Abstract
Background:
Several studies have assessed potential associations between use of weight loss products in the periconceptional period and neural tube defects (NTDs). However, the individual studies are inconclusive and there has not been a systematic review of this literature.
Methods:
We conducted a systematic search, using Ovid MEDLINE and PubMed, to identify studies that evaluated the association between products used for weight loss and the risk of NTDs. Because many studies of birth defects only evaluate a composite birth defect outcome, we evaluated studies that defined the outcome as “any major birth defect” or as NTDs. We abstracted data on study design, exposure definition, outcome definition, covariates and effect size estimates from each article that met our inclusion criteria. For studies that evaluated a composite birth defect outcome, we also abstracted the number of NTD cases included in the composite outcome. We used a modified version of the Newcastle-Ottawa Scale to assess the quality of each article.
Results:
We screened 865 citations and identified nine articles that met our inclusion criteria. The majority of studies reported positive associations between maternal use of weight loss products and birth defects (overall and NTDs). However, there were few significant associations and there was considerable heterogeneity in the specific exposures assessed across the nine studies.
Conclusion:
Our systematic review of weight loss products and NTDs indicates that the literature on this topic is sparse. Because several studies reported modest, positive associations between risk and use of weight loss products, additional studies are warranted.
Keywords: birth defects, dieting, neural tube defects, pregnancy, spina bifida, weight loss
Introduction
Weight loss activities are common in women of reproductive age, even among women who are not overweight or obese. In the 2003 Behavioral Risk Factor Surveillance System survey, 75% of obese, 65% of overweight and 30% of normal/underweight women reported that they were trying to lose weight (Katon et al., 2012). Consequently, many women may be engaged in weight loss activities at the time of conception and during early pregnancy, when such activities could have an adverse impact on the embryo. It is, therefore, important to understand the potential impact of different weight loss strategies on birth defect risk.
One weight loss strategy that is relatively common among reproductive-aged women is the use of weight loss products. For example, in a 2002 survey, 17% of women 18 to 34 years of age reported the use of a nonprescription weight loss product in the past year (Blanck et al., 2007). The use of weight loss products by reproductive aged females is of concern, because such products could have a direct teratogenic effect or indirectly influence embryonic develop by means of the weight loss mechanism (e.g., decreased caloric intake due to appetite suppression, decreased absorption of fats) or its sequelae (e.g., ketosis, micronutrient deficiencies).
Several studies have evaluated potential associations between maternal weight loss strategies and the risk of neural tube defects (NTDs) (e.g., Martin et al., 1988; Carmichael et al., 2003). NTDs are of particular interest as they are one of the most common groups of serious birth defects, with a prevalence of approximately 7/10,000 (Williams et al., 2015) births in the United States. In addition, there is a substantial body of literature suggesting a link between NTD risk and maternal nutritional and dietary parameters (Carmichael et al., 2003). However, the individual studies of maternal use of weight loss products and NTDs are inconclusive, and we are not aware of any systematic reviews of this literature. Consequently, the objective of this study was to assess the evidence for an association between a common weight loss strategy, use of weight loss products (e.g., drugs, supplements), and the risk of NTDs.
Materials and Methods
We conducted a systematic literature search using Ovid MEDLINE and PubMed to identify articles that reported on the association between maternal use of weight loss products and the risk of (1) NTDs or (2) the composite outcome, “any major birth defect.” The latter was included because, due to the relatively low prevalence of individual birth defects, many studies only assess a composite outcome.
The search included articles published from 1946 through June 20, 2016. We used combinations of keywords defining the outcome (e.g., spina bifida), exposure period (e.g., pregnancy), and exposure (e.g., anti-obesity drugs), including specific drugs (e.g., sibutramine) and supplements (e.g., garcinia cambogia) used for weight loss. The exposure search terms were obtained from relevant reviews (Bitsko et al., 2008; Egras et al., 2011; Finkelstein and Kruger, 2014; Yanovski and Yanovski, 2014; Rios-Hoyo and Gutierrez-Salmean, 2016) and Web sites (see the Links section). The Ovid MEDLINE search strategy is provided in Supplementary Appendix S1, which is available online.
We excluded articles that were reviews or abstracts, animal or in vitro studies, or case reports or series, as well as any study that did not involve a comparison group. We also excluded articles that did not evaluate: (1) NTDs or a composite birth defect outcome; (2) products used specifically for weight loss (e.g., a study of diuretic use for weight loss would have been included, whereas a study of diuretic use for treatment of hypertension would not); or (3) exposures during, at least part of, the period from the three months before conception through the first trimester of pregnancy.
Two reviewers (T.T.H., A.J.A.) screened the titles and abstracts of all identified articles. The same two individuals reviewed the full text of articles screened as potentially eligible by either reviewer. Discrepancies were resolved through discussion. For each article selected for inclusion, one author abstracted information on study design, exposure definition, outcome definition and covariates, as well as effect estimates and 95% confidence intervals (CIs). For studies that only evaluated a composite birth defect outcome, we also abstracted the number of NTDs included in each comparison group. When an article did not provide effect estimates, but did provide the necessary count data, effect estimates, and CIs were calculated using Stata v14.2 (Stata Corp, College Station, TX).
We abstracted adjusted relative risks (aRR) or odds ratios (aOR) when available and otherwise abstracted unadjusted relative risks (uRR) or odds ratios (uOR). For articles that reported on a composite birth defect outcome that included both major and minor birth defects, we only abstracted information on major birth defects. For articles that included information on multiple exposure windows, we abstracted information specifically for first trimester exposures when available and otherwise abstracted information on exposures that occurred during the 3 months before pregnancy and/or the first trimester. A second author confirmed the accuracy of all abstracted data.
Additional, potentially relevant articles were identified by reviewing the reference lists for all included articles and searching Scopus for articles that referenced the included articles. The full text of each potentially relevant article identified by these searches was reviewed as described above.
We modified the Newcastle-Ottawa Scale for the evaluation of nonrandomized studies for this review (Supplementary Appendices S2 and S3) (Wells et al., 2009) and used the modified scale to evaluate the quality of each included article. One author scored each article and a second confirmed the accuracy of each score. We based Newcastle-Ottawa quality scores solely on the content of the included article (i.e., we did not use information from related publications to supplement details in the included article).
Results
The Ovid MEDLINE and PubMed searches identified 865 unique citations, of which 72 were selected for full-text review. Following the full-text review, seven articles were selected for inclusion. Following review of the bibliographies from and citations to these seven articles, two additional articles were included in the review. The nine articles, including four cohort and five case–control studies, are summarized in Table 1.
TABLE 1.
Characteristics of Included Studies and Reported Associations between Maternal Use of Weight Loss Products and Risk of Major Birth Defects or NTDs
| Reference [quality score]a | Location/source (enrollment period) | Exposed or case, nb | Unexposed or control, nb | Outcome | Exposure | Exposure period | Results |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Major birth defects: cohort studies | |||||||
| Milkovich and van den Berg 1977 [5] | Child Health and Development Study of Oakland, CA (1959–1966) | 409 | 8,989 | Severe congenital anomalies diagnosed before 61 months of age | Amphetamine or other anorectic drug prescribed sometime during pregnancy Prescription information obtained from Kaiser Health Plan clinic and hospital records |
1st trimester | Rate of malformations in women exposed during the first trimester (3.9/100) was not significantly different (p > 0.05) from rate in unexposed women (3.6/100) 0 NTDs observed in exposed pregnancies |
| Jones et al. 2002 [3] | California Teratogen Information Service (1996–1998) | 84 | 201 | Major structural anomalies | Self-reported use of phentermine combined with fenfluramine | 1st trimester |
Phentermine/fenfluramine BD: uRR =3.6 (95% CI, 0.6–21.1) 0 NTDs observed in exposed pregnancies |
| Manakova et al. 2012 [2] | Czech Teratology Information Service (1997–2012) | 17 | 85 | Major congenital malformations | Self-reported use of appetite suppressants | 1st trimester | Distribution of pregnancies with and without any birth defect was not significantly different in exposed and unexposed infants (p = 0.55) 0 birth defects observed in the exposed pregnancies Only two appetite suppressants were reported, sibutramine (n = 9) and phentermine (n = 8) |
| Kalten 2014 [6] | Swedish Medical Birth Register (1998–2011) | 509 | 1,392,126c | Relatively severe congenital malformations | Self-reported use of anti-obesity drugs Information reported at first prenatal visit, usually at 10–12 weeks gestation |
Early pregnancy |
Any anti-obesity drugd,e BD: aOR = 1.4 (95% CI, 0.7–1.7) Sibutramine BD: aRR = 1.8 (95% CI, 1.0–3.0) 0 NTDs observed in infants exposed to sibutramine Orlistat BD: aRR = 0.4 (95% CI, 0.1–1.0) One case of anencephaly observed in 248 infants exposed to orlistat |
| Major birth defects: case–control studies | |||||||
| Nelson and Forfar 1971 [4] | Scotland (3 maternity wards over a 2-year period) | 175 | 911 | Major congenital abnormalities | Use of prescribed appetite suppressant Self-reported prior to hospital discharge following delivery Confirmed by general practitioner, hospital record or pricing bureau |
1st trimester | BD: uRR = 1.9 (95% CI, 0.4–6.1)f 35% of cases had anencephaly, spina bifida or encephaloceleg |
| Lacroix et al. 2012 [5] | France EFEMERIS, prescription database (2004–2008) | 943 | 39,412 | Congenital anomalies | Benfluorex Exposure information obtained from prescription database |
First 2 months of pregnancy |
Benfluorex BD: aRRh = 1.7 (95% CI, 0.4–6.9) No exposed case had an NTD |
| NTDs: case–control studies | |||||||
| Carmichael et al. 2003 [5] | California (population-based within select counties, 1989–1991) | 538 | 539 | Anencephaly, spina bifida cystica, craniorachischisis and iniencephaly | Dieting behaviors including the use of diet pills, diuretics, or laxatives Self-reported ∼5 months following delivery |
3 months before through the end of pregnancy |
Diet pills NTD: uOR = 1.3 (95% CI, 0.6–2.9) Diuretics NTD: uOR = 2.7 (95% CI, 0.7–10.2) Laxatives NTD: uOR = 0.9 (95% CI, 0.6–1.5) |
| Bitsko et al. 2008 [6] | USA National Birth Defects Prevention Study (1998–2003) | 9,672 (18 exposed NTDs) | 3,324 | 30 types of major birth defects, including spina bifida, anencephaly and other neural tube defects | Prescription and over the counter medication marketed for weight loss or containing a component marketed for weight loss Self-reported 6 weeks to 24 months after estimated date of delivery |
1 month before through 1st trimester |
Any weight loss producti NTD: aOR = 1.2 (95% CI, 0.7–2.0) SB: aOR = 0.7 (95% CI, 0.3–1.6) AN: aOR = 2.6 (95% CI, 1.3–5.3) Ephedra NTD: aOR = 1.2 (95% CI, 0.6–2.6) SB: aOR = 0.8 (95% CI, 0.3–2.3) AN: aOR = 2.8 (95% CI, 1.0–7.3) Other NTD: aOR = 1.1 (95% CI, 0.7–1.6) SB: not calculated (<4 exposed cases) AN: aOR = 1.6 (95% CI, 1.0–2.6) |
| Suarez et al. 2012 [5] | Texas Department of Health’s Texas-Mexico border NTD project (1995–2000) | 184 | 225 | Anencephaly, spina bifida or encephalocele | Use of weight reduction supplements Self-reported 1–3 months following delivery or termination |
3 months prior to pregnancy |
Diet pills NTD: uOR = 1.6 (95% CI, 0.7–3.5) |
BD, birth defect; uRR, unadjusted relative risk; aRR, adjusted relative risk; uOR, unadjusted odds ratio; aOR, adjusted odds ratio; NTD, neural tube defects; SB, spina bifida; AN, anencephaly.
The quality score is based on a modified Newcastle-Ottawa scale and ranges from 0 to 8 for cohort studies and 0 to 9 for case–control studies.
For cohort studies, n = number exposed/unexposed (unless otherwise noted). For case–control studies, n = number of cases/controls.
n = number of infants in full birth cohort.
Calculated RRs when the expected number of outcomes was <10. Otherwise, ORs were calculated.
Adjusted for year of birth, maternal age, parity, smoking, and body mass index.
Calculated using Stata v14.2; 95% CI calculated using Woolf approximation.
Data from Nelson and Forfar, 1969.
Adjusted for maternal age and maternal diabetes mellitus.
Adjusted for maternal body mass index, education, periconceptional smoking, alcohol use and caffeine use, as well as multiple births and pregnancy intention.
Quality scores ranged from two to six (out of eight) for the cohort studies and from four to six (out of nine) for the case–control studies. Common study limitations included the potential for error in exposure classification, lack of control for potential confounding, and failure to provide details about response rates and the comparability of nonrespondents and study participants.
MAJOR BIRTH DEFECTS
Six studies (four cohort, two case–control) evaluated the association between any major birth defect and weight loss products (Nelson and Forfar, 1971; Milkovich and van den Berg, 1977; Jones et al., 2002; Lacroix et al., 2012; Manakova et al., 2012; Kallen, 2014). Four of these studies evaluated a broad exposure category that included the use of any weight loss product or diet pill. However, the included products differed across the studies (e.g., some studies included only prescription products, whereas others included prescription and nonprescription products). Three studies evaluated specific weight loss products (e.g., sibutramine), but there was no overlap across studies in the products that were evaluated.
Effect size estimates were not provided and could not be calculated for two cohort studies (Milkovich and van der Berg, 1977; Manakova et al., 2012). However, neither reported significant differences in the risk of birth defects in exposed and unexposed infants and no NTDs were observed in the exposed infants. In the four studies for which effect estimates were provided or calculated, elevated risk ratios (range for unadjusted estimates: 1.9–3.6; range of adjusted estimates: 1.4–1.8) were reported for all exposures except orlistat (Table 1). The highest reported relative risk was for the combined use of phentermine and fenfluramine (uRR = 3.6; 95% CI, 0.6–21.1) (Jones et al., 2002), but this association was not statistically significant and did not adjust for potentially important covariates, such as body mass index. Furthermore, there were no NTDs in the exposed infants in this study. Sibutramine was the only weight loss product that was associated with a significantly increased risk of birth defects (aRR = 1.8; 95% CI, 1.0–3.0) (Kallen, 2014). This association was significant even after accounting for potentially confounding variables including maternal body mass index, and seemed to be primarily with heart defects (aRR = 2.2; 95% CI, 0.8–5.9). No NTDs were observed in the infants exposed to sibutramine. Orlistat was the only weight loss product that was not associated with an increased risk of birth defects (aRR = 0.4; 95% CI, 0.1–1.0) (Kallen, 2014). However, 1/248 (0.4%) infants exposed to orlistat had anencephaly.
NTDS
Three case–control studies included analyses specific to NTDs (Table 1) (Carmichael et al., 2003; Bitsko et al., 2008; Suarez et al., 2012). In an unadjusted analysis of data from a population-based case–control study conducted in California, 1989 to 1991, there was a modest, nonsignificant increase in the use of diet products in the mothers of NTD cases as compared to mothers of controls (uOR = 1.3; 95% CI, 0.6–2.9) (Carmichael et al., 2003). A similar nonsignificant association was also reported in a study based on data from the National Birth Defects Prevention Study, 1998 to 2003, which adjusted for body mass index and additional covariates (aOR = 1.2; 95% CI, 0.7–2.0) (Bitsko et al., 2008). Finally, a slightly higher, but still nonsignificant, effect size estimate was obtained in an unadjusted analysis of data from a study of Mexican-Americans on the Texas–Mexico border (uOR = 1.6; 95% CI, 0.7–3.5) (Suarez et al., 2012).
In the one study that evaluated anencephaly and spina bifida separately (Bitsko et al., 2008), only anencephaly was associated with the use of any weight loss product (aOR = 2.6; 95% CI, 1.3–5.3). Furthermore, in this study, an association with anencephaly was observed for products containing ephedra (aOR = 2.8; 95% CI, 1.0–7.3) as well as products without ephedra (aOR = 1.6; 95% CI, 1.0–2.6). These associations were all significant in analyses that adjusted for body mass index and other covariates. In the single study that assessed use of diuretics (Carmichael et al., 2003), this exposure was more common in mothers of NTD cases as compared to controls (uOR = 2.7; 95% CI, 0.7–10.2).
Discussion
The use of weight loss products is relatively common in reproductive-aged women. In a 2002 survey, 17% of women 18 to 34 years of age reported the use of a nonprescription weight loss product in the past year (Blanck et al., 2007) and 2.4% of control mothers in the National Birth Defects Prevention Study reported periconceptional use of any weight loss product (Bitsko et al., 2008). Thus, it is important to understand the potential risks of such exposures to the embryo. However, our systematic review indicates that the literature on this topic is sparse, especially for specific exposures. Only three studies evaluated individual products (sibutramine, orlistat, benfluorex) or product combinations (phentermine and fenfuramine), and there was no overlap in the products assessed across these studies. Furthermore, studies that considered broad categories of exposures differed in the products that were included (e.g., only prescription versus prescription and nonprescription products). This heterogeneity is not surprising, given the number of weight loss products that are available, as well as changes in the availability of specific products over time (e.g., fenfluramine entered the United States market in 1973 and was withdrawn in 1997) (Haslam, 2016).
Based on the limited literature on specific weight loss products, it is not possible to determine whether maternal use of phentermine plus fenfluramine, benfluorex, sibutramine or orlistat is associated with the overall risk of birth defects or the risk of NTDs. Orlistat is the only one of these products that is currently marketed in the United States and it is available as both a prescription (pregnancy risk category: X, contraindicated) and lower dose, nonprescription medication. It is reassuring that, in a large Swedish birth cohort, orlistat was not associated with an increased risk of birth defects. However, 1/248 (0.4%) exposed infants had anencephaly, which is higher than expected for a condition with a prevalence of ~2/10,000 in Sweden (ICBDSR, 2013).
A single reviewed study evaluated maternal use of diuretics for weight loss and reported a moderate association with NTDs (uRR = 2.7; 95% CI, 0.7–10.2). At least two additional studies have reported associations between diuretics and NTDs. In a study based on data from the Texas Birth Defects Registry (which was not included in our review because it was only described in an abstract), women who had an NTD affected pregnancy were twice as likely as women with unaffected pregnancies to report using diuretics (OR = 2.0; 95% CI, 0.5–9.0) (Waller et al., 2001). In addition, in a study based on data from the Collaborative Perinatal Project (which was not included in our review because it did not focus on products used for weight loss), diuretics and NTDs were significantly associated (OR = 6.1; 95% CI, 2.0–18.4) (Myrianthopoulos and Melnick, 1987).
Our review provides some evidence that the risk of birth defects and the risk of NTDs are modestly associated with broad exposures that include the use of any diet product or any diet pill. Each study that assessed such exposures and provided an effect size estimate (or for which an estimate could be calculated) reported a nonsignificant increase in the risk of birth defects (range: 1.4–1.9) or NTDs (range: 1.2–1.6) in exposed, as compared to unexposed infants. However, as all but one of these studies used a case–control design, these findings are subject to all of the concerns inherent to this design, including the potential for misclassification bias in the exposure assessment and uncontrolled confounding. Furthermore, given the range of products considered in these studies, it is difficult to speculate about the potential mechanisms that might underlie the observed associations.
Optimally, future studies of the association between weight loss products and birth defects will consider specific weight loss products and specific birth defects. Additional studies of orlistat and diuretics as well as studies of newer prescription weight loss products (e.g., lorcaserin), which have not been evaluated for potential associations with birth defects, are warranted. Commonly used nonprescription products should also be evaluated. Realistically, given the low prevalence of both specific exposures and specific birth defects, additional studies that consider composite exposure and outcome categories will be needed. Such studies should dis-aggregate categories to the extent possible (e.g., defect specific analyses as well as analyses of any birth defect). Furthermore, whether using individual or composite categories, future studies should include a thorough assessment of factors that may confound, mediate or moderate the association between exposure and outcome. Such factors include maternal body mass index, other weight loss strategies (e.g., caloric restriction) and lifestyle factors (e.g., smoking).
In summary, our systematic review indicates that there are substantial gaps in our understanding of the potential teratogenicity of weight loss products. Our review does not help to define specific guidance for women who are concerned about the risk of birth defects due to periconceptional exposure to weight loss products. However, our systematic review does support the clinical management guidelines from the American College of Obstetricians and Gynecologists, which indicate that medications for weight management are not recommended during the time of conception (ACOG, 2016). Furthermore, our findings suggest that, in addition to receiving information on the importance of achieving and maintaining a healthy weight through lifestyle choices that include healthy eating and regular physical activity (CDC, 2016), reproductive-age women should receive information on the potential (unknown) risks, to the embryo, of exposure to weight loss products.
Supplementary Material
FIGURE 1.
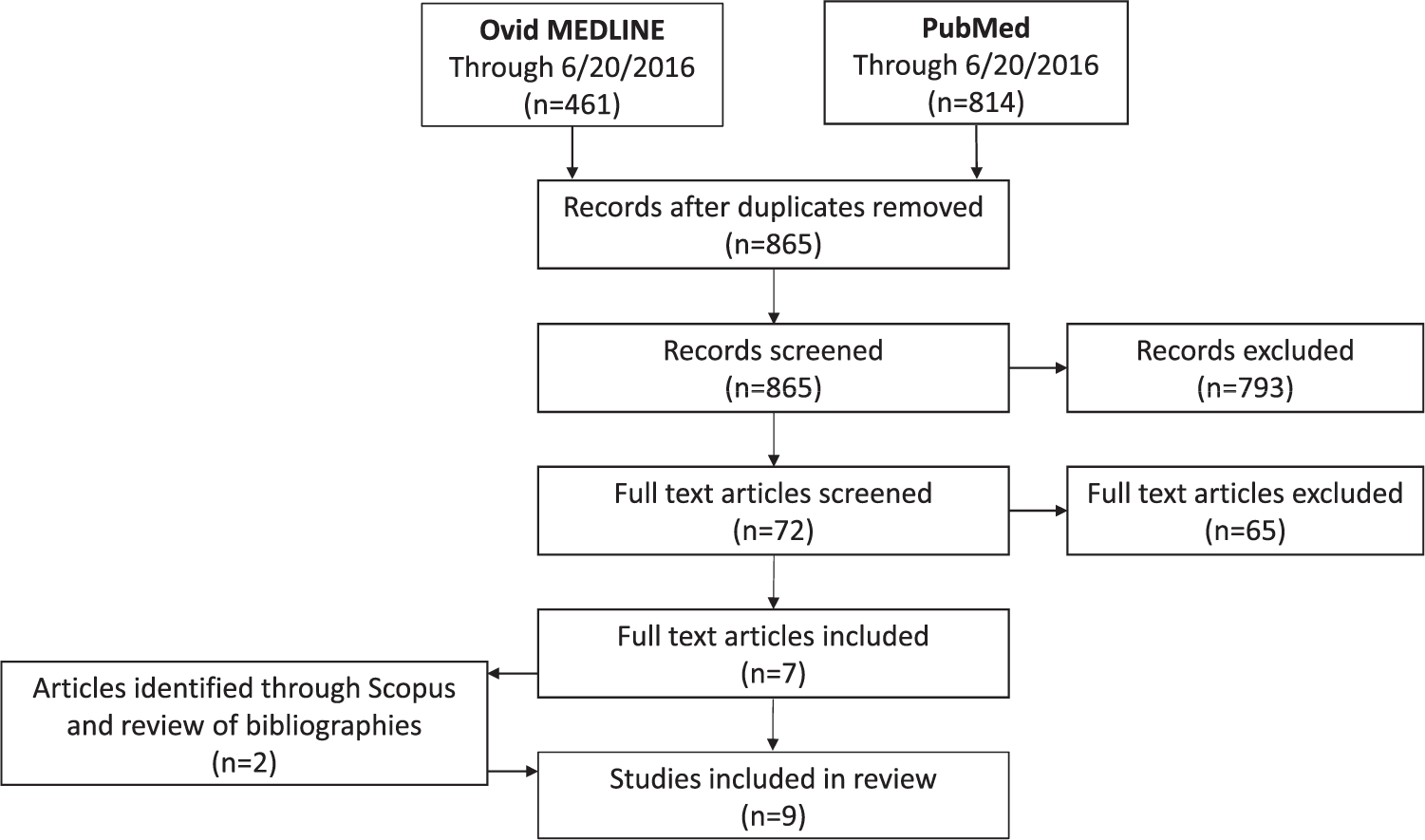
Flow diagram of article selection.
Acknowledgement
The authors thank Dr. Sarah C. Tinker for her comments and input on an earlier version of this manuscript.
Supported by Grant Number U01 DD 001179 funded by the Centers for Disease Control and Prevention.
Footnotes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Additional Supporting information may be found in the online version of this article.
Links
Mayo Clinic:
http://www.mayoclinic.org/healthy-lifestyle/weight-loss/in-depth/weight-loss/art-20046409?pg=2
National Institute of Health (NIH):
https://ods.od.nih.gov/factsheets/WeightLoss-HealthProfessional/
Report of top 10 weight loss products that people search on Google: https://sway.com/KTPEcrVWCfNUSYGw
Natural Database: http://naturaldatabase.therapeuticre-search.com/ce/ceCourse.aspx?s=ND&cs=&pc=15–112&cec=1&pm=5
References
- American Congress of Obstetricians and Gynecologists (ACOG). 2016. Practice bulletin No. 156: Obesity in pregnancy: correction. Obstet Gynecol 128:1450. [DOI] [PubMed] [Google Scholar]
- Bitsko RH, Reefhuis J, Louik C, et al. 2008. Periconceptional use of weight loss products including ephedra and the association with birth defects. Birth Defects Res A Clin Mol Teratol 82:553–562. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Serdula MK, Gillespie C, et al. 2007. Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc 107:441–447. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Schaffer DM, et al. 2003. Dieting behaviors and risk of neural tube defects. Am J Epidemiol 158:1127–1131. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2016. Preconception Health and Health Care. Altanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Egras AM, Hamilton WR, Lenz TL, et al. 2011. An evidence-based review of fat modifying supplemental weight loss products. J Obes 2011:pii: 297315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Kruger E. 2014. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity (Silver Spring) 22:1942–1951. [DOI] [PubMed] [Google Scholar]
- Haslam D 2016. Weight management in obesity - past and present. Int J Clin Pract 70:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Clearinghouse for Birth Defects Surveillance and ResearchI (ICBDSR). 2013. Annual report 2013. Roma, Italy: The International Centre on Birth Defects - ICBDSR Centre. [Google Scholar]
- Jones KL, Johnson KA, Dick LM, et al. 2002. Pregnancy outcomes after first trimester exposure to phentermine/fenfluramine. Teratology 65:125–130. [DOI] [PubMed] [Google Scholar]
- Kallen BA. 2014. Antiobesity drugs in early pregnancy and congenital malformations in the offspring. Obes Res Clin Pract 8:e571–576. [DOI] [PubMed] [Google Scholar]
- Katon J, Maynard C, Reiber G. 2012. Attempts at weight loss in U.S. women with and without a history of gestational diabetes mellitus. Womens Health Issues 22:e447–e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix I, Hurault-Delarue C, Montastruc JL, et al. 2012. Can benfluorex induce congenital malformations? Diabetes Metab 38: 373–374. [DOI] [PubMed] [Google Scholar]
- Manakova E, Kralova T, Hubickova Heringova L. 2012. Appetite suppressants in pregnancy. Neuro Endocrinol Lett 33(Suppl 3): 179–182. [PubMed] [Google Scholar]
- Martin L, Chavez GF, Adams MJ Jr., et al. 1988. Gastric bypass surgery as maternal risk factor for neural tube defects. Lancet 1: 640–641. [DOI] [PubMed] [Google Scholar]
- Milkovich L, van der Berg BJ. 1977. Effects of antenatal exposure to anorectic drugs. Am J Obstet Gynecol 129:637–642. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos NC, Melnick M. 1987. Studies in neural tube defects. I. Epidemiologic and etiologic aspects. Am J Med Genet 26:783–796. [DOI] [PubMed] [Google Scholar]
- Nelson MM, Forfar JO. 1971. Associations between drugs administered during pregnancy and congenital abnormalities of the fetus. Br Med J 1:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Hoyo A, Gutierrez-Salmean G. 2016. New dietary supplements for obesity: what we currently know. Curr Obes Rep 5:262–270. [DOI] [PubMed] [Google Scholar]
- Suarez L, Felkner M, Brender JD, et al. 2012. Dieting to lose weight and occurrence of neural tube defects in offspring of Mexican-American women. Matern Child Health J 16:844–849. [DOI] [PubMed] [Google Scholar]
- Waller DK, Anderson JL, Nembhard WN, et al. 2001. Dieting, diet-related behaviors and risk of neural tube defects: results from The Texas Birth Defects Research Center, 1996 to 2000. Frontiers in Fetal Health. p 54. [Google Scholar]
- Wells GA, Shea B, O’Connell D, et al. 2009. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: The Ottawa Health Research Institute. [Google Scholar]
- Williams J, Mai CT, Mulinare J, et al. 2015. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep 64:1–5. [PMC free article] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. 2014. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


