTs, including C10 and other medium‐chain fatty acids, remain to be explored. The BHB is one of the ketone bodies converted from fats and fatty acids. It has been recently reported that BHB induces BDNF expression in cortical neurons, 40 inhibits hippocampal neuroinflammation, 14 and has an antidepressant‐like behavioral effect.14, 15 In this study, acute injection of low‐dose C10 produced no significant differences in plasma BHB levels between treatment groups. Our results showed that the acute behavioral effects of low‐dose C10 were not associated with plasma BHB levels. In addition, BHB levels did not differ among groups of mice repeatedly administered with low‐dose C10 or vehicle for 4 weeks, when BHB were measured in plasma samples collected 24 hours after the last administration. These results also suggest that the behavioral effects of repeated C10 administrations may not be explained by plasma BHB levels during behavioral tests. However, since our study did not measure BHB at different time points after repeated C10 administrations, it does not exclude the possibility that some changes in BHB levels after injections might have contributed to the behavioral effects of C10. C10 is easily transported to the brain across the blood–brain barrier.13, 41 C10 stimulates glycolysis to produce lactate via mitochondria, which is considered an energy source for brain cells. 42 Lactate has been reported to promote panic‐like responses43, 44 or exert anxiolytic and antidepressant effects.45, 46 We recently showed increased lactate levels in the brain in various mouse models of neuropsychiatric disorders, including anxiety and depression models,47, 48 although it remains unknown whether the increased brain lactate is a pathogenic phenomenon or compensatory response. We hypothesize that some of the mouse models show a shared phenotype of brain hyperexcitability and altered energy metabolism,48, 49, 50, 51 as proposed to be essential features in some brain regions in neuropsychiatric disorders.52, 53, 54 KD and C10 are reported to contribute to the inhibition of excitatory neurotransmission via glutamatergic and GABAergic systems,4, 6, 55 which may be one of the potential mechanisms underlying the anxiolytic‐ and antidepressant‐like effects. One of the limitations of this study is that C10 and ketone bodies in the brain were not measured. Therefore, it is unknown whether oral C10 administration increased brain C10 and ketone bodies and whether such increases in the brain, if any, could directly or indirectly exert antidepressant effects. Another limitation was the use of normal mice. This study did not investigate the potential therapeutic effects of C10 using animal models for studying anxiety and depression. Thus, it is necessary to further study the action mechanisms of C10 and to verify whether C10 can attenuate increased anxiety‐ and depression‐related behaviors in animal models of anxiety and depression.
In conclusion, the present study demonstrated that acute oral administration of a high‐dose C10 markedly decreased locomotor activity and increased anxiety‐like behaviors in adult male C57BL/6J mice, possibly due to its adverse effects on physiological functions. Additionally, acute low‐dose administration of C10 induced a slight increase in locomotor activity. This study also showed that a low‐dose, long‐term oral administration of C10 caused a decrease in depression‐related behavior assessed in the tail suspension test. However, any of the results of acute and chronic effects of low‐dose C10 administration failed to reach study‐wide statistical significance. Taken together, these findings suggest that further study will be needed to replicate the results, and capric acid, which may have some antidepressant properties, holds therapeutic potential for the treatment of neuropsychiatric disorders.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
HS designed and performed the experiments, analyzed the data, and wrote manuscript. HK and TM coordinated the study, interpreted the data, and wrote manuscript. All authors read and approved the final manuscript.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
N/A
INFORMED CONSENT
N/A
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/A
ANIMAL STUDIES
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Fujita Health University.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S3
Data S1‐S3
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Tamaki Murakami for her assistance in animal husbandry and behavioral experiment. Behavioral analysis was carried out at the Joint Usage/Research Center for Genes, Brain and Behavior in the Institute for Comprehensive Medical Science at Fujita Health University, which is accredited by the Ministry of Education, Science, Sports and Culture of Japan. This study was supported by AMED under Grant Number JP20dm0107101.
Shoji H, Kunugi H, Miyakawa T. Acute and chronic effects of oral administration of a medium‐chain fatty acid, capric acid, on locomotor activity and anxiety‐like and depression‐related behaviors in adult male C57BL/6J mice. Neuropsychopharmacol Rep. 2022;42:59–69. 10.1002/npr2.12226
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921;2:307–8. [Google Scholar]
- 2. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3–5. [DOI] [PubMed] [Google Scholar]
- 3. Sampaio LPDB. Ketogenic diet for epilepsy treatment. Arq Neuropsiquiatr. 2016;74(10):842–8. [DOI] [PubMed] [Google Scholar]
- 4. Rho JM. How does the ketogenic diet induce anti‐seizure effects? Neurosci Lett. 2017;637:4–10. [DOI] [PubMed] [Google Scholar]
- 5. Huttenlocher PR, Wilbourn AJ, Signore JM. Medium‐chain triglycerides as a therapy for intractable childhood epilepsy. Neurology. 1971;21(11):1097–103. [DOI] [PubMed] [Google Scholar]
- 6. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. Mechanisms of action for the medium‐chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17(1):84–93. [DOI] [PubMed] [Google Scholar]
- 7. Rho JM, Stafstrom CE. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, et al. A ketone ester diet exhibits anxiolytic and cognition‐sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34(6):1530–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sussman D, Germann J, Henkelman M. Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain Behav. 2015;5(2):e00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Contreras CM, Rodríguez‐Landa JF, García‐Ríos RI, Cueto‐Escobedo J, Guillen‐Ruiz G, Bernal‐Morales B. Myristic acid produces anxiolytic‐like effects in Wistar rats in the elevated plus maze. Biomed Res Int. 2014;2014:492141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva DDC, Tavares MG, do Nascimento CKB, Lira EC, dos Santos ÂA, Maia LMSDS, et al. Can coconut oil and treadmill exercise during the critical period of brain development ameliorate stress‐related effects on anxiety‐like behavior and episodic‐like memory in young rats? Food Funct. 2018;9(3):1492–99. [DOI] [PubMed] [Google Scholar]
- 12. Hollis F, Mitchell ES, Canto C, Wang D, Sandi C. Medium chain triglyceride diet reduces anxiety‐like behaviors and enhances social competitiveness in rats. Neuropharmacology. 2018;138:245–56. [DOI] [PubMed] [Google Scholar]
- 13. Wlaź P, Socała K, Nieoczym D, Żarnowski T, Żarnowska I, Czuczwar SJ, et al. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog Neuro‐Psychopharmacol Biol Psychiatry. 2015;57:110–16. [DOI] [PubMed] [Google Scholar]
- 14. Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N, Wada N, et al. Beta‐hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress‐induced behavioral and inflammatory responses. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan S, Hu P, You Q, Chen J, Wu J, Zhang Y, et al. Evaluation of the antidepressive property of β‐hydroxybutyrate in mice. Behav Pharmacol. 2020;31(4):322–32. [DOI] [PubMed] [Google Scholar]
- 16. Shoji H, Miyakawa T. Increased depression‐related behavior during the postpartum period in inbred BALB/c and C57BL/6 strains. Mol Brain. 2019;12(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shoji H, Miyakawa T. Differential effects of stress exposure via two types of restraint apparatuses on behavior and plasma corticosterone level in inbred male BALB/cAJcl mice. Neuropsychopharmacol Rep. 2020;40(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology. 2001;155(1):110–12. [DOI] [PubMed] [Google Scholar]
- 19. Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–70. [DOI] [PubMed] [Google Scholar]
- 20. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. [DOI] [PubMed] [Google Scholar]
- 21. Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety‐like behavior: the elevated plus‐maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–205. [DOI] [PubMed] [Google Scholar]
- 22. Shoji H, Miyakawa T. Effects of test experience, closed‐arm wall color, and illumination level on behavior and plasma corticosterone response in an elevated plus maze in male C57BL/6J mice: a challenge against conventional interpretation of the test. Mol Brain. 2021;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porsolt RD, Bertin A, Jalfre MJAIP. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–36. [PubMed] [Google Scholar]
- 24. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–70. [DOI] [PubMed] [Google Scholar]
- 25. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. [DOI] [PubMed] [Google Scholar]
- 26. Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464. [DOI] [PubMed] [Google Scholar]
- 27. Murphy P, Likhodii S, Nylen K, Burnham WM. The antidepressant properties of the ketogenic diet. Biol Psychiatry. 2004;56(12):981–3. [DOI] [PubMed] [Google Scholar]
- 28. Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, et al. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158(1):123–32. [DOI] [PubMed] [Google Scholar]
- 29. Tomasiewicz HC, Mague SD, Cohen BM, Carlezon WA Jr. Behavioral effects of short‐term administration of lithium and valproic acid in rats. Brain Res. 2006;1093(1):83–94. [DOI] [PubMed] [Google Scholar]
- 30. Bourin M, Masse F, Hascoët M. Evidence for the activity of lamotrigine at 5‐HT1A receptors in the mouse forced swimming test. J Psychiatry Neurosci. 2005;30(4):275–82. [PMC free article] [PubMed] [Google Scholar]
- 31. de Assis Lima IV, Almeida‐Santos AF, Ferreira‐Vieira TH, Aguiar DC, Ribeiro FM, Campos AC, et al. Antidepressant‐like effect of valproic acid—Possible involvement of PI3K/Akt/mTOR pathway. Behav Brain Res. 2017;329:166–71. [DOI] [PubMed] [Google Scholar]
- 32. Denenberg VH. Open‐field behavior in the rat: what does it mean? Ann NY Acad Sci. 1969;159(3):852–9. [DOI] [PubMed] [Google Scholar]
- 33. Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011;55(1):8–10. [DOI] [PubMed] [Google Scholar]
- 34. De Kloet ER, Molendijk ML. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J, Li YQ, Wu CH, Zhang YL, Zhao ST, Chen YJ, et al. The effect of ketogenic diet on behaviors and synaptic functions of naive mice. Brain Behav. 2019;9(4):e01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin‐McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug‐resistant epilepsy. Cochrane Database Syst Rev. 2020;6:CD001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, et al. A high‐fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292(6):E1724–39. [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Mitchell ES. Cognition and synaptic‐plasticity related changes in aged rats supplemented with 8‐and 10‐carbon medium chain triglycerides. PLoS One. 2016;11(8):e0160159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mayr KA, Kwok CH, Eaton SE, Baker GB, Whelan PJ. The effects of a ketogenic diet on sensorimotor function in a thoracolumbar mouse spinal cord injury model. eNeuro. 2020;7(4):ENEURO.0178‐20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marosi K, Kim SW, Moehl K, Scheibye‐Knudsen M, Cheng A, Cutler R, et al. 3‐Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oldendorf WH. Carrier‐mediated blood‐brain barrier transport of short‐chain monocarboxylic organic acids. Am J Physiol. 1973;224(6):1450–53. [DOI] [PubMed] [Google Scholar]
- 42. Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, et al. Medium‐chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte‐neuron lactate and ketone body shuttle systems. FASEB J. 2016;30(5):1913–26. [DOI] [PubMed] [Google Scholar]
- 43. Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin‐releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100(1–2):207–15. [DOI] [PubMed] [Google Scholar]
- 44. Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann NY Acad Sci. 2003;985(1):308–25. [DOI] [PubMed] [Google Scholar]
- 45. Carrard A, Elsayed M, Margineanu M, Boury‐Jamot B, Fragnière L, Meylan EM, et al. Peripheral administration of lactate produces antidepressant‐like effects. Mol Psychiatry. 2018;23(2):392–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karnib N, El‐Ghandour R, El Hayek L, Nasrallah P, Khalifeh M, Barmo N, et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology. 2019;44(6):1152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hagihara H, Catts VS, Katayama Y, Shoji H, Takagi T, Huang FL, et al. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology. 2018;43(3):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hagihara H, Shoji H. International Brain pH Project Consortium, Miyakawa T. systematic analysis of brain lactate and pH levels in 65 animal models related to neuropsychiatric conditions. bioRxiv. 2021. [Google Scholar]
- 49. Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T. Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plast. 2013;2013:318596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hagihara H, Horikawa T, Irino Y, Nakamura HK, Umemori J, Shoji H, et al. Peripheral blood metabolome predicts mood change‐related activity in mouse model of bipolar disorder. Mol Brain. 2019;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murano T, Hagihara H, Tajinda K, Matsumoto M, Miyakawa T. Transcriptomic immaturity inducible by neural hyperexcitation is shared by multiple neuropsychiatric disorders. Commun Biol. 2019;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Binder MR. The multi‐circuit neuronal hyperexcitability hypothesis of psychiatric disorders. Am J Clin Exp Med. 2019;7(1):12–30. [Google Scholar]
- 53. Pruett BS, Meador‐Woodruff JH. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta‐analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr Res. 2020;223:29–42. [DOI] [PubMed] [Google Scholar]
- 54. Toniolo S, Sen A, Husain M. Modulation of brain hyperexcitability: potential new therapeutic approaches in Alzheimer’s disease. Int J Mol Sci. 2020;21(23):9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139(2):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S3
Data S1‐S3
Supplementary Material
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


