Abstract
As of September 18th, 2021, global casualties due to COVID-19 infections approach 200 million, several COVID-19 vaccines have been authorized to prevent COVID-19 infection and help mitigate the spread of the virus. Despite the vast majority having safely received vaccination against SARS-COV-2, the rare complications following COVID-19 vaccination have often been life-threatening or fatal. The mechanisms underlying (multi) organ complications are associated with COVID-19, either through direct viral damage or from host immune response (i.e., cytokine storm). The purpose of this manuscript is to review the role of imaging in identifying and elucidating multiorgan complications following SARS-COV-2 vaccination—making clear that, in any case, they represent a minute fraction of those in the general population who have been vaccinated. The authors are both staunch supporters of COVID-19 vaccination and vaccinated themselves as well.
Keywords: Covid-19, Vaccination, Adverse events
Key points
Different vaccines against SARS-COV-2 have been authorized in clinical practice.
Post-vaccination COVID-19 adverse events have been described.
Non-invasive imaging should be performed in patients with a clinical suspicion after vaccination.
Introduction
COVID-19 is a pandemic with dramatic consequences for global health leading to high death rates [1, 2].
Different vaccines against SARS-COV-2 have been authorized in clinical practice to prevent the spread of the disease pandemic and reduce mortality [3]. The spike protein of SARS-COV-2 represents the most suitable target and various vaccines have been developed with different platforms, including viral vector vaccines and mRNA vaccines [3].
The most commonly described side effects after COVID-19 vaccination are pain at the injection site, fever, muscle pain, fatigue, and headache [4].
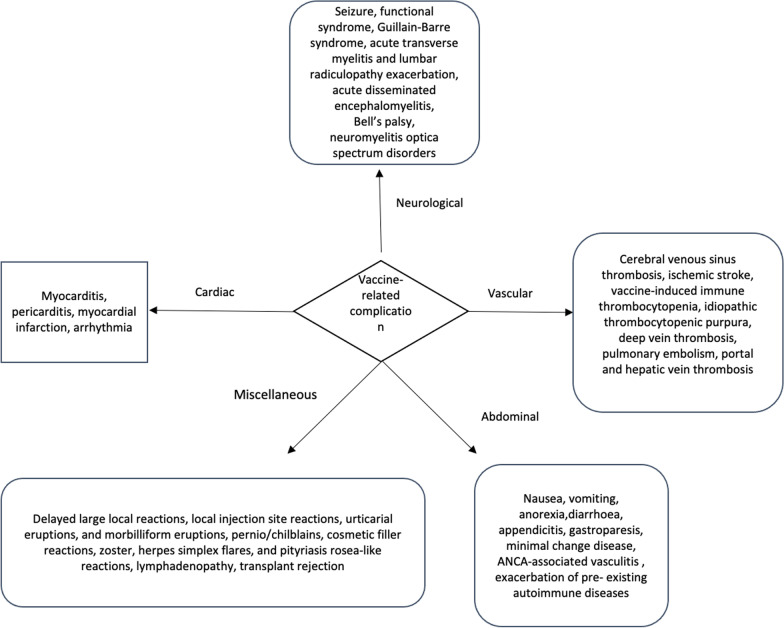
An adverse event following vaccination is referred to as any unpleasant medical event after vaccination, without a definite causal relationship to the vaccine whether it’s either local or systemic [5]. Despite clinical trial data regarding safety and efficacy data of COVID-19 vaccines, multiple case reports, and case series have described rare but serious adverse events, with multiorgan involvement including brain, heart, and vascular system (Fig. 1) [6–15].
Fig. 1.
Vaccine-related side effects in different organs
We present the complications reported following SARS-COV-2 vaccination and discuss both the adverse effects and their mechanisms in the current literature. This review also shed the light on the role of imaging for early detection of these potentially life-threatening complications. Even though we discuss the complications of the vaccine, the benefits outweigh the risk of being severely infected with SAR-COV-2.
Cardiac complications
Background
Vaccine-related cardiac complications have been reported as a rare adverse events after vaccination, especially Smallpox vaccination [16], Hepatitis B, Anthrax, and Haemophilus influenzae vaccination [17]. Myopericarditis was the most frequently described cardiac complication after vaccination. Before the COVID-19 pandemic, the Vaccine Adverse Event Reporting System (VAERS) reported 708 patients who met the diagnosis of myopericarditis among 620,195 individuals between 1990 and 2018, with a rate of 0.1% [17].
Eckart et al. evaluated 540,824 patients following Smallpox vaccination for vaccine-related myopericarditis. Among them, myocarditis was diagnosed in 67 patients, reporting an objective normalization of cardiac function at follow-up, and 20% of patients with persistent symptoms despite normal testing [18]. Given these findings, the authors suggested considering vaccine-related myocarditis in a patient with chest pain after vaccination [18].
As of August 4, 2021, a total of 4.27 billion doses of the COVID-19 vaccine were used, and thus far cardiac complications were reported all over the world during the first wave of COVID-19 vaccination.
Recently, the Center for Disease Control and Prevention (CDC) described a likely association between the mRNA vaccine and myocarditis and pericarditis, cataloged as “probable myocarditis”, “confirmed myocarditis”, and “acute pericarditis” [11]. CDC data suggested that myopericarditis was more common in young adults, male, and identified predominantly after the second vaccine dose, compared with the first [11].
Several mechanisms for post-vaccination myocarditis have been hypothesized: (1) mRNA vaccine can induce an aberrant non-specific innate response [11, 19] or (2) a molecular mimicry mechanism between the viral spike protein and cardiac protein [20, 21]; or (3) high antibody response may have been generated in a small group of subjects, leading to a hyperimmune response [21]; or (4) autoantibodies generation against several antigens with functional effects on cardiomyocytes in susceptible individuals after vaccination [11, 22].
The Medicines and Healthcare Products Regulatory Agency (MHRA) also described some cases of myocarditis and pericarditis with the viral vector vaccine (i.e. AstraZeneca) [23, 24]. Similar cases were also reported in the EudraVigilance database [25]. Through July 21st, 2021, the MHRA also reported rate and rhythm disorder as well as myocardial infarction. In total, there were 181 recorded deaths following a vaccine-related cardiac complication [24]. Several papers described myocarditis and pericarditis following vaccine as self-limited and transient conditions [13, 26, 27]. Notwithstanding only long-term follow-up can reveal with certainty the permanent impact of this cardiac injury. See Fig. 2
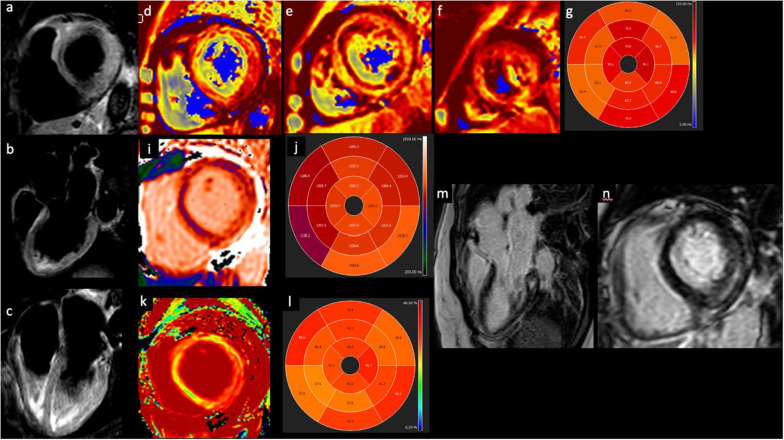
Fig. 2.
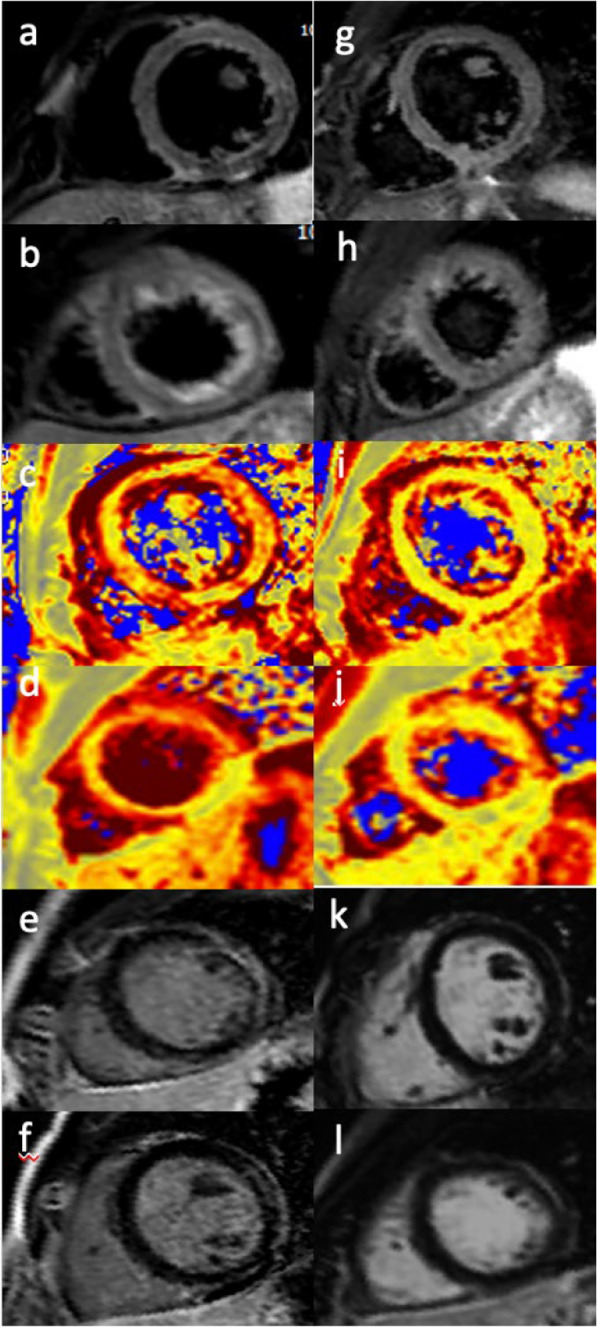
A 25-years-old-man with symptoms of fever, fatigue, shortness of breath and chest pain which developed one day following the second dose of COVID-19 vaccine. T2-short tau inversion recovery CMR short axis (a, b) demonstrating edema in the antero-lateral segments. T2 mapping short-axis view confirmed the presence of edema in the same segments (c, d). Late gadolinium enhancement short-axis view showed a subepicardial antero-lateral scar with an associated pericardial enhacement (e–f). Follow-up CMR was done 3 months from start of symptoms revealing the disappearance of edema in both the T2-short tau inversion recovery CMR short axis (g, h) and T2 mapping short-axis view (i, j). There is no evidence of focal areas of late gadolinium enhancement in the antero-lateral segments (k, l)
A review of the worldwide literature reveals some cases of myocardial infarction either after mRNA vaccines and after viral vector vaccines [23, 25, 28–31].
Tajstra et al. described a clinical case of an 86-years old-man with acute ST-segment elevation myocardial infarction (STEMI) around 30 min after the injection of the first dose of Pfizer–BioNTech vaccine [31]. Similar findings were also reported with the viral vector vaccine [23].
The possible link between acute coronary syndrome and vaccination is unclear. Some authors speculated different theories, such as (1) a vasospastic acute coronary syndrome, namely Kounis syndrome, that involves mast cell activation through the release of inflammatory cytokines, leading to coronary artery vasospasm or atherosclerotic plaque rupture [31]; (2) post-vaccine physiological stress can destabilization of chronic atherosclerotic plaque; or (3) an immunologic response triggering of plaque rupture [29].
Nevertheless, there is still a lack of large multicenter studies and little evidence to establish a direct correlation between myocardial injuries and COVID-19 vaccines. To overcome this gap and evaluate the prevalence of myocardial damage after mRNA vaccines, a prospective study has been implemented [32].
Table 1 summarized previous research regarding vaccine-related cardiac complications.
Table 1.
Previous case-report about vaccine-related cardiac complication
| Authors | Cardiac complication | Type of vaccine | Number of patients described |
|---|---|---|---|
| Tajstra et al. [31] | Myocardial infarction | mRNA vaccine | 1 |
| Muthukumar et al. [22] | Myocardial infarction | mRNA vaccine | 1 |
| Srinivasan et al. [29] | Myocardial infarction | mRNA and viral vector vaccine | 3 |
| Chamling et al. [25] | Myocarditis | mRNA and viral vector vaccine | 3 |
| Sung et al. [30] | Myocardial infarction | mRNA vaccine | 2 |
| Abou et al. [28] | Myocardial infarction | viral vector vaccine | 1 |
| Isaak et al. [38] | Myocarditis | mRNA vaccine | 1 |
| Montgomery et al. [12] | Myocarditis | mRNA vaccine | 23 |
| Kim et al. [13] | myocarditis | mRNA vaccine | 7 |
| Marshall et al. [14] | Myocarditis | mRNA vaccine | 7 |
| Starekova et al. [15] | Myocarditis | mRNA vaccine | 5 |
Imaging
Clinicians should suspect vaccine-related myocarditis or pericarditis in patients with chest pain and a rise in cardiac enzyme. The diagnostic gold standard for the diagnosis of myocarditis is endomyocardial biopsy [33], however this procedure is infrequently used in clinical practice due to its invasive nature and limitations (e.g. sampling errors caused by focal or patchy involvement of myocardium, variability histopathological interpretation) [34]. Due to this uncertainty, several non-invasive imaging modalities help to diagnose myocarditis [35–37]. Beyond the first-line echocardiography, cardiac magnetic resonance (CMR) has emerged as a key tool in the diagnosis of myocarditis [37]. CMR features of vaccine-related myocarditis are similar to other virus-associated with myocarditis [25, 38].
Based on the Lake Louis criteria, CMR can identify myocardial damage with a diagnostic accuracy of 78% [39]. In addition, adding the parametric mapping techniques, such as T1 mapping, T2 mapping, and ECV to the classic CMR protocol may improve its accuracy, provide additional disease characterization, and help the management of different cardiac injuries [40].
Figure 3 demonstrated an example of CMR in patients with vaccine-related myocarditis.
Fig. 3.
Myocarditis after COVID-19 vaccination. T2-short tau inversion recovery CMR short axis (a), three-chamber (b) and four-chamber view revealing edema in the infero-lateral basal segment. T2 mapping short-axis view and T2 mapping in AHA 16-segment model (d–g) showed altered values in all myocardial segments, especially in the infero-lateral basal segments. T1 mapping (i, j) and ECV (k, l) short-axis view confirming the altered values. Late gadolinium enhancement three chamber (m) and short-axis (n) view demonstrating an intramyocardial infero-lateral scar
There has been an association such other cardiac side effects, including myocardial infarction and arrhythmias [41].
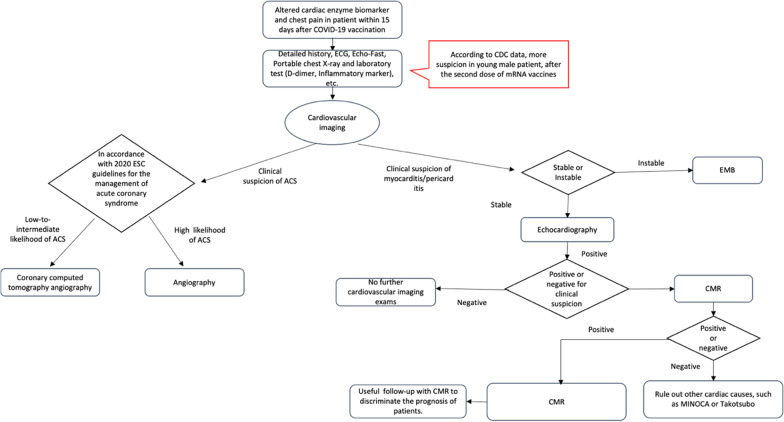
Similarly, myocardial infarction should be suspected among patients presenting with acute chest pain to the emergency department after the COVID-19 vaccination. For an initial evaluation, ECG and cardiac troponin levels should be obtained. In accordance with 2020 ESC guidelines for the management of acute coronary syndrome, coronary computed tomography angiography (CCTA) may be an option in patients with low-to-intermediate clinical likelihood of acute coronary syndrome thanks to its negative predictive value to exclude coronary artery disease [42].
As reported in the previous studies [29, 30], imaging features are suggestive of acute thrombotic events as the underlying mechanism. CCTA can easily detect the presence of an intracoronary filling defect, while also evaluating the status of an atherosclerosis plaque. Thus excluding the presence of a vulnerable plaque as a trigger for thrombus formation [43]. Figure 3 showed a proposed diagnostic flowchart for suspected myocardial damage post-COVID-19 vaccination (Fig. 4).
Fig. 4.
Diagnostic flowchart for suspected myocardial damage post COVID-19 vaccination
Neurological complications
Background
Neurological disorders after COVID-19 infection are well known, with a spectrum of pathologies ranging from mild to severe [44]. Neurological complications after COVID-19 vaccination have emerged at the end of 2020 after 2 patients developed transverse myelitis following viral vector vaccination [45]. To date, out of 9,442 adverse events following immunization reported in the VAERS data related to mRNA vaccines, 254 (2.69%) were neurological [10]. A nationwide descriptive study by García-Grimshaw et al. including data from 704,004 first-dose recipients reported 33 (4.7/100,000 doses) serious adverse events. Out of these, 17 (51.7%; 2.4/100,000 doses) were neurologic complications, including seizure (0.99/100,000 doses), functional syndrome (0.56/100,000 doses), Guillain–Barre syndrome (0.43/100,000 doses), acute transverse myelitis (0.28/100,000 doses), and lumbar radiculopathy exacerbation (0.14/100,000 doses) [46]. On the other hand, the overall incidence of non-serious neurologic events was 600.7 cases per 100,000 administered doses [46]. Mild neurological side effects reported were headache (62.2%; 577.7/100,000 doses), transitory sensory symptoms (3.5%; 32.9/100,000 doses) and weakness (1%; 9.1/100,000 doses).
According to a trial with the Sinovac and Sinopharm vaccine, the most common neurological side effect after vaccination was headache (68%), and myalgia (60%) [47]. Among the 9442 reports of adverse events, the VAERS described also cases of stroke (17 cases), Guillain–Barre syndrome (32 cases), Bell’s palsy (190 cases), transverse myelitis (9 cases), and acute disseminated encephalomyelitis (6 cases) [48].
A frequently reported neurological side effect was Bell’s palsy [48–50]. Reports from the mRNA vaccine trials described 7 cases of 37,000 vaccine recipients who developed Bell’s palsy [48].
One explanation for this phenomenon is transient lymphopenia due to Type I interferons action following vaccination [50].
Neuroimmune complications were also described in some clinical cases, including Guillain–Barre Syndrome, Neuromyelitis Optica Spectrum Disorders, and Traverse Myelitis [9, 10, 51–53]. The development of a post-vaccination neuroimmune syndrome may be related to overactivation of the immune system after vaccination or a cross-reaction between host antibodies and proteins present in the peripheral myelin [51, 52]. Subsequently, the Guillen-Barre syndrome and Chronic Inflammatory Demyelinating Polyneuropathy Foundation suggested that individuals that developed Guillen-Barre Syndrome after their first immunization should avoid the second dose [54].
A clinical case by Vogrig et al. described a 56-year old female patient who developed an acute disseminated encephalomyelitis after the first dose of mRNA COVID-19 vaccine [55]. Similar cases were also reported by the VAERS [54] and by a case report out of China [56].
Table 2 reported previous research regarding vaccine-related neurological complications.
Table 2.
Previous case-report about vaccine-related neurological complications
| Authors | Neurological complications | Type of vaccine | Number of patients described |
|---|---|---|---|
| Allen et al | Guillan-Barre Syndrome | Viral vector vaccine | 4 |
| Maramattom et al | Guillan-Barre Syndrome | Viral vector vaccine | 7 |
| Waheed et al | Guillan-Barre Syndrome | mRNA | 1 |
| García-Grimshaw et al | Adverse neurological events following vaccination | mRNA | 6503 |
| Chen et al | Neuromyelitis optica spectrum disorders | Viral vector vaccine | 1 |
| Malhotra et al | Trasverse myelitis | Viral vector vaccine | 2 |
| Roman et al | Trasverse myelitis | Viral vector vaccine | 3 |
| Vogrig et al | Acute disseminated encephalomyelitis | Viral vector vaccine | 1 |
| Cirillo et al | Bell’s palsy | mRNA vaccine and viral vector vaccine | |
| Soeiro et al | Bell’s palsy | mRNA vaccine | 9 |
| Mehta et al | Cerebral venous sinus thrombosis | Viral vector vaccine | 2 |
| Cao et al | Acute disseminated encephalomyelitis | Viral vector vaccine | 1 |
| Mayhani et al | Ischemic stroke | Viral vector vaccine | 3 |
| Blauenfeldt et al | Ischemic stroke | Viral vector vaccine | 1 |
| Suresh et al | Cerebral venous sinus thrombosis | viral vector vaccine | 1 |
Recommendations suggest that comprehensive surveillance systems be in place to ensure vaccine safety and that the benefit of vaccination overcomes the risks [54].
Imaging
Beyond the neuro-vascular adverse events following COVID-19 vaccination described in the following paragraph, other neurological adverse events were reported and should be managed with appropriate neuroimaging exams [9, 10, 54]. CT and MRI are the most important imaging techniques in the diagnosis of neurological disease. Most patients with neurological side effects related to the COVID-19 vaccination undergo neuroimaging and no specific findings are revealed [10, 53].
Guillain-Barré syndrome may occur following COVID-19 immunization. The classic neuroimaging pattern of this syndrome is characterized by cord T2 signal alteration and gadolinium enhancement of the caudal nerves roots. Waheed et al. reported a case of an 82-year-old highly functional female without significant comorbidities, with a suspected Guillain–Barre syndrome after mRNA vaccine, with typical MRI features of Guillain Barre syndrome [57].
Chen et al. described a patient who developed Neuromyelitis Optica Spectrum Disorder after vaccination for COVID-19, highlighting the usefulness of MRI in revealed area postrema and bilateral hypothalamus lesions without optic nerve and cervical spinal cord sparing [51].
The spectrum of neuroimaging abnormalities includes changes related to Guillain–Barre Syndrome, Bell’s palsy, Transverse Myelitis, Neuromyelitis Optica Spectrum Disorder, and acute disseminated encephalomyelitis [48, 54].
Figure 5 showed neuroimaging features following COVID-19 vaccination.
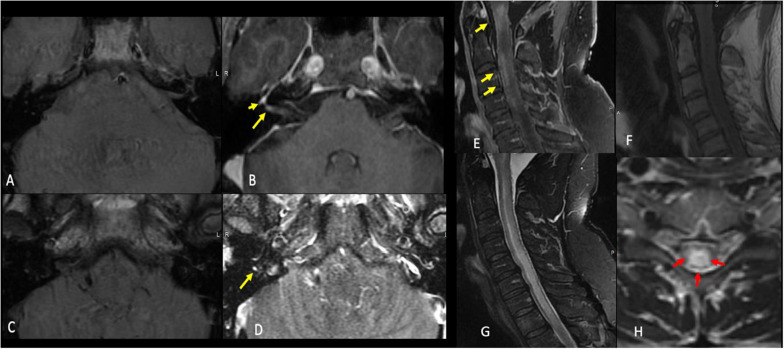
Fig. 5.
Neuroimaging features after COVID-19 vaccination. Case 1: A 46-year-old-man who presented with a rapid onset right-sided facial weakness after having COVID-19 vaccine. Axial T1 pre (a and c) and T1 postcontrast fat sat (b and d) demonstrate abnormal enhancement of the right facial nerve within the lateral right IAC (long yellow arrow in b) as well as asymmetric enhancement of the right geniculate ganglion (short yellow arrow in b) and tympanic portion of the facial nerve (yellow arrow in d). Findings are consistent with Bell's palsy. Case 2: A 51-year-old-man who presented with a sudden upper and lower limb weakness after having COVID-19 vaccine. Sagittal T1 postcontrast (e) T1 pre (f), STIR (g), and axial T2W (h) images demonstrate extensive T2 signal hyperintensity of the central cervical cord (red arrows) with patchy areas of enhancement at the levels of c1, c2, c3 and c4 (yellow arrows). There was no associated restricted diffusion. Findings were consistent with transverse myelitis
Vascular complications
Background
Recently, coagulopathy has been described after COVID-19 vaccination, especially following viral vector vaccine [58].
Mehta et al. reported two cases of cerebral venous sinus thrombosis after viral vector vaccine and proposed a potential immunological disorder supported by the presence of antibodies to platelet factor-4, with a mechanism similar to spontaneous heparin-induced thrombocytopenia. The authors suggest radiologists and neurologists be aware of this neurological complication after vaccination, particularly when comparing its management to traditional cerebral venous sinus thrombosis [59].
For an initial evaluation, beyond laboratory testing of blood count, non-contrast CT followed by CT venogram or magnetic resonance venography in selected patients is required [59].
Similar neurological complications were reported by the European Medicine Agency and by the MHRA [59]. The spectrum of vaccine-related coagulopathy was also shown by Al-Mayhani et al. [60], reporting three cases of ischemic stroke with large vessel occlusion after COVID-19 vaccination. Their observations suggest that immune-mediated coagulopathy, in addition to venous thrombosis, can involve arterial occlusion [60].
In view of several reports, the definition of a new syndrome has been proposed, namely vaccine-induced immune thrombocytopenia [59–66]. Additional cases have been also described for the mRNA vaccine by the European Medicine Agency, including at least 40 possible cases among 58 million recipients of the mRNA vaccine [67]. A recent autopsy report by Fanni et al. described a case of vaccine-induced immune thrombocytopenia in a 58-years old man 13 days after his first dose of the viral vector vaccine [61]. The report confirmed multiple microthrombi in unusual sites, including the heart, aortic vasa vasorum, lung, liver, kidney, and choroid plexus [61]. There have also been reports of post mRNA vaccination exacerbation of chronic idiopathic or immune thrombocytopenic purpura [68, 69]. The Scottish National Population-Based Database of 2.53 millions vaccinated individuals revealed a potential association between the viral vector vaccine and idiopathic thrombocytopenic purpura, with an incidence of 1.13 cases per 100,000 vaccinations [68].
Table 3 summarizes previous research regarding vaccine-related vascular complications.
Table 3.
Previous case-report about vaccine-related vascular complications
| Authors | Vascular complications | Type of vaccine | Number of patients described |
|---|---|---|---|
| Fanni et al | Thrombotic Thrombocytopenia | Viral vector vaccine | 1 |
| Schuktz et al | Thrombotic Thrombocytopenia | Viral vector vaccine | 5 |
| Greinacher et al | Thrombotic Thrombocytopenia | Viral vector vaccine | 11 |
| Wolf et al | Thrombotic Thrombocytopenia | Viral vector vaccine | 3 |
| Toom et al | Exacerbation of familiar thrombocytopenia | mRNA | 1 |
| Blauenfeldt et al | Thrombocytopenia | Viral vector vaccine | 1 |
| Suresh et al | Thrombotic Thrombocytopenia | viral vector vaccine | 1 |
Imaging
Several international consensus guidelines about vaccine-induced immune thrombocytopenia diagnosis and management have been proposed, These are based upon laboratory and clinical findings first described in case reports of vaccine-induced immune thrombocytopenia [66, 70–72].
Based on these consensus guidelines, the patients would be classified as “definite case,” “probable case,” “suspected case,” and “unlikely case”. For patients with a high clinical suspicion of vaccine-induced immune thrombocytopenia, the guidelines suggest ordering imaging studied based on the location of symptoms to confirm the site of thrombosis, keeping in mind that VITT is associated with both arterial and venous thrombosis in a variety of sites [6, 71, 72]. Modern CT scans, thanks to constantly advancing technology with an increasingly better spatial and temporal resolution, can provide optimal and rapid imaging of the vessel lumen [73, 74]. In addition, CT scan is able to rule out organ complications, including mesenteric ischemia, infarcted bowel, and solid organ infarcts [75]. Even ultrasound represents a suitable imaging analysis, allowing an assessment of abdominal, lower, and upper limb vessels [72]. (See Fig. 6).Finally, MRI is an accurate alternative non-invasive imaging that can be performed in patients with neurological symptoms when evaluating for cerebral venous thrombosis [72, 75].
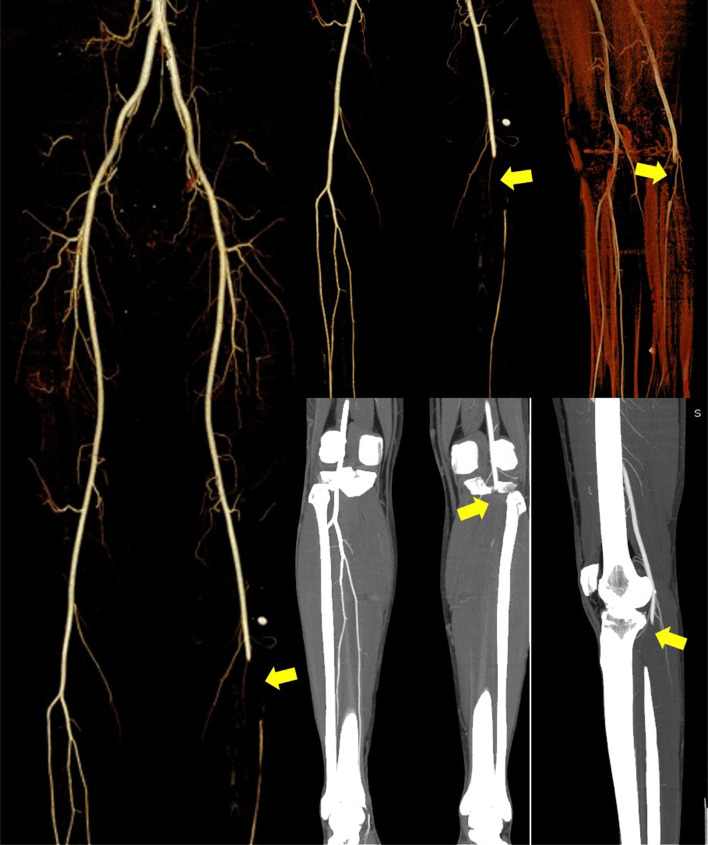
Fig. 6.
A 21-year-old, normal weight and non-smoker man presented to the emergency department with new onset of cool and painful left leg, ten days after having the second dose of COVID-19 vaccine from AstraZeneca. Volume Rendering and Maximum Intensity Projection CT angiography images show acute segmental thrombotic occlusion of the popliteal artery (yellow arrows). He had no underlying illnesses, trauma, surgery, infection, or immobilization. There was no known thrombophilia. Unfortunately, the patient underwent critical ischemia and urgent limb amputation
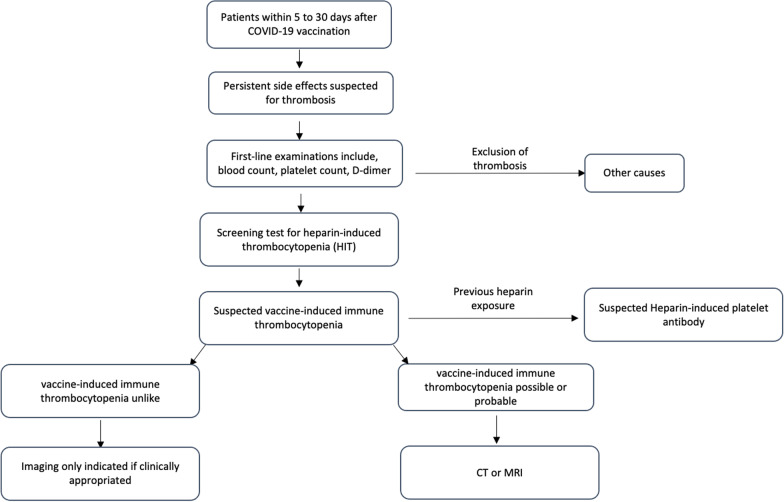
A diagnostic flowchart for suspected vaccine-induced immune thrombocytopenia was proposed in Fig. 7
Fig. 7.
Diagnostic flowchart for suspected vaccine-induced immune thrombocytopenia
Therefore, emergency clinicians should know this vascular complication when evaluating and managing patients after the COVID-19 vaccination, allowing a prompt diagnosis through non-invasive imaging to improve the patient outcomes.
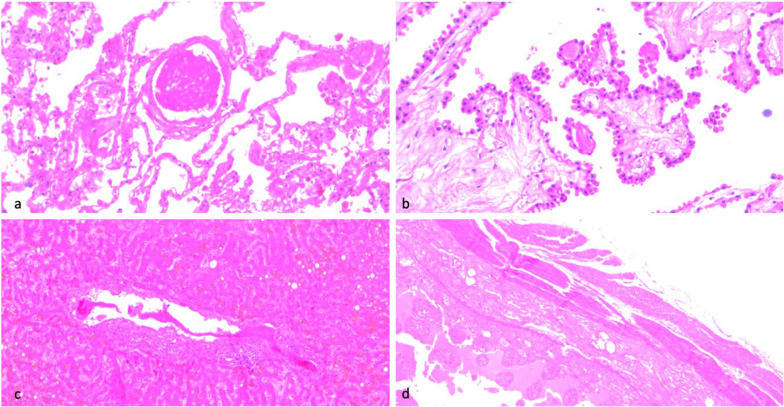
Figure 8 showed a histological sample in a patient who developed vaccine-induced immune thrombocytopenia after viral vector vaccine.
Fig. 8.
Histological sample of a patient who developed vaccine-induced immune thrombocytopenia after viral vector vaccine. a Lung. Thrombosis of a small arterial branch. Original magnification ×200. b Choroid plexuses. Multiple small thrombi are present inside the capillaries of choroid plexuses. H&E. Original magnification × 200. c Liver. Thrombosis of a portal vein branch. ×100. d Ileum. Diffuse severe hemorrhagic necrosis of the wall. ×50
Abdominal complications
Background
A high frequency of non-serious gastrointestinal adverse events was reported including nausea and vomiting, beyond these transitory post-vaccination side effects, some serious adverse events were described [24, 76, 77]. Of the more serious adverse events reported following vaccination, the most common was appendicitis, which was more frequent in younger populations [78]. These findings are in line with the CDC data, reporting appendicitis as the most common severe adverse event in the vaccine group compared with the placebo group [5]. A report by Scott et al. described a potential association between gastroparesis and mRNA vaccine in a previously healthy 57-years- old-man, that developed after both vaccine doses with refractory nausea and vomiting. A nuclear imaging study showed delayed gastric emptying, which improved after a course of prednisone. The authors concluded that the mechanism of action of mRNA vaccine may precipitate immune-mediated gastroparesis [79]. Several reports speculated that there may be a connection between COVID-19 vaccines and pre-existing autoimmune disease exacerbations [80–82]. COVID-19 vaccination has also been associated with the occurrence of glomerular diseases, including minimal change disease [83, 84]. Some authors formulated a hypothesis on how the COVID-19 vaccination can trigger glomerulonephritis, not implicating a direct action by the vaccine itself but rather a T-cell activation leading to podocyte injuries, or through molecular mimicry [84]. Other rare renal adverse events after vaccination against SARS-COV-2 were described as well. In particular, Shakoor reported a new onset renal limited ANCA-associated vasculitis in a 78- year- old woman with previously normal kidney function after receiving the mRNA vaccine [85]. Observational studies formulated a link between different infections and the development of vasculitis, with a poorly understood pathogenesis, that could involve a molecular mimicry mechanism between microbial peptides and antigens [86]. While there have been no reported cases of hepatitis in the registration trials [5, 24, 76], several reports have described biopsy proved autoimmune hepatitis in previously healthy patients [87, 88]. In a recent in-vitro study, a high affinity between antibodies against the spike protein S1 of SARS-COV-2 and human tissue proteins was reported—this included transglutaminase 3, transglutaminase 2, anti-extractable nuclear antigen, nuclear antigen, and myelin basic protein. Similarly, the mRNA vaccine codifying the same viral protein may uncover autoimmune diseases in predisposed patients [89]. These cases support the notion of COVID-19 vaccine-triggered autoimmune phenomena.
Table 4 described previous studies regarding vaccine-related abdominal complications.
Table 4.
Previous case-report about vaccine-related abdominal complications
| Authors | Abdominal complications | Type of vaccine | Number of patients described |
|---|---|---|---|
| Scott et al | Gastroparesis | mRNA | 1 |
| Terracina et al | Flare of rheumatoid arthritis | mRNA | 1 |
| Obeid et al | Reactivation of IgA vasculitis after | mRNA | 1 |
| Rahim et al | IgA nephropathy flare-up | mRNA | 1 |
| Salem et al | minimal change disease | mRNA | 3 |
| Leclerc et al | minimal change disease | Viral vector | 1 |
| Shakoor et al | ANCA-Associated Vasculitis | mRNA | 1 |
| Lodato et al | Autoimmune hepatitis | mRNA | 1 |
| Bril et al | Autoimmune hepatitis | mRNA | 1 |
Imaging
The possible abdominal manifestations and imaging features of abdominal post-vaccination complications are wide. However, to our knowledge, serious abdominal complications following COVID-19 vaccination could be categorized into two major categories: abdominal vascular complications and vaccine-triggered autoimmune phenomena. With regard to the first category, we discussed the potential imaging strategies in the previous section in accordance with published guidelines, that suggest the use of intravenous contrast-enhanced CT of the abdomen and pelvis for diagnosing vessel thrombosis and organ complications, in patients with “possible” or “probable” vaccine-induced immune thrombocytopenia [72, 75]. On the other hand, if vaccine-induced immune thrombocytopenia is “unlike”, abdominal imaging should be obtained if clinically appropriated [72, 75].
Vaccine-triggered abdominal autoimmune phenomena are presented with a wide spectrum of manifestations, from glomerular disease to autoimmune hepatitis [79, 80, 82–85, 87, 88]. In view of these potential manifestations, several non-invasive imaging studies should be performed, including abdominal ultrasound, CT, and MRI [79, 80, 82–85, 87, 88].
Miscellaneous
Among COVID-19 vaccination side effects, some authors described dermatological complications [90–95]. Devon E McMahon reported a spectrum of cutaneous reactions after the mRNA vaccine, such as delayed large local reactions, local injection site reactions, urticarial eruptions, morbilliform eruptions, pernio/chilblains, cosmetic filler reactions, zoster, herpes simplex flares, and pityriasis rosea-like reactions. The authors highlighted that all the skin reactions in the registry are self-limited and minor [92]. Similar results were reported in the literature review by Gronbeck et al., describing that skin reactions were more common following mRNA vaccine and widely self-limited [90].
Another COVID-19 side effect reported was lymphadenopathy [77, 96–99]. In particular, Ozutemiz presented a case series of 5 vulnerable oncologic patients with axillary lymphadenopathy after COVID-19 vaccination [98]. Their results indicated that the lymphadenopathy following immunization may constitute a benign and self-limited condition [98].
Cocco et al. investigated the multiparametric ultrasound findings of patients with post-vaccine lymphadenopathy, describing “worrisome” features, usually suspicious for malignancy including size, shape, morphology, cortex–hilum, SMI, and elastography. The authors highlighted the importance of knowledge of post-vaccination lymph node hypermetabolism, especially in cancer patients to avoid unnecessary biopsy, and appropriately select patients that need a short-term ultrasound follow-up [96].
Previous studies showed potential ocular side effects after COVID-19 vaccination, including panuveitis, acute macular neuroretinopathy, central serous retinopathy [100, 101]. Although no severe ocular complications were described in the registration trials, different vaccines have also been associated with ocular manifestation [100]. In addition, acute corneal graft rejection was described. A case of a 73-years-old man with a penetrating keratoplasty due to keratoconus presented with discomfort in his left eye 13 days after receiving the first dose of mRNA vaccine, with a subsequent diagnosis of corneal graft rejection which improved after drops and oral cortisone [102]. A 66-year-old Caucasian woman endothelial keratoplasty transplant recipient developed acute onset of blurred vision, redness, and photophobia after mRNA vaccine with a clinical appearance typical for acute endothelial graft rejection [103]. Transplant rejection has also been documented in other solid organs [104, 105]. Di Bello et al. reported a case of a 23-year-old who underwent a kidney transplant who presented with acute rejection after the second dose of the mRNA vaccine [104]. Similarly, Vyhmeister et al. described an episode of acute cellular rejection in a liver transplants recipient occurring after the first dose of the mRNA vaccine [105]. In this setting of rapid vaccine deployment, little is known of the efficacy and potential risks of novel SARS-CoV-2 vaccination in transplant recipients, whether this link might be causality or casualty.
Table 5 summarized previous research regarding unusual adverse events after COVID-19 vaccination.
Table 5.
Previous case-report about vaccine-related miscellaneous complications
| Authors | Others complications | Type of vaccine | Number of patients described |
|---|---|---|---|
| Blumenthalet al | Delayed Large Local Reactions | mRNA | 12 |
| Johnston et al | Delayed Localized Hypersensitivity Reactions | mRNA | 16 |
| Ackerman et al | Persistent maculopapular rash | mRNA | 1 |
| Ohsawa et al | Morbilliform rash | mRNA | 1 |
| Ozutemiz et al | Lymphadenopathy | mRNA | 5 |
| Singh et al | Lymphadenopathy | mRNA | 1 |
| Fernández-Prada et al | Lymphadenopathy | mRNA | 20 |
| Fowler et al | central serous retinopathy | mRNA | 1 |
| Mudie et al | Panuveitis | mRNA | 1 |
| Wasser et al | Keratoplasty Rejection | mRNA | 2 |
| Phylactou et al | endothelial corneal transplant rejection | mRNA | 2 |
| Del Bello et al | Transplant rejection | mRNA | 1 |
| Vyhmeister et al | Transplant rejection | mRNA | 1 |
Conclusion
Although multiorgan adverse events have been reported with the COVID-19 vaccines, the benefits of immunization in preventing severe morbidity and mortality overcomes the risk of vaccinations against SARS-COV-2. However, clinicians should be aware of these potential complications when evaluating and managing patients after COVID-19 vaccination, allowing a prompt diagnosis to improve the patient outcomes.
Abbreviations
- CCTA
Coronary computed tomography angiography
- CDC
Center for Disease Control and Prevention
- CMR
Cardiac magnetic resonance
- COVID-19
Coronavirus disease 2019
- CTA
Computed tomography angiography
- MHRA
Medicines and Healthcare Products Regulatory Agency
- MRA
Magnetic resonance angiography
- SARS
Severe acute respiratory syndrome
- STEMI
ST-segment elevation myocardial infarction
- VAERS
Vaccine Adverse Event Reporting System
Authors' contributions
All authors contributed equally as authors to this work. The scientific guarantor of this publication is the corresponding author. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data of this manuscript are available.
Declarations
Ethics approval and consent to participate
Ethics approval and consent to participate approval are not required for this manuscript.
Consent for publication
All authors agreed with the content and gave consent to submit and publish.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cau R, Falaschi Z, Paschè A, et al. CT findings of COVID-19 pneumonia in ICU-patients. J Public health Res. 2021 doi: 10.4081/jphr.2021.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cau R, Pacielli A, Fatemeh H, et al. Complications in COVID-19 patients: characteristics of pulmonary embolism. Clin Imaging. 2021;77:244–249. doi: 10.1016/j.clinimag.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Xiong W, Mu J, et al. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol Scand. 2021;144(1):3–12. doi: 10.1111/ane.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 Vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fc
- 6.Oldenburg J, Klamroth R, Langer F et al (2021) Erratum: Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie. 41(3):e1. 10.1055/s-0041-1729135 [DOI] [PubMed]
- 7.Morais S, Cruz E. Trombose, hemorragia e trombocitopenia Induzidas pelas Vacinas contra a COVID-19: Protocolo de Atuação. Acta Med Port. 2021;34(13):1–5. doi: 10.20344/amp.16602. [DOI] [PubMed] [Google Scholar]
- 8.Parums DV. Editorial: SARS-CoV-2 mRNA vaccines and the possible mechanism of Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Med Sci Monit. 2021;27:1–2. doi: 10.12659/MSM.932899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12(April):1–11. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra HS, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. Ann Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA Vaccines. Circulation. 2021 doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 15.Starekova J, Bluemke DA, Bradham WS, Grist TM, Schiebler ML, Reeder SB. Myocarditis associated with mRNA COVID-19 vaccination. Radiology. 2021 doi: 10.1148/radiol.2021211430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalgaard JB. Fatal myocarditis following smallpox vaccination. Am Heart J. 1957;54(1):156–157. doi: 10.1016/0002-8703(57)90090-x. [DOI] [PubMed] [Google Scholar]
- 17.Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. 2021;39(5):839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination11 the views expressed in this study are those of the authors and do not reflect the official policy or position of the US Government. J Am Coll Cardiol. 2004;44(1):201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khedkar PH, Patzak A. SARS-CoV-2: what do we know so far? Acta Physiol. 2020 doi: 10.1111/apha.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muthukumar A, Narasimhan M, Li Q-Z, et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation. 2021 doi: 10.1161/circulationaha.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur R, Dutta S, Charan J, et al. Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med. 2021;14(July):3909–3927. doi: 10.2147/ijgm.s324349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medicines and Healthcare Products Regulatory Agency (MHRA). Coronavirus vaccine- weekly summary of yellow card reporting. Update 30 July 2021 https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-su
- 25.Chamling B, Vehof V, Drakos S, Weil M, Stalling P, Vahlhaus C (2021) Occurrence of acute infarct ‑ like myocarditis following COVID ‑ 19 vaccination : just an accidental co ‑ incidence or rather vaccination ‑ associated autoimmune myocarditis? Clin Res Cardiol (0123456789). doi:10.1007/s00392-021-01916-w [DOI] [PMC free article] [PubMed]
- 26.Snapiri O, Rosenberg Danziger C, Shirman N, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. 2021;40(10):e360–e363. doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parmar K, Mekraksakit P, Del Rio-Pertuz G, et al. Myocarditis following COVID-19 mRNA vaccination. Baylor Univ Med Cent Proc. 2021 doi: 10.1080/08998280.2021.1990743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abou R, Hwa K, Joo Y, et al (2020) Myocardial infarction after COVID-19 vaccination-casual or causal? 2020–2022
- 29.Srinivasan KN, Sathyamurthy I, Neelagandan M (2020) Relation between COVID-19 vaccination and myocardial infarction e Casual or coincidental?
- 30.Sung JG, Sobieszczyk PS, Bhatt DL. Acute myocardial infarction within 24 hours after COVID-19 vaccination. Am J Cardiol. 2020;2021:4–6. doi: 10.1016/j.amjcard.2021.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajstra M, Jaroszewicz J, Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14(9):e103–e104. doi: 10.1016/j.jcin.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevalence of perimyocarditis after covid-19 vaccine. https:// clini caltr ials. gov/ ct2/ show/ NCT04 865900
- 33.Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz A, Klingel K, Kandolf R, Sechtem U. Imaging in inflammatory heart disease: from the past to current clinical practice. Hellenic J Cardiol. 2009;50(6):449–460. [PubMed] [Google Scholar]
- 35.Cau R, Bassareo P, Cherchi V, et al. Early diagnosis of chemotherapy-induced cardiotoxicity by cardiac MRI. Eur J Radiol. 2020;130:109158. doi: 10.1016/j.ejrad.2020.109158. [DOI] [PubMed] [Google Scholar]
- 36.Cau R, Bassareo P, Saba L (2020) Cardiac Involvement in COVID-19—assessment with echocardiography and cardiac magnetic resonance imaging. SN Compr Clin Med. doi:10.1007/s42399-020-00344-7 [DOI] [PMC free article] [PubMed]
- 37.Cau R, Bassareo PP, Mannelli L, Suri JS, Saba L. Imaging in COVID-19-related myocardial injury. Int J Cardiovasc Imaging. 2020 doi: 10.1007/s10554-020-02089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaak A, Feisst A, Luetkens JA. Myocarditis following COVID-19 vaccination. Radiology. doi:10.1148/radiol.2021211766 [DOI] [PMC free article] [PubMed]
- 39.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 41.Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503–1510. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 42.Collet J-P, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 43.Al NK. Acute intra-coronary thrombus on cardiac CT imaging. J Saudi Hear Assoc. 2011;23(1):49–50. doi: 10.1016/j.jsha.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H, Sun T, Feng J. Complications and pathophysiology of COVID-19 in the nervous system. Front Neurol. 2020;11:573421. doi: 10.3389/fneur.2020.573421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen A, Szabo L, Kaiser Health News (2020) NIH “Very Concerned” aboutSerious Side Effect in Coronavirus Vaccine Trial [Internet]. Scientific American. Available at: https:www.scientificamerican.com/article/nih-very-concerned-about-serious-side-effect-in-c.
- 46.García-Grimshaw M, Ceballos-Liceaga SE, Hernández-Vanegas LE, et al (2021) Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin Immunol 229(June). doi:10.1016/j.clim.2021.108786 [DOI] [PMC free article] [PubMed]
- 47.Bhopal SS, Olabi B BR. Bhopal SS, Olabi B, Bhopal R (2021) Vaccines for COVID- 19: learning from ten phase II trials to inform clinical and public health vacci- nation programmes. Public Health 29(193):57–60. 10.1016/j.puhe.2021.01.011. [DOI] [PMC free article] [PubMed]
- 48.Finsterer J, Scorza FA. SARS-CoV-2 vaccines are not free of neurological side effects. Acta Neurol Scand. 2021;144(1):109–110. doi: 10.1111/ane.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powezka K, Khan T, Narlawar R, Antoniou GA (2020) Bell’s palsy and SARS-CoV-2 vaccines [DOI] [PMC free article] [PubMed]
- 50.Soeiro T, Salvo F, Pariente A, Grandvuillemin A, Jonville-Béra AP MJ (2021) Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell’s palsy. Therapie 76(4):365–367 [DOI] [PMC free article] [PubMed]
- 51.Chen S, Fan X-R, He S, Zhang J-W, Li S-J (2021) Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci [DOI] [PMC free article] [PubMed]
- 52.Allen CM, Ramsamy S, Tarr AW, et al. Guillain–Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 53.Maramattom BV, Krishnan P, Paul R, et al. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 54.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D’Agostini S, Fabris M VM (2020) Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol neuroinflammation. 7(5). doi:10.1212/NXI.0000000000000797
- 56.Cao L, Ren L (2021) Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. doi:10.1007/s13760-021-01608-2 [DOI] [PMC free article] [PubMed]
- 57.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain–Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2):e13426–e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markus HS (2021) Ischaemic stroke can follow COVID-19 vaccination but is much more common with COVID-19 infection itself. J Neurol Neurosurg Psychiatry. 10.1136/jnnp-2021-327057 [DOI] [PubMed]
- 59.Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination: a report of two UK cases. Brain Behav Immun. 2021;95:514–517. doi: 10.1016/j.bbi.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Mayhani T, Saber S, Stubbs MJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 61.Fanni D, Saba L, Demontis R, Gerosa C, Chighine A, Nioi M (2021) Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: a report of an autoptic case and review of the literature. Eur Rev Med Pharmacol Sci 25(15):5063–5069 [DOI] [PubMed]
- 62.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med 10(8). doi:10.3390/jcm10081599 [DOI] [PMC free article] [PubMed]
- 65.Suresh P, Petchey W (2021) ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep 14(6). doi:10.1136/bcr-2021-243931 [DOI] [PMC free article] [PubMed]
- 66.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas A-M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toom S, Wolf B, Avula A, Peeke S, Becker K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am J Hematol. 2021;96(5):E134–E135. doi: 10.1002/ajh.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.International Society on Thrombosis and Haemostasis (ISTH). ISTH guidance on vaccine-induced immune thrombotic thrombocytopenia. 2021 [cited 2021 April 20]. Available at: https://cdn.ymaws.com/www.isth.org/resource/resmgr/news/ISTH_VITT_Flow_Chart_Final.p
- 71.Lavin M, Elder PT, O’Keeffe D, et al (2021) Vaccine-induced immune thrombotic thrombocytopenia (VITT) – a novel clinico-pathological entity with heterogeneous clinical presentations. Br J Haematol. doi:10.1111/bjh.17613 [DOI] [PMC free article] [PubMed]
- 72.COVID-19 rapid guideline: vaccine-induced immune thrombocytopenia and thrombosis (VITT) NICE guideline. Published: 29 July 2021. www.nice.org.uk/guidance/ng200 [PubMed]
- 73.Saba L, Agarwal N, Cau R, et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS Vasc Sci. 2021 doi: 10.1016/j.jvssci.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cademartiri F, Balestrieri A, Cau R, et al. Insight from imaging on plaque vulnerability: similarities and differences between coronary and carotid arteries—implications for systemic therapies. Cardiovasc Diagn Ther. 2020;10(4):1150–1162. doi: 10.21037/cdt-20-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imaging Recommendations for Patients Suspected of VITT, The Royal Australian and New Zealand College of Radiologists (RANZCR). June 2021. https://www.ranzcr.com/our-work/coronavirus/position-statements-and-guidance
- 76.Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karron RA, Key NS, Sharfstein JM (2021) Assessing a rare and serious adverse event following administration of the Ad26.COV2.S vaccine. JAMA 325(24):2445–2447. doi:10.1001/jama.2021.7637 [DOI] [PubMed]
- 78.Li X, Ostropolets A, Makadia R, et al (2021) Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv Prepr Serv Heal Sci. doi:10.1101/2021.03.25.21254315 [DOI] [PMC free article] [PubMed]
- 79.Scott J, Anderson J, Mallak N, Beitinjaneh B (2021) Gastroparesis after Pfizer- BioNTech Vaccination. Published online 2021:1–2 [DOI] [PubMed]
- 80.Rahim SEG, Lin JT, Wang JC. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100(1):238. doi: 10.1016/j.kint.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3(7):e469–e470. doi: 10.1016/S2665-9913(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obeid M, Fenwick C, Pantaleo G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021;9913(21):9913. doi: 10.1016/s2665-9913(21)00211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salem F, Rein J, Mon-Wei Yu S, Abramson M, Cravedi P, Chung M (2021) Report of three cases of minimal change disease following the second dose of mRNA SARS-CoV-2 vaccine. Kidney Int Rep. Published online August 9, 2021. doi:10.1016/j.ekir.2021.07.017 [DOI] [PMC free article] [PubMed]
- 84.Leclerc S, Royal V, Lamarche C, Laurin L-P. Minimal change disease with severe acute kidney injury following the Oxford-AstraZeneca COVID-19 vaccine: a case report. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Timmeren MM, Heeringa P, Kallenberg CGM. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26(4):416–423. doi: 10.1097/BOR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 87.Lodato F, Larocca A, D’Errico A, Cennamo V (2021) an anusual case of acute cholestatic hepatitis after m-RNABNT162b2 (COMIRNATY) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug related liver injury? J Hepatol. Published online August 10, 2021. doi:10.1016/j.jhep.2021.07.005 [DOI] [PMC free article] [PubMed]
- 88.Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gronbeck C, Grant-Kels JM. Attention all anti-vaccinators: the cutaneous adverse events from the mRNA COVID-19 vaccines are not an excuse to avoid them! Clin Dermatol. 2021 doi: 10.1016/j.clindermatol.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cazzato G, Romita P, Foti C, et al (2021) Purpuric skin rash in a patient undergoing pfizer-BioNTech COVID-19 vaccination: histological evaluation and perspectives. Vaccines 9(7). doi:10.3390/vaccines9070760 [DOI] [PMC free article] [PubMed]
- 94.Ohsawa R, Sano H, Ikeda M, Sano S (2021) Clinical and histopathological views of morbilliform rash after COVID-19 mRNA vaccination mimic those in SARS-CoV-2 virus infection-associated cutaneous manifestations. J Dermatol Sci. Published online June 2021. doi:10.1016/j.jdermsci.2021.06.006 [DOI] [PMC free article] [PubMed]
- 95.Ackerman M, Henry D, Finon A, Binois R, Esteve E. Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(7):e423–e425. doi: 10.1111/jdv.17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cocco G, Delli Pizzi A, Fabiani S, et al (2021) Lymphadenopathy after the Anti-COVID-19 Vaccine: multiparametric ultrasound findings. Biology (Basel) 10(7). 10.3390/biology10070652 [DOI] [PMC free article] [PubMed]
- 97.Singh B, Kaur P, Kumar V, Maroules M. COVID-19 vaccine induced Axillary and Pectoral Lymphadenopathy on PET scan. Radiol Case Rep. 2021;16(7):1819–1821. doi: 10.1016/j.radcr.2021.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300(1):E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernández-Prada M, Rivero-Calle I, Calvache-González A, Martinón-Torres F (2021) Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill 26(10):2100193. 10.2807/1560-7917.ES.2021.26.10.2100193 [DOI] [PMC free article] [PubMed]
- 100.Fowler N, Mendez Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Reports. 2021;23:101136. doi: 10.1016/j.ajoc.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mudie LI, Zick JD, Dacey MS, Palestine AG (2021) Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. Published online July 2, 2021:1–2. doi:10.1080/09273948.2021.1949478 [DOI] [PubMed]
- 102.Wasser LM, Roditi E, Zadok D, Berkowitz L, Weill Y. Keratoplasty rejection after the BNT162b2 messenger RNA vaccine. Cornea. 2021;40(8):1070–1072. doi: 10.1097/ICO.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phylactou M, Li J-PO, Larkin DFP (2021) Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol 105(7):893 LP–896. doi:10.1136/bjophthalmol-2021-319338 [DOI] [PubMed]
- 104.Del Bello A, Marion O, Delas A, Congy-Jolivet N, Colombat M, Kamar N. Acute rejection after anti-SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021;100(1):238–239. doi: 10.1016/j.kint.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vyhmeister R, Enestvedt CK, VanSandt M, Schlansky B (2021) Steroid-resistant acute cellular rejection of the liver after severe acute respiratory syndrome coronavirus 2 mRNA vaccination. Liver Transpl. doi:10.1002/lt.26097 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this manuscript are available.