Abstract
Introduction
Herpes simplex virus (HSV) may be involved in Alzheimer's disease (AD) pathophysiology. The antiviral valacyclovir inhibits HSV replication.
Methods
This phase‐II pilot trial involved valacyclovir administration (thrice daily, 500 mg week 1, 1000 mg weeks 2–4) to persons aged ≥ 65 years with early‐stage AD, anti‐HSV immunoglobulin G, and apolipoprotein E ε4. Intervention safety, tolerability, feasibility, and effects on Mini‐Mental State Examination (MMSE) scores and cerebrospinal fluid (CSF) biomarkers were evaluated.
Results
Thirty‐two of 33 subjects completed the trial on full dosage. Eighteen percent experienced likely intervention‐related mild, temporary adverse events. CSF acyclovir concentrations were mean 5.29 ± 2.31 μmol/L. CSF total tau and neurofilament light concentrations were unchanged; MMSE score and CSF soluble triggering receptor expressed on myeloid cells 2 concentrations increased (P = .02 and .03).
Discussion
Four weeks of high‐dose valacyclovir treatment was safe, tolerable, and feasible in early‐stage AD. Our findings may guide future trial design.
Keywords: Alzheimer's disease, apolipoprotein E ε4, feasibility study, herpes simplex, pilot project, valacyclovir
1. BACKGROUND
Since its presentation in 1982, the hypothesized involvement of herpes simplex virus (HSV) in the etiology of Alzheimer's disease (AD) has been supported by accumulating preclinical and epidemiological evidence. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 This theory entails exciting prospects for AD treatment and prevention among HSV carriers, as indicated by considerable reductions in the risk of later dementia and AD diagnoses achieved by anti‐herpes virus treatment in registry and cohort studies. 15 , 16 , 17 , 18 Such treatment is used widely, administered easily, and generally well tolerated.
Valacyclovir, an oral antiviral agent, is effective against herpesvirus infections (e.g., herpes labialis, herpes encephalitis, and herpes zoster). The oral administration of 1 g valacyclovir twice daily was shown to effectively treat the signs and symptoms of herpes infection, 19 and 500 mg valacyclovir twice daily suppressed asymptomatic herpes infection reactivation. 20 Valacyclovir also may effectively suppress intracerebral HSV reactivation, 21 , 22 but a higher dose would be required due to its low central nervous system (CNS) penetration (mean ± standard deviation [SD], 19% ± 3%). 23 The safety, tolerability, and feasibility of 1 g valacyclovir administered three times daily have been evaluated in small populations, with satisfactory results, 21 , 22 , 23 , 24 , 25 but have not been assessed among patients with AD.
As HSV carriership is highly prevalent, any benefit achieved with its treatment may apply to a large portion of the population. 26 Concurrent carriership of HSV type 1 (HSV‐1) and apolipoprotein E (APOE) ε4 is associated strongly with AD, 6 , 7 suggesting an increased probability of HSV‐driven AD development. Randomized controlled trials (RCTs) are needed to evaluate anti‐herpes virus treatment effectiveness in terms of AD symptoms and pathology. Here, we report the outcome of a phase II pilot trial involving high‐dose valacyclovir administration to patients with early‐stage AD and anti‐HSV immunoglobulin (Ig)G and APOE ε4 positivity. In addition to the treatment's safety, tolerability, and feasibility, we assessed its clinical efficacy, as reflected by Mini‐Mental State Examination (MMSE) scores and biological effects reflected by cerebrospinal fluid (CSF) biomarkers. The trial results may guide the design of future RCTs.
2. METHODS
2.1. Design
In this open phase II dual‐site pilot trial, high‐dose valacyclovir was administered to patients with early‐stage AD. No placebo was administered. The trial was approved by the Regional Ethics Review Board of Umeå (nos. 2016‐390‐31 M and 2018‐93‐32 M), the Swedish Medical Products Agency (nos. 5.1‐2016‐71047 and 5.1‐2018‐53658, Eudra‐CT 2016‐002317‐22), and Region Västerbotten's Radiation Protection Committee (no. 1623). It was conducted in accordance with the Declaration of Helsinki, as revised in 2013, and registered prior to its initiation (ClinicalTrials.gov: NCT02997982).
In addition to participation in the present trial, patients at the University Hospital of Umeå were initially offered participation in a joint trial of the safety and feasibility of 9‐(4‐[18F]fluoro‐3‐(hydroxymethyl)butyl)guanine ([18F]FHBG)‐positron emission tomography/computed tomography (PET/CT) examination to assess the potential use of this probe for HSV‐1 thymidine kinase imaging during virus replication. That trial was discontinued due to the lack of significant cerebral uptake. Its methodology is described in supporting information.
2.2. Sample size
The predetermined optimal sample was 36 participants, with assessment of eligibility of up to 120 persons. As no data on which to base a power calculation were available, a minimum sample of 30 participants was chosen to allow for invocation of the central limit theorem, with an additional 20% to allow for potential drop‐outs.
2.3. Setting
This study was conducted at the University Hospital of Umeå and Uppsala University Hospital, Sweden. Data were collected in the hospitals’ memory clinics and the University Hospital of Umeå’s Neurology Clinic and Unit of Nuclear Medicine.
RESEARCH IN CONTEXT
Systematic review: Traditional sources, such as PubMed, were used for the literature review. No previous trial examining antiviral therapy for patients with Alzheimer's disease (AD) was identified.
Interpretation: We present novel findings of the high safety, tolerability, and feasibility of 4 weeks of antiviral therapy for patients with early‐stage AD, in line with previous findings from other patient groups. These findings enable the design of efficient randomized controlled trials examining the effects of such treatment on AD symptoms and pathology.
Future directions: Randomized controlled trials are needed to assess the effects of the intervention on AD symptoms and pathology. This paper provides guidelines for the design of such studies.
HIGHLIGHT
We evaluated a 4‐week high‐dose oral valacyclovir treatment regimen.
Participants were ≥65 years old with early Alzheimer's disease, HSV, and APOE ɛ4.
The intervention was safe, tolerable, and feasible.
Cognitive performance and sTREM2 levels increased during the intervention.
Our findings can aid the design of RCTs on antivirals in Alzheimer's disease.
2.4. Participants
Potential participants were recruited via advertisements and during regular health‐care visits. They provided written informed consent to participation after having been provided information by a participating physician. Potential participants’ eligibility was then further assessed using the criteria listed in Table 1 through interviews, medical records reviews, and blood assays.
TABLE 1.
Eligibility criteria
| Inclusion criteria |
|---|
| 1. Provision of informed consent. |
| 2. Age ≥ 65 years. |
| 3. Sufficient cognitive performance to provide informed consent, as determined by clinical evaluation. |
| 4. Diagnosis of late‐onset AD or mild cognitive impairment of the AD type, supported by typical clinical presentation and/or progression and at least one objective finding (e.g., significantly decreased perfusion or metabolism in both brain hemispheres in the temporal lobes or hippocampal atrophy, as determined by CT, MRI, PET/CT, or SPECT; pathological concentrations of AD biomarkers in cerebrospinal fluid [low Aβ42 concentration, low Aβ42/40 ratio, and/or high p‐tau concentration, as determined by laboratory analysis]; or absence of other significant pathology). Diagnostic support from at least one cerebral imaging modality (CT, MRI, PET/CT, or SPECT). Patients with normal white‐matter changes for their age were not excluded. |
| 5. Serum or plasma anti‐herpes simplex virus IgG positivity. |
| 6. Hetero‐ or homozygous APOE ε4 genotype carriership. |
| 7. At least 1 month stability of medication regimens for other conditions. |
| Exclusion criteria |
|---|
| 1. Known allergy to valacyclovir or acyclovir. |
| 2. Inability to adhere to a treatment regimen. Assistance from non‐study personnel involved in the daily administration of medication was encouraged and was not a reason for exclusion. |
| 3. Kidney failure or decreased kidney function (estimated glomerular filtration rate < 30 ml/min/1.73 m2). a |
| 4. Ongoing anticoagulant treatment, excluding the use of antiplatelet drugs in normal doses. |
| 5. Unstable or life‐threatening disease with expected survival time < 1 year. |
| 6. Severe somatic condition projected to obstruct study participation. |
| 7. Diagnosis of major neurocognitive disorder other than AD. |
| 8. Known severe neurological or neurocognitive disease. |
| 9. Psychiatric condition requiring treatment that is likely to obstruct study participation (e.g., depression or psychosis). |
| 10. Current or recent (in the last 5 years) history of substance addiction. |
| 11. Condition rendering examination in the supine position impossible. b |
| 12. Claustrophobia or other contraindication to PET/CT examination. b |
Calculated using the Chronic Kidney Disease Epidemiology Collaboration equation from the serum creatinine level, sex, and age.
Only for participation at the University Hospital of Umeå study site.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; CT, computed tomography; IgG: immunoglobulin G; MRI, magnetic resonance imaging; PET, positron emission tomography; p‐tau, phosphorylated tau; SPECT, single‐photon emission computed tomography.
2.5. Intervention
The study substance was valacyclovir (Valtrex; GlaxoSmithKline), ingested orally as 500‐mg tablets (three times daily; one tablet on days 1–7, two tablets on days 8–28). Patients were given all doses on day 1 and instructed in the self‐administration of the drug. They were encouraged to involve their next‐of‐kin or other supporting individuals in drug administration and to bring these individuals to study visits to receive instruction. The information was also repeated during check‐ups conducted by telephone 1 and 2 weeks after intervention initiation.
Information on adverse events (AEs) and serious adverse events (SAEs) was recorded from intervention day 1 to 30 days after intervention termination. A participating physician determined the degree to which AEs and SAEs were related to the intervention. Clinically relevant AEs and SAEs suspected to be related to the intervention were followed until their resolution. The intervention was terminated early in cases of withdrawn consent or the occurrence of a possibly related AE that rendered continuation unsuitable.
2.6. Procedure
Baseline clinical information, including participants’ age, sex, height, and weight, was collected during initial screening visits. Participants’ blood was sampled during screening and on intervention day 28 using a standardized clinical procedure for venipuncture. The blood was collected into ethylenediaminetetraacetic acid tubes, which were inverted 10 times, and into serum tubes, which were incubated at room temperature for 60 minutes and then centrifuged at 2000 g for 10 minutes. The supernatant was collected and stored at –80°C.
Participants’ CSF was sampled by lumbar puncture, using a standardized clinical procedure, on intervention days 0 and 28. A sterile atraumatic (pencil‐point) spinal needle (22 G, 0.7 × 90 mm; MEDIQ) was used to collect the first 10 ml of CSF into two polypropylene tubes. The samples were centrifuged at 2000 g for 10 minutes, and the supernatant was collected and stored at –80°C, according to a standardized procedure. Participants on antiplatelet drugs were instructed to temporarily discontinue those drugs the day before lumbar puncture.
2.7. Outcome measures
The primary outcomes were the intervention's feasibility, tolerability, and safety, and changes in the CSF levels of total tau (t‐tau) and neurofilament light chain (NfL) during the intervention. The secondary outcomes were changes in the MMSE score, anti‐HSV IgG titers, and CSF levels of amyloid beta (Aβ)42, Aβ40, phosphorylated tau (p‐tau), soluble triggering receptor expressed on myeloid cells 2 (sTREM2), YKL‐40, glial fibrillary acidic protein (GFAP), interleukin (IL)‐6, IL‐8, IL‐1β, and tumor necrosis factor (TNF)‐α during the intervention; and the detection, magnitude, and location of replicating herpesvirus in the CNS by [18F]FHBG PET/CT.
The intervention's feasibility and tolerability were assessed by calculating the proportion of participants who completed it on a full or reduced valacyclovir dosage and by assessing the CSF acyclovir concentration on intervention day 28. The CSF and serum concentrations of acyclovir and the metabolite 9‐carboxymethoxymethylguanine (9‐CMMG) were analyzed using accredited liquid chromatography‐tandem mass spectrometry, as described in the supporting information, at the Pharmacological Laboratory, Division of Clinical Pharmacology, Karolinska University Hospital, Sweden. CSF acyclovir concentrations were compared to the half maximal inhibitory concentration (IC50) for HSV‐1 clinical isolates (mean ± SD, 1.69 ± 1.02 μmol/L). 27 The intervention's safety was assessed by evaluating the frequency and severity of AEs and SAEs considered to be related to the intervention occurred during and 30 days after the intervention, and by assessing changes in the serum creatinine concentration during the intervention.
A participating physician or registered nurse with experience in the procedure administered the revised Swedish version of the MMSE according to the instructions with the instrument. 28 , 29 , 30 The assessments were performed before participants took their first valacyclovir doses on day 0, and after they had taken their last doses on day 28. The assessment environment was controlled, with only the assessor, patient, and, optionally, a close relative who was not allowed to help the patient, present. Care was taken to vary the use of alternative versions of items 11 and 12.
Anti‐HSV IgG titers were determined using an in‐house enzyme‐linked immunosorbent assay (ELISA) at the Department of Clinical Microbiology, Section of Virology, Umeå University, Sweden. The anti‐HSV IgG titer was defined as the highest positive dilution in CSF and serum with an absorbance value (optical density [OD]) ≥ 0.150, except for 1/100 serum dilutions, for which OD ≥ 0.200 was used. Serum/CSF anti‐HSV IgG titer ratios < 100 were considered to be pathological and CSF/serum anti‐HSV IgG titer ratios > 1% were considered to indicate blood‐brain barrier disruption or intrathecal antibody production.
CSF samples obtained on intervention days 0 and 28 were analyzed to determine the t‐tau, NfL, Aβ42, Aβ40, p‐tau, sTREM2, YKL‐40, GFAP, IL‐6, IL‐8, IL‐1β, and TNF‐α concentrations at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. The Aβ42, Aβ40, t‐tau, and p‐tau concentrations were measured using automated sandwich immunoassays on a LUMIPULSE G600II instrument (Fujirebio). Aβ42/40 ratios ≤ 0.072 were considered to be pathological. NfL and GFAP concentrations were measured using in‐house ELISAs, as described previously. 31 , 32 sTREM2 concentrations were measured using an in‐house immunoassay with electrochemiluminescence detection. 33 YKL‐40 concentrations were measured using a commercially available ELISA (R&D Systems). The IL‐6, IL‐8, IL‐1β, and TNF‐α concentrations were measured using an MSD 4‐plex kit (Meso Scale Discovery). The plasma t‐tau and NfL concentrations were measured on an HD‐X Analyzer using commercially available single‐molecule‐array assays, as described by the manufacturer (Quanterix Corporation). Board‐certified laboratory technicians who were blinded to the clinical data performed all measurements in one round of experiments using one batch of reagents. Intra‐assay coefficients of variation were < 10%.
2.8. Other measures
Blood samples for routine clinical analysis of the creatinine, albumin, and C‐reactive protein (CRP) concentrations and the erythrocyte sedimentation rate (ESR) were collected from the participants during screening and on intervention day 28, and analyzed at accredited clinical laboratories (Laboratoriemedicin, University Hospital of Umeå; Laboratoriemedicin, Skellefteå Lasarett; Akademiska laboratoriet, Uppsala Research Hospital). The prothrombin time‐international normalized ratio, activated partial thromboplastin time, and complete blood count were determined to ascertain whether participants’ coagulation was normal before lumbar puncture.
HSV and cytomegalovirus (CMV) serology and polymerase chain reaction (PCR) were performed at the Department of Clinical Microbiology, Section of Virology, Umeå University. Serum anti‐HSV IgG positivity was assessed using an in‐house HSV‐1 whole cell lysate antigen‐based ELISA, modified from that described by Juto and Settergren, 34 and the Umeå clinical isolate HSV‐1 1351‐95 for antigen production, as described previously. 1 Anti‐HSV IgG‐positive samples were subtype characterized using commercial ELISA kits (HerpeSelect 1 and HerpeSelect 2; Focus Diagnostics). Anti‐CMV IgG positivity was assessed using the same in‐house ELISA method, with the CMV 169 laboratory strain for antigen production. 35 All analyses were performed in duplicate.
DNA was purified from participants’ whole blood on a magLEAD 12gC platform with MagDEA Dx SV reagent kits (Precision System Science). For each quantitative polymerase chain reaction (qPCR), 50 ng purified DNA was used. All qPCR reactions and analyses were performed on a Quantstudio5 platform (Thermo Fisher Scientific).
Participants’ APOE genotypes, based on the single nucleotide polymorphisms rs429358 and rs7412 (ε2, ε3, ε4), were determined using a protocol based on differential amplification by allele‐specific primers. 36 Reaction mixes consisted of primers as referenced, sample DNA, and Power SYBR Green PCR Master Mix (Thermo Fisher Scientific; final volume, 25 μl). Manual melt‐curve analysis, which enables unambiguous allotype discrimination, was performed.
Participants’ γ marker (GM) 3/17 genotypes were determined using a custom‐designed TaqMan assay (Applied Biosystems Inc.) and 5Prime HotMaster Mix (QuantaBio), as described previously. 37 Paired immunoglobulin‐like type 2 receptor alpha (PILRA) rs1859788 genotypes were determined using a custom‐designed TaqMan assay, as described previously. 38 The reaction mixes consisted of primers and probes as referenced (0.2‐ and 0.1‐μM concentrations, respectively), sample DNA, and TaqMan GT Master Mix (Thermo Fisher Scientific; final volume, 20 μl). Allelic discrimination was performed using standard genotyping settings. GM 3/17 and PILRA genotypes were categorized as reflecting GM 17/17 or PILRA A/A carriership (homozygotic) or non‐carriership (including heterozygotic carriership).
2.9. Statistical analyses
Prior to analysis, all variables were examined for missing values, outliers, and normality using standardized scores and the z test. Variables with > 5% missing values were investigated to detect patterns. The data preparation is described in detail in the supporting information. Changes occurring during the intervention were evaluated using the paired‐samples t test and Wilcoxon signed rank test for normally and non‐normally distributed variables, respectively.
Bivariate correlations were examined using Pearson and Spearman analyses for normally and non‐normally distributed variables, respectively. Associations of baseline values with absolute changes in variables that changed significantly during the intervention were examined using linear regression. Non‐independence of errors in the models was assessed using the Durbin‐Watson statistic (acceptable, 1.0–3.0), and outliers in the solutions were identified using the Mahalanobis distance (α = 0.001). The normality, linearity, and homoscedasticity of residuals were assessed using scatterplots, P‐P plots, and histograms. Models including acyclovir or 9‐CMMG concentrations were adjusted for the time (in minutes) since the last valacyclovir dose.
All tests were two‐tailed and P < .05 was considered to reflect significance. Complete case analysis was used. The analyses were performed using IBM SPSS Statistics (ver. 24.0 for Windows; IBM Corporation).
3. RESULTS
Figure 1 shows the flow of participants through the study. In total, 33 participants (100% of included participants, 45% of persons whose eligibility was assessed) completed the 4‐week valacyclovir treatment; 32 participants were on the full dosage and 1 was on a reduced dosage due to the adverse reaction of headache. On average, 147.5 ± 8.6 tablets per participant were taken. The recruitment period lasted 3 years (December 1, 2016–December 4, 2019; follow‐up January 9, 2017–March 11, 2020). Twenty‐six of 33 participants were recruited and treated at the University Hospital of Umeå. The study was terminated prematurely due to the low recruitment rate, after having obtained a sufficient sample (> 30 participants) for statistical analysis.
FIGURE 1.
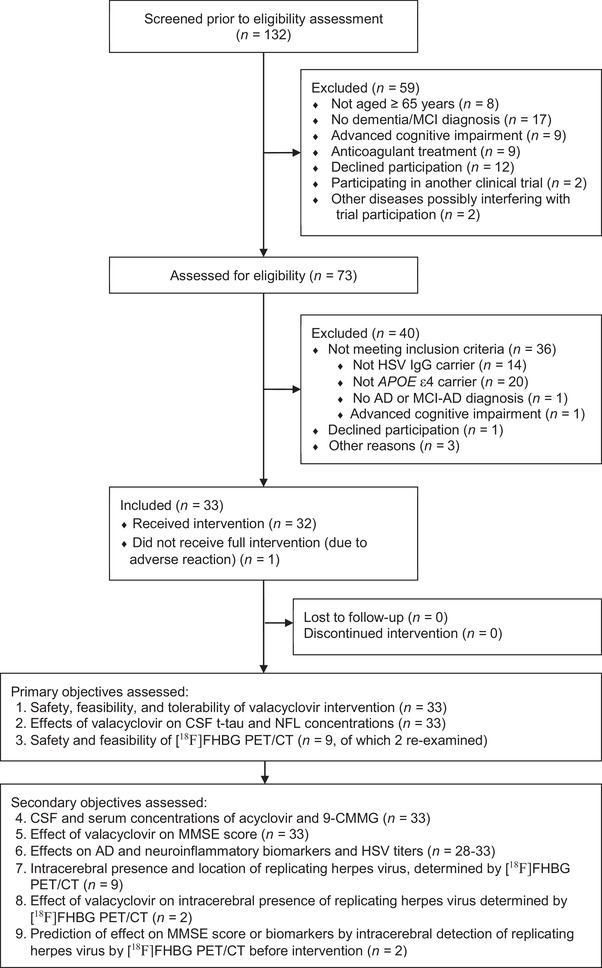
Participant flow. [18F]FHBG PET/CT, 9‐(4‐[18F]fluoro‐3‐(hydroxymethyl)butyl) guanine positron emission tomography/computed tomography; 9‐CMMG, 9‐carboxymethoxymethylguanine; AD, Alzheimer's disease; APOE, apolipoprotein E; CSF, cerebrospinal fluid; HSV, herpes simplex virus; IgG, immunoglobulin G; MCI, mild cognitive impairment; NfL, neurofilament light chain; MMSE, Mini‐Mental State Examination; t‐tau, total tau.
Nine participants underwent brain [18F]FHBG PET/CT examinations before the intervention, and two of these participants were re‐examined after the intervention. Preliminary analyses indicated likely [18F]FHBG distribution to the CSF, but no clear cerebral CNS uptake, in any participant.
Fourteen AEs in 11 participants and two SAEs in one participant were reported. Ten AEs and no SAE in six participants (18.2% of all) were considered related to the valacyclovir intervention (fatigue, headache [n = 2 each]; thirst, nausea, loose stools, mild depressive symptoms, mild tremor, polyuria [n = 1 each]). One AE (panic attack) related to the [18F]FHBG PET/CT intervention occurred.
Table 2 shows the participants' baseline demographic and clinical characteristics and the acyclovir and 9‐CMMG concentrations on intervention day 28. The mean CSF acyclovir concentration was 5.29 ± 2.31 μmol/L. The median CSF/serum acyclovir ratio was 0.20 (interquartile range [IQR] 0.15–0.32).
TABLE 2.
Baseline demographic and clinical characteristics and 28‐day acyclovir and 9‐CMMG concentrations
| Characteristics | Baseline assessment (n = 33) |
|---|---|
| Age (years), mean ± SD | 74.4 ± 4.3 |
| Sex (women), n (%) | 12 (36.4) |
| APOE genotype | |
| ε2/ε4, n (%) | 2 (6.1) |
| ε3/ε4, n (%) | 24 (72.7) |
| ε4/ε4, n (%) | 7 (21.2) |
| PILRA genotype | |
| AA, n (%) | 5 (15.2) |
| AG, n (%) | 12 (36.4) |
| GG, n (%) | 16 (48.5) |
| GM 3/17 genotype | |
| 3/3, n (%) | 10 (30.3) |
| 3/17, n (%) | 19 (57.6) |
| 17/17, n (%) | 4 (12.1) |
| Serum anti‐HSV‐1 IgG‐positive, n (%) | 31 (93.9) |
| Serum anti‐HSV‐2 IgG‐positive, n (%) | 9 (27.3) |
| Serum anti‐CMV IgG‐positive, n (%) | 27 (81.8) |
| MMSE score, median (IQR) | 23 (19‐26) |
| CSF Aβ42/40 ratio ≤ 0.072, n (%) | 31 (96.9) a |
| 28‐day assessment (n = 33) | |
|---|---|
| Serum acyclovir, μmol/L, mean ± SD | 25.94 ± 14.48 |
| CSF acyclovir, μmol/L, mean ± SD | 5.29 ± 2.31 |
| CSF/serum acyclovir ratio, median (IQR) | 0.20 (0.15–0.32) |
| Serum 9‐CMMG, μmol/L, mean ± SD | 3.86 ± 2.07 |
| CSF 9‐CMMG, μmol/L, median (IQR) | 0.00 (0.00–0.00) b |
| CSF/serum 9‐CMMG ratio, median (IQR) | 0.00 (0.00–0.00) b |
| Time from last valacyclovir dose, min, median (IQR) | 150 (82.5‐240) |
Abbreviations: 9‐CMMG, 9‐carboxymethoxymethylguanine; Aβ, amyloid beta; APOE, apolipoprotein E; CMV, cytomegalovirus; CSF, cerebrospinal fluid; GM, γ marker; HSV, herpes simplex virus; IgG, immunoglobulin G; IQR, interquartile range; MMSE, Mini‐Mental State Examination; PILRA, paired immunoglobulin‐like type 2 receptor alpha; SD, standard deviation.
Total n = 32.
Only four participants had detectable CSF 9‐CMMG concentrations (0.1640, 0.1706, 0.1930, and 0.4816 μmol/L, respectively), with corresponding CSF/serum 9‐CMMG ratios of 0.0212, 0.0274, 0.0357, and 0.0578, respectively.
Participants’ GFAP levels were not analyzed due to the lack of CSF volume. The CSF IL‐1β and TNF‐α concentrations could not be analyzed statistically due to the small numbers of measurable samples (supporting information). Correlations between plasma and CSF NfL concentrations were significant on intervention day 1 (rho = –0.63, P < .001) and on day 28 (rho = 0.37, P = .03). Correlations between plasma and CSF t‐tau concentrations were not significant. Bivariate correlations between participants’ MMSE scores and CSF levels of AD and neuroinflammation biomarkers at baseline are shown in Table S1 in supporting information.
Table 3 shows participants’ MMSE scores, CSF AD and neuroinflammation biomarker levels, and anti‐HSV IgG titers before and after the intervention, with correlations and associations between baseline and end‐of‐study values presented. Figure 2 shows individual changes in t‐tau, NfL, p‐tau, YKL‐40, and sTREM2 concentrations and MMSE scores during the intervention. After the removal of data from three participants with undetectable CSF IgG, the median CSF/serum anti‐HSV IgG titer ratio was 0.25% (range 0.25%–1.00%). The serum creatinine, albumin, and CRP levels and ESR did not change significantly during the intervention, but seven participants experienced temporary creatinine increases > 10%; all of these participants’ creatinine levels had normalized at the time of subsequent medical records reviews. Pre‐intervention serum creatinine was median (IQR) = 78.0 (69.5–92.0) μmol/L and post‐intervention 77.0 (67.5–102.5) μmol/L (P for change = .09). Pre‐intervention estimated glomerular filtration rate was mean = 72.1 ± 15.4 ml/min/1.73 m2 and post‐intervention 70.2 ± 17.3 ml/min/1.73 m2 (P for change = .05).
TABLE 3.
MMSE scores, CSF levels of AD and neuroinflammation biomarkers, and anti‐HSV IgG titers at baseline and after 28 days of high‐dose valacyclovir treatment
| Correlation | ||||||
|---|---|---|---|---|---|---|
| Biomarker | n pairs | Before treatment | After treatment | P value for difference | r | P value |
| MMSE score, median (IQR) a | 33 | 23 (19–26) | 24 (20–27) | .023 | 0.864 | < .001 |
| CSF Aβ42, pg/ml, mean ± SD | 32 | 363.2 ± 130.0 | 376.1 ± 115.4 | .204 | 0.902 | < .001 |
| CSF Aβ40, pg/ml, mean ± SD | 33 | 10344.9 ± 3715.7 | 10650.1 ± 3502.5 | .245 | 0.918 | < .001 |
| CSF Aβ42/40 ratio, median (IQR) | 32 | 0.34 (0.29–0.42) | 0.33 (0.30–0.42) | .112 | 0.990 | < .001 |
| CSF t‐tau, pg/ml, mean ± SD | 33 | 720.8 ± 342.8 | 727.7 ± 326.6 | .653 | 0.967 | < .001 |
| CSF p‐tau, pg/ml, mean ± SD | 32 | 97.1 ± 47.1 | 97.6 ± 46.55 | .819 | 0.971 | < .001 |
| CSF NfL, pg/ml, mean ± SD | 32 | 1768.8 ± 680.4 | 1816.3 ± 702.4 | .520 | 0.822 | < .001 |
| CSF sTREM2, pg/ml, mean ± SD | 33 | 2483.6 ± 917.4 | 2674.6 ± 889.3 | .028 | 0.861 | < .001 |
| CSF YKL40, ng/ml, mean ± SD | 28 | 179.0 ± 65.0 | 183.0 ± 61.9 | .488 | 0.893 | < .001 |
| CSF IL‐6, pg/ml, mean ± SD | 32 | 0.93 ± 0.34 | 0.86 ± 0.23 | .568 | 0.334 | .062 |
| CSF IL‐8, pg/ml, mean ± SD | 32 | 38.9 ± 9.9 | 41.7 ± 13.3 | .374 | 0.581 | < .001 |
| Serum anti‐HSV IgG, titer, median (IQR) | 33 | 6400 (6400–25600) | 6400 (6400–25600) | .157 | 0.921 | < .001 |
| CSF anti‐HSV IgG, titer, median (IQR) b | 32 | 64 (16–64) | 16 (16–64) | .083 | 0.870 | < .001 |
| Serum/CSF anti‐HSV IgG ratio, median (IQR) b | 31 | 400 (100–400) | 400 (100–400) | .655 | 0.701 | < .001 |
Note: The data include repeat assessments. Mean differences and correlations were tested using the paired‐samples t test and Pearson analysis, respectively, except for the MMSE score; CSF Aβ42/40 ratio; CSF IL‐6, IL‐8, and anti‐HSV IgG levels; serum anti‐HSV IgG level; and serum/CSF‐anti‐HSV IgG ratio, for which the Wilcoxon signed rank test and Spearman correlation analysis were used to accommodate ordinal data or non‐normality.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; CSF, cerebrospinal fluid; HSV, herpes simplex virus; IgG, immunoglobulin G; IL, interleukin; IQR, interquartile range; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; p‐tau, phosphorylated tau; SD, standard deviation; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t‐tau, total tau.
Mean MMSE change = 0.88 ± 1.96 points.
Three participants had undetectable CSF anti‐HSV IgG concentrations, recorded as 0.
FIGURE 2.
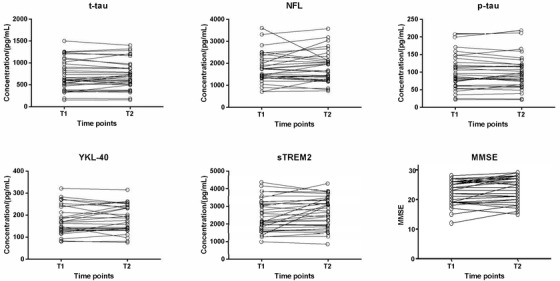
Individual changes in t‐tau, NfL, p‐tau, YKL‐40, and sTREM2 concentrations and MMSE scores between day 0 (T1) and day 28 (T2) of the intervention. MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; p‐tau, phosphorylated tau; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t‐tau, total tau.
Table 4 shows associations of study variables with changes in the MMSE score and CSF sTREM2 level during the intervention, as determined by linear regression. No violation of assumptions was found in any model. Differences between participants with MMSE and sTREM2 increases > 1 SD and the other participants are presented in the supporting information.
TABLE 4.
Associations of study variables with changes in the MMSE score and CSF sTREM2 level during 28 days of high‐dose valacyclovir treatment (linear regression analysis)
| MMSE change | CSF sTREM2 change | |||||
|---|---|---|---|---|---|---|
| Baseline assessment | B (95% CI) | Stand. β | P value | B (95% CI) | Stand. β | P value |
| Age (years) | 0.13 (−0.29–0.03) | −0.28 | .109 | 35.60 (−2.58–73.79) | 0.32 | .067 |
| Sex (women) | −1.24 (−2.64–0.16) | −0.31 | .081 | 142.86 (−211.55–497.27) | 0.15 | .417 |
| PILRA A/A | 0.14 (−1.83–2.12) | 0.03 | .884 | −456.47 (−907.09–−5.85) | −0.35 | .047 |
| GM 17/17 | 0.42 (−1.74–2.59) | 0.07 | .694 | 99.39 (−427.39–626.16) | 0.07 | .703 |
| Serum anti‐HSV‐2 IgG‐positive | 0.32 (−1.27–1.91) | 0.07 | .684 | −18.67 (−405.56–368.23) | −0.02 | .922 |
| Serum anti‐CMV IgG‐positive | 1.07 (−0.72–2.87) | 0.21 | .231 | 0.26 (−6.45–6.98) | −0.07 | .938 |
| MMSE score | −0.07 (−0.26–0.11) | −0.15 | .419 | 17.28 (−27.47–62.03) | 0.14 | .437 |
| CSF Aβ42, pg/ml | −1.43 × 10−3 (−7.11 × 10−3–4.26 × 10−3) | −0.09 | .612 | −1.58 (−2.84–0.32) | −0.42 | .016 |
| CSF Aβ40, pg/ml | −0.11 × 10−3 (−0.30 × 10−3–0.08 × 10−3) | −0.21 | .235 | −0.05 (−0.10–−0.01) | −0.40 | .020 |
| CSF Aβ42/40 ratio c | −2.26 (−8.13–3.62) | −0.14 | .439 | 152.03 (−1286.49–1590.54) | 0.04 | .831 |
| CSF t‐tau, pg/ml | −0.72 × 10−3 (−2.80 × 10−3‐1.37 × 10−3) | −0.13 | .487 | −0.42 (−0.90‐0.07) | −0.30 | .093 |
| CSF p‐tau, pg/ml | −0.01 (−0.02–0.01) | −0.13 | .465 | −2.12 (−5.85–1.62) | −0.21 | .256 |
| CSF NfL, pg/ml | −1.20 × 10−3 (−2.18 × 10−3–−0.22 × 10−3) | −0.41 | .019 | −0.17 (−0.43–0.08) | −0.24 | .178 |
| CSF sTREM2, pg/ml | −0.66 × 10−3 (−1.41 × 10−3–0.09 × 10−3) | −0.31 | .081 | −0.17 (−0.35–0.02) | −0.32 | .071 |
| CSF YKL40, ng/ml | −0.02 (−0.03–−0.01) | −0.48 | .006 | −2.43 (−5.20–0.33) | −0.32 | .082 |
| CSF IL‐6 a , pg/ml | −2.27 (−6.59–2.05) | −0.19 | .291 | −420.34 (−1476.99–636.30) | −0.15 | .423 |
| CSF IL‐8 a , pg/ml | −0.31 (−1.28–0.66) | −0.12 | .519 | −168.37 (−396.16–59.43) | −0.27 | .142 |
| Serum anti‐HSV IgG, titer | 0.02 × 10−3 (0.05 × 10−3–0.09 × 10−3) | 0.12 | .499 | 0.02 (0.00–0.03) | 0.37 | .036 |
| CSF anti‐HSV IgG, titer b | 0.04 (−0.23–0.31) | 0.06 | .757 | 3.66 (−3.08–10.40) | 0.20 | .276 |
| Serum/CSF anti‐HSV IgG ratio b , c | 1.98 (−1.05–5.01) | 0.16 | .191 | 714.77 (−12.25–1441.79) | 0.25 | .054 |
| MMSE change | CSF sTREM2 change | |||||
|---|---|---|---|---|---|---|
| 28‐day assessment d | B (95% CI) | Stand. β | p value | B (95% CI) | Stand. β | P value |
| Serum acyclovir, μmol/L | −0.03 (−0.09–0.02) | −0.25 | .188 | −7.37 (−19.88–5.15) | −0.22 | .238 |
| CSF acyclovir, μmol/L | −0.08 (−0.40–0.25) | −0.09 | .642 | 6.07 (−72.34–84.48) | 0.03 | .875 |
| CSF/serum acyclovir ratio c | 2.56 (−0.49–5.61) | 0.38 | .096 | 376.00 (−376.29–1128.30) | 0.23 | .316 |
| Serum 9‐CMMG, μmol/L | −0.07 (−0.43–0.28) | −0.08 | .675 | −10.39 (−95.25–74.48) | −0.05 | .804 |
Abbreviations: 9‐CMMG, 9‐carboxymethoxymethylguanine; Aβ, amyloid beta; CI, confidence interval; CMV, cytomegalovirus; CSF, cerebrospinal fluid; GM, γ marker; HSV, herpes simplex virus; IgG, immunoglobulin G; IL, interleukin; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; PILRA, paired immunoglobulin‐like type 2 receptor alpha; p‐tau, phosphorylated tau; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t‐tau, total tau.
Square root‐transformed.
Three participants had undetectable CSF anti‐HSV IgG concentrations, recorded as 0.
Base‐10 log‐transformed.
Models adjusted for the time (in minutes) since the last valacyclovir dose (log 10‐transformed).
Repeated analyses performed without data from the two participants with normal Aβ42/40 ratios produced essentially identical results (not shown).
4. DISCUSSION
This report describes the results of an open phase II dual‐site pilot trial of a 4‐week high‐dose valacyclovir intervention for subjects with early‐stage AD. The intervention was found to be feasible, tolerable, and safe for the duration of the trial. The CSF levels of t‐tau and NfL (neurodegeneration markers) did not change significantly during the intervention, possibly because the intervention did not affect AD pathophysiology or because these markers were not suitable for measurement of the dynamics of such effects. Minor changes in these biomarkers, whose half‐lives are suspected to exceed 20 days, would have been difficult to detect over the 4‐week period. 39 , 40 Additionally, a longer treatment period may be required to affect neurodegeneration. The mean MMSE score increased significantly during the intervention. The CSF sTREM2 level also increased during the intervention; other biomarker levels remained unchanged.
The average CSF acyclovir concentrations were higher than the previously reported IC50 value for HSV‐1 and comparable to those reported from a study in which HSV‐1 encephalitis was treated successfully with the same intervention. 22 The AEs that occurred were mild and did not result in intervention discontinuation. Although 4 weeks may be considered a short period for assessment of the safety and tolerability of this high‐dose treatment regimen, indications of its long‐term (6‐month) safety and tolerability have been observed in a small sample of patients with stable multiple sclerosis. 23
An increase in the MMSE score was predicted by lower baseline CSF NfL and YKL‐40 levels, which in turn have been associated with slower AD progression. 41 , 42 CSF NfL is a marker of large‐caliber myelinated axon damage and is associated with cognitive deterioration and structural brain changes over time. 42 CSF YKL‐40 is a biomarker of neuroinflammation and may be involved in the downregulation of glial phagocytic activities. 41 Although these findings need to be replicated in RCTs, participants with less axonal damage and neuroinflammation, as indicated by lower baseline CSF NfL and YKL‐40 levels, may be more likely to respond to this short‐term antiviral intervention. The relatively small SD for the change in MMSE score (1.96 points) is consistent with a previously determined measurement error (within‐subject SD) of 1.44 points for this score. 43 In relation to an average yearly MMSE decline of about 2 points among patients with early‐stage AD, 44 this finding suggests that the MMSE is an effective measure of cognition in clinical AD trials, as argued previously. 45
CSF sTREM2 is considered to be a marker of microglial activity. 46 Higher CSF sTREM2 levels have been associated with increased gray‐matter volume, slower rates of decline in hippocampal volume and memory, and slower rates of clinical progression in patients with early‐stage AD and mild cognitive impairment. 47 , 48 Thus, an increase in the sTREM2 level may indicate a favorable change in neuroinflammation with antiviral treatment. However, these results must be interpreted with caution.
An increase in the sTREM2 level was predicted by lower baseline CSF Aβ42 and Aβ40 levels, PILRA A/A non‐carriership, and higher baseline serum anti‐HSV IgG titers. Lower CSF Aβ (especially Aβ42) concentrations indicate a greater brain amyloid load. Aβ also has been shown to have antimicrobial activities against various pathogens, such as HSV, potentially through microbe entrapment. 12 Although such activities may theoretically consume Aβ, relationships between Aβ concentrations and antimicrobial activity levels have not been investigated. PILRA was analyzed in this study because of its connection to AD. 38 As PILRA A/A is the AD‐protective variant, different pathological processes may be involved in AD development among carriers and non‐carriers. PILRA is a cellular entry co‐receptor for HSV‐1, 49 and A/A carriers may have reduced infection by reactivated HSV‐1. 38 PILRA A/A carriers may thus be less likely to develop HSV‐1–associated AD. A high baseline serum anti‐HSV IgG titer may reflect greater exposure to HSV‐1, possibly due to poor immunological control over HSV reactivation. The increasing sTREM2 concentrations with the suppression of HSV reactivation, accentuated among individuals with potentially greater HSV exposure, may indicate that HSV is involved in microglial activity inhibition.
As this study had no placebo arm, we could not determine the causes of the observed effects on the MMSE score and biomarker levels. This limitation must be taken into account when interpreting the study results. Normally, MMSE score fluctuations over such a short period would be expected to balance out at the group level, and the instrument's test–retest reliability is high. 50 A training effect could partially explain the MMSE score increase, but such an effect may not be significant among cognitively impaired individuals; the MMSE training effect with administration at a 2.3‐day interval was 0.28 points in another study. 43
This trial demonstrated that a 4‐week high‐dose oral valacyclovir regimen was feasible, tolerable, and safe for patients with early‐stage AD, HSV, and APOE ε4. These findings may guide the design of RCTs for the assessment of antiviral treatment effectiveness for such patients in terms of AD symptoms, progression, and pathology.
CONFLICTS OF INTEREST
Kaj Blennow has served as a consultant, advisory board member, or data‐monitoring committee member for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Henrik Zetterberg has served on scientific advisory boards for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx, and Red Abbey Labs; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). Martin Ingelsson is a paid consultant for BioArctic AB and has received support from Forschungszentrum Jülich and Liber. Torbjörn Sundström has served as an advisory board member for Pfizer and given lectures in symposia sponsored by Pfizer. The other authors (Bodil Weidung, Eva‐Stina Hemmingsson, Jan Olsson, Fredrik Elgh, and Hugo Lövheim) declare that they have no competing financial interest.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors thank the study nurses Eva‐Lise Lundberg, Käthe Ström, and Malin Edén at the Uppsala University Hospital and Johanna Emanuelsson, Mona Dahlberg, and Lise‐Lotte Mannberg at the University Hospital of Umeå for their help with data collection and participant management; laboratory technician Emma Honkala, Department of Clinical Microbiology, Umeå University, for performing the laboratory analyses; radiochemists Margareta Ögren and Mattias Ögren at the Department of Radiation Sciences, Umeå University, for documentation and preparation of the radioligand; and Kristina Öjbrandt, Clinical Trial Unit, University Hospital of Umeå, for monitoring and support. This work was financially supported by the Wallenberg Centre for Molecular Medicine in Umeå (grant number RV‐767061); the Swedish Dementia Association; the Swedish Alzheimer Fund (grant number AF‐648851, AF‐930991); Region Västerbotten (grant number RV‐941800, RV‐932300, RV‐930361, RV‐860141, RV‐771851, RV‐680051, RV‐676151, RV‐644931, RV‐581621, RV‐581611); the Umeå University Foundation for Medical Research; the Swedish Society of Medicine (grant number SLS‐694001); Märta Lundqvists stiftelse; Region Uppsala; and The Swedish Brain Foundation (grant number PS2019‐0054). Kaj Blennow received funding from the Swedish Research Council (grant number 2017‐00915); the Swedish Alzheimer Foundation (grant number AF‐742881); Hjärnfonden (grant number FO2017‐0243); and the Swedish state under the ALF agreement between the Swedish government and county councils (grant number ALFGBG‐715986). Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (grant number 2018‐02532), the European Research Council (grant number 681712), Swedish State Support for Clinical Research (grant number ALFGBG‐720931), the Alzheimer Drug Discovery Foundation (grant number 201809‐2016862), the AD Strategic Fund and the Alzheimer's Association (grant numbers ADSF‐21‐831376‐C, ADSF‐21‐831381‐C, and ADSF‐21‐831377‐C), the Olav Thon Foundation, the Erling‐Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, The Swedish Brain Foundation (grant number FO2019‐0228), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. Martin Ingelsson received funding from the Swedish Research Council, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, and the Swedish Parkinson Foundation.
Weidung B, Hemmingsson E‐S, Olsson J, et al. VALZ‐Pilot: High‐dose valacyclovir treatment in patients with early‐stage Alzheimer's disease. Alzheimer's Dement. 2022;8:e12264. 10.1002/trc2.12264
REFERENCES
- 1. Lövheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimers Dement (N Y). 2015;11:593‐599. [DOI] [PubMed] [Google Scholar]
- 2. Lövheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F. Herpes simplex infection and the risk of Alzheimer's disease: a nested case‐control study. Alzheimers Dement (N Y). 2015;11:587‐592. [DOI] [PubMed] [Google Scholar]
- 3. Letenneur L, Peres K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population‐based cohort study. PLoS One. 2008;3:e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer's disease. J Alzheimers Dis. 2016;51:979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steel AJ, Eslick GD. Herpes viruses increase the risk of Alzheimer's disease: a meta‐analysis. J Alzheimers Dis. 2015;47:351‐364. [DOI] [PubMed] [Google Scholar]
- 6. Lopatko Lindman K, Weidung B, Olsson J, et al. A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex—associated Alzheimer's disease. Alzheimer Dement. 2019;5:697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lövheim H, Norman T, Weidung B, et al. Herpes simplex virus, APOE ɛ4, and cognitive decline in old age: results from the Betula cohort study. J Alzheimers Dis. 2019;67:211‐220. [DOI] [PubMed] [Google Scholar]
- 8. Ball MJ. Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can J Neurol Sci. 1982;9(3):303‐306. [DOI] [PubMed] [Google Scholar]
- 9. Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009;217:131‐138. [DOI] [PubMed] [Google Scholar]
- 10. Readhead B, Haure‐Mirande J‐V, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta‐amyloid accumulation and secretase upregulation. Neurosci Lett. 2007;429:95‐100. [DOI] [PubMed] [Google Scholar]
- 12. Eimer WA, Kumar V, Kumar D, et al. Alzheimer's disease‐associated β‐amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99(1):56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997;349:241‐244. [DOI] [PubMed] [Google Scholar]
- 14. Lövheim H, Olsson J, Weidung B, et al. Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer's disease development. J Alzheimers Dis. 2018;61(3):939‐945. [DOI] [PubMed] [Google Scholar]
- 15. Lopatko Lindman K, Hemmingsson ES, Weidung B, et al. Herpesvirus infections, antiviral treatment, and the risk of dementia‐a registry‐based cohort study in Sweden. Alzheimers Dement (N Y). 2021;7:e12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tzeng N‐S, Chung C‐H, Lin F‐H, et al. Anti‐herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections: a nationwide, population‐based cohort study in Taiwan. Neurotherapeutics. 2018;15:417‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bae S, Yun S‐C, Kim M‐C, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population‐based cohort study. Eur Arch Psychiatry Clin Neurosci. 2020;271, 987–997. 10.1007/s00406-020-01157-4 [DOI] [PubMed] [Google Scholar]
- 18. Hemmingsson ES, Hjelmare E, Weidung B, et al. Antiviral treatment associated with reduced risk of clinical Alzheimer's disease—a nested case‐control study. Alzheimer's Dement. 2021;7:e12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyring SK, Douglas JM, Corey L, et al. A randomized, placebo‐controlled comparison of oral valacyclovir and acyclovir in immunocompetent patients with recurrent genital herpes infections. Arch Dermatol. 1998;134:185‐191. [DOI] [PubMed] [Google Scholar]
- 20. Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374‐1381. [DOI] [PubMed] [Google Scholar]
- 21. McLaughlin MM, Sutton SH, Jensen AO, Esterly JS. Use of high‐dose oral valacyclovir during an intravenous acyclovir shortage: a retrospective analysis of tolerability and drug shortage management. Infect Dis Ther. 2017;6:259‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pouplin T, Pouplin JN, Van Toi P, et al. Valacyclovir for herpes simplex encephalitis. Antimicrob Agents Chemother. 2011;55:3624‐3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lycke J, Malmeström C, Ståhle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother. 2003;47:2438‐2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9:863‐869. [DOI] [PubMed] [Google Scholar]
- 25. Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546‐1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olsson J, Kok E, Adolfsson R, Lövheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immun Ageing. 2017;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sangdara A, Bhattarakosol P. Acyclovir susceptibility of herpes simplex virus isolates at King Chulalongkorn Memorial Hospital, Bangkok. J Med Assoc Thai. 2008;91:908‐912. [PubMed] [Google Scholar]
- 28. Palmqvist S, Terzis B, Strobel C, Wallin A. Mini Mental State Examination: Svensk Revidering (MMSE‐SR).. Svensk Förening för Kognitiva sjukdomar; 2012. [Google Scholar]
- 29. Palmqvist S, Terzis B, Strobel C, Wallin A. MMSE‐SR Mini Mental State Examination ‐ Svensk Revidering: MANUAL. 2nd ed.. Svensk Förening för Kognitiva sjukdomar; 2013. [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 31. Gaetani L, Höglund K, Parnetti L, et al. A new enzyme‐linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosengren LE, Ahlsén G, Belfrage M, Gillberg C, Haglid KG, Hamberger A. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of children. J Neurosci Methods. 1992;44:113‐119. [DOI] [PubMed] [Google Scholar]
- 33. Banerjee G, Ambler G, Keshavan A, et al. Cerebrospinal fluid biomarkers in cerebral amyloid angiopathy. J Alzheimers Dis. 2020;74:1189‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Juto P, Settergren B. Specific serum IgA, IgG and IgM antibody determination by a modified indirect ELISA‐technique in primary and recurrent herpes simplex virus infection. J Virol Methods. 1988;20(1):45‐55. [DOI] [PubMed] [Google Scholar]
- 35. Sjöström S, Hjalmars U, Juto P, et al. Human immunoglobulin G levels of viruses and associated glioma risk. Cancer Causes Control. 2011;22:1259‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calero O, Hortiguela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost‐effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183:238‐240. [DOI] [PubMed] [Google Scholar]
- 37. Pandey JP, Olsson J, Weidung B, et al. An Ig gamma marker genotype is a strong risk factor for Alzheimer disease, independent of apolipoprotein E epsilon4 genotype. J Immunol. 2020;205:1318‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rathore N, Ramani SR, Pantua H, et al. Paired immunoglobulin‐like type 2 receptor alpha G78R variant alters ligand binding and confers protection to Alzheimer's disease. PLoS Genet. 2018;14:e1007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barry DM, Millecamps S, Julien J‐P, Garcia ML. New movements in neurofilament transport, turnover and disease. Exp Cell Res. 2007;313:2110‐2120. [DOI] [PubMed] [Google Scholar]
- 40. Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97:1284‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lananna BV, McKee CA, King MW, et al. Chi3l1/YKL‐40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer's disease pathogenesis. Sci Transl Med. 2020;12(574):eaax3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zetterberg H, Skillbäck T, Mattsson N, et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hörnsten C, Littbrand H, Boström G, et al. Measurement error of the Mini‐Mental State Examination among individuals with dementia that reside in nursing homes. Eur J Ageing. 2021;18:109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lladó A, Froelich L, Khandker RK, et al. Assessing the progression of Alzheimer's disease in real‐world settings in three European countries. J Alzheimers Dis. 2021;80:749‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider LS. Pragmatic trials and repurposed drugs for Alzheimer disease. JAMA Neurol. 2020;77:162‐163. [DOI] [PubMed] [Google Scholar]
- 46. Suárez‐Calvet M, Kleinberger G, MÁ AraqueCaballero, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early‐stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8:466‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gispert JD, Suárez‐Calvet M, Monté GC, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer's disease. Alzheimers Dement (N Y). 2016;12:1259‐1272. [DOI] [PubMed] [Google Scholar]
- 48. Ewers M, Franzmeier N, Suárez‐Calvet M, et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer's disease. Sci Transl Med. 2019;11:eaav6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Satoh T, Arii J, Suenaga T, et al. PILRalpha is a herpes simplex virus‐1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132:935‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the Mini‐Mental State Examination and the Standardized Mini‐Mental State Examination: implications for clinical practice. App Nurs Res. 2000;13:209‐213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


