Abstract
Sotrovimab (Xevudy®) is a recombinant human monoclonal antibody targeted against the severe acute respiratory syndrome coronavirus 2. It is being developed by Vir Biotechnology in collaboration with GlaxoSmithKline for the treatment of coronavirus disease 2019 (COVID-19). Sotrovimab received its first emergency use authorization in May 2021 for the treatment of COVID-19 in the USA, with interim, emergency or conditional authorizations subsequently granted in several other countries. In December 2021, sotrovimab received its first full approval in the EU for use in adolescents (aged ≥ 12 years and weighing ≥ 40 kg) and adults with COVID-19 who do not require oxygen supplementation and who are at high risk of progressing to severe COVID-19. This article summarizes the milestones in the development of sotrovimab leading to this first approval.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01690-7.
| Digital Features for this AdisInsight Report can be found at 10.6084/m9.figshare.19172528 |
Sotrovimab (Xevudy®): Key Points
| A recombinant human monoclonal antibody is being developed by Vir Biotechnology and GlaxoSmithKline for the treatment of COVID-19 |
| Received its first full approval on 17 December 2021 in the EU |
| Approved for the treatment of COVID-19 in adolescents (aged ≥ 12 years and weighing ≥ 40 kg) and adults who do not require oxygen supplementation and who are at high risk of progressing to severe COVID-19 |
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative virus of coronavirus disease 2019 (COVID-19), it has spread rapidly around the world, resulting in the currently ongoing COVID-19 pandemic [1]. Symptoms of COVID-19 can vary in severity, from being asymptomatic to being fatal, with older age and certain comorbidities, such as obesity, diabetes, chronic obstructive pulmonary disease and chronic kidney disease, being risk factors for severe COVID-19 [1]. The National Institutes of Health guideline recommends monoclonal antibodies for the treatment of non-hospitalized adults with mild to moderate COVID-19 who are at high risk of disease progression [2].
Sotrovimab (Xevudy®), a recombinant human monoclonal antibody targeted against the SARS-CoV-2, is being developed by Vir Biotechnology in collaboration with GlaxoSmithKline for the treatment of COVID-19. Sotrovimab neutralizes SARS-CoV-2 by binding to a highly conserved epitope located on the receptor binding domain (RBD) of the virus’ spike protein [3]. Preclinical studies indicate that the antibody provides a high barrier against viral escape and retains antiviral activity against newer SARS-CoV-2 variants that are associated with increased transmissibility and immune invasion, including Omicron variant (Sect. 2.1) [3, 4].
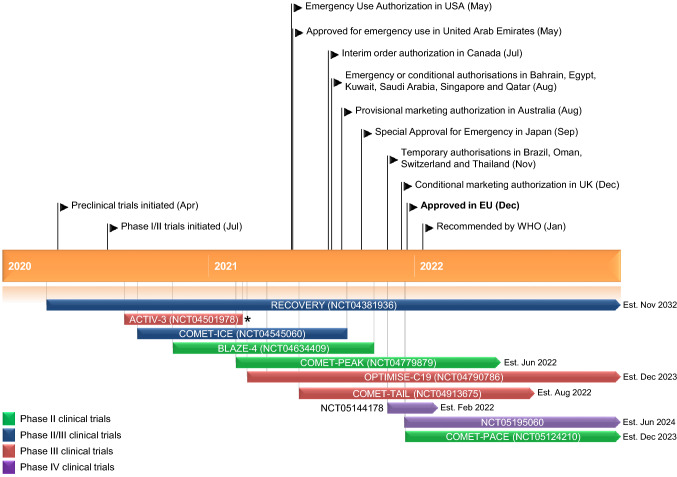
Key milestones in the development of sotrovimab for the treatment of COVID-19. *Evaluation of other monoclonal antibodies is ongoing; the estimated completion date of ACITV-3 is July 2022.
Sotrovimab received its first emergency use authorization in May 2021 in the USA for the treatment of mild to moderate COVID-19 in patients who are at high risk for progressing to severe COVID-19 [5, 6], with similar interim, emergency or conditional authorizations subsequently granted in a number of other countries, including Bahrain, Brazil, Canada, Egypt, Japan, Kuwait, Saudi Arabia, Singapore, Qatar, Switzerland, Thailand and the United Arab Emirate [7–10]. On 17 December 2021, sotrovimab received its first full approval in the EU for the treatment of COVID-19 in adolescents aged ≥ 12 years and weighing ≥ 40 kg and adults who do not require oxygen supplementation and who are at high risk of progressing to severe COVID-19 [11, 12]. Sotrovimab was also granted provisional marketing authorization in Australia [13] and conditional marketing authorization in the UK [14].
The recommended dose of sotrovimab is 500 mg, administered as a single 30 min intravenous (IV) infusion; the solution must be diluted with 0.9% sodium chloride injection or 5% dextrose for injection prior to administration [11]. Sotrovimab is recommended to be administered within 5 days of COVID-19 symptom onset and should be administered in healthcare facilities where patients can be monitored during and at least 1 h after administration. The efficacy and safety of sotrovimab have not yet been established in children aged < 12 years or those weighing < 40 kg [11]. The recommended administration of sotrovimab (e.g. IV infusion duration and timing of administration) may differ between countries; please see local prescribing information for administration details.
In addition to IV administration, intramuscular (IM) administration of sotrovimab for the treatment of COVID-19 is being evaluated to increase patient access and convenience. In January 2022, an application for sotrovimab was submitted in the USA to expand its emergency use authorization to include IM administration [15].
Company Agreements and Patent Information
In February 2020, Vir Biotechnology and WuXi Biologics entered into a development and manufacturing collaboration for production of human monoclonal antibodies for the treatment of COVID-2019 [16]. Under the terms of the agreement, the companies will work together to develop, manufacture and commercialize Vir biotechnology’s proprietary antibodies. Once approved, Vir Biotechnology has the rights to commercialize products worldwide, except in Greater China where WuXi Biologics has the rights to commercialize products [16].
In March 2020, Vir Biotechnology entered into a license agreement with Xencor to gain a non-exclusive access to Xencor’s Xtend™ XmAb® Fc technology to extend the half-life of antibodies that are being investigated as potential COVID-19 treatments [17]. In March 2020, Vir Biotechnology also entered into a research collaboration with the National Institutes of Health Vaccine Research Center to characterize and develop antibodies against coronavirus, including SARS-CoV-2 [18].
In April 2020, Vir Biotechnology and GlaxoSmithKline signed a binding agreement to research and develop solutions for coronaviruses, including SARS-CoV-2 [19]. The collaboration will use Vir Biotechnology’s proprietary monoclonal antibody platform technology to identify and develop antibodies that could be used for the prevention and treatment of COVID-19 and other coronaviruses [19]. The collaboration agreement was expanded in February 2021 to include the research and development of new therapies for influenza and other respiratory viruses [20]. Under the terms of agreements, GlaxoSmithKline will make an equity investment in Vir Biotechnology [19]. In April 2020, Vir Biotechnology and Samsung Biologics entered into a manufacturing agreement under which the latter will manufacture SARS-CoV-2 antibodies VIR-7831 (sotrovimab) and VIR-7832 for the treatment of COVID-2019 [21].
In May 2020, Vir Biotechnology and Biogen entered a development and manufacturing agreement under which the latter will perform process development activities and provide specified manufacturing and process transfer services to enable commercial supply of Vir Biotechnology’s SARS-CoV-2 monoclonal antibody candidates, including sotrovimab [22]. The agreement complements its existing manufacturing agreements with WuXi Biologics and Samsung Biologics [22].
In January 2021, Eli Lilly entered an agreement with Vir Biotechnology and GlaxoSmithKline to evaluate the efficacy of bamlanivimab in combination with sotrovimab in the ongoing BLAZE-4 trial (completed in October 2021) in low risk patients with mild to moderate COVID-2019 [23, 24].
In Jul 2021, Vir Biotechnology and GlaxoSmithKline announced a joint procurement agreement with the European Commission to provide up to 220,000 doses of sotrovimab to participating member states [25]. As of January 2022, Vir Biotechnology and GlaxoSmithKline have announced purchase agreements of sotrovimab with numerous countries, including Australia [26], Canada [27], Singapore [28] and the USA [29, 30]. Through national agreements, the antibody is also supplied in Japan, Switzerland, UK and the United Arab Emirates [30].
As of December 2020, Vir technology has multiple US patent applications in the sotrovimab intellectual property portfolio with claims to the compositions of matters, pharmaceutical composition and methods of treatment, which are presently estimated to expire in 2041, absent any available patent term adjustments or extensions [31]. The patent portfolio also includes non-exclusively in-licensed patents and applications from Xencor, including 98 issued patents and 4 pending patent applications in various countries and jurisdictions for composition, methods of extending antibody serum half-life claims, pharmaceutical composition claims, method of treatment claims and process (methods of producing) claims, which are presently estimated to expire between 2021 and 2028, absent any available patent term adjustments or extensions [31].
Scientific Summary
Pharmacodynamics
Sotrovimab is a recombinant human monoclonal immunoglobulin G1 antibody that has been engineered with Xencor’s proprietary Xtend™ technology to enhance distribution in the lungs and to extend antibody half-life [4]. Sotrovimab binds with high affinity to a highly conserved epitope of the SARS-CoV-2 spike protein RBD (dissociation constant Kd = 0.21 nM), thereby suppressing viremia and accelerating infected cell clearance [32]. The antibody targets a RBD epitope that is outside the receptor binding motif and therefore does not compete with angiotensin converting enzyme 2 binding [3, 32].
In vitro, sotrovimab neutralized wild-type SARS-CoV-2 virus in a concentration-dependent manner, with a half maximal effective concentration (EC50) of 100.1 ng/mL [3, 32]. In a SARS-CoV-2 infected hamster model, sotrovimab significantly decreased weight loss (a surrogate for clinical disease), and total viral load and infectious virus levels in the lung [3, 32].
In vitro, sotrovimab displayed a high barrier to resistance to COVID-19 and neutralized pseudotyped viruses expressing spike monoclonal antibody resistance mutations that confer reduced susceptibility to bamlanivimab, casirivimab and/or imdevimab [3, 32]. Of note, reduced susceptibility to sotrovimab (as measured by changes in EC50 values) was observed with epitope variants K356T, P337H/L/R/T and E340A/K/G; however, the clinical impact of these variants is not yet known [3, 32].
On the basis of preclinical studies, the neutralization potency of sotrovimab is anticipated to be retained against a number of SARS-CoV-2 variants of interest, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Epsilon (B.1.427/B.1.429), Iota (B.1.526), Kappa (B.1.617.1), Delta (B.1.617.2), Lambda (C.37). Delta Plus (AY.1/AY.2), Mu (B.1.621) and Omicron (B.1.1.529) [3, 4, 11, 33, 34]. Notably, of all the tested monoclonal antibodies, including bamlanivimab, casirivimab, cilgavimab, imdevimab, sotrovimab and tixagevimab, only sotrovimab demonstrated significant neutralisation of Omicron variant in vitro [3, 33–36].
Pharmacokinetics
Following a single 1 h IV infusion of sotrovimab 500 mg, the mean maximum concentration (Cmax) was 117.6 µg/mL and the mean concentration on day 29 was 24.5 µg/mL; the median time to Cmax was 0.042 days [11, 32]. Based on non-compartmental analysis, the mean steady-state volume of distribution, the mean systemic clearance and the median terminal half-life was 8.1 L, 125 mL/day and ≈ 49 days, respectively [11]. Sotrovimab is degraded by proteolytic enzymes that are widely distributed in the body [11]. Of note, a phase I single-dose study (NCT04988152) to investigate the pharmacokinetics, safety and tolerability of IV or IM administration of sotrovimab versus placebo in Japanese and Caucasian participants was completed in December 2021.
Patient characteristics, including age and kidney or hepatic impairments, do not appear to have any clinically significant impact on the pharmacokinetics or elimination of sotrovimab [11]. Given that sotrovimab is not metabolized by CYP450 enzymes or renally excreted, drug-drug interactions between sotrovimab and drugs that are substrates, inducers or inhibitors of CYP450 enzymes or that are renally excreted are unlikely [11].
Features and properties of sotrovimab
| Alternative names | GSK-4182136; VIR-7831; WBP 2275; Xevudy |
| Class | Antivirals; Monoclonal antibodies |
| Mechanism of action | Virus internalization inhibitor |
| Route of administration | Intravenous infusion; intramuscular |
| Pharmacodynamics | Binds to a highly conserved epitope located on the spike protein receptor binding domain of SARS-CoV-2 |
| Pharmacokinetics | Reaches maximum plasma concentration within 0.042 days; volume of clearance 8.1 L, clearance 125 mL/day; terminal half-life ≈ 49 days; degraded by proteolytic enzymes |
| Adverse events | Diarrhoea, hypersensitivity reactions, infusion-related reactions |
| ATC codes | |
| WHO ATC code | J06B-D05 (Sotrovimab) |
| EphMRA ATC code | J5 (Antivirals for Systemic Use) |
Therapeutic Trials
Sotrovimab reduced the risk of disease progression in non-hospitalized adults with symptomatic, mild to moderate COVID-19 who are at high risk of progressing to severe disease in a multicenter, double-blind, placebo-controlled phase II/III COMET-ICE trial (NCT04545060) [37, 38]. Enrolled patients were aged ≥ 18 years, had tested positive for SARS-CoV-2 infection, confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) or antigen test, and had symptom onset within 5 days prior to randomization. Patients were also required to have ≥ 1 of the risk factors: aged ≥ 55 years, diabetes, obesity (body mass index > 30 kg/m2), chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73 m2), congestive heart failure (New York Heart Association class II–IV), chronic obstructive pulmonary disease or moderate to severe asthma. Patients with severe COVID-19 symptoms (shortness of breath at rest, < 94% oxygen saturation or requiring supplemental oxygen) were among those excluded [37, 38].
Patients were randomized to a single dose of sotrovimab 500 mg (n = 528) or placebo (n = 529), administered as a 1 h IV infusion [37, 38]. Randomization was stratified by age, duration of COVID-19 symptoms and region. Baseline demographic and disease characteristics were generally similar between the groups. In the intent-to-treat population, the most common pre-defined risk factors were obesity (63%), followed by aged ≥ 55 years (47%), diabetes (22%) and moderate to severe asthma (17%). Treatment was administered within 3 days or 4–5 days of COVID-19 symptom onset in 59% and 41% of patients, respectively [37, 38].
The proportion of patients who progressed to all-cause hospitalization for > 24 h or death due to any cause through day 29 (primary endpoint) was 1% in the sotrovimab group versus 6% in the placebo group, a significant (p < 0.001) relative risk reduction of 79% (95% CI 50–91%), with 0 and 14 patients in the respective groups requiring oxygen support or mechanical ventilation [38]. By day 29, there were no deaths in the sotrovimab group and 2 deaths in the placebo group [38].
Sotrovimab also provided significant (p ≤ 0.007) benefits over placebo for the first four key hierarchically tested secondary endpoints, which included all-cause emergency room visits, hospitalizations of any duration or deaths (adjusted relative risk reduction 66%; 95% CI 37–81%), change in viral load from baseline to day 8 (least square mean difference − 0.232 log10 copies/mL; 95% CI − 0.399 to − 0.065) in virology population (n = 733), progression to severe/critical respiratory COVID-19 requiring supplemental oxygen (adjusted relative risk reduction 74%; 95% CI 41–88%) and change in symptoms as measured by inFLUenza Patient-Reported Outcome (FLU-PRO) Plus total score from baseline to day 7 (least square mean difference − 1.07; 95% CI − 1.38 to − 0.76) [38]. All-cause mortality, one of the key hierarchical secondary endpoints, was not formally analysed due to a fewer number of anticipated deaths [38].
Primary endpoint was met in an ongoing, multicenter, randomized, open-label phase III COMET-TAIL trial (NCT04913675) indicating that IM administration of sotrovimab was noninferior to IV administration for the treatment of mild to moderate COVID-19 in non-hospitalized, high-risk adolescents aged ≥ 12 years and adults (n = 983) [10]. Following IM or IV administration of sotrovimab 500 mg, progression to all-cause hospitalization for > 24 h or death due to any cause through day 29 occurred in 2.7% and 1.3% of patients, respectively; the adjusted difference between the administrations was 1.07% and the upper bound of the 95% CI did not exceed the predetermined noninferiority margin of 3.5% (primary endpoint) [10].
In a prospective, propensity-matched observational cohort study in non-hospitalized patients with mild to moderate COVID-19 due to Delta variant (n = 3069), casirivimab plus imdevimab or sotrovimab treatment significantly reduced the risk of hospitalization or death by 28 days compared with no treatment (risk ratio 0.4; 95% CI 0.28–0.57), with the comparative effectiveness between the treatments appearing to be similar [39]. In addition, in an ongoing randomized, parallel-assignment, open-label phase III OPTIMISE-C19 trial (NCT04790786) in patients with COVID-19 who received casirivimab plus imdevimab (n = 2454) or sotrovimab (n = 1104), the median hospital-free days were 28 days [39].
Interim futility analyses of two neutralizing monoclonal antibody therapies, namely sotrovimab and BRII-196 plus BRII-198, in a multinational, double-blind, randomized placebo-controlled phase III ACTIV-3 trial (NCT04501978) in hospitalized adults with COVID-19 have been reported [40]. There was no significant (based on 95% CI) difference between sotrovimab (n = 182) and placebo (n = 178) recipients with respect to a more favourable category on the pulmonary scale at day 5 (adjusted odds ratio 1.07; 95% CI 0.74–1.56), a more favourable category on the pulmonary-plus complication scale at day 5 (1.08; 0.74–1.58) and a more favorable sustained clinical recovery by day 90 (adjusted rate ratio 1.12; 0.91–1.37). Enrolment in the sotrovimab arm was halted on the basis of the analysis [40].
Preliminary positive topline results from a randomized, double-blind, placebo-controlled phase II BLAZE-4 trial (NCT04634409) in low-risk adult patients with mild to moderate COVID-19 have been reported [41]. Combination therapy of bamlanivimab 700 mg with sotrovimab 500 mg significantly (p < 0.001) reduced the percentage of patients with high viral load (i.e. viral load > 5.27) by 70% at day 7 versus placebo (primary endpoint). In addition, there were no COVID-19 related hospitalization or death by day 29 and the combination therapy significantly reduced the mean SARS-CoV-2 viral load relative to placebo from baseline to days 3, 5 and 7 [41].
Key clinical trials of sotrovimab
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor (collaborator) |
|---|---|---|---|---|---|---|
| Sotrovimab | Treatment of COVID-19 (non-hospitalized) | IV | Recruiting | United Arab Emirates | NCT05144178, No108/2021EHS | Emirates Health Services |
| Sotrovimab, casirivimab/imdevimab, molnupiravir | Treatment of COVID-19 (observational cohort) | IV | Recruiting | Netherlands | NCT05195060, NL78705-018-21, TURN-COVID | Academisch Medisch Centrum—Universiteit van Amsterdam |
| Sotrovimab (IM) and (IV) | Treatment of COVID-19 (non-hospitalized) | III | Ongoing | Multinational | NCT04913675, EudraCT2021-000623-13, P024-2021, VIR7831-5008, COMET-TAIL | GlaxoSmithKline, Vir Biotechnology |
| Sotrovimab, amubarvimab, bamlanivimab, cilgavimab/tixagevimab, ensovibep, romlusevimab | Treatment of COVID-19 (hospitalized) | III | Completeda | Multinational | NCT04501978, EudraCT2020-003278-37, 014ACTIV3, INSIGHT014, ACTIV-3 | AstraZeneca, Brii Biosciences, Eli Lilly, GlaxoSmithKline, Molecular Partners AG, Novartis, NRx Pharmaceuticals, Pfizer, Vir Biotechnology |
| Sotrovimab, bamlanivimab, bamlanivimab + etesevimab, casirivimab/imdevimab | Treatment of COVID-19 (non-hospitalized) | III | Ongoing | USA | NCT04790786; OPTIMISE-C19 | University of Pittsburgh, Berry consultants |
| Sotrovimab (IV), placebo | Treatment of COVID-19 (non-hospitalized) | II/III | Completed | Multinational | NCT04545060, GSK-Study214367, VIR7831-5001, COMET-ICE | GlaxoSmithKline, Vir Biotechnology |
| Sotrovimab, aspirin, casirivimab/imdevimab, colchicine, corticosteroid, immunoglobulin, tocilizumab, anakinra; azithromycin; convalescent plasma, baricitinib, empagliflozin, hydroxychloroquine, lopinavir/ritonavir, infliximab | Treatment of COVID-19 (hospitalized) | II/III | Recruiting | Multinational | NCT04381936, EudraCT2020-001113-21, 50189673, RECOVERY | University of Oxford, UK research and innovation, National Institute for Health Research, Bill and Melinda Gates Foundation |
| Sotrovimab (IV), bamlanivimab (IV), bebtelovimab (IV), etesevimab (IV), placebo | Treatment of COVID-19 (non-hospitalized) | II | Completed | Multinational | NCT04634409, 18160, J2X-MC-PYAH, BLAZE-4 | Abcellera, Eli Lilly, GlaxoSmithKline, Junshi Biosciences—Impact Therapeutics, Shanghai Junshi Biosciences, Vir Biotechnology |
| Sotrovimab (IV) and (IM) | Treatment of COVID-19 (non-hospitalized) | II | Ongoing | Multinational | NCT04779879, EudraCT2021-000724-35, 216912, VIR7831-5006, GSK-Study216912, COMET-PEAK | GlaxoSmithKline, Vir Biotechnology |
| Sotrovimab (IV) and (IM) | Treatment of COVID-19 (non-hospitalized children) | II | Recruiting | Multinational | NCT05124210, EudraCT2021-003717-18, 215226, 215226VIR7831-5005, P468/2021, COMET-PACE | GlaxoSmithKline, Vir Biotechnology |
| Sotrovimab (IV) | Prevention of COVID-19 | II | Recruiting | USA | NCT05210101 | GlaxoSmithKline |
| Sotrovimab (IV) | Prevention of COVID-19 | I | Recruiting | USA | NCT05135650; COVIDMAB | Vir Biotechnology |
COVID-19 coronavirus disease 2019, IM intramuscular, IV intravenous
aEvaluation of other monoclonal antibodies is ongoing
Adverse Events
In the COMET-ICE study in patients with mild to moderate COVID-19, the overall incidence of adverse events (AEs) in the sotrovimab (n = 523) and placebo (n = 526) groups was 22% and 23%, with the most common (> 1%) AE occurring more frequently with sotrovimab than placebo being diarrhoea (2% vs < 1%) [38]. Serious AEs and grade 3 or 4 AEs occurred in 2% and 3% of sotrovimab recipients versus 6% and 7% of placebo recipients, respectively; no serious AEs were considered to be related to sotrovimab. During the study (including follow-up period), there were no deaths in the sotrovimab group and four deaths in the placebo group (due to COVID-19 pneumonia, pneumonia or respiratory failure). The incidence of systemic infusion-related reactions (including hypersensitivity reactions), an AE of special interest, was low and similar between the treatment groups (1% in each group), with all reactions being grade 1 or 2 in severity. Results from the COMET-ICE study suggest that sotrovimab is not associated with antibody-dependent enhancement [38].
In the COMET-TAIL study, serious AEs and grade 3 or 4 AEs were rare in patients receiving IM or IV administration of sotrovimab [10].
Ongoing Clinical Trials
In addition to the ongoing phase III COMET-TAIL (NCT04913675; Sect. 2.3) and OPTIMISE-C19 (NCT04790786; Sect. 2.3) trials, a multinational, randomized, double-blind phase II trial (COMET-PEAK; NCT04779879) to evaluate the safety and pharmacokinetics of a second generation sotrovimab in non-hospitalized patients with mild to moderate COVID-19 is ongoing. Recruitment is underway in a multinational, randomized, open-label phase II/III trial (RECOVERY; NCT04381936) to evaluate the safety and efficacy of multiple treatments, including sotrovimab, in hospitalized patients with COVID-19. Also recruiting is an open-label phase II trial (COMET-PACE; NCT05124210) to evaluate the pharmacokinetics, pharmacodynamics and safety of sotrovimab in paediatric patients aged < 18 years with mild to moderate COVID-19 who are at high risk of disease progression.
A prospective observational study (TURN-COVID; NCT05195060) in Netherlands is recruiting patients to evaluate the use of neutralizing monoclonal antibodies, including sotrovimab, and other antiviral agents for the treatment of COVID-19. Recruitment is also underway in a retrospective observational study (NCT05144178) in the United Arab Emirates to evaluate the efficacy of sotrovimab in ambulatory patients with early symptomatic COVID-19 who are at high risk for hospitalization.
Sotrovimab is also being investigated for the prevention of COVID-19. An open-label phase II trial (NCT05210101) is recruiting immunocompromised patients with impaired humoral immunity against SARS-CoV-2 to examine the tolerability of IV sotrovimab when administered as prophylaxis for COVID-19. A phase I study (COVIDMAB; NCT05135650) is recruiting patients undergoing hematopoietic stem cell transplantation to evaluate the pharmacokinetics of sotrovimab when administered as pre-exposure prophylaxis for COVID-19.
Current Status
Sotrovimab received its first full approval on 17 December 2021 for the treatment of COVID-19 in the EU [11, 12].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Young-A Heo is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
References
- 1.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/. Accessed 15 Feb 2022.
- 3.Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2021:2021.03.09.434607.
- 4.GlaxoSmithKline. Preclinical studies demonstrate sotrovimab retains activity against the full combination of mutations in the spike protein of the Omicron SARS-CoV-2 variant [media release]. 8 Dec 2021. http://www.gsk.com.
- 5.US Food & Drug Administration. Emergency use authorization: sotrovimab. 2021. https://www.fda.gov. Accessed 25 Jan 2022.
- 6.GlaxoSmithKline. GSK and Vir Biotechnology announce sotrovimab (VIR-7831) receives emergency use authorization from the US FDA for treatment of mild-to-moderate COVID-19 in high-risk adults and pediatric patients [media release]. 26 May 2021. http://www.gsk.com.
- 7.GlaxoSmithKline. Japan approves second drug for mildly ill COVID-19 patients [media release]. 30 Sep 2021. http://www.gsk.com.
- 8.GlaxoSmithKline. GSK receives authorization under health Canada's interim order for sotrovimab for injection to treat COVID-19 in high-risk adults and adolescents [media release]. 30 Jul 2021. http://www.gsk.ca.
- 9.Vir Biotechnology. Vir Biotechnology provides corporate update and reports second quarter 2021 financial results [media release]. 5 Aug 2021. http://www.vir.bio.
- 10.GlaxoSmithKline. Primary endpoint met in COMET-TAIL phase 3 trial evaluating intramuscular administration of sotrovimab for early treatment of COVID-19 [media release]. 15 Nov 2021. http://www.gsk.com.
- 11.GlaxoSmithKline. Xevudy 500 mg concentrate for solution for infusion: summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf. Accessed 25 Jan 2022.
- 12.GSK. Xevudy (sotrovimab) granted marketing authorisation by the European Commission for the early treatment of COVID-19 [media release]. 17 Dec 2021. https://www.gsk.com/en-gb/media/press-releases/xevudy-sotrovimab-granted-marketing-authorisation-by-the-european-commission-for-the-early-treatment-of-covid-19/.
- 13.Therapeutic Goods Administration. TGA provisionally approves GlaxoSmithKline's COVID-19 treatment: sotrovimab (Xevudy) [media release]. 25 Aug 2021. http://www.tga.gov.au.
- 14.Mahase E. Covid-19: UK approves monoclonal antibody sotrovimab for over 12s at high risk. BMJ. 2021;375:n2990. doi: 10.1136/bmj.n2990. [DOI] [PubMed] [Google Scholar]
- 15.GlaxoSmirthKline. GSK and Vir submit emergency use authorization application to FDA for intramuscular administration of sotrovimab for the early treatment of COVID-19 [media release]. 13 Jan 2022. http://us.gsk.com.
- 16.Vir Biotechnology. Vir Biotechnology and WuXi Biologics announce collaboration for global development of antibodies to treat COVID-19 [media release]. 26 Feb 2020. http://www.vir.bio.
- 17.Xencor, Vir Biotechnology. Xencor and Vir Biotechnology enter license agreement for use of Xtend(T) XmAb(R) antibody technology in investigational antibodies to treat COVID-19 [media release]. 26 Mar 2020. http://www.xencor.com.
- 18.Vir Biotechnology. Vir Biotechnology announces research collaboration with the National Institutes of Health Vaccine Research Center on antibodies against coronaviruses [media release]. 12 Mar 2020. http://www.vir.bio.
- 19.GlaxoSmithKline, Vir Biotechnology. GSK and Vir Biotechnology enter collaboration to find coronavirus solutions [media release]. 6 Apr 2020. http://www.gsk.com.
- 20.GlaxoSmithKline. GSK and Vir Biotechnology expand coronavirus collaboration to advance new therapeutics for influenza and other respiratory viruses [media release]. 18 Feb 2021. http://www.gsk.com.
- 21.Samsung BioLogics. Samsung Biologics and Vir Biotechnology enter into agreement for large scale manufacture of SARS-COV-2 antibodies for potential COVID-19 treatment [media release]. 13 Apr 2020. http://www.samsungbiologics.com.
- 22.Vir Biotechnology., Biogen. Vir Biotechnology and Biogen execute agreement to manufacture SARS-CoV-2 antibodies for potential COVID-19 treatment [media release]. 1 Jun 2020. http://www.vir.bio.
- 23.Eli Lilly. Lilly, Vir Biotechnology and GSK announce first patient dosed in expanded BLAZE-4 trial evaluating bamlanivimab (LY-CoV555) with VIR-7831 (GSK4182136) for COVID-19 [media release]. 28 Jan 2021. http://www.lilly.com.
- 24.Abcellera. AbCellera-discovered Antibody, bamlanivimab, to be developed with VIR-7831 for the treatment of COVID-19 [media release]. 27 Jan 2021. http://www.abcellera.com.
- 25.GlaxoSmithKline. GSK and Vir Biotechnology announce joint procurement agreement with European Commission for COVID-19 treatment, sotrovimab [media release]. 29 Jul 2021. http://www.gsk.com.
- 26.Vir Biotechnology. Vir Biotechnology announces first marketing authorization for its first commercial product, sotrovimab, granted in Australia [media release]. 24 Aug 2021. http://www.vir.bio.
- 27.GlaxoSmithKline. GSK announces purchase agreement with the government of Canada for COVID-19 monoclonal antibody therapy, sotrovimab [media release]. 5 Oct 2021. http://www.ca.gsk.com.
- 28.GlaxoSmithKline. GSK Singapore and Vir Biotechnology announce advance purchase agreement with the government of Singapore for monoclonal antibody sotrovimab (VIR-7831) [media release]. 5 Jul 2021. http://www.gsk.com.
- 29.GlaxoSmithKline, Vir Biotechnology. GSK and Vir Biotechnology announce United States government agreements to purchase sotrovimab, a COVID-19 treatment [media release]. 18 Nov 2021. http://www.gsk.com.
- 30.GlaxoSmirthKline. GSK and Vir Biotechnology announce United States government agreement to purchase additional supply of sotrovimab, authorized for the early treatment of COVID-19 [media release]. 11 Jan 2022. https://www.gsk.com/.
- 31.United States securities and exchange commission. VirBio Sec 2021, Form 10K. Internet-Doc. 2020. https://investors.vir.bio/static-files/d3b18b23-68f8-484d-adb4-cd17a663087f. Accessed 28 Jan 2022.
- 32.European Medicines Agency. Assessment report: procedure under Article 5(3) of Regulation (EC) No 726/2004-GlaxoSmithKline use of sotrovimab (VIR-7831/GSK4182136) for the treatment of COVID-19. 2021. https://www.ema.europa.eu. Accessed 27 Jan 2022.
- 33.Ikemura N, Hoshino A, Higuchi Y, et al. SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.13.21267761. [DOI] [Google Scholar]
- 34.Aggarwal A, Stella AO, Walker G, et al. SARS-CoV-2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv. 2021 doi: 10.1101/2021.12.14.21267772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022 doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2021 doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of the neutralizing SARS-CoV-2 antibody sotrovimab in preventing progression of COVID-19: a randomized clinical trial. medRxiv. 2021 doi: 10.1101/2021.11.03.21265533. [DOI] [Google Scholar]
- 39.Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial. medRxiv. 2021:2021.12.23.21268244. [DOI] [PMC free article] [PubMed]
- 40.ACTIV-3/Therapeutics for Inpatients with COVID-19 Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GlaxoSmithKline. Lilly, Vir Biotechnology and GSK announce positive topline data from the phase 2 BLAZE-4 trial evaluating bamlanivimab with VIR-7831 in low-risk adults with COVID-19 [media release]. 30 Mar 2021. http://www.gsk.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.