Abstract
Purpose
Fatigue and pain are common among women with breast cancer, and often related to depressive symptoms. Social support may influence levels of fatigue, pain interference, and depressive symptoms. We tested a theory-based, structural model examining the relationship between social support (i.e., emotional and instrumental) and depressive symptoms via fatigue and pain interference in women with breast cancer.
Methods
Women (N = 327) with stages I–III breast cancer were enrolled in a randomized trial investigating a behavioral pain intervention. Measures of social support, fatigue, pain interference, and depressive symptoms were completed at enrollment. Data were analyzed using structural equation modeling to test direct and indirect pathways relating social support, fatigue, pain interference, and depressive symptoms.
Results
Our model evidenced good fit. Significant direct effects emerged linking higher levels of emotional support with lower levels of fatigue (β = −.30), pain interference (β = −.32), and depressive symptoms (β = −.31). More instrumental support was significantly associated with more depressive symptoms (β = .11), but not fatigue or pain interference. Higher levels of fatigue (β = .30) and pain interference (β = .34) were significantly related to higher levels of depressive symptoms. More emotional support related to less depressive symptoms via lower levels of fatigue (β = −.09) and pain interference (β = −.11).
Conclusion
Women reporting higher levels of emotional support endorsed fewer depressive symptoms, and that relationship was driven by lower levels of fatigue and pain interference. Our results highlight novel pathways that healthcare professionals can leverage to optimize social support topics in psychosocial interventions targeting breast cancer symptoms. This model should be replicated using longitudinal data.
Keywords: Breast cancer, Emotional support, Instrumental support, Fatigue, Pain interference, Depression
Introduction
There are nearly four million women in the USA with a history of breast cancer [1]. Survivors of breast cancer endorse physical and psychological sequelae following diagnosis and treatments [2]. Fatigue and pain are common and described as some of the most distressing aspects of breast cancer [3, 4]. Fatigue and pain cause ongoing disruptions in patients’ lives and are associated with symptoms of depression during and after breast-conserving surgeries and adjuvant treatments (i.e., chemotherapy, radiation, hormonal therapy) [3–5].
Fatigue, the subjective experience of feeling worn out or tired, is experienced by approximately one-third of women with breast cancer [6]. In a review of patients with cancer, the average correlation between fatigue and depressive symptoms was large (r = .56, 95% CI [.54, .58]), and fatigue shared approximately 31% of its variance with depression [5]. This effect has been shown within breast cancer samples specifically, where more fatigue is associated with significantly more symptoms of depression [4–7]. Pain is also consistently related to higher levels of depression [8, 9]. Patients with pain often describe it as significantly interfering with daily activities such as work, enjoyment of life, and relations with others [10]. Higher pain interference has emerged as a correlate of depressive symptoms in heterogeneous samples of cancer survivors [11, 12]. In a sample of women with breast cancer reporting pain after surgery, approximately 37% also endorsed pain interference to daily activities [13]. Women with breast cancer can report varying levels of pain interference independent of pain intensity. Psychosocial factors, such as social support, may impact patients’ levels of pain interference.
Social support is often conceptualized based on its structure (i.e., quantity) and function (i.e., type) [14]. Functional distinctions of social support are especially relevant within the context of stress (e.g., breast cancer; [16]). For example, when stress is high, women with breast cancer may require emotional support from a partner (e.g., verbal encouragement), as well as instrumental support from a friend (e.g., transportation). Findings on the unique effects of emotional and instrumental support on psychological and physical symptoms are inconsistent. In patients with various chronic illnesses (e.g., cancer, chronic pain), researchers have found significant associations between more emotional and instrumental support and less depressive symptoms [15–17]. Yet, others have observed that only more emotional support relates to less depressive symptoms [18–20]. Relationships between emotional and instrumental support with fatigue and pain interference are also unclear. Some research suggests that more emotional support is related to less fatigue and pain interference among cancer and chronic pain populations [15, 21]. However, this finding has not been consistently replicated for emotional and instrumental support, and the nature of these relationships among survivors of breast cancer specifically is not well-established [16, 18, 22].
Given observed links between social support, fatigue, pain interference, and depressive symptoms, it is possible that fatigue and pain interference operate as intermediary variables relating social support (i.e., emotional and instrumental) with symptoms of depression. Ferrans’ and colleagues’ (2005) model for health-related quality of life provides a theoretical underpinning for such relationships. Their model posits that individual and environmental characteristics (i.e., social support systems) influence subjective well-being through critical biopsychosocial variables, such as physical symptom experiences of fatigue and pain, which might then influence overall health-related quality of life [23]. To date, there is little research exploring the specific paths within this model relating social support to depressive symptoms via the mechanistic variables of fatigue and pain interference [18]. In particular, more research is needed to confirm these effects within breast cancer samples where fatigue, pain interference, and depressive symptoms are common.
The current study tested a theory-based, structural model examining the relationship between social support (i.e., emotional and instrumental) and depressive symptoms via intermediary pathways of fatigue and pain interference in women with breast cancer and pain. First, we hypothesized that more emotional and instrumental support would relate to less fatigue, pain interference, and depressive symptoms. Second, we hypothesized that higher levels of fatigue and pain interference would relate to more depressive symptoms. Third, we hypothesized that the relationships between emotional and instrumental support and depressive symptoms would be driven by fatigue and pain interference as intermediary variables. This study was an exploratory secondary analysis of a randomized trial (N = 327) that investigated a Pain Coping Skills Training (PCST) protocol in women with breast cancer.
Methods
Participants
Women with breast cancer (N = 327) were recruited from 2017 to 2020. Eligibility criteria included the following: (1) diagnosis of stages I–IIIC breast cancer within the past 2 years; (2) ≥ 18 years of age; (3) life expectancy of ≥ 12 months; and (4) pain severity rating ≥ 5 out of 10. Patients were excluded if they reported (verified by medical chart review) (1) cognitive impairment; (2) brain metastases; (3) severe psychiatric disorder (e.g., psychotic disorder) or condition (e.g., suicidal intent) that would contraindicate safe participation; and/or (4) current or past (< 6 months) engagement in Pain Coping Skills Training (PCST) for cancer pain. The parent study was a randomized trial of PCST approved by the Duke University Institutional Review Board (IRB #: Pro00070823) and registered on ClinicalTrials.gov (NCT02791646).
Procedures
The study staff reviewed electronic medical records to assess eligibility. If initial inclusion criteria were met, a potential participant was mailed a recruitment letter signed by their oncologist and the principal investigator. The letter informed the patient that they may qualify for a randomized controlled trial of Pain Coping Skill Training [24] and provided them with a phone number for opting out. Patients who did not opt out were scheduled for a phone call with a study staff member to receive additional information and, if still interested, complete informed consent. As part of the larger trial, participants completed a baseline assessment via Qualtrics consisting of self-report questionnaires measuring social support, physical symptoms, and depressive symptoms. The current study is a secondary analysis of baseline data.
Measures
Demographic and medical characteristics
Participants’ demographic and medical characteristics were collected through self-report and electronic medical records. At the time of enrollment, information was collected regarding demographics (e.g., partner status, education, employment, income) and medical history (e.g., cancer stage, surgeries and treatment received, functional status, use of antidepressant and/or pain medication, pain severity). The 4-item pain severity subscale of the Brief Pain Inventory (Cronbach’s α = .86) and 9-item Functional Status Questionnaire (Cronbach’s α = .85) were assessed as covariates [10, 25].
Social support
Perceived social support was assessed utilizing the 8-item Medical Outcomes Study Social Support Survey (MOS-SS; [27]). The MOS-SS was validated in a sample of women with breast cancer and is often used to measure perceived social support [26–28]. The parent trial used the MOS-SS because it distinguishes between emotional and instrumental support. The emotional support subscale asked participants if they had someone available to “have a good time with” and “turn to for suggestions” etc. The instrumental support subscale ask participants if they had someone available to help if “they were confided to bed” and “needed to visit a doctor” etc. Response options range from 1 (none of the time) to 5 (all of the time). Results were averaged to create composite scores for both emotional support and instrumental support subscales. Higher scores indicated higher levels of emotional and instrumental support. Reliability for the present sample was excellent (Cronbach’s α = .90 and .94, for emotional and instrumental support respectively).
Fatigue
Fatigue was assessed utilizing the 7-item Patient-Reported Outcome Measurement Information System-Fatigue (PROMIS-Fatigue) scale [29, 30]. Participants were asked to identify the number of times during the past week they experienced tiredness, extreme exhaustion, lack of energy, and limitations in performing work/house work due to fatigue, as well as how many times during the past week they felt too tired to think clearly, bathe/shower, and whether they had enough energy to exercise strenuously. Response options range from 1 (never) to 5 (always). Items were summed and then converted to T-scores. Higher T-scores indicated higher levels of fatigue. The PROMIS-Fatigue scale is commonly used in cancer samples [30, 31] and demonstrated adequate reliability in the present sample (Cronbach’s α = .78).
Pain interference
Pain interference was assessed with seven items from the Brief Pain Inventory (BPI) [10]. Items ask participants to rate the degree to which, over the past week, pain has interfered with daily activities (i.e., general activity, mood, walking ability, normal work/house work, relations with others, sleep, enjoyment of life). Response options range from 0 (does not interfere) to 10 (completely interferes). The seven items were averaged for a composite score, with higher scores reflecting higher levels of pain interference (Cronbach’s α = .91) [32, 33]. The BPI is recommended for use in all clinical trials assessing pain, and has been readily used in cancer samples [33, 34].
Depressive symptoms
Depressive symptoms were assessed utilizing the 20-item Center for Epidemiological Studies Depression Scale (CES-D) [35]. Participants were asked to rate the number of times during the previous week they experienced depressive symptoms (e.g., low mood, anhedonia, lack of appetite, difficult concentrating). Response options range from 0 (rarely or none of the time) to 3 (all of the time). Items were summed for a total score with higher scores indicating higher levels of depressive symptomology. The CES-D is frequently used to assess depressive symptoms among survivors of breast cancer [36, 37] and demonstrated excellent reliability in the present sample (Cronbach’s α = .90).
Analytic strategy
Preliminary descriptive analyses were conducted using Statistical Package for the Social Sciences Version 27 (SPSS 27). All variables of interest were screened for outliers, and distributions were inspected for skewness, kurtosis, and multivariate assumptions of normality. Subsequent analyses were performed using Mplus version 7 [38].
Path analysis was conducted using structural equation modeling (SEM) in Mplus to test the direct and indirect pathways relating social support (i.e., emotional and instrumental), fatigue, pain interference, and depressive symptoms. Correlations were specified between emotional and instrumental support, as well as fatigue and pain interference. Theoretically supported demographic (i.e., age) and medical factors (i.e., pain severity, cancer stage, receipt of surgery and/or adjuvant treatment during week before baseline assessment, functional status, and use of antidepressant and pain medication) were included as covariates and regressed on the outcome variable. Receipt of surgery and/or adjuvant treatment (i.e., chemotherapy, radiation, endocrine therapy) during the week before baseline assessment was categorized dichotomously (no = 0 vs. yes = 1). Missing data across study variables were minimal (0–.09%) and estimated using full information maximum likelihood (FIML), which derives population estimates using all observed data. Four indices were estimated and interpreted for model fit: chi-square test (χ2) >.05, confirmatory fit index (CFI) >.95, root mean squared error of approximation (RMSEA) <.06, and standardized root mean square residual (SRMR) <.08 [39]. Standardized coefficients were examined as measures of effect sizes as follows: .1 = small; .3 = medium; .5 = large [40].
Results
Participant characteristics
Women were an average of 57.19 (SD = 11.87) years old, and over half were partnered (59.6%). Over one-third of the total sample was a member of a racial minority group (35.6%). Additional demographic characteristics are reported in Table 1.
Table 1.
Demographic characteristics
| N (%) | M (SD) | |
|---|---|---|
| Age (years) | 57.19 (11.87) | |
| Race | ||
| White/Caucasian | 203 (62.1%) | |
| Black/African American | 97 (29.7%) | |
| Two or more races | 9 (2.8%) | |
| Asian | 9 (2.8%) | |
| American Indian or Alaskan Native | 1 (0.3%) | |
| Other | 3 (0.9%) | |
| Not reported/declined | 5 (1.5%) | |
| Ethnicity | ||
| Non-Hispanic | 311 (95.1%) | |
| Hispanic or Latino | 5 (1.5%) | |
| Hispanic Mexican | 2 (0.6%) | |
| Hispanic Cuban | 3 (0.9%) | |
| Hispanic Puerto Rican | 2 (0.6%) | |
| Hispanic Other | 4 (1.2%) | |
| Education | ||
| Less than high school diploma | 7 (2.1%) | |
| High school diploma | 41 (12.5%) | |
| Some college | 106 (32.4%) | |
| Bachelor’s degree | 102 (31.2%) | |
| Graduate degree | 71 (21.7%) | |
| Partner status | ||
| Single | 40 (12.2%) | |
| Married | 191 (58.4%) | |
| Divorced | 62 (19.0%) | |
| Separated | 6 (1.8%) | |
| Widowed | 24 (7.3%) | |
| Life-/long-term partner | 4 (1.2%) | |
Note. M, mean; SD, standard deviation
At the time of enrollment, women were on average 10 months post-diagnosis (SD = 6.21). For the majority of the sample (97.2%), this was an initial breast cancer diagnosis. Women were mostly diagnosed with stage I (56.0%) or II (34.6%) disease. Most women underwent a surgical procedure (mastectomy = 59.7%, lumpectomy = 72.8%), and adjuvant therapy was common. Approximately 8.3% (N = 27) reported receipt of chemotherapy during the week before the baseline assessment, while 10.8% (N = 35) reported receipt of radiation. Further information regarding medical characteristics of the sample is reported in Table 2.
Table 2.
Medical characteristics
| N (%) | M (SD) | |
|---|---|---|
| Cancer diagnosis | ||
| First/initial | 317 (97.2%) | |
| Recurrence | 9 (2.8%) | |
| Months since diagnosis | 10.11 (6.21) | |
| Stage | ||
| I | 183 (56.0%) | |
| II | 113 (34.6%) | |
| III | 31 (9.5%) | |
| Receipt of surgery | ||
| Yes | 22 (6.7%) | |
| No | 304 (93.3%) | |
| Receipt of chemotherapy | ||
| Yes | 27 (8.3%) | |
| No | 299 (91.7%) | |
| Receipt of radiation | ||
| Yes | 35 (10.8%) | |
| No | 290 (89.2%) | |
| Receipt of endocrine therapy | ||
| Yes | 49 (15.1%) | |
| No | 275 (84.9%) | |
| Use of antidepressant medication | ||
| Yes | 123 (37.7%) | |
| No | 203 (62.3%) | |
| Use of pain medication | ||
| Yes | 212 (64.8%) | |
| No | 115 (35.2%) | |
Receipt of surgery, chemotherapy, radiation, and endocrine therapy is for a week before baseline assessment
Note. M, mean; SD, standard deviation
Mean levels of emotional (M = 3.88, SD = 1.00) and instrumental (M = 3.83, SD = 1.21) support were comparable and suggested that women in the current sample could count on someone to provide emotional and/or instrumental support “most of the time.” Participants reported moderate pain intensity (M = 4.04, SD = 1.73) and pain interference (M = 4.07, SD = 2.43). Two hundred and forty two women (74.5%) rated their pain intensity at or above a validated cutoff for clinically significant pain (i.e., ≥ 3) [13] (Table 3). The average T-score for fatigue intensity was 56.00 (SD = 7.11) which falls in the 73rd percentile and “average” range. Approximately half of our sample (50.6%) scored above an established cutoff on the CES-D (≥ 16) that indicates clinically significant symptoms of depression [41].
Table 3.
Means (M), standard deviations (SD), and correlation matrix for the main study variables
| Variable | Emotional Support | Instrumental Support | Fatigue | Pain Interference | Depressive Symptoms |
|---|---|---|---|---|---|
| M (SD) | 3.88 (1.00) | 3.83 (1.21) | 56.00 (7.11) | 4.07 (2.43) | 17.60 (10.30) |
| Emotional Support | 1 | - | - | - | - |
| Instrumental Support | .67** | 1 | - | - | - |
| Fatigue | −.25** | −.13* | 1 | - | - |
| Pain Interference | −.28** | −.16** | .53** | 1 | - |
| Depressive Symptoms | −.43** | −.20** | .59** | .58** | 1 |
Note. M = mean; SD = standard deviation
Fatigue scores shown as T-score
p < .05
p < .01
Relationships between social support, fatigue, pain interference, and depressive symptoms
A structural model assessed proposed relationships between social support (i.e., emotional and instrumental), fatigue, pain interference, and depressive symptoms (Fig. 1). Pain severity, age, cancer stage, receipt of surgery, and/or adjuvant treatment during week before baseline assessment, functional status, and use of antidepressant and pain medication were included as covariates. Fit indices indicated that the specified model was consistent with the data, χ2 (10) = 11.00, p = .36; RMSEA = .02, (90% CI [.00, .06]); CFI = 1.00; SRMR = .02.
Fig. 1.
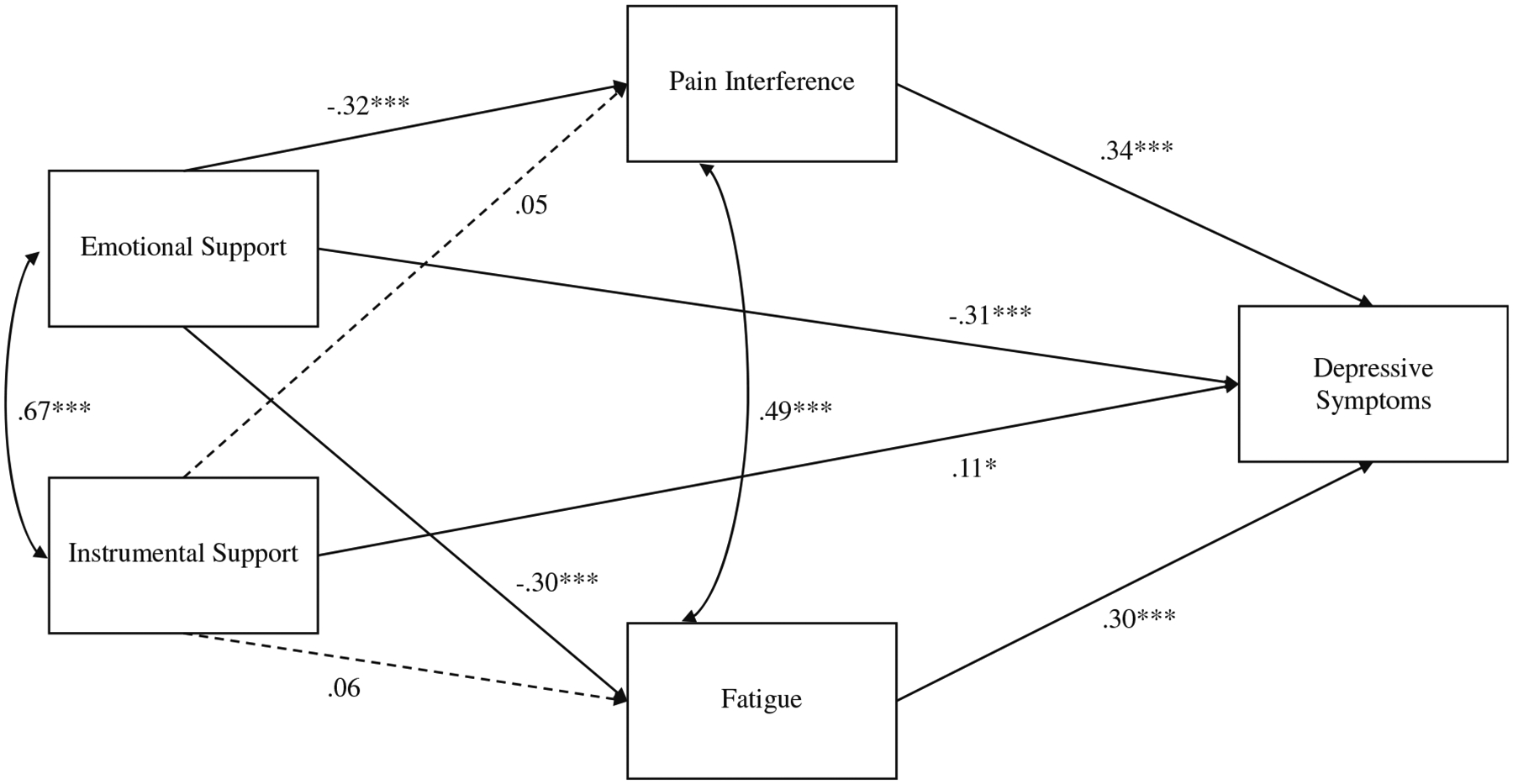
Structural model. Note. Pain severity, age, cancer stage, receipt of surgery and/or adjuvant treatment during a week before baseline assessment, functional status, and use of antidepressant and pain medication were included as covariates; paths not shown for simplicity. Standardized parameter estimates are shown. Dashed lines indicate non-significant paths; solid lines indicate significant paths; *p < .05; ***p < .001
First, significant direct effects emerged linking more emotional support with less fatigue (B = −2.08, p < .001, 95% CI [−3.08, −1.08], β = −.30), pain interference (B = −.76, p < .001, 95% CI [−1.10, −.43], β = −.32), and depressive symptoms (B = −3.17, p < .001, 95% CI [−4.23, −2.11], β = −.31). Effect sizes for these associations were medium. Conversely, instrumental support was not significantly related to fatigue (B = .37, p = .38, 95% CI [−.46, 1.21], β = .06) or pain interference (B = .09, p = .53, 95% CI [−.19, .37], β = .05); however, more instrumental support was significantly associated with more depressive symptoms (B = .91, p < .05, 95% CI [.07, 1.75], β = .11). The effect size for this association was small.
Second, more fatigue was significantly associated with more depressive symptoms (B = .43, p < .001, 95% CI [.30, .56], β = .30). Likewise, higher levels of pain interference were significantly associated with more symptoms of depression (B = 1.44, p < .001, 95% CI [.96, 1.92], β = .34). Effect sizes for these relationships were medium, and significance was achieved above and beyond the effect of covariates (pain severity, age, cancer stage, receipt of surgery and/or adjuvant treatment during week before baseline assessment, functional status, and use of antidepressant and pain medication) included in the structural model. The only covariates significantly associated with depressive symptoms were age (B = −.10, p < .01, 95% CI [−.17, −.03], β = −.12) and use of antidepressants (B = 3.43, p < .001, 95% CI [1.82, 5.04], β = .16).
Finally, small indirect effects linking more emotional support to less depressive symptoms via less fatigue (B = −.90, p < .001, 95% CI [−1.40, −.40], β = −.09) and less pain interference (B = −1.10, p < .001, 95% CI [− 1.71, −.49], β = −.11) emerged, suggesting partial mediation. The same paths relating instrumental support to depressive symptoms via fatigue (B = .16, p = .38, 95% CI [−.20, .52], β = .02) and pain interference (B = .13, p = .52, 95% CI [−.28, .54], β = .02) were not significant.
Discussion
This is an observational study using data from a randomized controlled trial of PCST for women with breast cancer and pain [24]. We tested a theory-based [25], structural model examining the relationship between social support (i.e., emotional and instrumental) and depressive symptoms via fatigue and pain interference. We found that women with breast cancer who reported higher levels of emotional support were more likely to report significantly less fatigue, pain interference, and depressive symptoms. Additionally, we found that women with breast cancer who endorsed higher levels of fatigue and pain interference were significantly more likely to endorse higher levels of depressive symptoms. Lastly, we observed significant indirect effects linking higher levels of emotional support to lower levels of depressive symptoms via lower levels of fatigue and pain interference.
The finding that more emotional support related to less fatigue, pain interference, and depressive symptoms offers partial confirmation of our first hypothesis. We extended prior literature by demonstrating such relationships occur within a large sample of women with breast cancer and pain. The same associations did not emerge for instrumental support. In fact, more instrumental support was significantly related with more depressive symptoms, as well as more fatigue and pain interference (though the latter two associations were not significant). Van Dyke and colleagues (2018) cited a similar pattern among patients with chronic pain, wherein receipt of more instrumental support longitudinally predicted increased depressive symptoms and pain interference [42]. It is plausible that if women with breast cancer receive too much instrumental support (e.g., tangible help with meals, daily chores), they may feel less confident in their ability to manage symptoms of fatigue, pain interference, and depression on their own. Over time, this dynamic may result in elevated symptomology. These concepts should be further explored as some research in breast cancer samples has found that more instrumental support relates to less physical and psychological symptoms among patients with cancer [15, 22].
We observed higher levels of fatigue and pain interference related to higher levels of depressive symptoms, supporting our second hypothesis. Our data confirm existing findings on the relationship between fatigue and depressive symptoms [4–7], and add to a growing literature on the role of pain interference among women with breast cancer [3, 11, 12]. Pain is a multi-dimensional experience best explained by two distinct domains: (1) the severity of pain and (2) the degree to which the pain interferes with functioning [43]. There is increasing interest in the role of pain interference as a correlate of breast cancer symptoms [11, 12]. We demonstrated that more pain interference is associated with more depressive symptoms, above and beyond pain severity and other relevant covariates. This underscores that pain interference uniquely influences breast cancer survivors’ emotional well-being. As such, pain interference (not just pain severity) should be attended to throughout the illness experience.
Lastly, in partial support of our third hypothesis, we found significant indirect effects linking more emotional support to less depressive symptoms via reductions in fatigue and pain interference. Since there was a significant direct relationship between higher levels of emotional support and lower levels of depressive symptoms, this indirect effect suggests partial mediation. There was no evidence of mediation for instrumental support. Our observation aligns with Ferrans’ and colleagues’ (2015) model; we confirmed that environmental characteristics, such as emotional support, can influence psychological well-being (i.e., depressive symptoms) through physical symptoms such as fatigue and pain interference. To date, there has been limited research exploring such a theory-based, structural model in breast cancer samples [18]. Results from the current study reveal novel pathways (via fatigue and pain interference) by which emotional support may influence depressive symptoms.
Our results should be considered in light of the following limitations. First, this was a cross-sectional analysis of baseline data from a larger randomized controlled trial. Future research should investigate these pathways longitudinally to confirm formal mediation. Additionally, alternative structural models should assess the potential for a bidirectional relationship wherein more fatigue and pain interference necessitate more instrumental support, leading to more depressive symptoms. Second, participants were mostly White, non-Hispanic women who self-selected into the parent trial. Women who opted into the trial might have differed from women who did not, particularly on the basis of social support (i.e., quantity, perception of importance). This selection bias might explain the high levels of social support observed in the current study. Furthermore, this relatively homogenous sample may limit generalizability to less-represented groups. Social support varies based on race and/or ethnicity [44]. Future work should explore the possibility that race and/or ethnicity moderate the relationships between social support, fatigue, pain interference, and depressive symptoms.
Despite these limitations, our study had several strengths. We leveraged robust statistical methodology to examine a theory-based, structural model in a large sample (N = 327) of women with breast cancer and pain. Structural equation modeling provides the flexibility to account for missing data using FIML, which reinforces confidence in parameter estimates. Another strength of this study was the use of the Medical Outcomes Study Social Support Survey (MOS-SS; [31]) to distinguish between emotional and instrumental support. This allowed for a nuanced investigation of the unique roles of these two types of social support within the context of breast cancer. Future work should continue to explore the distinction between emotional and instrumental support, as well as other support subtypes (e.g., tangible support, affection, trust, security), sources (e.g., significant other, friend), and broader social networks [45]. Likewise, findings regarding specific physical and psychological symptom experiences should be explored within the broader context of health-related quality of life.
Our results have meaningful clinical implications. First, the observation that more emotional support (and not instrumental support) related to less fatigue, pain interference, and depressive symptoms highlights the relevance of prioritizing this type of support throughout the breast cancer experience. Psychosocial interventions, particularly those involving a patient’s partner or caregiver, should include instruction on seeking and providing effective emotional support. It is possible that such a skillset may influence critical physical and psychological symptoms. Second, our finding that higher levels of emotional support related to less depressive symptoms indirectly through reduced fatigue and pain interference suggests that these physical symptoms may be important mechanistic variables driving positive effects of emotional support. As such, individual and dyadic psychosocial interventions for women with breast cancer might emphasize the ways in which receipt of emotional support may empower women to better manage their fatigue and pain interference, and in turn, reduce depressive symptoms.
Funding
This study was funded through an NIH/NCI 1R01CA202779-01 awarded to senior author, Tamara J. Somers, PhD. The work of Joseph G. Winger, PhD, was supported, in part, by a Kornfeld Scholars Program Award from the National Palliative Care Research Center.
Footnotes
Ethics approval The procedures complied with ethical guidelines and received Duke University Institutional Review Board approval (Pro00070823).
Consent to participate Informed consent was obtained from all individual participants included in this study.
Consent for publication The authors affirm that human research participants provided informed consent for publication of the data included in this publication.
Competing interests The authors declare no competing interests.
References
- 1.DeSantis CE et al. (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6):438–451 [DOI] [PubMed] [Google Scholar]
- 2.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA (2016) American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 34(6):611–635 [DOI] [PubMed] [Google Scholar]
- 3.Bamonti PM, Moye J, Naik AD (2018) Pain is associated with continuing depression in cancer survivors. Psychol Health Med 23(10):1182–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H (2018) The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev 63:1–11 [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Kroenke K (2009) Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics 50(5):440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Asher A, Garet D, Petersen L, Ganz PA, Irwin MR, Cole SW, Hurvitz SA, Crespi CM (2019) Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125(4):633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderon C, Carmona-Bayonas A, Hernández R, Ghanem I, Castelo B, Martinez de Castro E, Ferreira E, Ciria L, Muñiz M, Jimenez-Fonseca P (2019) Effects of pessimism, depression, fatigue, and pain on functional health-related quality of life in patients with resected non-advanced breast cancer. Breast 44:108–112 [DOI] [PubMed] [Google Scholar]
- 8.Brandao T, Schulz MS, Matos PM (2017) Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psychooncology 26(7):917–926 [DOI] [PubMed] [Google Scholar]
- 9.Teo I, Novy DM, Chang DW, Cox MG, Fingeret MC (2015) Examining pain, body image, and depressive symptoms in patients with lymphedema secondary to breast cancer. Psychooncology 24(11):1377–1383 [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 23(2):129–138 [PubMed] [Google Scholar]
- 11.Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L (2006) Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs 29(5):400–405 [DOI] [PubMed] [Google Scholar]
- 12.Wood R, Mitra D, de Courcy J, Iyer S (2017) Patient-reported pain severity, pain interference and health status in HR+/HER2-advanced/metastatic breast cancer. ESMO Open 2(3):e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmond SN, Shelby RA, Keefe FJ, Fisher HM, Schmidt JE, Soo MS, Skinner CS, Ahrendt GM, Manculich J, Sumkin JH, Zuley ML, Bovbjerg DH (2017) Persistent breast pain among women with histories of breast-conserving surgery for breast cancer compared with women without histories of breast surgery or cancer. Clin J Pain 33(1):51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helgeson VS (2003) Social support and quality of life. Qual Life Res 12(Suppl 1):25–31 [DOI] [PubMed] [Google Scholar]
- 15.Soares A, Biasoli I, Scheliga A, Baptista RL, Brabo EP, Morais JC, Werneck GL, Spector N (2013) Association of social network and social support with health-related quality of life and fatigue in long-term survivors of Hodgkin lymphoma. Support Care Cancer 21(8): 2153–2159 [DOI] [PubMed] [Google Scholar]
- 16.Cook SA, Salmon P, Hayes G, Byrne A, Fisher PL (2018) Predictors of emotional distress a year or more after diagnosis of cancer: a systematic review of the literature. Psychooncology 27(3):791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong AJ, Scarapicchia TMF, McDonough MH, Wrosch C, Sabiston CM (2017) Changes in social support predict emotional well-being in breast cancer survivors. Psychooncology 26(5):664–671 [DOI] [PubMed] [Google Scholar]
- 18.Heo S, Lennie TA, Moser DK, Kennedy RL (2014) Types of social support and their relationships to physical and depressive symptoms and health-related quality of life in patients with heart failure. Heart Lung 43(4):299–305 [DOI] [PubMed] [Google Scholar]
- 19.Morelli SA, Lee IA, Arnn ME, Zaki J (2015) Emotional and instrumental support provision interact to predict well-being. Emotion 15(4):484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon T et al. (2017) Breast cancer survivors’ contribution to psychosocial adjustment of newly diagnosed breast cancer patients in a computer-mediated social support group. J Mass Commun Q 94(2): 486–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matos M, Bernardes SF, Goubert L (2017) Why and when social support predicts older adults’ pain-related disability: a longitudinal study. Pain 158(10):1915–1924 [DOI] [PubMed] [Google Scholar]
- 22.Mardanian-Dehkordi L, Kahangi L (2018) The relationship between perception of social support and fatigue in patients with cancer. Iran J Nurs Midwifery Res 23(4):261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL (2005) Conceptual model of health-related quality of life. J Nurs Scholarsh 37(4):336–342 [DOI] [PubMed] [Google Scholar]
- 24.Kelleher SA, Dorfman CS, Plumb Vilardaga JC, Majestic C, Winger J, Gandhi V, Nunez C, van Denburg A, Shelby RA, Reed SD, Murphy S, Davidian M, Laber EB, Kimmick GG, Westbrook KW, Abernethy AP, Somers TJ (2017) Optimizing delivery of a behavioral pain intervention in cancer patients using a sequential multiple assignment randomized trial SMART. Contemp Clin Trials 57:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jette AM, Davies AR, Cleary PD, Calkins DR, Rubenstein LV, Fink A, Kosecoff J, Young RT, Brook RH, Delbanco TL (1986) The Functional Status Questionnaire: reliability and validity when used in primary care. J Gen Intern Med 1(3):143–149 [DOI] [PubMed] [Google Scholar]
- 26.Moser A, Stuck AE, Silliman RA, Ganz PA, Clough-Gorr KM (2012) The eight-item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. J Clin Epidemiol 65(10):1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke CH, Hershman DL, Gomez SL, Adams SR, Eldridge EH, Kwan ML, Ergas IJ, Kubo A, Kushi LH (2018) Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res Treat 170(3):623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astrup GL, Hofsø K, Bjordal K, Rustøen T (2020) Cancer patients’ diagnosis and symptoms and their family caregivers’ self-efficacy and social support are associated with different caregiver reactions. Eur J Cancer Care (Engl) 29(6):e13311. [DOI] [PubMed] [Google Scholar]
- 29.Riley WT, Rothrock N, Bruce B, Christodolou C, Cook K, Hahn EA, Cella D (2010) Patient-reported outcomes measurement information system (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual Life Res 19(9):1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella D, Lai JS, Jensen SE, Christodoulou C, Junghaenel DU, Reeve BB, Stone AA (2016) PROMIS fatigue item bank had clinical validity across diverse chronic conditions. J Clin Epidemiol 73: 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Spenner A, Vicari S, Rao K, Courneya KS, Hoelzer K, Robbs R, Verhulst S (2015) Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial. Psychooncology 24(3):302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J (2003) Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106(3):337–345 [DOI] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9(2):105–121 [DOI] [PubMed] [Google Scholar]
- 34.Somers TJ, Abernethy AP, Edmond SN, Kelleher SA, Wren AA, Samsa GP, Keefe FJ (2015) A pilot study of a mobile health pain coping skills training protocol for patients with persistent cancer pain. J Pain Symptom Manag 50(4):553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radloff LS (1977) The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- 36.Stagl JM, Bouchard LC, Lechner SC, Blomberg BB, Gudenkauf LM, Jutagir DR, Glück S, Derhagopian RP, Carver CS, Antoni MH (2015) Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer 121(11):1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hann D, Winter K, Jacobsen P (1999) Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res 46(5):437–443 [DOI] [PubMed] [Google Scholar]
- 38.Muthén L, Muthén B (2012) MPlus user’s guide, 6th edn. Muthén & Muthén, Los Angeles [Google Scholar]
- 39.Hu LT, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model Multidiscip J 61(1):1–55 [Google Scholar]
- 40.Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Academic Press, New York [Google Scholar]
- 41.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB (1997) Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12(2):277–287 [DOI] [PubMed] [Google Scholar]
- 42.Van Dyke B, Kim M, Thorn B (2018) Longitudinal relationships between perceived social support and quality-of-life among patients with chronic pain. J Pain 19(3):S39 [Google Scholar]
- 43.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS (1995) When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61(2):277–284 [DOI] [PubMed] [Google Scholar]
- 44.Sammarco A, Konecny LM (2010) Quality of life, social support, and uncertainty among Latina and Caucasian breast cancer survivors: a comparative study. Oncol Nurs Forum 37(1):93–99 [DOI] [PubMed] [Google Scholar]
- 45.Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, Hershman D, Kushi LH (2013) Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat 139(2):515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]


