Abstract
Background
Coronavirus disease 2019 (COVID‐19) is known to cause not only respiratory but also neuropsychiatric symptoms, which are assumed to be derived from a cytokine storm and its effects on the central nervous systems. Patients with COVID‐19 who develop severe respiratory symptoms are known to show severe neuropsychiatric symptoms such as cerebrovascular disease and encephalopathy. However, the detailed clinical courses of patients with neuropsychiatric symptoms caused by mild or asymptomatic COVID‐19 remain poorly understood. Here, we present a case of COVID‐19 who presented with severe and prolonged neuropsychiatric symptoms subsequent to mild respiratory symptoms.
Case presentation
A 55‐year‐old female with COVID‐19 accompanied by mild respiratory symptoms showed delusion, psychomotor excitement, and poor communication ability during quarantine outside the hospital. Considering her diminished respiratory symptoms, her neuropsychiatric symptoms were initially regarded as psychogenic reactions. However, as she showed progressive disturbance of consciousness accompanied by an abnormal electroencephalogram, she was diagnosed with post‐COVID‐19 encephalopathy. Although her impaired consciousness and elevated cytokine level improved after steroid pulse therapy, several neuropsychiatric symptoms, including a loss of concentration, unsteadiness while walking, and fatigue, remained.
Conclusions
This case suggests the importance of both recognizing that even apparently mild COVID‐19‐related respiratory symptoms can lead to severe and persistent neuropsychiatric symptoms, and elucidating the mechanisms, treatment, and long‐term course of COVID‐19‐related neuropsychiatric symptoms in the future.
Keywords: case report, COVID‐19, cytokines, encephalopathy, long COVID
A 55‐year‐old female with COVID‐19 accompanied by mild respiratory symptoms was diagnosed with post‐COVID‐19 encephalopathy. Although her impaired consciousness and elevated cytokine level improved after steroid pulse therapy, several neuropsychiatric symptoms remained. This case suggests the importance of both recognizing that even apparently mild COVID‐19‐related respiratory symptoms can lead to severe and persistent neuropsychiatric symptoms.

1. INTRODUCTION
In December 2019, a cluster of patients with pneumonia of unknown cause led to the identification of a novel virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), leading to the development of coronavirus disease 2019 (COVID‐19). Although respiratory complications are at the forefront of the clinical presentation of this disease, COVID‐19 is also known to cause neuropsychiatric symptoms.1, 2 One putative pathogenesis of neuropsychiatric symptoms is derived from the excessive release of pro‐inflammatory factors, the so‐called cytokine storm, 3 following changes in the permeability of the blood‐brain barrier (BBB), which initiates a neuroinflammatory process. 4 Patients with severe COVID‐19 are reported to show severe neuropsychiatric symptoms that could lead to higher mortality, such as encephalopathy. 5 However, the detailed clinical courses of patients who develop neuropsychiatric symptoms after mild or asymptomatic COVID‐19 remain poorly understood. Therefore, neuropsychiatric symptoms occurring after mild or asymptomatic COVID‐19 with respiratory symptoms could be missed. To improve the knowledge of neuropsychiatric symptoms from mild or asymptomatic COVID‐19, we present a case of COVID‐19 with mild respiratory symptoms following severe encephalopathy with prolonged neuropsychiatric sequelae.
2. CASE PRESENTATION
The patient was a 55‐year‐old Japanese female who worked part‐time and had no medical history other than that described below. She had been briefly admitted to a psychiatric hospital at age 16 years because of depressive symptoms, but she was never followed up or given treatment after discharge. After graduating from high school, she started to work part‐time.
After her husband, with whom she was cohabitating, was diagnosed with COVID‐19, she underwent a polymerase chain reaction (PCR) test for SARS‐CoV‐2. On day 4, after experiencing her first mild respiratory symptoms, the results of the PCR test confirmed a diagnosis of COVID‐19. She had not received the COVID‐19 vaccine prior to infection. She was ordered to be quarantined at a hotel near her home without treatment because her general and respiratory symptoms were mild. During the quarantine period, she experienced insomnia and poor communication ability. After consulting with the outpatient psychiatric clinic, she was diagnosed with depression due to the psychological burden of having COVID‐19. However, a few days later, she showed delusion and disorganized behavior with agitation. On day 15, she was admitted to the psychiatric hospital to ensure her safety, and antipsychotic treatment was started. Then, due to difficulties in communicating and eating, she was transferred to the psychiatric ward in the general hospital for further examination and treatment after confirming a negative result for a subsequent PCR test for SARS‐CoV‐2. The clinical course of this case is shown in Figure 1.
FIGURE 1.
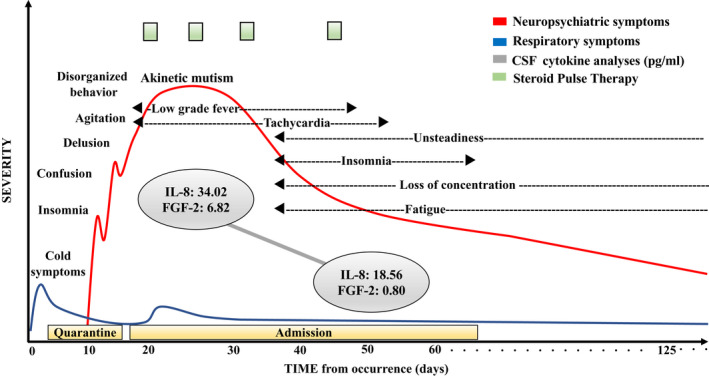
Clinical course of neuropsychiatric symptoms after a mild case of COVID‐19. The figure shows the clinical features, treatment, cerebrospinal fluid (CSF) cytokine analyses, and neuropsychiatric symptoms. Admission was on the 15th day after the initial symptoms. Discharge was on the 67th day. While the severity of the respiratory symptoms was mild, the neurological symptoms were severe. Steroid pulse therapy (three consecutive days with methylprednisolone 1000 mg per week) improved the neuropsychiatric symptoms and reduced the level of cytokines. However, various neurological deficits persisted, some of which are still present
On admission to the general hospital, she presented with poor communication ability accompanied by catatonic features, dehydration symptoms, tachycardia, and low‐grade fever. We observed consolidation in both lower lobes on lung computed tomography (Figure. S1A, B) and increased inflammation markers in laboratory findings (white blood cell count, 11 700/μL; C‐reactive protein, 1.15 mg/mL). In addition, the level of several cytokines in the cerebrospinal fluid (CSF) and plasma was increased on admission (Figure 2 and Supplementary Methods). However, no abnormal findings were seen in several brain imaging tests (Figure S2), no increased cell counts were observed in the CSF, and no SARS‐CoV‐2 was found in the CSF by PCR. She subsequently showed progressive consciousness disturbance leading to akinetic mutism and found it nearly impossible to communicate. An electroencephalogram (EEG) showed a generalized slowing δ wave with frontal lobe dominance (Figure 3A). These findings led to a diagnosis of encephalopathy, and as a result, three courses of steroid pulse therapy were started (three consecutive days with methylprednisolone 1000 mg per week), with reference to former reports.6, 7 Brain single‐photon emission computed tomography findings were within the normal limits (Figure S3). Although she showed mild pulmonary embolism after starting steroid treatment (Figure S4), her consciousness disturbance gradually improved. Moreover, the slow EEG waves were decreased (Figure 3B), as were the levels of several cytokines, including interleukin‐8 (IL‐8) and fibroblast growth factor‐2 (FGF‐2), in the CSF and plasma after the third course of steroid pulse therapy compared with at admission (Figure 2). When she was able to communicate, she showed memory loss from the middle of the quarantine period.
FIGURE 2.
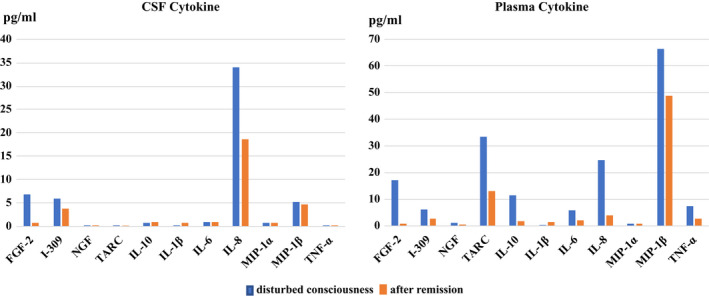
Cytokine levels during disturbed consciousness and after remission. We compared cerebrospinal fluid (CSF) and plasma cytokine levels during and after treatment for encephalopathy. The results showed increased levels of most cytokines during disturbed consciousness. In many categories, cytokines are reduced with improvements in disturbed consciousness. The concentrations of cytokines/chemokines, including fibroblast growth factor‐2 (FGF‐2), I‐309 (chemokine (C‐C motif) ligand 1 [CCL1]), nerve growth factor (NGF), thymus and activation‐regulated chemokine (TARC), interleukin (IL)‐10, IL‐1β, IL‐6, macrophage inflammatory protein‐1α (MIP‐1α), MIP‐1β, and tumor necrosis factor (TNF)‐α, were measured using a multiplex assay system. The concentration of IL‐8 in both CSF and plasma was measured using an enzyme‐linked immunosorbent assay kit
FIGURE 3.
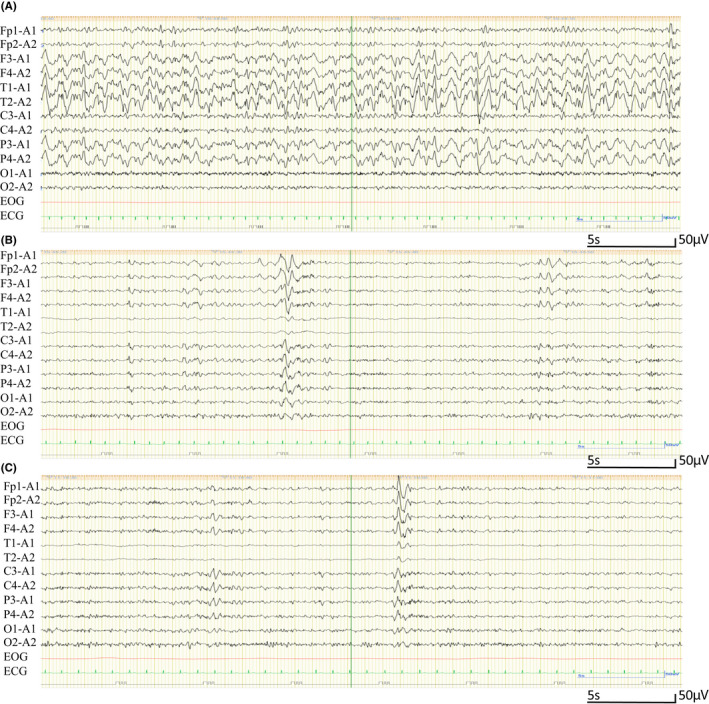
Electroencephalogram (EEG) alterations. A, Acute phase in the akinetic mutism state with a diffuse δ wave with frontal lobe dominance. B, Reduced slow wave and reorganization of the background α wave and reactivity after the third course of steroid pulse treatment. C, Remaining slow waves at 4 months after onset. EEG acquisition settings: referential montage; recording speed: 30 s/page; sensitivity: 7 μV/mm; time constant: 0.1 s; high‐frequency filter: 15 Hz
When she began oral intake and getting out of bed by herself, she experienced weakness and unsteadiness while walking, as well as insomnia and a lack of concentration. An examination of these symptoms revealed postural tachycardia and impaired concentration; these residual symptoms are summarized in Table 1. A variety of antibody and physiological tests did not suggest any neuromuscular or collagen diseases. We administered the fourth course of steroid pulse therapy for her neuropsychiatric symptoms, but no clear effect was seen. Although several sequelae persisted, she was discharged on day 67. Her frontal lobe function, which could have been related to her impaired concentration, gradually improved until she was able to communicate with her family normally, as shown in Table S1. However, she still could not start working for 3 months after discharge because she continued to experience several persistent neuropsychiatric symptoms.
TABLE 1.
Persisting neuropsychiatric symptoms after encephalopathy
| Neuropsychiatric symptoms | Examination | Outcome |
|---|---|---|
| Tachycardia | ・Autonomic nerve test (head‐up tilt test) demonstrated postural tachycardia | ・Tachycardia improved until the effect no longer interfered with daily life at discharge |
| Unsteadiness | ・Cerebellar ataxia suggested an inability to coordinate balance, but nerve conduction studies were normal | ・Unsteadiness gradually decreased ・Unsteady balance at 4 months after occurrence |
| Insomnia | ・Nocturnal awakening was improved by suvorexant | ・Nocturnal awakening was improved at discharge without drug therapy |
| Loss of concentration | ・FAB showed decreased frontal lobe function (13/18) | ・FAB showed improvement to 17/18 at 4 months after occurrence |
Detailed results of a frontal assessment battery (FAB) are shown in Table S1.
3. DISCUSSION
Here, we reported a case with severe and long‐lasting neuropsychiatric symptoms after mild respiratory symptoms caused by COVID‐19. Neuropsychiatric symptoms related to COVID‐19 have been reported,1, 2 and patients with severe COVID‐19 have also shown a high frequency of severe neuropsychiatric symptoms (Table S2), including encephalopathy, that could lead to higher mortality. 5 However, the severity and detailed clinical courses of patients with neuropsychiatric symptoms caused by mild or asymptomatic COVID‐19 remain poorly understood. Therefore, severe neuropsychiatric symptoms occurring after mild respiratory symptoms could be missed, as seen in this case. Actually, only few case reports 8 have shown detailed clinical courses in nonsevere COVID‐19. In the present case report, we have described the detailed long clinical course of neuropsychiatric symptoms including cytokine measurement and the evaluation of neuropsychiatric sequelae from mild COVID‐19.
Based on accumulated knowledge regarding COVID‐19, one putative pathogenesis of neuropsychiatric symptoms is the excessive release of cytokines from SARS‐CoV‐2‐infected cells, which can alter the permeability of the BBB, 4 thereby initiating neuroinflammation. 9 Although both brain imaging tests and PCR testing for SARS‐CoV‐2 in the CSF were negative in this case, considering the slow EEG waves (Figure 3A) that have previously been reported in cases of COVID‐19, 10 putative mechanisms underlying the neuropsychiatric symptoms observed in this case could be derived from microvascular regions that can only be detected by high‐power magnetic resonance imaging. 11
Furthermore, in this case, elevated cytokine levels, including IL‐8, which is reportedly increased in patients with COVID‐19‐related encephalopathy, 12 suggesting increased vascular permeability due to inflammatory cytokines, 13 and FGF‐2, which is reportedly increased in local hypoxia, suggesting microcirculatory disturbance, 14 were found in the CSF (Figure 2). However, in this case, cytokine levels were low compared with previous studies describing COVID‐19 with severe respiratory symptoms.7, 15 Therefore, considering the severe neuropsychiatric symptoms observed in this case, the association between cytokine levels in the CSF and the neuropsychiatric symptoms should be investigated more thoroughly in a future study. The fact that the present case had severe neuropsychiatric symptoms, even though here cytokines were lower than in previous studies, suggests that she could have been vulnerable to neuropsychiatric symptoms after COVID‐19. Although the details were unclear, she had a history of admission to a psychiatric hospital for depressive symptoms in adolescence, which suggests that she may have had some kind of brain vulnerability. Actually, it has recently been reported that impaired immune responses are related to the pathogenesis of psychiatric disorders, 16 and an integrated model of the immune system and genetic risk factors has been considered to gain a better understanding of the pathogenesis of psychiatric disorders and facilitate the development of novel diagnostic systems and drugs. 17
In addition to during the acute phase, we followed the patient to monitor her numerous residual neuropsychiatric symptoms, such as insomnia, fatigue, loss of concentration, and unsteadiness while walking, which have been reported as neuropsychiatric sequelae of COVID‐19. 18 For these residual symptoms, we observed several abnormal findings, such as slow EEG waves (Figure 3B, C), postural tachycardia, and disturbed frontal lobe function. Considering the putative pathophysiology of encephalopathy in the acute phase, the mechanisms underlying these persisting neuropsychiatric symptoms could have derived from an increased inflammatory state leading to a pathophysiology related to demyelination, 19 autonomic dysfunction, 20 and neuromuscular diseases. 21 Steroid pulse therapy (fourth steroid pulse therapy) after brain encephalopathy did not show any immediate effects on these neuropsychiatric sequelae. These neurological symptoms could lead to fatigue and a depressed mood, which have been reported as long‐lasting sequelae in COVID‐19, or so‐called “long COVID.” 22 However, the frequency of headache and depression has been reported to be reduced at 1 year after admission in cases of COVID‐19 undergoing steroid therapy. 23
In conclusion, we have reported a case with severe neuropsychiatric symptoms after mild respiratory symptoms from COVID‐19. Considering the substantial impact on daily life after remission, attention should be paid to the possibility of severe neuropsychiatric symptoms from apparently mild COVID‐19. Therefore, the underlying mechanisms, treatment modalities, and long‐term clinical courses of neuropsychiatric symptoms related to the COVID‐19 should be more clearly elucidated in the future.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
RJ, Hiroki K, TU, Masato K, KK, Haruki K, and TI acquired the case data. HF, YY, and KS performed the cytokine analysis. RJ, HK, TU, Masahisa K, and NO drafted the manuscript. All authors read and approved the final manuscript.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
This study was approved by the Ethics Review Committee of the Nagoya University Graduate School of Medicine based on the guidelines on “Case Reports Treated as Research” from The Japanese Society of Psychiatry and Neurology.
INFORMED CONSENT
Informed consent was obtained from this case directly. This study was conducted in accordance with the Helsinki Declaration of 1975 and its later amendments or comparable ethical standards.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
n/a
ANIMAL STUDIES
n/a
Supporting information
Supplementary Material
ACKNOWLEDGMENT
We thank the patient for her participation in this study.
Jozuka R, Kimura H, Uematsu T, Fujigaki H, Yamamoto Y, Kobayashi M, et al. Severe and long‐lasting neuropsychiatric symptoms after mild respiratory symptoms caused by COVID‐19: A case report. Neuropsychopharmacol Rep. 2022;42:114–119. 10.1002/npr2.12222
Funding information
This research was supported by the AMED under Grant Nos. JP21dk0307099 and JP21dk0307103, and supported in part by a grant‐in‐aid from an intramural fund from NCNP (3‐8). Support was also provided by the Japan Society for the Promotion of Science (JSPS) KAKENHI under Grant No. 21H02848
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its supplementary information files.
REFERENCES
- 1. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar‐Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7(7):611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the Nervous System. Cell. 2020;183(1):16–27 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, et al. Frequent neurologic manifestations and encephalopathy‐associated morbidity in Covid‐19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pugin D, Vargas MI, Thieffry C, Schibler M, Grosgurin O, Pugin J, et al. COVID‐19‐related encephalopathy responsive to high‐dose glucocorticoids. Neurology. 2020;95(12):543–6. [DOI] [PubMed] [Google Scholar]
- 7. Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elfil M, Selby L, Van Schooneveld TC, Fadul N. Acute psychosis associated with recent SARS‐CoV‐2 infection: a case report. Idcases. 2021;24:e01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Valle DM, Kim‐Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med. 2020;26(10):1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canham LJW, Staniaszek LE, Mortimer AM, Nouri LF, Kane NM. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid‐19: a case series. Clin Neurophysiol Pract. 2020;5:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular Injury in the Brains of Patients with Covid‐19. N Engl J Med. 2021;384(5):481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Espindola OM, Gomes YCP, Brandao CO, Torres RC, Siqueira M, Soares CN, et al. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann Neurol. 2021;89(5):1041–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, et al. Blood‐brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14(12):1640–50. [DOI] [PubMed] [Google Scholar]
- 14. Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8(10):483–9. [DOI] [PubMed] [Google Scholar]
- 15. Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas‐Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID‐19. BMC Neurol. 2020;20(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry ‐ novel perspectives on brain disorders. Nat Rev Neurol. 2019;15(6):317–28. [DOI] [PubMed] [Google Scholar]
- 17. Kimura H, Mori D, Aleksic B, Ozaki N. Elucidation of molecular pathogenesis and drug development for psychiatric disorders from rare disease‐susceptibility variants. Neurosci Res. 2021;170:24–31. [DOI] [PubMed] [Google Scholar]
- 18. Carfi A, Bernabei R, Landi F. Gemelli against C‐P‐ACSG. persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Espindola OM, Brandao CO, Gomes YCP, Siqueira M, Soares CN, Lima M, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID‐19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn DI, Jaradeh S. A case report of postural tachycardia syndrome after COVID‐19. Clin Auton Res. 2020;30(5):449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirayama T, Hongo Y, Kaida K, Kano O. Guillain‐Barre syndrome after COVID‐19 in Japan. BMJ Case Rep. 2020;13(10):e239218. doi: 10.1136/bcr-2020-239218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home‐isolated patients. Nat Med. 2021;27(9):1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catalan IP, Marti CR, Sota DP, Alvarez AC, Gimeno MJE, Juana SF, et al. Corticosteroids for COVID‐19 symptoms and quality of life at 1 year from admission. J Med Virol. 2021, 94(1):205–10. doi: 10.1002/jmv.27296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files.


