Summary
Background
Amoxicillin crystalluria (AC), potentially responsible for acute kidney injury (AKI), is reported more and more frequently in patients treated with high doses of intravenous amoxicillin (HDIVA). The main objective of this study was to evaluate AC incidence in these patients. The secondary objectives were to identify factors associated with AC and to evaluate its impact on the risk of AKI.
Methods
This multicentre, observational, cohort study was conducted between Mar 18, 2014 and Aug 16, 2019 in Dijon, Nancy, and Reims University Hospitals as well as Châlon-sur-Saône, Charleville-Mézières, and Troyes general hospitals in France. Adult patients (≥18 years) treated with HDIVA and having been tested for AC at least once during treatment were included. Clinical, biological, and therapeutic characteristics of the patients were collected. A univariable mixed logistic regression model assessed the factors associated with AC. A multivariable Cox model with AC as a time-dependent variable assessed the prognostic factors for AKI. ClinicalTrials.gov number: NCT02853292.
Findings
Of the 112 included patients, 27 (24.1%, 95% CI [16.2-32.0]) developed at least one episode of AC within a mean of 5.1 days. The factors associated with its occurrence were the concomitant use of angiotensin converting enzyme (ACE) inhibitors (OR=4.6, 95% CI [2.2-9.3], p<0.0001) and the decrease of urinary pH (OR=2.1 for one pH point decrease, 95% CI [1.2-3.7], p=0.009). 20 patients (17.9%) presented with AKI, within a mean time of 10.9 days. The main factor associated with the occurrence of AKI was the occurrence of AC (aHR=7.4, 95% CI [2.5-22.2], p=0.0003).
Interpretation
AC occurred in a quarter of patients treated with HDIVA and was highly prognostic of AKI.
Funding
None.
Keywords: Amoxicillin, crystalluria, acute kidney injury, incidence, cohort study
Research in context.
Evidence before this study
We searched for articles in all languages in MEDLINE (via PubMed) using the following search request: “(ampicillin or amoxicillin) and crystalluria” before Nov 25, 2021 and found 29 articles. Amoxicillin is known to cause urine crystallization. Amoxicillin crystalluria (AC) usually occurs with high-dose amoxicillin therapy, in urines with high density and low pH. It can be asymptomatic or can be responsible for hematuria or acute renal failure. Mechanisms are currently uncertain but it may be attributable to intratubular precipitation or urinary tract obstruction. However, its incidence has been unknown up until now.
Added value of this study
In a cohort of 112 patients treated with high doses of intravenous amoxicillin and tested systematically for AC, 27 developed at least one episode of AC, which was very strongly predictive of the subsequent occurrence of acute kidney injury.
Implications of all the available evidence
These results may encourage clinicians to change their attitude toward the screening and prevention of this underrated adverse effect of a frequently prescribed drug. Future research is needed for the prevention of this event and to assess whether angiotensin-converting enzyme inhibitors are associated with its occurrence.
Alt-text: Unlabelled box
Introduction
Amoxicillin, because of its wide range of indications, is one of the most widely prescribed antibiotics worldwide.1 In France, it accounts for 65.2% and 50.9% of antibiotic use in general practitioners’ practices and hospitals, respectively, in 2018.2 Its main side effects are digestive (diarrhea, nausea) and cutaneous (skin rash, mostly allergic).3 Occasionally, it can have renal adverse reactions: allergic acute tubulointerstitial nephritis and amoxicillin crystalluria (AC).3
AC was described for the first time in 1985 in a study evaluating renal excretion of intravenous (IV) amoxicillin: immediately after administration of 2.3 g of amoxicillin over 20 minutes, a healthy 26-year-old volunteer presented macroscopic crystalluria, resolved spontaneously in 3 hours without renal function damage.4 AC stems from urine oversaturation favored by high doses of amoxicillin, dehydration and low urine output that induce high density urine, acidic urinary pH or hypoalbuminemia. AC is described in patients treated with amoxicillin, with or without clavulanic acid, dispensed by mouth or intravenous, for curative or prophylactic use. It can present with macroscopic hematuria and/or acute kidney injury (AKI), which can be obstructive (lithiasis or amoxicillin “sludge”) and/or intrinsic5. The microscopic examination of first morning urine, voided less than 2 hours before analysis and kept at room temperature, can find trihydrated amoxicillin crystals shaped like thin needles or rods, aggregated into “broom bush-like” forms and highly birefringent under polarized light.6
Treatment consists of discontinuing amoxicillin or reducing the dosage, hydration, and urine alkalinization. Dialysis or urinary diversion is sometimes necessary. The outcome is mostly positive and without sequelae.5 In most cases, AC disappears in less than 24 hours, macroscopic hematuria in 1 to 3 days and AKI in 3 to 17 days.7
To this day, about twenty case reports or small case series have been reported worldwide.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Since 2010, the French regional pharmacovigilance center's mean notification rate of sodium amoxicillin crystalluria cases, either proved or suspected, for all the marketing authorization holders, increased significantly, rising from 8.7 to 124 cases for 100 000 treated patient-years.5 This is probably underestimated since one study estimated that only 37.5% of AC-linked nephropathies were even declared to a pharmacovigilance center.23 A rise in AKI cases since the mid-2010s has been shown in a French series of patients treated with high doses of intravenous amoxicillin (HDIVA) for osteoarticular infection, regardless of any practice change. Indeed, this complication affected 4% of 228 patients between 2004 and 2014, as compared to 18% of 56 patients between 2014 and 2015.24,25 A French retrospective single-center study evaluated AC nephropathy incidence at 4.5% in patients treated with HDIVA, but there was no systematic testing for AC and no data on AC incidence.23 This consistent signal of crystal nephropathy with amoxicillin emerged in France but also in the FDA Adverse Event Reporting System.26
Few studies have evaluated the incidence of this phenomena, described more and more frequently and involving an antibiotic frequently prescribed at high dosage and intravenously, nor the predictive impact of AC on the risk of AKI.
The main objective of this study was to assess AC incidence in hospitalized patients treated with HDIVA, which is the situation most likely to result in crystalluria.27 The secondary objectives were to identify the factors associated with the occurrence of AC and to evaluate its impact on the risk of AKI.
Methods
Study design
This was a multicentre, observational historical-prospective cohort study with descriptive and analytical aims carried out between MAR 18, 2014 and AUG 16, 2019 (start of the prospective phase in May 2016) in the internal medicine and infectious diseases departments of Dijon, Nancy and Reims University Hospitals as well as Châlon-sur-Saône, Charleville-Mézières, and Troyes general hospitals, in France.
Study population
Included patients were adults (≥18 years), treated with HDIVA (≥150 mg/kg/day of IV amoxicillin, like most indications for prolonged intravenous amoxicillin, which is the main at-risk population for AC, lead to dosages of around 200 mg.kg/day), initiated in the recruiting department or within 48 hours of patient admission to this department (to ascertain that the patient was in a participating department when Day 3 AC sampling was possible). Dialysis or kidney transplant patients were not included, as their renal function generally does not allow for receiving HDIA treatment, and as they may be anuric or have a modified urinary sediment. Patients who did not benefit from an AC test while on HDIVA were secondarily excluded. If a patient had more than one hospitalization while receiving HDIVA and was screened for AC, only the first hospitalization was considered, as we expected very few of these patients. The patients were followed up until the end of their hospitalization or at the latest until the 28th day.
Data collection
Since the observation of a case in one of the participating departments14 and a pharmacovigilance alert sent out over the infectious disease specialist network (infectioflash) in 2015, participating departments have implemented systematic urine sampling for AC testing into clinical practice on D3 ± 1 day, D7 ± 2 days and/or D14 ± 2 days, from the start of HDIVA and as long as the treatment lasts. A symptomatic patient was defined by the occurrence of macroscopic hematuria and/or AKI. In this case, additional testing for AC could be carried out by the unit responsible for the patient. The collection and analysis techniques were as follows: fresh first morning urine was collected, transported at room temperature without adding any preservative, and underwent laboratory analysis within 2 hours of voiding: investigation of AC, urine dipstick, a cytobacteriological examination (CBEU) and urine pH measurement. The observational study design and need for a quick analysis of the samples used in clinical care meant that AC investigation was not performed in a blind manner. Renal function was monitored regularly, at the discretion of the practitioner in charge of the patient.
Data was collected using a standardized case report form from patient medical records: socio-demographic data (age and sex); weight and height; medical history (urinary lithiasis, chronic renal failure, diabetes, hypertension, myeloma); start and end dates of hospitalization; start and end dates of HDIVA; indications, dosage and number of injections per day of amoxicillin; treatments in progress when starting HDIVA; investigation of AC (date of sampling, context, presence or absence of AC), cytobacteriological examination, urine dipstick, urine pH by pHmetry; serum creatinine and albumin measurement at the initiation of treatment, peak serum creatinine within 28 days of treatment initiation, serum creatinine level on day 28; occurrence of AKI defined by an increase of more than 50% in serum creatinine at the start of treatment (Kidney Disease: Improving Global Outcomes (KDIGO)≥1); patient's situation at the end of hospitalization or at the latest on the 28th day.
The data was acquired retrospectively during the historical phase and prospectively during the prospective phase.
The diagnosis of AC was made using direct examination of urine under polarized optical microscopy, upon detection of fine non-polarizing needles with pointed ends, or thicker rods with monochrome polarization, isolated or asymmetrically aggregated in a "broom bush-like” fashion.6
The primary outcome was the incidence of the occurrence of AC during treatment with HDIVA. The secondary outcomes were the factors associated with AC and the impact of this AC on the subsequent risk an in-hospital AKI episode.
Statistical analysis
To detect a risk of AC of 10% – which we considered the minimum clinically significant value given the absence of published data at that time – with an accuracy of 5%, and an alpha risk at 5%, we planned to include 137 patients. Considering a 10% exclusion rate, we increased this number to include 153 subjects. Qualitative variables were described under frequency (percentage) and quantitative variables under mean ± standard deviation. The incidence of AC was expressed as a percentage with its 95% confidence interval.
For the analysis of the factors associated with the occurrence of AC, as there was potentially several samples for each patient and some explanatory variables were measured at the same time as AC, a repeated measures univariable logistic regression model was performed to obtain odds ratios (OR) and their confidence intervals.
For the analysis of the factors associated with the occurrence of an episode of intra-hospital AKI, we generated Kaplan Meier curves representing the risk of AKI over time as a function of the occurrence of AC and associated 95% confidence interval. The Kaplan Meier curves and the corresponding results of the log rank test were intended only for presentation purpose, as a Cox model using the occurrence of AC as a time-dependent explanatory variable would lead to a less-biased association between the two variables. Therefore, a Cox model was used considering the occurrence of AC as a time-varying covariate, allowing the estimation of hazard ratios (HR) and 95% confidence intervals. For multivariable analysis, variable selection was performed including all variables with a p-value lower than 0.20 then using a backward method with a p-value for the removal of 0.10. For this analysis, patients who presented with AKI before the first AC test were not included as AC results were not known before AKI occurrence. To assess whether the exclusion of patients who developed AKI without knowledge of AC status may have significantly altered the results, we performed a “worst case scenario” sensitivity analysis including these patients and considering they had no AC before AKI occurrence. To assess whether the presence of AC investigations performed in symptomatic patients could also alter these findings, we performed another sensitivity analysis not taking into account AC investigations performed in symptomatic patients. No imputation was performed for missing data. A value of p <0.05 was considered significant. Analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical considerations
This study obtained the favorable opinion of the Institutional Review Board of the University Hospital of Reims (January 2015), the favorable opinion of the Advisory Committee on the Processing of Information in Research in the field of Health (CCTIRS - No. 15.380 of 06/10/2015) and the authorization of the French Data Protection Authority (CNIL - decision DR-2015-618 of 12/24/2015). It is listed on clinicaltrials.gov under the number NCT02853292. All patients received an information notice, either prospectively or retrospectively, in accordance with French legislation.
Role of the funding source
There was no funding source for this study. All authors confirm they had full access to all the data in the study and accept responsibility to submit for publication.
Results
Study population
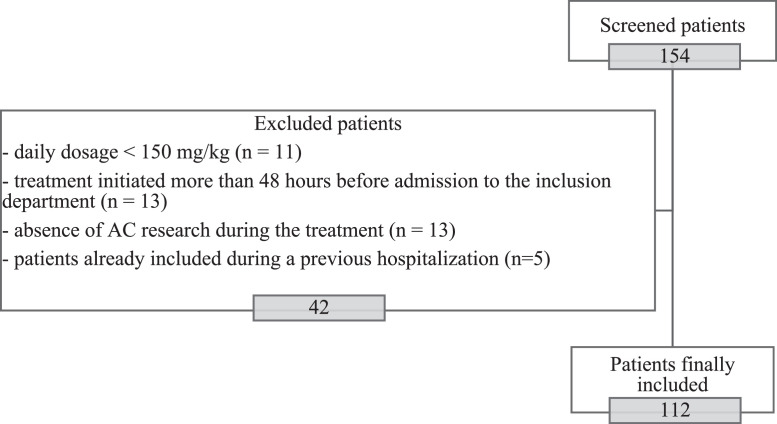
Among the 154 patients initially recruited, 42 were excluded for the following reasons: dosage less than 150 mg/kg/day, treatment initiated more than 48 hours before admission to the inclusion department, no investigation of AC during the treatment, or patients already included during a previous hospitalization. 112 patients were finally included. Forty-two patients were recruited retrospectively and 70 were recruited prospectively. The flow chart is presented in Figure 1.
Figure 1.
Flow chart of the 112 patients who received high-dose intravenous amoxicillin treatment, included between 2014 and 2019 in participating centers in north-eastern France.
The main clinical characteristics of the 112 patients included are presented in Table 1 and the main characteristics of the 186 urine samples taken from the 112 patients included are presented in Supplementary table 1. The patients included were predominantly men (65.2%), with a mean age of 66.9 ± 16.0 years and a mean body mass index (BMI) of 28.0 ± 6.9 kg/m2, frequently hypertensive (48.2%), diabetic (33.9%) and/or with chronic renal failure (22.3%).
Table 1.
Clinical characteristics of the 112 included patients receiving high doses of intravenous amoxicillin.
| Clinical characteristics (N=112) |
Missing data | ||
|---|---|---|---|
| N | (%) | ||
| Age (years) (mean±sd) | 66.9 | ±16.0 | 0 |
| Male gender | 73 | (65.2) | 0 |
| Weight (kg) (mean±sd) | 80.5 | ±21.6 | 3 |
| BMI (kg/m²) (mean±sd) | 28.0 | ±6.9 | 16 |
| History of | |||
| Urinary lithiasis | 6 | (5.4) | 0 |
| Chronic renal insufficiency (KDIGO classification) | 25 | (22.3) | 0 |
| Mild (G1/G2) | 11 | (9.8) | |
| Moderate (G3) | 10 | (8.9) | |
| Severe (G4) | 3 | (2.7) | |
| Terminal (G5) | 0 | (0.0) | |
| Diabetes mellitus | 38 | (33.9) | 0 |
| Hypertension | 54 | (48.2) | 0 |
| Multiple myeloma | 4 | (3.6) | 0 |
| Known indications for high-dose amoxicillin* | 112 | (100.0) | 0 |
| Infectious endocarditis | 92 | (75.4) | |
| Osteoarticular infections | 26 | (23.2) | |
| Meningitis | 8 | (7.1) | |
| Other | 5 | (4.5) | |
| Initial amoxicillin daily dosage (g per day) (mean±sd) | 14.0 | ±3.2 | 0 |
| Number of injections per day (mean±sd) | 5.0 | ±1.4 | 8 |
| Intravenous treatment duration (days) (mean±sd) | 22.5 | ±21.0 | 2 |
| Treatment discontinuation before 28th day of treatment | 73 | (67.0) | 3 |
| Reason: | |||
| Switched to another antibiotic | 20 | (17.9) | |
| Side effects | 10 | (8.9) | |
| End of treatment | 19 | (17.0) | |
| Death | 2 | (1.8) | |
| Oral amoxicillin | 10 | (8.9) | |
| Unknown | 12 | (10.7) | |
| Associated antibiotics during amoxicillin course | |||
| Aminoglycosides | 71 | (63.4) | 0 |
| Ceftriaxone | 20 | (17.9) | 0 |
| Vancomycin | 2 | (1.8) | 0 |
| Other | 32 | (28.6) | 0 |
| Other treatments received at amoxicillin initiation | |||
| Diuretics | 47 | (42.0) | 0 |
| Loop diuretics | 37 | (33.0) | |
| Thiazide diuretics | 8 | (7.1) | |
| Potassium-sparing diuretics | 5 | (4.5) | |
| ACE inhibitors | 23 | (20.5) | 0 |
| Angiotensin II receptor blockers | 16 | (14.3) | 0 |
All these patients were included between 2014 and 2019 in the participating centers of Northeast France.
ACE: angiotensin-converting enzyme.
BMI: body mass index.
sd: standard deviation.
Some patients had several indications for high-dose amoxicillin. Initial reasons for hospitalization were not recorded.
The main indications for HDIVA were infectious endocarditis (IE) (75.4%) and bone and joint infections (23.2%). The mean daily dose of HDIVA was 14.0 ± 3.2 g, with a mean number of daily injections of 5.0 ± 1.4, for a mean duration of 22.5 ± 21.0 days. No patient received continuous infusions. HDIVA was associated with an aminoglycoside in 63.4% of patients (for a mean duration of 9.4 ± 6.1 days), and with at least one diuretic in 42.0% of patients.
Incidence and factors associated with amoxicillin crystalluria in patients receiving HDIVA
The characteristics of AC research are presented in Table 2. Twenty-seven patients (24.1%, 95% CI [16.2-32.0]) developed at least one episode of AC, within a mean of 5.1 ± 3.6 days of treatment initiation. No significant difference of AC incidence was observed between the retrospective and the prospective phase of the study: 11/42 (26.2%) and 16/70 (22.9%) for the retrospective and the prospective phases, respectively, p=0.69. There was also no increasing trend for the AC occurrence between the first half of patients included and the second half: (12/56 (21.4%) versus 15/56 (26.8%) respectively, p=0.51). AC was identified in 12.9% of systematic samples and 21.4% of samples taken from symptomatic patients. Of the 29 positive AC, only 3 (10.3%) were from symptomatic patients. Eighty-three patients (74.1%) were tested for AC at D3 ± 1 day, 18 (21.7%) of whom were positive. Sixty-four patients (57.1%) were tested for AC at D7 ± 2 days, of whom 6 (9.4%) were positive. Thirty-one patients (27.7%) were tested for AC at D14 ± 2 days, of whom 4 (12.9%) were positive. Eight patients (7.1%) received additional AC investigation, which was positive in one (12.5%). Among the 112 included patients, 16 were tested for AC at each time point, 36 twice and 60 once.
Table 2.
Characteristics of the different AC studies carried out on samples from the 112 included patients receiving high doses of intravenous amoxicillin.
| Crystalluria |
Missing data | ||
|---|---|---|---|
| N | % [95%CI] | ||
| At least one positive crystalluria | 27 | 24.1 [16.2-32.0] | 0 |
| On systematic samples (n= 124) | 16 | 12.9 [7.0-18.8] | |
| On symptomatic samples* (n= 14) | 3 | 21.4 [0.0-42.9] | |
| On unknown context (n= 48) | 10 | 20.8 [9.3-32.3] | |
| Positive crystalluria at D3 ± 1d | 18 | 21.7 [12.8-30.6] | 29 |
| Positive crystalluria at D7 ± 2d | 6 | 9.4 [2.2-16.5] | 48 |
| Positive crystalluria at D14 ± 2d | 4 | 12.9 [1.1-24.7] | 81 |
| Positive crystalluria in other searches | 1 | 12.5 [0.0-35.4] | 104 |
All these patients were included between 2014 and 2019 in the participating centers of Northeast France.
samples collected from patients presenting macroscopic hematuria and/or AKI
D3 ± 1d: third day after initiation of amoxicillin, with a delay of less or more than one day
CI: confidence interval.
The univariable analysis of factors associated with the occurrence of AC is presented in Table 3. Significantly associated factors were the use of angiotensin-converting enzyme (ACE) inhibitor (OR = 4.55, 95% CI [2.22-9.34], p <0.0001), the urinary dipstick pH (OR = 2.13 by reduction of a pH point, 95% CI [1.21 - 3.74], p = 0.009), age (OR = 1.04, 95% CI [1.01-1.06], p = 0.01) and hematuria on CBEU (defined by > 10 red blood cells/mm3) (OR = 1.01, 95% CI [1.00-1.02], p = 0.03).
Table 3.
Factors associated in univariable analysis with the occurrence of AC in the 112 included patients receiving high doses of intravenous amoxicillin.
| OR [95%CI] | p value | Missing data | |
|---|---|---|---|
| Clinical characteristics (N=112) | |||
| Male gender | 1.68 [0.72-3.94] | 0.23 | 0 |
| Age (per increase of 1 year) | 1.04 [1.01-1.06] | 0.01 | 0 |
| BMI (per increase of 1 kg/m2) | 1.01 [0.93-1.09] | 0.90 | 16 |
| Chronic renal insufficiency | 1.45 [0.60-3.48] | 0.41 | 0 |
| Diabetes mellitus | 1.27 [0.61-2.64] | 0.51 | 0 |
| Aminoglycosides | 1.45 [0.60-3.46] | 0.41 | 0 |
| Vancomycin | 2.83 [0.70-11.46] | 0.14 | 0 |
| Diuretics | 1.44 [0.67-3.09] | 0.35 | 0 |
| Loop diuretics | 1.63 [0.74-3.60] | 0.22 | 0 |
| ACE inhibitors | 4.55 [2.22-9.34] | < 0.0001 | 0 |
| Angiotensin II receptor blockers | 0.65 [0.21-2.02] | 0.45 | 0 |
| Initial amoxicillin daily dosage (per increase of 1 g/day) | 1.06 [0.96-1.17] | 0.24 | 0 |
| Number of injections per day (per increase of 1 injection/day) | 0.98 [0.73-1.32] | 0.90 | 8 |
| Initial blood creatinine value (per increase of 1 µmol/L) | 1.00 [0.99-1.01] | 0.94 | 3 |
| Albuminemia (per increase of 1 g/L) | 1.08 [0.97-1.21] | 0.17 | 44 |
| Sample characteristics (N=186) | |||
| Negative direct exam (CBEU) | 1.88 [0.16-22.21] | 0.62 | 59 |
| Sterile culture (CBEU) | 1.62 [0.55-4.76] | 0.38 | 20 |
| Leucocyturia (per increase of 1/mm3) (CBEU) | 1.00 [1.00-1.00] | 0.72 | 12 |
| Hematuria (per increase of 10/mm3) (CBEU) | 1.01 [1.00-1.02] | 0.03 | 12 |
| Proteinuria (urinary dipstick) (per increase of 1 cross) | 1.61 [0.69-3.74] | 0.27 | 49 |
| Urinary density (urinary dipstick) (per increase of 0,001 point) | 2.25 [0.00-2.78] | 0.19 | 56 |
| Urinary pH (urinary dipstick) (per decrease of 1 point of pH) | 2.13 [1.21-3.74] | 0.009 | 52 |
| Urinary pH (pHmetry) (per decrease of 1 point of pH) | 1.57 [0.90-2.74] | 0.11 | 32 |
All these patients were included between 2014 and 2019 in the participating centers of Northeast France.
ACE: angiotensin-converting enzyme.
BMI: body mass index.
CBEU: cytobacteriological exam of urine.
CI: confidence interval.
OR: odds ratio.
Occurrence and associated factors with AKI in patients receiving HDIVA
Mean serum creatinine at the start of treatment was 86.6 ± 49.6 µmol/L and peak serum creatinine during the 28 days after initiation of treatment was 151.1 ± 142.0 µmol/L. Of the 112 patients, 20 (17.9%) developed an AKI within the first 28 days of treatment, with a mean duration of 10.9 ± 6.3 days after initiation of HDIVA. The serum creatinine at the end of hospitalization or at the latest on the 28th day of hospitalization was 97.8 ± 76.4 µmol/L.
Five patients developed AKI before the first screening for AC and were not included in the analysis of the impact of the occurrence of AC on the occurrence of AKI. Of the remaining 107 patients, 15 (14.0%) developed an AKI within 28 days of starting treatment with HDIVA, with a mean duration of 10.4 ± 6.0 days after initiation of HDIVA. In these patients, mean peak serum creatinine was 353 ± 202 µmol/L. Of the fifteen patients, 7 presented stage 2 AKI and the other 8 presented stage 3 AKI, based on KDIGO classification.
Kaplan Meier curves for the association between the risk of AKI over time and the occurrence of AC are available as supplementary material. The analysis of the factors associated with the occurrence of AKI is presented in Table 4. The factors significantly associated in univariable analysis were the occurrence of AC (HR = 8.65, 95%CI [2.91-25.67], p = 0.0001), the presence of hypertension (HR = 4.41, 95%CI [1.25-15.65], p = 0.02), increase in BMI (HR = 1.08 per one point increase, 95%CI [1.01-1.16], p = 0.03) and increase in age (HR = 1.05 per one year, 95%CI [1.00-1.10], p = 0.04). In multivariable analysis, the variables retained in the model were the occurrence of AC (HR = 7.41, 95% CI [2.48-22.16], p = 0.0003) and hypertension (HR = 3.43, 95%CI [0.96-12.28], p = 0.06).
Table 4.
Factors associated in univariable and multivariable analyses with the occurrence of AKI in the 112 included patients receiving high doses of intravenous amoxicillin.
| Univariate analysis |
Multivariate analysis |
Missing data | |||
|---|---|---|---|---|---|
| HR [95%CI] | p value | HR [95%CI] | p value | ||
| Female gender | 0.49 [0.14-1.73] | 0.26 | 0 | ||
| Age (per increase of 1 year) | 1.05 [1.00-1.10] | 0.04 | 0 | ||
| BMI (per increase of 1 kg/m2) | 1.08 [1.01-1.16] | 0.03 | 16 | ||
| Chronic renal insufficiency | 1.81 [0.62-5.30] | 0.28 | 0 | ||
| Diabetes mellitus | 2.20 [0.79-6.07] | 0.13 | 0 | ||
| Hypertension | 4.41 [1.25-15.65] | 0.02 | 3.43 [0.96-12.28] | 0.06 | 0 |
| Infectious endocarditis | 2.50 [0.33-19.11] | 0.38 | 0 | ||
| Aminoglycosides | 1.73 [0.48-6.19] | 0.40 | 0 | ||
| Vancomycin | 3.80 [0.50-29.07] | 0.20 | 0 | ||
| Diuretics | 2.22 [0.79-6.24] | 0.13 | 0 | ||
| Loop diuretics | 1.39 [0.49-3.90] | 0.53 | 0 | ||
| ACE inhibitors | 1.46 [0.50-4.29] | 0.49 | 0 | ||
| Angiotensin II receptor blockers | 0.91 [0.21-4.03] | 0.90 | 0 | ||
| NSAIDs | 2.09 [0.27-15.99] | 0.48 | 0 | ||
| Initial amoxicillin daily dosage (per increase of 1 g/day) | 1.09 [0.95-1.25] | 0.23 | 0 | ||
| Initial blood creatinine value (per increase of 1 µmol/L) |
1.00 [0.99-1.01] | 0.98 | 3 | ||
| Amoxicillin crystalluria | 8.65 [2.91-25.67] | 0.0001 | 7.41 [2.48-22.16] | 0.0003 | 0 |
All these patients were included between 2014 and 2019 in the participating centers of Northeast France.
Amoxicillin crystalluria was fitted as a time-varying covariate.
ACE: angiotensin-converting enzyme.
BMI: body mass index.
CI: confidence interval.
HR: hazard ratio.
NSAIDs: non-steroidal anti-inflammatory drugs.
In sensitivity analyses, considering patients who did not have AC investigation before the occurrence of AKI as having no crystalluria (worst-case scenario), the only variable retained in the model was the occurrence of amoxicillin crystalluria (HR=5.00, 95%CI[2.00 – 12.49], p=0.0006]. Excluding crystalluria testing performed in symptomatic patients, the two variables retained in the model were the occurrence of AC (HR = 6.70, 95% CI [2.38-18.85], p = 0.0003) and hypertension (HR = 3.75, 95% CI [1.06-13.27], p = 0.04).
Other outcomes
Mean hospital stay was 26.1 ± 27.3 days. By day 28, 8 patients (7.1%) had died, 4 of whom presented with AC followed by AKI, 3 had neither AC nor AKI and 1 had developed AKI without documented AC.
Discussion
In this study of 112 patients treated with HDIVA who benefited from at least one systematic investigation of AC, we showed that AC was a frequent phenomenon since it was found in about a quarter of patients, that it seemed to be associated with both taking ACE inhibitors and urinary pH, and that its occurrence was the main predictor of the development of in-hospital AKI.
We found AC incidence to be 24.1% (95% CI [16.2-32.0]) in patients treated with HDIVA. Little data on incidence is currently available in the literature. One other study, with similar methodology but of smaller size, involving 32 patients treated with HDIVA for suspected IE, estimated the incidence of AC at 43.8%.28 Another French single-center prospective study involving 32 patients found the incidence of AC during treatment with HDIVA to be 21.9%, the diagnosis being retained in the event of evidence of AC but also in the event of obstructive AKI without other obvious identified cause.29
In univariable analysis, factors associated with the occurrence of AC were the presence of hematuria in the cytobacteriological examination, acidification of pH on urinary dipstick, increased age and use of ACE inhibitors. Hematuria appears to be more of a consequence, through tubular damage and medullary congestion secondary to intrarenal crystal precipitation, than a cause of AC. The acidity of urine pH has long been known to favor AC in patients treated with amoxicillin, because it increases the proportion of amoxicillin excreted in the urine in the unmetabolized trihydrate form 19, which is less soluble than the sodium form11. This data is corroborated by a recent study that found the acidification of urine pH (OR = 0.78 by one increasing pH point, 95% CI [0.62-0.97]) 28 and the increase in amoxicillin blood concentration (OR = 1.03, 95% CI [1.01-1.05]) as risk factors for AC. ACE inhibitors, by vasodilation of the efferent arteriole, induce a decrease in glomerular filtration rate and therefore a decrease in urine output, a factor known to promote AC.5 However, we have not found a similar association with angiotensin II receptor antagonists whose effect on renal physiology is similar. Taking ACE inhibitors could be epidemiologically associated with conditions favoring urinary acidification such as obesity 30 or heart failure, given that ACE inhibitors may be used preferentially in situations of heart failure.31 The effect of ACE inhibitors on the occurrence of AC has never been described previously in the literature and should be considered, subject to confirmation by further studies.
In our study, 17.9% of patients developed AKI within 28 days of starting treatment. In a retrospective study of 358 patients treated with HDIVA, 20.4% of patients developed an episode of AKI, attributed to AC or not.23 By multivariable analysis, we found that the main factor associated with the occurrence of AKI was the occurrence of AC. This impact was potentially underestimated because the detection of asymptomatic AC could lead to corrective measures (stopping or reducing the dosage of amoxicillin, increasing hydration, alkalinization of the urine). The impact of the occurrence of AC on the occurrence of AKI, widely assumed since the mid-1980s 13, is supported by our study as well as another study using similar methodology which found a predictive impact of the same order (cause-specific hazard = 7.44, p = 0.005).28
In our study, AC occurred within a mean time of 5 days after treatment initiation and AKI occurred within a mean time of 10 days after treatment initiation. Only 10.3% of positive AC tests occurred in symptomatic patients. It may seem relevant to systematically check for AC during the first week of treatment to anticipate and prevent the occurrence of AKI through corrective measures, whose effectiveness remains to be demonstrated. However, the implementation AC investigation can be complicated: urine samples must be analyzed by an experienced operator within two hours of voiding, and cannot be collected in a borate tube. Availability of AC investigation depends on the center and is often not feasible on weekends and public holidays.
An interesting predictive marker for AC could be the amoxicillin residual dosage, performed just before the subsequent amoxicillin injection (not analyzed in our study due to the lack of usable data). It is easily performed in clinical practice in centers where it is available and would allow practitioners to optimize amoxicillin doses rapidly in terms of PK / PD according to the minimum inhibitory concentrations of the identified pathogens.28 However, this analysis is not available in all centers and may be subject to a long rendering time, while AC testing can be performed in all laboratories using a simple microscope with the results being immediate.
Our study has several limitations. First, some of the data was collected retrospectively, which resulted in missing data and may have introduced classification bias. Unfortunately, most of the amoxicillin blood concentration dosages corresponded to peak concentrations rather than residual concentrations. The lack of reliable data for amoxicillin residual dosage precluded analysis of this variable. As the biologists who carried out the AC testing were not blinded from the clinical context, classification bias may have arisen, although AC investigation is generally easy to perform. The analysis of the factors associated with the occurrence of AC probably suffered from a lack of power, because of the number of patients included, but this was not the main objective of our study. The number of excluded patients, larger than anticipated, limited the power of the study and may have resulted in selection bias (for example, patients who were excluded because they were not tested for AC may be at lower risk of AC). Exclusion criteria may also alter the generalizability of the findings; however, we restricted our population to those who would most benefit from systematic AC investigation. Most patients did not benefit from systematic AC investigation at each stage and therefore the incidence of its occurrence, which may be transient, could be an underestimate. It must be acknowledged that our study could not determine a relationship of causality between AC and subsequent AKI, as the patients were severe cases whose AKI could have other causes. Characteristics of AKI episodes were not collected, whereas these could have elucidated the link with AC, for example the simultaneous occurrence of macroscopic hematuria.
Our study has several strengths. AC investigation was carried out systematically, regardless of the presence of symptoms. This allowed for a more accurate analysis of the impact of AC and its impact on the occurrence of AKI. To our knowledge, among the studies of similar methodology published to date, ours has the largest number of patients.
In conclusion, in our study, almost a quarter of patients treated with HDIVA developed AC, which was very strongly correlated with the subsequent occurrence of AKI, advocating for systematic investigation of this frequent and potentially serious complication of the widespread amoxicillin antibiotic therapy. Future research should focus on the means of preventing AC.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgements
We are indebted to Ottavia Pelissero and Grace Stockton for their precious help during the writing of this article.
CRISTAMOX study group
DE CHAMPS Christophe, VERNET-GARNIER Véronique, BRASME Lucien, GUILLARD Thomas, ROBBINS Ailsa, DIDIER Kevin, ORQUEVAUX Pauline, TROMEUR Thibault, BERMEJO Messaline, BRUNET Aurélie, ROMARU Juliette, NOEL Violaine, MINARD Geoffrey, BERGER Jean-Luc, GILTAT Aurélien, LAMBERT Dorothée, SCHVARTZ Betoul, COUSSON Joël, DJERADA Zoubir, THOUVENIN Maxime, PERNAS Patrick, LACOSTE Marion (Troyes), MAY Thiery, RABAUD Christian, GOEHRINGER François, JACQUET Caroline, DOUSSET Brigitte (Nancy), CHAVANET Pascal, MASSON David, BLOT Matthieu, GOHIER Sandrine (Dijon), GALEMPOIX Jean-Marc, MENDES-MARTINS Lucile, GALLON Olivier, LEBRUN Delphine, PRIEUR Nathalie, MEURICE Julie, MATEU Philippe, FEDUN Samuel, BRENKLE Klaus, COMANDINI Michel, ROSMAN Jeremy (Charleville-Mézières), Dr MARTHA Benoit, OGIER-DESSEREY Agathe, KARSENTY Judith (Chalon-Sur-Saône).
Contributors
MH, SD and MD designed the study; MH, SD, SM, AC, EB, SP collected the data; MH and SD verified the underlying data. MH performed the statistical analysis; MH and SD drafted the manuscript. All authors contributed to data interpretation and analysis and revised the manuscript. All authors confirm they had full access to all the data in the study and accept responsibility to submit for publication.
Funding
None.
Data sharing statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be made available, as will be the study protocol (in French), to researchers who provide a methodologically sound proposal, up to 36 months following study publication. Proposals should be directed to mhentzien@chu-reims.fr. To gain access, data requestors will need to sign a data access agreement.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2022.101340.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; Genève, Suisse: 2018. WHO report on surveillance of antibiotic consumption : 2016-2018 early implementation. [Google Scholar]
- 2.Maugat S, Berger-Carbonne A. Consommation d'antibiotiques et résistance aux antibiotiques en France : une infection évitée, c'est un antibiotique préservé ! https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques/documents/rapport-synthese/consommation-d-antibiotiques-et-resistance-aux-antibiotiques-en-france-une-infection-evitee-c-est-un-antibiotique-preserve. Accessed 8 Aug 2021.
- 3.Vidal France. Vidal. https://www.vidal.fr. Accessed 8 Aug 2021.
- 4.Sjovall J, Westerlund D, Alvan G. Renal excretion of intravenously infused amoxycillin and ampicillin. Br J Clin Pharmacol. 1985;19:191–201. doi: 10.1111/j.1365-2125.1985.tb02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agence nationale de sécurité du médicament et des produits de santé. Enquête de pharmacovigilance : amoxicilline IV et risque de cristallurie et d'atteinte rénale. https://ansm.sante.fr/actualites/rappel-du-bon-usage-de-lamoxicilline-injectable-pour-diminuer-le-risque-de-cristalluries. Accessed 8 Aug 2021.
- 6.Daudon M. Cristallurie. Néphrologie Thérapeutique. 2015;11:174–190. doi: 10.1016/j.nephro.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Fogazzi GB. Amoxycillin, a rare but possible cause of crystalluria. Nephrol Dial Transplant. 2003;18:212–214. doi: 10.1093/ndt/18.1.212. [DOI] [PubMed] [Google Scholar]
- 8.Belko J, Urueta G, Emre U. Amoxicillin overdose manifested by hematuria and acute renal failure. Pediatr Infect Dis J. 1995;14:917–919. [PubMed] [Google Scholar]
- 9.Bright DA, Gaupp FB, Becker LJ, Schiffert MG, Ryken TC. Amoxicillin overdose with gross hematuria. West J Med. 1989;150:698–699. [PMC free article] [PubMed] [Google Scholar]
- 10.Couto J, Pontes dos Santos L, Carlos Alves J, López R, Maldonaldo C. Amoxicillin crystalluria : a rare side-effect of a commonly prescribed antibiotic. Eur J Case Rep Intern Med. 2017;4 doi: 10.12890/2017_000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckart P, Brouard J, Duhamel J. Cristalluries secondaires aux aminopénicillines. A propos de cinq observations pédiatriques. Arch Pédiatrie. 1995;2:811. [Google Scholar]
- 12.Fritz G. Amoxicillin-induced acute renal failure. Nephrol Dial Transplant. 2003;18:1660–1662. doi: 10.1093/ndt/gfg236. [DOI] [PubMed] [Google Scholar]
- 13.Geller RJ, Chevalier RL, Spyker DA. Acute Amoxicillin Nephrotoxicity Following an Overdose. J Toxicol Clin Toxicol. 1986;24:175–182. doi: 10.3109/15563658608990456. [DOI] [PubMed] [Google Scholar]
- 14.Hentzien M, Lambert D, Limelette A, et al. Macroscopic amoxicillin crystalluria. The Lancet. 2015;385:2296. doi: 10.1016/S0140-6736(14)62001-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones DP, Gaber L, Nilsson GR, Brewer ED, Bruder Stapleton F. Acute renal failure following amoxicillin overdose. Clin Pediatr (Phila) 1993;32:735–739. doi: 10.1177/000992289303201205. [DOI] [PubMed] [Google Scholar]
- 16.Kleppe DM, Patel AD, Goodin J, et al. Amoxicillin-induced crystalline nephropathy presenting as ureteral obstruction. Clin Pediatr (Phila) 2020;59:614–617. doi: 10.1177/0009922820912214. [DOI] [PubMed] [Google Scholar]
- 17.Labriola L, Jadoul M, Daudon M, Pirson Y, Lambert M. Massive amoxycillin crystalluria causing anuric acute renal failure. Clin Nephrol. 2003;59:455–457. doi: 10.5414/cnp59455. [DOI] [PubMed] [Google Scholar]
- 18.Moesch C, Ravasse P, Leroyer R, Rince M. Various types of crystalluria due to amoxicillin. Ann Biol Clin (Paris) 1990;48:331–332. [PubMed] [Google Scholar]
- 19.Moesch C, Rince M, Raby C, Denis F, Leroux-Robert C. Crystalluria following aminopenicillin therapy. Clin Nephrol. 1985;23:318–319. [PubMed] [Google Scholar]
- 20.Rafat C, Haymann J-P, Gaudry S, et al. AKI in a patient with suspected meningoencephalitis. Kidney Int. 2014;86:1065–1066. doi: 10.1038/ki.2013.446. [DOI] [PubMed] [Google Scholar]
- 21.Trillaud E, Bendib I, Arrestier R, Razazi K. Macroscopic amoxicillin crystalluria. Intensive Care Med. 2020;46:1616–1617. doi: 10.1007/s00134-020-05970-2. [DOI] [PubMed] [Google Scholar]
- 22.van Noord C, Wulkan RW, van den Dorpel MA. Crystalluria. Neth J Med. 2012;70:84–87. [PubMed] [Google Scholar]
- 23.Garnier A-S, Dellamaggiore J, Brilland B, et al. High incidence of amoxicillin-induced crystal nephropathy in patients receiving high dose of intravenous amoxicillin. J Clin Med. 2020;9:2022. doi: 10.3390/jcm9072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeller V, Puyraimond-Zemmour D, Sené T, Lidove O, Meyssonnier V, Ziza J-M. Amoxicillin crystalluria, an emerging complication with an old and well-known antibiotic. Antimicrob Agents Chemother. 2016;60 doi: 10.1128/AAC.00359-16. 3248–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousseaux C, Rafat C, Letavernier E, et al. Acute Kidney Injury After High Doses of Amoxicillin. Kidney Int Rep. 2021;6:830–834. doi: 10.1016/j.ekir.2020.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatti M, Fusaroli M, Raschi E, Capelli I, Poluzzi E, De Ponti F. Crystal nephropathy and amoxicillin: insights from international spontaneous reporting systems. J Nephrol. 2021 doi: 10.1007/s40620-021-01191-y. published online Nov 11. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L, Le Beller C, Trenque T, et al. Amoxicillin-induced crystal nephropathy : a nationwide French pharmacovigilance databases study. Br J Clin Pharmacol. 2020;86:2256–2265. doi: 10.1111/bcp.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamme M, Oliver L, Ternacle J, et al. Amoxicillin crystalluria is associated with acute kidney injury in patients treated for acute infective endocarditis. Nephrol Dial Transplant. 2021:1–11. doi: 10.1093/ndt/gfab074. [DOI] [PubMed] [Google Scholar]
- 29.Tamisier N, Maillard N, Brunel P, Roussel M, Botelho-Nevers E, Gagneux-Brunon A. Incidence de la cristallurie à l'amoxicilline dans le traitement des endocardites infectieuses (EI) en 2018 au sein d'un CHU. Médecine Mal Infect. 2019;49:S58. [Google Scholar]
- 30.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CYC. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 31.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.