Abstract
Background
The decision to initiate clozapine treatment should be made on an individual basis and may be closely related to the early detection of treatment‐resistant schizophrenia (TRS), although there is evidence that the early use of clozapine results in a better response to treatment. Therefore, we investigated the relationship between the examination rate of TRS and the prescription rate of clozapine.
Methods
After attending a 1‐day educational program on schizophrenia based on the "Guidelines for the Pharmacological Treatment of Schizophrenia," we asked the participating facilities to submit records of whether or not TRS was evaluated for each patient. We calculated the clozapine prescription rate from the schizophrenic patients prescribed clozapine and all of the schizophrenic patients. Forty‐nine facilities in 2017 were included in the study.
Results
There were dichotomous distributions in the examination rate of TRS and a non‐normal distribution in the prescription rate of clozapine. There was a significant correlation between the prescription rate of clozapine and the examination rate of TRS (r s = 0.531, P = 1.032 × 10−4). A significant difference was found in the prescription rate of clozapine between the three groups of facilities according to the examination rate of TRS.
Conclusion
As a preliminary problem for the use of clozapine, in Japan, the examination rate of TRS varies, and there are many facilities that typically do not consider the possibility of TRS; this trend leads to a low rate of clozapine use. Clearly, further clinician training is needed for the early detection and appropriate management of TRS that includes an explanation of TRS and how to introduce clozapine therapy to patients and their families.
Keywords: clozapine, examination rate, prescription rate, treatment‐resistant schizophrenia
A significant difference was found (P = .041) in the prescription rate of clozapine between the three groups according to the examination rate of TRS. Post hoc analysis revealed that the prescription rate of clozapine in facilities with a high examination rate (P = .014) was significantly higher than that in facilities with a low examination rate of TRS.
1. INTRODUCTION
The prevalence of schizophrenia is <1%, and it occurs mainly in adolescence and young adulthood. 1 Symptoms include positive symptoms such as hallucinations, delusions, and excitement, and negative symptoms such as emotional flatness, loss of motivation, and autism. 2 Antipsychotics are effective in improving positive symptoms and preventing relapse and are therefore used worldwide as first‐line drugs for schizophrenia.3, 4 However, treatment‐resistant schizophrenia (TRS), that is, schizophrenia that does not respond to antipsychotics, occurs in approximately 30% of patients. 5 TRS can be divided into two categories: poorly responsive schizophrenia and poorly tolerated schizophrenia. Poorly responsive schizophrenia is defined as schizophrenia that has not responded to two or more adequate doses of antipsychotic medications (600 mg of chlorpromazine or an equivalent/day or more, including one or more atypical antipsychotics) for a sufficient period of time (4 weeks or more) with appropriate medication compliance, unless tolerated. 5
Clozapine is currently the only indication for TRS. Siskind et al 6 reported a meta‐analysis (N = 25, n = 2364) comparing the efficacy and tolerability of clozapine and other antipsychotics in treatment‐resistant schizophrenic patients. On the other hand, when deciding on the use of clozapine, it is necessary to consider the benefits of reducing psychosis and the potential for serious side effects, such as suicide burden, weight gain, diabetes, and other harmful side effects. 6 Bachmann et al 7 reported that as of 2014, the highest incidence of clozapine prescriptions was in Finland (189 per 100 000), followed by New Zealand (116 per 100 000). However, Japan was the country with the lowest number of prescriptions (0.6 per 100 000). The number of clozapine prescriptions in Japan was 2975 in 2014,7 and even when adjusted for the 10 110 prescriptions in 2020, the prescription rate was 2.0 per 100 000, which is extremely low compared with other countries.
In Japan, the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) Psychiatric Treatment Project was launched in 2016. The purpose of the EGUIDE project was to disseminate the guidelines for the treatment of schizophrenia and major depressive disorder (MDD) through educational programs for psychiatrists. The EGUIDE project developed a 2‐day educational course (1 day for each disorder) for psychiatrists to learn about the Japanese treatment guidelines for schizophrenia and MDD.8, 9, 10, 11, 12, 13, 14, 15, 16 In addition, this study examined the effectiveness of the guideline education program by assessing participants' prescribing behaviors (prescribing at discharge for patients with moderate to severe schizophrenia or MDD). The EGUIDE study found antipsychotic polypharmacy for schizophrenia and antidepressant polypharmacy for MDD. In the EGUIDE study, there was interinstitutional variations in antipsychotic polypharmacy for schizophrenia 9 and antidepressant polypharmacy for MDD 10 and interdrug variation. 11
The decision to initiate clozapine should be made on an individual basis and may be closely related to the early detection of TRS, as there is evidence that the early use of clozapine results in a better response to treatment. Therefore, we investigated the relationship between the examination rate of TRS and the prescription rate of clozapine.
2. METHODS
Psychiatrists were recruited beginning in October 2016. Written informed consent was obtained from all participants after the procedures had been fully explained by a chief researcher at the facility.8, 9, 10, 11, 12, 13, 14, 15, 16 This study was approved by the ethics committees of the National Center of Neurology and Psychiatry (A2017‐105) and each participating university/hospital/clinic. The study procedures were conducted according to the Declaration of Helsinki. The protocol of this study was registered in the University Hospital Medical Information Network registry (UMIN000022645).
The participating psychiatrists attended a 1‐day educational program on schizophrenia based on the "Guidelines for the Pharmacological Treatment of Schizophrenia" of the Japanese Society of Neuropsychopharmacology. In the educational program, psychiatrists learned how to diagnose and treat schizophrenia based on the guidelines and then learned how to understand and implement the guidelines by discussing how to address two cases of schizophrenia. From the discharge summaries of the inpatients who the participating psychiatrists were treating, the relationship between the presence of a treatment‐resistant diagnosis of schizophrenia and the content of treatment was investigated. Beginning in 2016, the prescriptions at discharge at each participating institution from April to September for both years were gathered from participants attending the course using a standardized data collection method that involved participants checking their medical records and manually entering them into an Excel spreadsheet, followed by double‐checking by the data manager. This study applied the definition of TRS by Clozapine Appropriate Use Committee in Japan, which is included in the insert package (Table S1). 16
The program was run from October 2016 to the end of February 2017. 8 The total number of participants who took the course was 272. After excluding 49 participants who later expressed their intention to withdraw their consent, 223 participants (155 males and 68 females) were included in the analysis of the EGUIDE program. The mean age (± standard deviation) of the participants was 34.0 ± 7.0 years, and they had been physicians for 6.9 ± 5.8 years or psychiatrists for 5.0 ± 5.6 years. Fifty‐seven of the 327 EGUIDE‐participating centers were clozapine‐enabled, and 8 of the clozapine‐enabled centers had a registered caseload of <5 cases. The remaining 49 sites were eligible for inclusion in the study. Of a total of 1852 inpatients at these 49 sites, 115 (6.2%) were treated with clozapine. Of the 49 centers, 218 physicians had received training. Cases were collected of patients that were discharged between April and September 2017, with a length of stay of at least 4 days. Admissions for laboratory tests only or for treatment of physical complications, as well as discharges for death, were excluded.
We calculated the clozapine prescription rate from the number of schizophrenic patients receiving clozapine and the number of all schizophrenic inpatients. In addition, we asked the facilities to submit a record of whether the psychiatrist in charge of the patients examined/diagnosed the patient as having TRS. We defined the examination rate of TRS based on the record that was submitted. We checked the types and dosages of all psychotropic drugs in 2017, including clozapine, and examined each patient for TRS.
The correlation between the prescription rate of clozapine and the examination rate of TRS was performed using the Spearman rank test. We compared the percentage of subjects who were prescribed clozapine among three groups of facilities based on the examination rate of TRS (0%, 1%‐99%, and 100%). The chi‐square test, Shapiro‐Wilk normality test, and Kruskal‐Wallis test were used, and a P value of <0.05 (two tailed) was considered statistically significant. The Mann‐Whitney U test, followed by Bonferroni's correction, was applied for multiple comparisons in which P < 0.033 (0.05/3) was judged to be significant.
3. RESULTS
Forty‐nine out of the 327 facilities with both clozapine prescription rate and TRS examination rate data were included in the study. Eight facilities with fewer than five patients with schizophrenia were removed from the data. A total of 270 facilities were not allowed to use clozapine. The 49 facilities were divided into groups of university hospitals, public general hospitals, public psychiatric hospitals, and private psychiatric hospitals, and their characteristics are shown in Table 1. Significant differences were found in number of hospital beds (P = 2.7 × 10−4), average bed occupancy rate (P =0.010), average number of days spent in the hospital (P = 2.7 × 10−3), and Electroconvulsive Therapy (ECT) available (P = 1.9 × 10−4).
TABLE 1.
The characteristics of the 49 facilities included in the survey
| University hospitals | Public general hospitals | Public psychiatric hospitals | Private psychiatric hospitals | |
|---|---|---|---|---|
| Number of hospitals | n = 31 | n = 6 | n = 5 | n = 7 |
| Number of hospital beds | 56 (48) | 78 (33) | 147 (91) | 349 (238) |
| Average bed occupancy rate (%) | 76 (12) | 72 (10) | 82 (11) | 91 (2) |
| Average number of days spent in the hospital (days) | 57 (24) | 110 (81) | 75 (20) | 256 (117) |
| ECT available (%) | 97 | 100 | 100 | 43 |
| TRS examination rate (%) | 46 (45) | 22 (39) | 4 (9) | 29 (48) |
| Clozapine prescription rate (%) | 6.6 (5.7) | 3.7 (6.3) | 4.4 (4.2) | 6.3 (16.8) |
Data are average (standard deviation). Significant differences were found in number of hospital beds, average bed occupancy rate, average number of days spent in the hospital, and ECT available.
Abbreviation: TRS, treatment‐resistant schizophrenia.
There were dichotomous distributions in the examination rate of TRS and a non‐normal distribution in the prescription rate of clozapine (Figures 1 and 2). The median (range) and coefficient of variation were 6.9% (0%‐100%) and 121% for the examination rate of TRS and 4.5% (0%‐43%) and 76% for the prescription rate of clozapine, respectively. There was a significant correlation between the prescription rate of clozapine and the examination rate of TRS (r s = 0.531, P = 1.032 × 10−4). A significant difference was found (P = 0.041) in the prescription rate of clozapine between the three groups according to the examination rate of TRS (Figure 3). Post hoc analysis revealed that the prescription rate of clozapine in facilities with a high examination rate (P = 0.014) was significantly higher than that in facilities with a low examination rate of TRS (Figure 3).
FIGURE 1.
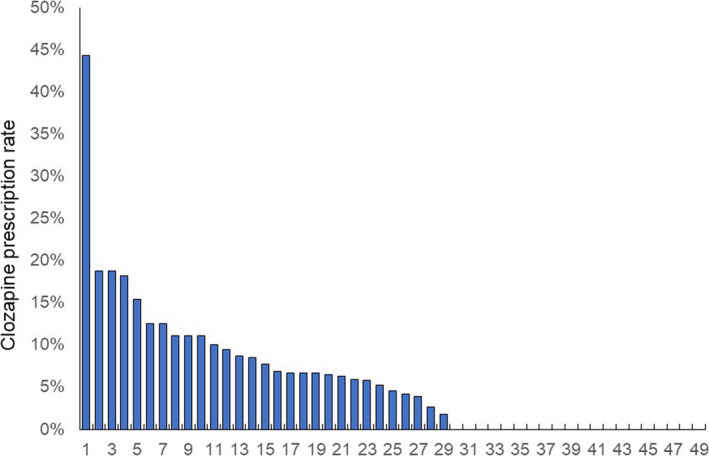
Proportion of patients with schizophrenia who had examinations for treatment‐resistant schizophrenia in 49 facilities. This figure shows that the distribution is dichotomous
FIGURE 2.
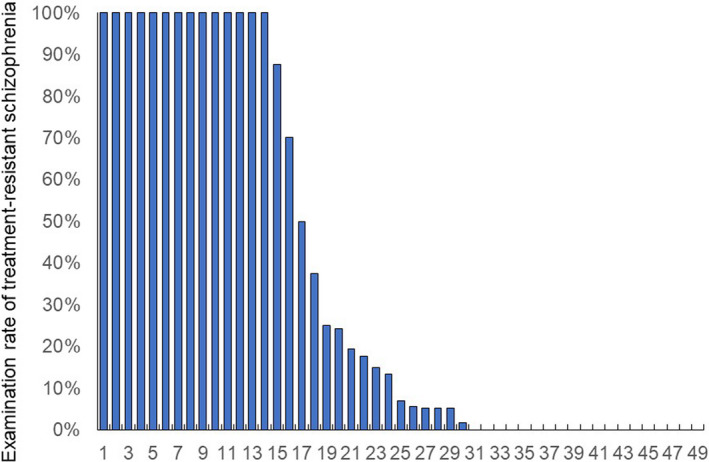
Proportion of patients with schizophrenia prescribed clozapine in 49 facilities. This figure shows that the distribution is non‐normal
FIGURE 3.
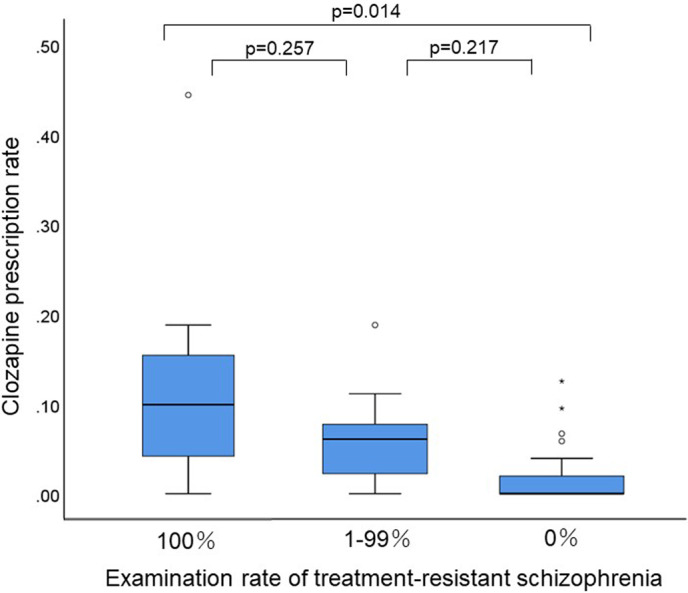
Clozapine prescription rates among the 3 facility groups, classified based on treatment‐resistant schizophrenia examination rates. This box‐and‐whisker plot shows the distribution of clozapine prescription rates. The centerline of the box shows the median, and the top and bottom sides of the box show the quartile ranges. Error bars indicate the maximum and minimum values. Outliers are plotted as points because the beard length only extends to 1.5 times the quartile range
4. DISCUSSION
The main result of our study was finding dichotomous distributions in the TRS examination rate and non‐normal distributions in the prescription rate of clozapine, both of which were strongly correlated. Not reporting the results of TRS examination means that clinicians are not considering whether patients have TRS. As a preliminary problem for the use of clozapine, in Japan, the examination rate of TRS varies, and there are many facilities that have a trend of not detecting it, which naturally leads to a low rate of clozapine use although there is clinical guidance on the detection and management of TRS. Clearly, further clinician training is needed for the early detection and appropriate management of TRS that includes an explanation of TRS and how to introduce clozapine therapy to patients and their families.
Most of the participating facilities were university hospitals, and the participants were young psychiatrists (Table 1). This may be because most university hospitals in Japan use a group practice system, and the opinions of physicians that are educated in the EGUIDE program are not often reflected in treatment decisions. In the future, it may be necessary to require group leaders to attend this education program. It may be necessary to require group leaders to take the course as well, or by the time the participating physicians become group leaders, the rate of clozapine prescriptions will have equalized.
Despite the strong evidence supporting the use of clozapine for TRS, its use in actual clinical practice is limited. 7 Worldwide, the initiation of clozapine is quite delayed because high‐dose antipsychotic treatment and multidrug combinations are often used prior to the initiation of clozapine. 17 In a UK naturalistic study, 56% (99/176) of patients were detected to have TRS in the community mental health team, and 52% (51/99) of these patients had never been treated with clozapine. 18 Thus, although TRS is common, a large proportion of patients with TRS have not received clozapine. 7 In addition, there may be some factors, such as access to and continuity of comprehensive care, that affect the use of clozapine. There are two factors that hinder the use of clozapine in Japan. Japan has also introduced the Clozaril Patient Monitoring Service, but there are some differences from other countries. 18 The first factor is that Japan is the only country that requires hospitalization at the time of clozapine introduction, while other countries do not have such a requirement. In addition, the second factor is that the criteria for the induction of clozapine are more stringent in Japan, with a required white blood cell count of more than 4000/mm3 compared with more than 3500/mm3 in other countries. Because of these factors, the criteria for the introduction of clozapine in Japan face two hurdles, namely, hospitalization and white blood cell count, 19 although these may have ensured the safety of clozapine use in Japan. Japan has a barrier to not only clozapine‐licensed facilities but also clozapine‐licensed doctors (Table S2). 20
There are several limitations to this study. First, the severity of symptoms of schizophrenia was not assessed using rating scales, such as the Brief Psychiatric Rating Scale, Positive and Negative Syndrome Scale, or electroencephalography, 21 to investigate the possibility that clozapine is not being prescribed despite the severity of the disease. Second, although the data collection method was standardized, the data were basically collected from medical records obtained by the collaborators in their daily clinical practice, which may affect the results. Third, although the data were collected from all over Japan, the participating facilities were those that voluntarily cooperated with the study, and there is a possibility of selection bias. Finally, there was also a bias for the type of hospital, with nearly half of the hospitals being university hospitals, so it may be difficult to generalize the results, even if significant differences were found by hospital type. Further studies are needed to overcome the limitations mentioned above.
5. CONCLUSION
In Japan, it was found that the examination rate of TRS varied among medical facilities and that the use of clozapine was low in facilities with low examination rates. For the appropriate management of TRS, it is important to diagnose TRS and explain clozapine treatment to patients and their families, and clinicians need to be trained on how to do so.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
NYF, HM, and NH were involved in data collection and data analysis and wrote the first draft of the manuscript. SO and SN were involved in the data analysis and contributed to the interpretation of the data and writing the manuscript. HH, AH, TO, KO, NH, TN, YT, TI, HT, TT, CK, RF, J‐II, HI, KM, JM, HY, and KS contributed to the interpretation of the data and data collection. KW and KI were involved in the study design and contributed to the interpretation of the data. RH supervised the entire project, collected the data, and was involved in the design, analysis, and interpretation of the data. All authors contributed to and approved the final article.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEW BOARD
This study was approved by the ethics committees of the National Center of Neurology and Psychiatry (A2017‐105) and each participating university, hospital, and clinic.
INFORMED CONSENT
All participants provided their written informed consent. The public availability of raw data was not planned in the research protocol that was approved by an Institutional Review Board. We did not obtain informed consent for public availability.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
Trial registration: The Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment, UMIN000022645, Registered 15 June 2016, https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000026044&type=summary&language=J.
ANIMAL STUDIES
Not available.
Supporting information
TABLES S1‐S2
ACKNOWLEDGEMENTS
This study was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP18dk0307060, JP19dk0307083, and JP20dk0307081, Health and Labor Sciences Research Grants (H29‐Seishin‐Ippan‐001, 19GC1201), the Japanese Society of Neuropsychopharmacology, and the Japanese Society of Mood Disorders. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Yasui‐Furukori N, Muraoka H, Hasegawa N, Ochi S, Numata S, Hori H, et al. Association between the examination rate of treatment‐resistant schizophrenia and the clozapine prescription rate in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. 2022;42:3–9. 10.1002/npr2.12218
Contributor Information
Norio Yasui‐Furukori, Email: furukori@dokkyomed.ac.jp.
Ryota Hashimoto, Email: ryotahashimoto55@ncnp.go.jp.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy and ethical restrictions (ie, we did not obtain informed consent for the public availability of raw data).
REFERENCES
- 1. Wyatt RJ, Alexander RC, Egan MF, Kirch DG. Schizophrenia, just the facts. What do we know, how well do we know it? Schizophr Res. 1988;1:3–18. [DOI] [PubMed] [Google Scholar]
- 2. Barch DM, Bustillo J, Gaebel W, Gur R, Heckers S, Malaspina D, et al. Logic and justification for dimensional assessment of symptoms and related clinical phenomena in psychosis: relevance to DSM‐5. Schizophr Res. 2013;150:15–20. [DOI] [PubMed] [Google Scholar]
- 3. Gaebel W, Weinmann S, Sartorius N, Rutz W, McIntyre JS. Schizophrenia practice guidelines: international survey and comparison. Br J Psychiatry. 2005;187:248–55. [DOI] [PubMed] [Google Scholar]
- 4. Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment‐resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first‐ and second‐generation antipsychotics in treatment‐refractory schizophrenia: systematic review and meta‐analysis. Br J Psychiatry. 2016;209:385–92. [DOI] [PubMed] [Google Scholar]
- 7. Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51. [DOI] [PubMed] [Google Scholar]
- 8. Takaesu Y, Watanabe K, Numata S, Iwata M, Kudo N, Oishi S, et al. Improvement of psychiatrists' clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the 'Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)' project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci. 2019;73:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ichihashi K, Hori H, Hasegawa N, Yasuda Y, Yamamoto T, Tsuboi T, et al. Prescription patterns in patients with schizophrenia in Japan: first‐quality indicator data from the survey of "Effectiveness of Guidelines for Dissemination and Education in psychiatric treatment (EGUIDE)" project. Neuropsychopharmacol Rep. 2020;40:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iida H, Iga J, Hasegawa N, Yasuda Y, Yamamoto T, Miura K, et al. Unmet needs of patients with major depressive disorder ‐ findings from the 'Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)' project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci. 2020;74:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashimoto N, Yasui‐Furukori N, Hasegawa N, Ishikawa S, Numata S, Hori H, et al. Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real‐world evidence from the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) psychiatric treatment project. Asian J Psychiatr. 2021;63:102744. [DOI] [PubMed] [Google Scholar]
- 12. Numata S, Nakataki M, Hasegawa N, Takaesu Y, Takeshima M, Onitsuka T, et al. Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. 2021;41:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogasawara K, Numata S, Hasegawa N, Nakataki M, Makinodan M, Ohi K, et al. The subjective assessment of participants in education programs on clinical practice guidelines in the field of psychiatry. 2021. (in submission). [DOI] [PMC free article] [PubMed]
- 14. Iida H, Okada T, Nemoto K, Hasegawa N, Numata S, Ogasawara K, et al. Satisfaction with web‐based courses on clinical practice guidelines for psychiatrists: findings from the 'Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)' Project. 2021. (in submission). [DOI] [PMC free article] [PubMed]
- 15. Yamada H, Motoyama M, Hasegawa N, Miura K, Matsumoto J, Ohi K, et al. Improvement of psychiatrists’ clinical behaviors in accordance with the treatment guidelines for schizophrenia and major depressive disorders using the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)’ project: nationwide dissemination, education, and evaluation. 2021. (in submission).
- 16. Clozaril package insert. [Accessed 2021 Sept 23]. Available from https://pins.japic.or.jp/pdf/newPINS/00056940.pdf
- 17. Yada Y, Yoshimura B, Kishi Y. Correlation between delay in initiating clozapine and symptomatic improvement. Schizophr Res. 2015;168:585–6. [DOI] [PubMed] [Google Scholar]
- 18. Beck K, McCutcheon R, Stephenson L, Schilderman M, Patel N, Ramsay R, et al. Prevalence of treatment‐resistant psychoses in the community: a naturalistic study. J Psychopharmacol. 2019;33:1248–53. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang Y‐T, Schulte PFJ, et al. Worldwide differences in regulations of clozapine use. CNS Drugs. 2016;30:149–61. [DOI] [PubMed] [Google Scholar]
- 20. The Expert Committee for Clozaril Patient Monitoring Service. [Accessed 2021 Sept 23]. Available from http://www.clozaril‐tekisei.jp/index.html
- 21. Edgar JC. Identifying electrophysiological markers of autism spectrum disorder and schizophrenia against a backdrop of normal brain development. Psychiatry Clin Neurosci. 2020;74:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLES S1‐S2
Data Availability Statement
The data are not publicly available due to privacy and ethical restrictions (ie, we did not obtain informed consent for the public availability of raw data).


