Abstract
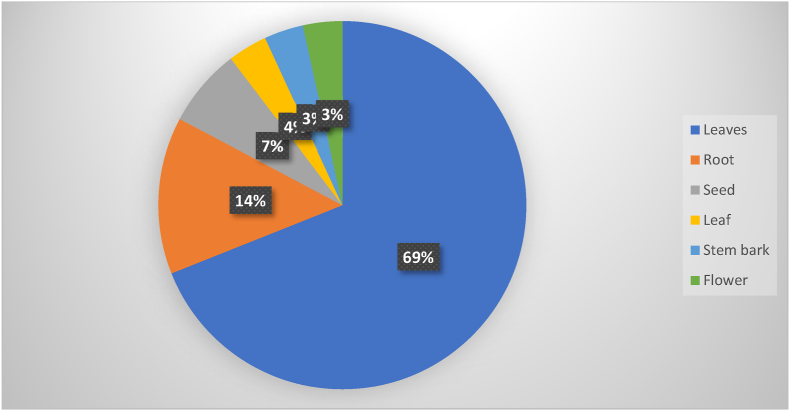
Diabetes mellitus is a serious, chronic disease that occurs either when the pancreas does not produce enough insulin, or when the body can't effectively use insulin. Herbal medicines have been commonly used by diabetic patients for the treatment of diabetes mellitus. To include findings from different studies, publications related to in vivo and invitro antidiabetic activities of medicinal plants in Ethiopia were searched from different databases, such as Web of Science, Google Scholar, Medline, Scopus, and PubMed, using English key terms. Different medicinal plant parts were used experimentally for antidiabetic effects in Ethiopia. Among these, leaves (69%) were the most commonly investigated medicinal plant parts followed by roots (14%) and seeds (7%). Most of the investigations were completed with hydro-methanolic extracts to obtain a higher percentage of yield. Medicinal plants such as Thymus schemperi R, Thymus vulgaris L, Hagenia abyssinica, Aloe megalacantha baker, Aloe moticola Reyonolds, Aloe pulecherrima Gilbert & sebseb, Bersama abyssinica fresen, and Rubus Erlangeri Engl have shown in vitro α-amylase inhibitory activity. However, only Hagenia abyssinica, Thymus schemperi R, and Thymus vulgaris L have exhibited α-glucosidase inhibitory activity. Likewise, only the extract of Aloe pulecherrima Gilbert & sebseb posses’ maltase and sucrose inhibitory activity. In vivo antidiabetic activity were conducted for the extract of medicinal plants such as A. remota, S. rebaudiani, T. schemperi, T. vulgaris, H. abyssinica, C. aurea, D. stramonium, A. megalacantha, A. moticola, A.integrifolia, A. pulecherrima, B. grandiflorum, B. abyssinica, P. schimperiana, M. stenopetala, C. aure, J. schimperiana, T. brownie, C. macrostachys, I. spicata, O. integrifolia, C. abyssinica, R. Erlangeri, L. culinaris, A. camperi, A. polystachyus, A. ilicifalius, C. tomentosa, and C. Edulis. This review gives collective evidence on the potential antidiabetic activities of medicinal plants in Ethiopia. Moreover, further studies are recommended to substantiate the use of these medicinal plants as an antidiabetic agent.
Keywords: Antidiabetic, Diabetes mellitus, Ethiopia, Medicinal plant
1. Introduction
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin action, insulin secretion, or both. Diabetic complications could be linked to alteration in the body’s antioxidant defense system, increased oxidative stress, and dyslipidemia [1].
According to the International Diabetes Federation (IDF) report, there are 451 million (age 18–99 years) people with diabetes worldwide. These figures were expected to increase to 693 million) by 2045. It was estimated that almost half of all people (49.7%) living with diabetes are undiagnosed. Moreover, there were an estimated 374 million people with impaired glucose tolerance (IGT) and it was projected that almost 21.3 million live births to women were affected by some form of hyperglycemia in pregnancy. In 2017, approximately 5 million deaths worldwide were attributable to diabetes in the 20–99 years age range [2]. Several studies reported the prevalence of diabetes mellitus complications varying from 20 to 90.5% [[3], [4], [5], [6], [7]]. Previous studies in Ethiopia revealed that hypertension, visual disturbance, nephropathy, and neuropathy were the highest four chronic complications diagnosed in diabetic patients [[8], [9], [10], [11]].
Generally, there are four classifications of diabetes mellitus such as Type I DM, Type II DM, gestational GDM, and specific types of DM (drug-induced diabetes, latent autoimmune diabetes in adults, cystic fibrosis diabetes, monogenic diabetes [12]. Oxidative stress is believed to be the basic cause of tissue damage, organ dysfunctions, and cellular injury usually linked to diabetic complications. Oxidative stress refers to elevated intracellular levels of reactive oxygen species that cause impairment to biological molecules like DNA, lipids, and proteins [13]. Oxidative stress could be decreased to a significant level by the action of numerous antioxidant enzymes including glutathione peroxidase, catalase, glutathione reductase, and superoxide dismutase [14].
Diabetes mellitus may cause acute complications like hyperosmolarity and ketoacidosis [15]. Its cause is obscure but appears to be precipitated by the same factors as ketoacidosis, especially those resulting in dehydration [16]. Diabetes mellitus also causes chronic complications like renal impairment, retinopathy, cardiovascular disorder, and foot ulcer. Patients with diabetes have an increased occurrence of arterial, cerebrovascular, peripheral, and atherosclerotic cardiovascular diseases [17].
No successful cure for diabetes mellitus has yet been found but can be managed using oral anti-diabetic agents, insulin, and diet modification. Medicinal plants may provide alternative management [18]. Affordability, accessibility, cost, tolerability, and Compromised effectiveness are some of the limitations of current conventional anti-diabetic drugs. African medicinal plants are frequently used in the treatment of diabetes mellitus and deliver an alternative therapy [19].
In Ethiopia, there are several medicinal plants used for the treatment of diabetes mellitus and a number of these were examined for their antihyperglycemic effect. About 80–90% of Ethiopians use medicinal plants as a primary form of health care [20,21]. The uses of plant-based medicine have continued to be a good foundation of natural products for the management of different illnesses. Several plant species were investigated and the majority of them have important phytoconstituents and the use of novel compounds from plants for pharmaceutical purposes has been steadily increasing [22]. There are preliminary studies on the scientific evidence of commonly used medicinal plants in Ethiopia though the evidence was not synthesized and research is required on different herbal formulations and indigenous plants. Thus, this study aimed to review the in vivo and in vitro antidiabetic activity of medicinal plants used for diabetic management in Ethiopia.
2. Pharmacological management of diabetes mellitus
Insulin replacement therapy is the mainstay for patients with type I DM, Insulin is also important in type II DM when blood glucose levels cannot be controlled by exercise, weight loss, diet, and oral medications [23].
The most common treatment strategy has been the combination of once or twice-daily injections of long-acting insulin-like glargine or insulin detemir and short-acting insulin-like lispro, aspart, glulisine, and neutral insulin. Detemir and glargine insulin are usually favored over neutral protamine hagedorn insulin since their use is linked to lower rates of nocturnal and severe hypoglycemia [24]. Novel ultra-long-acting insulin analogs are being developed. Insulin degludec delivers basal insulin coverage for more than 40 h and attains comparable glycemic control with less overnight hypoglycemia than glargine [25].
Oral hypoglycemic agents include drugs that decrease hepatic glucose production like biguanides, drugs that stimulate insulin secretion from the β-cells like sulphonylureas, drugs targeting the Glucagon-Like Peptide-1 axis like GLP-1 receptor agonists, drugs that delay carbohydrate uptake in the gut like a-glucosidase inhibitors, drugs that improve insulin action like thiazolidinediones, sodium-glucose cotransporter 2 inhibitors, bile acid sequestrants, and dopamine agonists [26,27]. Clinically, there are also untoward side effects, enormous cost, and noticeable treatment failures associated with conventional antidiabetic drugs. Thus, generating an urgent need and desire for alternative treatments is required [28].
3. Medicinal plants in diabetes mellitus management
Herbal medicines have been used by a large number of diabetic patients (80–85%) for the treatment of diabetes mellitus [29,30]. Ethnobotanical studies revealed that more than 1200 medicinal plants have been used for the management of diabetes mellitus [31]. The plant-derived medicines may correct metabolic abnormalities and delay the development of diabetic complications [32]. In the previous studies, the new bioactive drugs isolated from plants with hypoglycemic effects revealed antidiabetic effects with more efficacy than conventional medication used for the management of diabetes mellitus [33,34].
The world health organization suggested that plant-based medicines be further studied as they are frequently considered to be less toxic and side effects [35]. Globally, several extracts of medicinal plants have been used for the management of diabetes mellitus, and these are considered relatively less toxic, side effects, and are inexpensive [36]. Numerous bioactive compounds were isolated from plant extracts for direct use, or as a lead compound [37]. For instance, Metformin is an oral hypoglycemic agent synthesized from Galega officinalis that was used traditionally for the management of diabetes mellitus [37].
Different medicinal plant parts were used experimentally for antidiabetic effects in Ethiopia. Among these, leaves were the most commonly investigated medicinal plant part. Plant-based products which are rich in phytoconstituents like flavonoids, coumarins, terpenoids, phenolic compounds, and other bioactive compounds have revealed the blood-glucose-lowering effect [38].
Some traditional medicines used for the treatment of diabetes mellitus include Vernonia amygdalina (Asteraceae) [39,40], Justicia schimperiana (Acanthaceae) [39], Croton macrostachys (Euphorbiaceae) [[39], [40], [41]], Aloe vera (Aloceae) [40,41], Momordica Charantia Linn (Cucurbitaceae) [42,43], Moringa Oleifera (Moringacea) [44], Trigonellafoenum-GraecumL (Fabaceae) [40,41,45], Euphorbia sp. Gmel, (Euphorbiace) [40,46], and AlliumSativum (Amaryllidaceae) [45].
4. Methods
Previously published articles were searched using Medline, Google Scholar, SCOPUS, Web of Science, and PubMed databases to extract the antidiabetic activities of medicinal plants done in Ethiopia. The search terms used were “medicinal plants,” “hypoglycemic activity,” “antihyperglycemic activity,” “antidiabetic effect,” “antidiabetic potential,” “hypoglycemic effect,” “diabetes in Ethiopia” “antihyperglycemic effect,” “blood glucose-lowering effect,” and “antidiabetic activity”. Only experimental investigations done in Ethiopia were contained within using English keywords. Published articles available online before May 30, 2020, were included in the current study. A total of 38, 347 published articles were collected through database searching. All published papers not conducted in Ethiopia were removed, and we obtained 711 articles. Forty-two research articles were identified after removing the 669 duplicate articles. Finally, these full-text published articles were evaluated for eligibility, and data were extracted from the remaining 28 experimentally investigated medicinal plants.
5. Extraction of medicinal plants
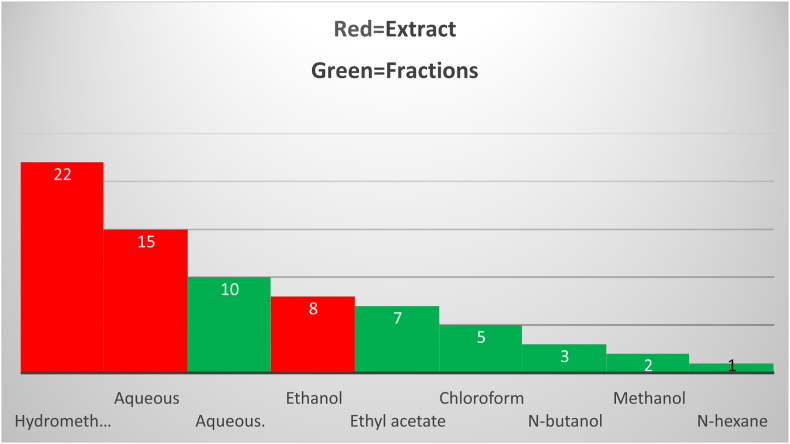
The antidiabetic activities of the crude extracts and solvent fractions of different medicinal plant parts using different chemicals were investigated as displayed in Fig. 1. Most of the investigations were completed with hydro-methanolic extracts to obtain a higher percentage of yield. Prominently, 80% methanol is more efficient in the cell wall and seed degradation as well as having low or no enzyme activity when compared with water. Furthermore, the methanolic extract of the medicinal plant comprises a wide variety of polar (and moderately nonpolar) constituents [[47], [48], [49]]. Among solvents, hydro-methanol was the most commonly used solvent for the extraction of medicinal plants, followed by aqueous and ethanol solvents. Regarding the solvent fractionation, chloroform was the most commonly used solvent for fractionation. Different medicinal plant parts were used experimentally for antidiabetic effects in Ethiopia. Among these, leaves (69%) were the most commonly investigated medicinal plant parts followed by roots (14%) and seeds (7%) as shown in Fig. 2.
Fig. 1.
Extracted and fractionated antidiabetic plants in Ethiopia.
Fig. 2.
Medicinal plants parts used for antidiabetic activities in Ethiopia.
6. In vitro studies
Medicinal plants such as Hagenia abyssinica [50], Aloe megalacantha and Aloe moticola Reyonolds [51,52], Aloe pulecherrima Gilbert & sebseb [53], Thymus schemperi and Thymus vulgaris [54,55], Bersama abyssinica fresen [56], and Rubus Erlangeri Engl 22], have shown in vitro α-amylase inhibitory activity. However, only Hagenia abyssinica [50], Thymus schemperi R, and Thymus vulgaris L [54,55], have exhibited α-glucosidase inhibitory activity. Likewise, only the extract of Aloe pulecherrima Gilbert & sebseb posses’ maltase and sucrose inhibitory activity [53]. All the crude extract and solvent fractions exhibited less activity when compared with the reference drug (acarbose). The in vitro antidiabetic activities of medicinal plants, which have been investigated in Ethiopia, are summarized in Table 1.
Table 1.
Summary of medicinal plants with confirmed antidiabetic activity.
| Scientific name | Family | Local name | In vivo Test | In vitro Test | Plant part | Effect | References | |
|---|---|---|---|---|---|---|---|---|
| Ajuga remota | Lamiaceae | Akoraracha | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGL (P < 0.0001) at aqueous extracts 300 and 500 mg/kg by 27.83 ± 2.96% and 38.98 ± 0.67%, respectively. ↓BGL (P < 0.05) at 70% ethanol extracts 300 and 500 mg/kg by 27.94 ± 1.92% and 28.26 ± 1.82%, respectively. | [107] | |
| Satvia rebaudiani | Asteraceae | Sugar leaf | OGTT and Alloxan induced DM | – | Leaf | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | [85] | |
| Thymus schemperi and Thymus vulgaris | Lamiaceae | Tosign | Alloxan induced DM | α-amylase α-glucosidase | Leaf | ↓BGL at all doses of the extract (P < 0.05) at days 7, 14, and 21. Antidiabetic activity (P < 0.05) exhibited by 400 mg/kg compared to 100 mg/kg. It also showed significant α-amylase α-glucosidase inhibitory activities. |
[54,55] | |
| Hagenia abyssinica | Rosaceae | Kosso | Normoglycemic, OGTT and STZ induced DM | α-amylase | Flower | Inhibited α-amylase activity by 54.23% with IC50 20.78 μg/mL at 800 μg/mL ethyl acetate fraction. Inhibited α–amylase activity with IC50 52.11 ± 0.63, 49.08 ± 0.97 μg/mL, and 28.09 ± 0.75 μg/mL of water and chloroform fraction, and crude extract, respectively. | [108] | |
| Hagenia abyssinica | Rosaceae | Kosso | STZ induced DM | – | Leaf | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | [50] | |
| Calpurnia aurea | Fabaceae | Digta | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓Hyperglycemia (P < 0.05) with 5.5 and 11 mg/kg at 2 h in OGTT mice. ↓BGL with 2.75 (P < 0.05), 5.5 (P < 0.01) and 11 mg/kg (P < 0.001) extract on the 7th and 14th day of repeated doses in diabetic mice. ≥175 [52] (14) Calpurnia aurea (Ait.) Benth. Hydromethanolic leaf extract STZ-induced diabetic mice 100, 200, and 400 GL Phenols, alkaloids, terpenoids, and flavonoids ↓Hyperglycemia (P < 0.05) at al | [109] | |
| Datura stramonium | Solanaceae | Astenagir | Normoglycemic, OGTT and STZ induced DM | – | Seed | BGL (P < 0.05) at 100 mg/kg (P < 0.01) and 200 and 400 mg/kg. ↓BGL (P < 0.0l) at all doses of extract on day 7 and 14. ↓BGL (P < 0.05) at doses of 200 and 400 mg/kg extract. Improved BW of diabetic mice on day 7 and 14. | [110] | |
| Aloe megalacantha and Aloe moticola | Aloeceae and Asparagaceae, respectivily | Eret | Normoglycemic, OGTT and STZ induced DM | α-amylase inhibitory | Leaf latex | ↓BGL (P < 0.05 and P < 0.001) with 100, 200, and 400 mg/kg doses at the 7th and 14th days, respectively. Possessed α-amylase suppression activity at both the leaf latex and the fraction (Rf value of 0.49) with IC50 value of 74.76 ± 1.98 and 96.75 ± 1.98 μg/mL, respectively (P < 0.001). | [51,52] | |
| Ajuga integrifolia | Lamiaceae | Anamaro | OGTT and STZ induced DM | Root | The extract and aqueous fraction of A. integrifolia exhibited a significant BGL reduction effect at all tested doses. Both the repeated daily doses of the crude extract and aqueous fraction of A. integrifolia showed similar activity in reducing the fasting BGL in streptozotocin-induced diabetic mice models | [91] | ||
| Aloe pulecherrima | Aloeceae | Eret | Normoglycemic, OGTT and STZ induced DM | α-amylase, Sucrose and Maltase inhibitory | Leaf latex | Inhibited sucrase, maltase, and α-amylase. ↓BGL (P < 0.05) in OGTT mice. ↓BGL of diabetic mice (P < 0.05) on week 1 and 2. ↓BGL with increasing the doses on week 1 (P < 0.05 (200 mg/kg), P < 0.01(400 mg/kg), and P < 0.001 (600 mg/kg)). | [53] | |
| Bacium grandiflorum Lam | Lamiaceae | Mentesie | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | [100] | |
| Bersama abyssinica | Melianthaceae | Azamira | Normoglycemic, OGTT and STZ induced DM | α-amylase | Leaf | ↓BGL by 25.71, 33.27, 40.71, and 48.39% at 400 mg/kg chloroform, ethyl acetate and aqueous fraction, and crude extract, respectively, in diabetic mice. Inhibited α-amylase with different IC50 values of crude extract, water fraction, ethyl acetate fraction, and the chloroform fraction. | [56] | |
| Pentas schimperiana | Rubiaceae | Not stated | Normoglycemic, OGTT and Alloxan-induced DM | – | Leaf | ↓BGL at a dose of 1,000 mg/kg for fresh leaf hydroalcoholic and dried leaf aqueous extracts by 26.7% (P < 0.01) and 26.97% (P < 0.001), respectively. ↓BGL with hydroalcoholic dried leaf extract by 19.27% (P < 0.001) at 1,000 mg/kg dose on 3 h ↓BGL with methanol and aqueous at a dose of 500 mg/kg (P < 0.001). | [111] | |
| Moringa stenopetala | Moringaceae | Shifraw | OGTT and STZ induced DM | – | Leaf | ↓BGL for ethanol extract at 60 (P < 0.05) and 120, 180, and 240 min (P < 0.001). ↓BGL for aqueous extract at 120 min (P < 0.01) and 180 and 240 min (P < 0.001) of single dose in diabetic mice. ↓BGL for the ethanol extract (P < 0.001) at 3rd day. ↓BGL for aqueous extract on the 3rd (P < 0.01) and 5th and 8th days (P < 0.001). ↓BGL for chloroform and butanol fractions on 5th day (P < 0.01) and 8th day (P < 0.001) in diabetic mice. | ||
| Calpunia aure | Fabaceae | Ligita | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGL at all doses of the extract (P < 0.05) at days 7, 14, and 21. Antidiabetic activity (P < 0.05) exhibited by 400 mg/kg compared to 100 mg/kg. | [109] | |
| Thymus schimperi | Lamiaceae | Tosign | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | ||
| Justicia schimperiana | Acanthaceae | Smiza | Normoglycemic, OGTT and STZ induced DM | – | Leaf | Showed significant tolerance (P < 0.05) at 1 and 2 h ↓BGL (P < 0.05) at 4 h in normoglycemic mice. ↓BGL (P < 0.05) at 400 mg/kg extract at 2, 3, and 4 h of treatment in diabetic mice. | [112] | |
| Terminalia brownie | Combrefaceae | Abalo | Normoglycemic, OGTT and STZ induced DM | Stem bark | ↓Hyperglycemia with OGTT by the crude extract at a dose of 500 mg/kg (P < 0.01), 750 (P < 0.05) after 60 min, and 750 mg/kg (P < 0.01) after 120 min ↓BGL (P < 0.01) with ethyl acetate and aqueous fractions at 500 mg/kg in diabetic model. | [113] | ||
| Croton macrostachys | Euphorbiaceae | Bisana | Normoglycemic, OGTT and STZ induced DM | – | Root | ↓Hyperglycemia by 300 mg/kg compared to 100 (P < 0.001) and 200 mg/kg (P < 0.01) in diabetic mice. ↓BGL in OGTT at doses of 100 (P < 0.01), 200 (P < 0.001), and 300 mg/kg (P < 0.001) after 60, 90, and 120 min of glucose loading. | [114] | |
| Indigofera spicata | Fabaceae | Not stated | Normoglycemic, OGTT and Alloxan-induced DM | – | Leaf | ↓BGL at 200 and 400 mg/kg in normoglycemic mice (P < 0.05). ↓BGL (P < 0.05) in only 400 mg/kg exposed groups at the 120 min of postexposure in OGTT model. ↓BGL (P < 0.05) at all doses of the extract at 4, 6, and 10 h on diabetic mice. | [115] | |
| Otostegia integrifolia | Lamiaceae | Tinjut | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGLs at 200 mg/kg extract in the hypoglycemic and OGTT models. ↓Fasting BGL (P < 0.001) at 100 and 200 mg/kg doses at 4 h in diabetic mice. | [116] | |
| Caylusea abyssinica | Resedaceae | Akorarach | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓BGL by 100 (P < 0.05) and 300 mg/kg extract (P < 0.01) starting from the 3rd h, and by 200 mg/kg (P < 0.001) as early as the 2 nd h in diabetic mice. ↓BGL by 100 mg/kg extract (P < 0.01) at 120 min and 200 mg/kg (P < 0.001) at 60 min in OGTT. | [117] | |
| Rubus Erlangeri | Rosaceae | Not stated | Normoglycemic, OGTT and STZ induced DM | α-amylase | Leaf | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | [118] | |
| Lens culinaris | Leguminosae | Not stated | Normoglycemic, OGTT and STZ induced DM | – | Seed | ↓BGL at all doses of the extract (P < 0.05) at days 7, 14, and 21. Antidiabetic activity (P < 0.05) exhibited by 400 mg/kg compared to 100 mg/kg. | [119] | |
| Aloe camperi | Asphadelaceae | Ere | OGTT and Alloxan-induced DM | – | Leaf | Showed a significant (P<0.001) reduction of BGL as compared to the diabetic control group. | [120] | |
| Acanthus polystachyus | Acanthaceae | Not stated | Normoglycemic, OGTT and STZ induced DM | – | Root | ↓BGL (P < 0.05) at the extract (100, 200, and 400 mg/kg) 14 days. | ||
| Capparis tomentosa | Capparaceae | Gumero | Alloxan-induced DM | – | Root | ↓BGL at all doses of the extract (P < 0.05) at days 7, 14, and 21. Antidiabetic activity (P < 0.05) exhibited by 400 mg/kg compared to 100 mg/kg. | [121] | |
| Catha Edulis | Celastracea | Khat | Normoglycemic, OGTT and STZ induced DM | – | Leaf | ↓Fasting BGL from 223.7 ± 27.6 to 106 ± 18.2 mg/dl, at the end of study (P < 0.05). | [122] | |
Note: SZT: Streptozotocin; +: in vivo antidiabetic activity; IC50: inhibitory concentration; BGL: blood glucose level; OGTT: oral glucose tolerance test; DM: Diabetes mellitus.
7. IN VIVO studies
There was significant variance in the duration of treatment among in vivo studies, ranging from 4 h to 30 days. Most of the in vivo studies used mice, and a few studies used rats as experimental animals. Noteworthy glycemic control was observed with Terminalia brownie Fresen for 14 days, better blood glucose level control when compared with diabetic control. Three similar studies [[57], [58], [59]], also revealed significant blood glucose level reduction when compared with diabetic control. These studies were done respectively for 14, 15, and 30 days in Calpurnia aurea, Thymus schimperi, and Persea Americana. Comparable antidiabetic activities to the reference drug (Glibenclimide) were reported in Moringa stenopetala and Persea Americana [58,[60], [61], [62]]. Significant acute blood glucose level control was reported in Urtica simensis Hochst.ex. A. Rich, Thymus schimperi, and Indigofera spicata Forssk [[63], [64], [65]]. The in vivo antidiabetic activities of medicinal plants, which have been investigated in Ethiopia, are summarized in Table 1.
8. Possible mechanism of actions of medicinal plants for DM management
Phytoconstituents that are obtained from various medicinal plants have shown significant blood glucose-lowering activities [[66], [67], [68], [69], [70]]. The mechanism of reducing the blood glucose level could be due to stimulation of glycogenesis, reduction of glucose absorption, activation of releasing insulin from ß-cells, and/or increment of glucose use [59,63,71]. In addition to reducing the elevated blood glucose level, bioactive compounds obtained from medicinal plants can terminate oxidative stress on ß-cells and restore the impaired ß-cells [[66], [67], [68], [69],72]. Moreover, enhancing the metabolic rate of oxygen consumption [73], inhibiting cellular apoptosis, reducing renal glucose reabsorption [66,67,73], and promoting translocation of GLUT-4 and glucose transporter (GLUT-2) expression [67], are also important mechanisms demonstrated with certain phytoconstituents that are accountable for antihyperglycemic activities [67]. Stimulating cyclic adenosine monophosphate (cAMP), providing some essential elements such as magnesium, calcium, manganese, zinc, and copper for the β-cell [66], and Blocking pancreatic β-cell K+ channel [74], are also some mechanisms that are possibly participated in β-cell dysfunction found in diabetes mellitus [66,74]. The enzymatic inhibition of α-glycosidase and α-amylase enzymes, which are crucial for carbohydrate digestion, is used as an alternative treatment modality for diabetes mellitus. Folkloric medicinal plants with antidiabetic activities through inhibition of these enzymes and their free radical scavenging potentials are becoming hopeful approaches in the management of diabetes mellitus and its associated complications [70]. Medicinal plants have a significant role in the discovery of potential antidiabetic activities and have begun to get greater attention as sources of bioactive constituents as well as antioxidants. The antioxidant effect of medicinal plants has protective activity in restoring β-cell function in DM. As free radicals are known to damage and mutation of cells, and hence, oxidative stress has a significant role in the pathogenesis of diabetes mellitus and diabetic complications. Thus, medicinal plants with antioxidant activity will have significant importance in managing diabetes mellitus and its complications through scavenging free radicals [66].
Generally, the mechanisms of action could be grouped as preventing oxidative stress that is possibly involved in pancreatic β-cell dysfunction; pancreatic β-cell potassium channel blocking; cyclic adenosine monophosphate stimulation; providing certain necessary elements like zinc, calcium, manganese, copper, and magnesium for the β-cells; Inhibition of α-glucosidase and β-galactosidase [75]; inhibition of glycogenolysis and gluconeogenesis; stimulation of glycolysis, citric acid cycle, glycogenesis, and hexose monophosphate shunt [76]; enhancement indigestion along with a reduction in urea and blood sugar; promotion of regeneration and protection of destruction of the β-cells, initiate insulin release; reduction in insulin resistance and/or inhibition in renal glucose reabsorption [77]. Table 1 summarizes the in vitro and in vivo antidiabetic activities of several medicinal plants in Ethiopia.
9. Toxicological profile of medicinal plants
Acute toxicity testes via in vivo model confirmed the relative safety of the medicinal plant's extract. Seven plants, Ajuga remota, Hagenia abyssinica, Datura stramonium, Aloe megalacantha, Ajuga integrifolia, Bacium grandiflorum Lam, Bersama abyssinica, Justicia schimperiana, Terminalia brownie, Indigofera spicata, Capparis tomentosa, Capparis tomentosa, Aloe camperi, and Acanthus polystachyus showed LD50 greater than 2000 mg/kg [71,[78], [79], [80], [81], [82], [83], [84]]. Other plants such as Satvia rebaudiani, Croton macrostachys, and Otostegia integrifolia revealed LD50 greater than 5000 mg/kg [64,[85], [86], [87], [88]]. The LD50 of Moringa stenopetalla were 50.6 g/kg [85] and 50 g/kg [88]. The LD50 of Pentas schimperiana was greater than 4000 mg/kg [89]. The sub-chronic toxicity of Moringa Stenopetala exhibited normal hematological, significantly higher platelet counts compared to controls, significant changes were observed in the clinical chemistry parameters such as (CA125, urea, TSH, FT3, ALT, creatinine, cholesterol, TGs, and AST) were significantly higher, and FT4 significantly reduced in the mice received the treatment [90] Table 2.
Table 2.
Phytochemical screening and toxicity study of medicinal plants used for diabetes mellitus management.
| Scientific name | LD50 (mg/kg) | Phytochemical constituents | References |
|---|---|---|---|
| Ajuga remota | >2,000 | Saponins, phenolic compounds, steroids, flavonoids, and tannins | [92] |
| Satvia rebaudiani | >5,000 | Triterpenes, rebaudioside A-F, steviolbioside, sterols, ducloside A, stevioside, and flavonoids | [123] |
| Hagenia abyssinica | >2,000 | Tannins, anthraquinones, terpenoids, steroids, flavonoids, glycosides, phenols, and saponins | [108] |
| Datura stramonium | >2,000 | Alkaloids, saponins, steroids, phenols, flavonoids, tannins, glycosides, and terpenoids | [110] |
| Aloe megalacantha | >2,000 | Terpenoids, alkaloids, tannins, flavonoids, saponins, anthraquinones, and phenolic compounds | [52] |
| Ajuga integrifolia | >2,000 | Alkaloids, terpenoids, flavonoids, glycosides, phenols, steroids, tannins, and saponins | [124] |
| Aloe pulecherrima | ND | Nataloin, chrysophanol, 7-hydroxyaloin, and aloesaponarin | [125]. |
| Bacium grandiflorum Lam | >2,000 | Anthraquinones, saponins, phytosterols tannins, alkaloids, terpenoids, flavonoids, glycosides, and coumarins | [126] |
| Bersama abyssinica | >2,000 | Steroids, glycosides, terpenes, carotenoids, alkaloids, phenols, anthraquinones, tannins, triterpene, flavonoids, fatty acids, coumarins, and vitamins | [127,128] |
| Pentas schimperiana | >4,000 | phenolic compounds, flavonoids, saponins, steroidal, and tannins | [89] |
| Moringa stenopetala | >50 g/kg | Alkaloid, flavonoids, glycoside, flavanol, glycosinolate, and sterol | [129] |
| Justicia schimperiana | >2,000 | Triterpenes, polyphenols, flavonoids, alkaloids, saponins, glycosides, quinines, and phytosterols | [130] |
| Terminalia brownie | >2,000 | Flavonoids, phytosterols, polyphenols, saponins, and tannins | [131] |
| Croton macrostachys | >5,000 | Flavonoids, phenolic compounds, alkaloids, terpenoids, tannins, and saponins | [114] |
| Indigofera spicata | >2,000 | Glycoside, alkaloid, saponin, tannins, diterpenes, phytosterol, and flavonoids | [115] |
| Otostegia integrifolia | >5,000 | Saponins, flavonoids, reducing sugars, and phenolic compounds. gas chromatography, GC-mass spectrometry and NMR techniques confirms the identification of many constituents such as stigmasterol, pentatriacontane, (15, 16-epoxy3a, 9a-dihydroxy-labda-13(16) & 14-diene and 9(13), (+)-1-methyl-4-(5, 9-dimethyl1-methylene-deca-4, 8-dienyl)-cyclohexene41, 15(16) - diepoxy-3a-hydroxy-16- dihydrolabda-14-ene] | [89,116] |
| Caylusea abyssinica | >2,000 | Reducing sugars, saponins, tannins, alkaloids, steroidal compounds, flavonoids, phenolic compounds, and cardiac glycosides | [117] |
| Aloe camperi | >2,000 | Saponins, phenols, steroids, alkaloids, glycosides, phenols, tannins, terpenoids, flavonoids, coumarins, proteins, and carbohydrates | [132] |
| Acanthus polystachyus | >2,000 | Flavonoids, alkaloids, tannins, steroidal compounds, polyphenols, glycosides, saponins, anthraquinones, and terpenoids | [133] |
Note: LD50: lethal dose 50; ND: result not determined.
10. Phytochemistry of medicinal plants
Medicinal plants were screened phytochemically and reported that phenolic compounds, terpenoids, saponins, glycosides, tannins, flavonoids, glycolipids, dietary fibers, alkaloids, carotenoids, and anthocyanins were most commonly isolated biologically active principles responsible for its medicinal properties [[90], [91], [92]].
Phenols and Tannins [72,93] might contribute to antidiabetic effects due to their potential to possess insulin-like effects or stimulate insulin secretion [63], prevent β-cells impairment through free radical scavenging effects [94,95], enhance β-cells propagation and restoration [95], and reduce carbohydrate absorption by impeding α-amylase and α-glucosidase [72].
Flavonoids and other polyphenols showed antidiabetic activities through enhancing insulin release [59,[96], [97], [98], [99]], enhancing the expression and promoting translocation of GLUT-4 [67,96,97], and enhancing GLUT-2 expression in pancreatic β-cells [67,97], which can increase glucose uptake by the liver, adipose tissue, and muscle [97,98]. Flavonoids also reduce aldose reductase [59], inhibit α-glycosidase [59,97], and α-amylase [97], retard the gastric emptying rate [59], increase calcium ion uptake [59], and regenerate pancreatic beta cells [100,101].
Saponins display their antidiabetic activities through the possible mechanisms of ameliorating insulin resistance [58], stimulating insulin release/secretion, and protecting pancreas β-cells [59,102].
Alkaloids have received extra attention due to their potential role in the management of diabetes mellitus through inhibition of dipeptidyl peptidase-4 (DPP-4), protein tyrosine phosphatase 1B (PTP1B), AGEs [103], and α-amylase and α-glucosidase [104]. In addition, they also activate GLUT-4 translocation and 50 adenosine monophosphate-activated protein kinases (AMPK). Alkaloids have shown a significant effect on insulin release, pancreatic regeneration, and protective effects on oxidative tissue damage [103,105].
Triterpenoids seem to have promising antihyperglycemic effects through inhibition of aldose reductase, hepatic glycogen phosphorylase [106], and α-amylase and α-glucosidase [104,106]. In addition, they increase insulin-stimulated GLUT-4 translocation, prevent pancreatic β-cell dysfunction, decrease body weight, decrease oxidative stress and agonistic properties of emerging G-protein-coupled receptor (TGR5). Triterpene compounds have also shown a significant effect on the formation of advanced glycation end products (AGEs) and are promising agents in the prevention and management of diabetes mellitus complications [106]. Several medicinal plants summarized in this review contain various phytoconstituents Table 2.
11. Strengths and limitations of the studies
The evidence synthesized from this review will have paramount for further investigations in human studies. It will show directions of further the studies and promote the traditional use. Although we reviewed all previous antidiabetic studies that were conducted in Ethiopia, some limitations could limit the findings of this review. The methods used for the induction of DM were STZ or alloxan which mostly induces T1DM. The challenge in an in vivo study is as the induction method mostly induces T2DM. Most of the medicinal plants included in this study lack identification and isolation of the active constituents that could join the adventure of modern drug discovery. No clinical trials were conducted and also no clearly defined preparation for clinical trials in Ethiopia. Moreover, most of the studies didn't report the standardization protocols, the composition of the formulation, and preparation procedures.
12. Conclusion
Herbal medicines are gaining importance as they are cost-effective and also display improved therapeutic effects with lesser side effects. In Ethiopia, most medicinal plants with antidiabetic claims were studied in different animal models such as normoglycemic mice, oral glucose-loaded mice, streptozotocin-induced diabetic mice, and alloxan-induced diabetic mice. However, in vitro antidiabetic activity was conducted for a few of them. Medicinal plants which are rich in phytoconstituents like flavonoids, coumarins, terpenoids, phenolic compounds, and other bioactive compounds have revealed a significant blood-glucose-lowering activity. Further in vitro studies and bioassay-guided isolation and characterization of the active principle responsible for the antidiabetic activity of the medicinal plants are recommended to substantiate the use of the plant as a potential target for the development of antidiabetic agents. Antidiabetic medicinal plants used in Ethiopia denote a key role in the future development of novel antidiabetic agents. To this end, more toxicological and pharmacological investigations need to be considered to prove the safety of bioactive compounds obtained from these medicinal plants. Finally, we recommend ensuring future success in the clinical study and development of novel medicines for diabetes management from these medicinal plants.
Availability of data and materials
Most of the data is included in the manuscript. Additional can be found from the corresponding author based on reasonable request.
Funding
There is no funding to report.
Ethics approval and consent to participate
Not applicable.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to acknowledge University of Gondar.
References
- 1.Association A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Cho N.H., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Korsa A.T., et al. Diabetes mellitus complications and associated factors among adult diabetic patients in selected hospitals of west Ethiopia. Open Cardiovasc Med J. 2019;13(1) [Google Scholar]
- 4.Okafor C., Ofoegbu E. Indications and outcome of admission of diabetic patients into the medical wards in a Nigerian tertiary hospital. Niger Med J. 2011;52(2) [Google Scholar]
- 5.Ali A., et al. Prevalence of microvascular complications in newly diagnosed patients with type 2 diabetes. Pakistan J. Med. Sci. 2013;29(4):899. doi: 10.12669/pjms.294.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olamoyegun M., et al. Burden and pattern of micro vascular complications in type 2 diabetes in a tertiary health institution in Nigeria. Afr Health Sci. 2015;15(4):1136–1141. doi: 10.4314/ahs.v15i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adogu P.O.U., et al. The prevalence and presentation pattern of diabetes mellitus in patients at imo state university teaching hospital (IMSUTH) orlu and imo state specialist hospital (IMSSH) umuguma owerri (A 10-year retrospective study: 1st november 2004 to 31st october 2013) J Diabetes Mellitus. 2015;5(2):49. [Google Scholar]
- 8.Worku D., Hamza L., Woldemichael K. Patterns of diabetic complications at jimma university specialized hospital, southwest Ethiopia. Ethiopian. J. Health Sci. 2010;20(1) doi: 10.4314/ejhs.v20i1.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abejew A.A., Belay A.Z., Kerie M.W. Diabetic complications among adult diabetic patients of a tertiary hospital in northeast Ethiopia. Adv.Public Health. 2015 2015. [Google Scholar]
- 10.Dejene S., et al. Depression and diabetes in jimma university specialized hospital, Southwest Ethiopia. J Psychiatry. 2014;17(126):2. [Google Scholar]
- 11.Gebre M.W. Diabetes mellitus and associated diseases from Ethiopian perspective: systematic review. Ethiop J Health Dev. 2013;27(3):249–253. [Google Scholar]
- 12.Association A.D. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Supplement 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 13.Mahdi A.A., et al. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem. 2003;18(2):8–15. doi: 10.1007/BF02867361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souza M., Rao V., Silveira E. Inhibition of lipid peroxidation by ternatin, a tetramethoxyflavone from Egletes viscosa L. Phytomedicine. 1997;4(1):27–31. doi: 10.1016/S0944-7113(97)80024-4. [DOI] [PubMed] [Google Scholar]
- 15.Kitabchi A.E., et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.K T. Essentials of medical pharmacology. sixth ed. ed. Jaypee Brothers Medical Publishers; New Delhi: 2008. [Google Scholar]
- 17.Surya S., et al. Diabetes mellitus and medicinal plants-a review. Asian.Pacific J.Trop. Dis. 2014;4(5):337–347. [Google Scholar]
- 18.Patel D., et al. Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012;2(5):411–420. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenya S., Potgieter M., Erasmus L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J Ethnopharmacol. 2012;141(1):440–445. doi: 10.1016/j.jep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Shyam K., Kadalmani B. Antidiabetic activity of Bruguiera cylindrica (Linn.) leaf in Alloxan induced diabetic rats. Int J Curr Res Biosci Plant Biol. 2014;1:56–60. [Google Scholar]
- 21.Soumya D., Srilatha B. Late stage complications of diabetes and insulin resistance. J Diabetes Metabol. 2011;2(9) [Google Scholar]
- 22.Krogsgaard-Larsen P., Christensen S., Kofod H. Natural Products and Drug Development; 1994. The role of Medicinal plants in drug development; pp. 34–45. [Google Scholar]
- 23.Bastaki A. Diabetes mellitus and its treatment. Int J Diabetes Metabol. 2005;13(3):111. [Google Scholar]
- 24.Kyi M., et al. Recent advances in type 1 diabetes. Med J Aust. 2015;203(7):290–293. doi: 10.5694/mja14.01691. [DOI] [PubMed] [Google Scholar]
- 25.Garber A.J., et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498–1507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 26.DiPiro Joseph T., R L T, Yee Gary C., Matzke Gary R., Wells Barbara G., Posey L. Michael. Pharmacotherapy: a pathophysiologic approach. ninth ed. ed. kMcGraw-Hill Education; New Yor: 2014. [Google Scholar]
- 27.Krentz A.J., Patel M.B., Bailey C.J. New drugs for type 2 diabetes mellitus. Drugs. 2008;68(15):2131–2162. doi: 10.2165/00003495-200868150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Suneetha B., Sujatha D., Prasad K. Antidiabetic and antioxidant activities of stem juice of musa paradisiaca on alloxan induced diabetic rats. IJAPS. 2010;1(2):167–174. [Google Scholar]
- 29.Rizvi S.I., Matteucci E., Atukeren P. Traditional medicine in management of type 2 diabetes mellitus. J Diabetes Res. 2013:2013. doi: 10.1155/2013/580823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elujoba A.A., Odeleye O., Ogunyemi C. 2005. Traditional medicine development for medical and dental primary health care delivery system in Africa. [Google Scholar]
- 31.Dey L1 A.A., Yuan C.S. Alternative therapies for type 2 diabetes. Alternative Med Rev. 2002;7(1) [PubMed] [Google Scholar]
- 32.Radwan H., et al. Complementary and alternative medicine use among patients with type 2 diabetes living in the United Arab Emirates. BMC.Complementary Med.Ther. 2020;20(1):1–12. doi: 10.1186/s12906-020-03011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bnouham M., et al. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metabol. 2006;14(1):1. [Google Scholar]
- 34.Koski R.R. Practical review of oral antihyperglycemic agents for type 2 diabetes mellitus. Diabetes Educat. 2006;32(6):869–876. doi: 10.1177/0145721706294260. [DOI] [PubMed] [Google Scholar]
- 35.Organization W.H. World Health Organization; 2016. Global report on diabetes. [Google Scholar]
- 36.Gupta R., et al. An overview of Indian novel traditional medicinal plants with anti-diabetic potentials. Afr J Tradit, Complementary Altern Med. 2008;5(1):1. [PMC free article] [PubMed] [Google Scholar]
- 37.Oubre A., et al. From plant to patient: an ethnomedical approach to the identification of new drugs for the treatment of NIDDM. Diabetologia. 1997;40(5):614–617. doi: 10.1007/s001250050724. [DOI] [PubMed] [Google Scholar]
- 38.Rao M.U., et al. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010;2(3):1883–1892. [Google Scholar]
- 39.Meresa Asfaw, Gemechu Worku, Basha Hirut, Fekadu Netsanet, Teka Firehiwot, Ashebir Rekik, Tadele A. 2017. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents; pp. 1–25. Research Gate. [Google Scholar]
- 40.Limenih Y., Umer S., Wolde-Mariam A.M. AMHARA REGION; NORTH ETHIOPIA: 2015. Ethnobotanical study ON traditional medicinal plants IN dega damot woreda. [Google Scholar]
- 41.Zewude S. 2005. Ethnobotanical study of medicinal plants in Chilga district ,North Western Ethiopia. Research Gate. [Google Scholar]
- 42.Mahomoodally M.F. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid base Compl Alternative Med. 2013 doi: 10.1155/2013/617459. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modak D. A review: anti-diabetic activity of herbal drugs. Pharma Tutor. 2015;3(9):36–42. [Google Scholar]
- 44.Bansal P. Ethno-potential of medicinal herbs in skindiseases: an overview. Rsearch Gate. 2010;3(3):1–8. [Google Scholar]
- 45.Yagi S.M., Ahmed Y. Traditional medicinal plants used for the treatment of diabetes in the Sudan: a review. Afr. J.Pharm.Pharmacol. 2018;12(3):1–14. [Google Scholar]
- 46.Limenih Y., Umer S., Mariam M.W. Ethnobotanical study on traditional medicinal plants in Dega Damot woreda, Amhara region, North Ethiopia. Int J Res Pharm Chem. 2015;5(2):1–16. [Google Scholar]
- 47.Derebe D., Wubetu M., Alamirew A. Hypoglycemic and antihyperglycemic activities of 80% methanol root extract of Acanthus polystachyus delile (Acanthaceae) in type 2 diabetic rats. J Clin Pharmacol. 2020;12:149. doi: 10.2147/CPAA.S273501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiwari P., et al. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 49.Jones W.P., Kinghorn A.D. Natural products isolation; 2012. Extraction of plant secondary metabolites; pp. 341–366. [DOI] [PubMed] [Google Scholar]
- 50.Kifle Z.D., Belayneh Y.M. Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of hagenia abyssinica (rosaceae) leaves in streptozotocin-induced diabetic mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:4085. doi: 10.2147/DMSO.S279475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tekulu G.H., Araya E.M., Mengesha H.G. In vitro α-amylase inhibitory effect of TLC isolates of Aloe megalacantha baker and Aloe monticola Reynolds. BMC Compl Alternative Med. 2019;19(1):206. doi: 10.1186/s12906-019-2622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammeso W.W., et al. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evid base Compl Alternative Med. 2019 doi: 10.1155/2019/8263786. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amare G.G., Meharie B.G., Belayneh Y.M. Evaluation of antidiabetic activity of the leaf latex of aloe pulcherrima Gilbert and sebsebe (aloaceae) Evid base Compl Alternative Med. 2020:2020. doi: 10.1155/2020/8899743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dessalegn E., et al. Evaluation of in vitro antidiabetic potential of Thymus schimperi R. And Thymus vulgaris L. Evaluation. 2019;69 [Google Scholar]
- 55.Taye G.M., et al. In vivo antidiabetic activity evaluation of aqueous and 80% methanolic extracts of leaves of Thymus schimperi (Lamiaceae) in alloxan-induced diabetic mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:3205. doi: 10.2147/DMSO.S268689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kifle Z.D., Anteneh D.A., Atnafie S.A. Hypoglycemic, anti-hyperglycemic and anti-hyperlipidemic effects of Bersama abyssinica fresen (melianthaceae) leaves’ solvent fractions in normoglycemic and streptozotocin-induced diabetic mice. J Exp Pharmacol. 2020;12:385. doi: 10.2147/JEP.S273959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belayneh Y., Birru E. Benth. Subspecies aurea; 2018. Anti-diabetic activities of hydromethanolic leaf extract of Calpurnia aurea (Ait. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao U.M., Adinew B. Remnant B-cell-stimulative and anti-oxidant effects of Persea americana fruit extract studied in rats introduced into streptzotocin-induced hyperglycaemic state. Afr J Tradit, Complementary Altern Med. 2011;8(3) doi: 10.4314/ajtcam.v8i3.65277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shewasinad A., et al. Antidiabetic activity of methanol extract and fractions of Thymus schimperi ronniger leaves in normal and streptozotocin induce diabetic mice. Iran J Pharmacol Ther. 2019;1(8) [Google Scholar]
- 60.Toma A., et al. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Compl Alternative Med. 2015;15(1):1–8. doi: 10.1186/s12906-015-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajanandh M., et al. Moringa oleifera Lam. A herbal medicine for hyperlipidemia: a pre–clinical report. Asian.Pacific J.Trop. Dis. 2012;2:S790–S795. [Google Scholar]
- 62.Nardos A., Makonnen E., Debella A. Effects of crude extracts and fractions of Moringa stenopetala (Baker f) Cufodontis leaves in normoglycemic and alloxan-induced diabetic mice. Afr J Pharm Pharmacol. 2011;5(20):2220–2225. [Google Scholar]
- 63.Shewamene Z., Abdelwuhab M., Birhanu Z. Methanolic leaf exctract of Otostegia integrifolia Benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Compl Alternative Med. 2015;15(1):1–7. doi: 10.1186/s12906-015-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsegaye W., Urga K., Asres K. Antidiabetic activity of samma (Urtica simensis hochst. Ex. A. Rich.) in streptozotocin-induced diabetic mice. Ethiop Pharmaceut J. 2008;27:75–82. [Google Scholar]
- 65.Liu J., et al. Effect of Tai Chi on mononuclear cell functions in patients with non-small cell lung cancer. BMC Compl Alternative Med. 2015;15(1):1–8. doi: 10.1186/s12906-015-0517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kifle Z.D., Enyew E.F. Evaluation of in vivo antidiabetic, in vitro α-amylase inhibitory, and in vitro antioxidant activity of leaves crude extract and solvent fractions of Bersama abyssinica fresen (melianthaceae) J Evid Base Integr Med. 2020;25 doi: 10.1177/2515690X20935827. 2515690X20935827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belayneh Y.M., et al. Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L.(Solanaceae) J Exp Pharmacol. 2019;11:29. doi: 10.2147/JEP.S192264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sayem A.S.M., et al. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258. doi: 10.3390/molecules23020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Upadhyay S., Dixit M. Oxidative medicine and cellular longevity; 2015. Role of polyphenols and other phytochemicals on molecular signaling. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tekulu G.H., Araya E.M., Mengesha H.G. In vitro α-amylase inhibitory effect of TLC isolates of Aloe megalacantha baker and Aloe monticola Reynolds. BMC Compl Alternative Med. 2019;19(1):1–7. doi: 10.1186/s12906-019-2622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seifu D., et al. Antidiabetic and gastric emptying inhibitory effect of herbal Melia azedarach leaf extract in rodent models of diabetes type 2 mellitus. J Exp Pharmacol. 2017;9:23. doi: 10.2147/JEP.S126146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kifle Z.D., Yesuf J.S., Atnafie S.A. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae) J Exp Pharmacol. 2020;12:151. doi: 10.2147/JEP.S249964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Debecho, D.A., et al., Effect of fresh juice of Khat (Catha edulis) on blood glucose levels of normoglycemic and streptozocin-induced diabetic rats. International Journal of Pharmaceutical Sciences and Research, 2028. 9(2): p. 784-789.
- 74.Herrington J., et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes. 2006;55(4):1034–1042. doi: 10.2337/diabetes.55.04.06.db05-0788. [DOI] [PubMed] [Google Scholar]
- 75.Jarald E., Joshi S.B., Jain D.C. Diabetes vs herbal medicines. Iran J Pharmacol Ther. 2008;7(1):97. [Google Scholar]
- 76.Thomson I., Khanavi Mahnaz, Taheri Marzieh, Rajabi Afsaneh, Fallah-Bonekohal Saeed, Baeeri Maryam, Mohammadirad Azadeh, Amin Gholamreza, Abdollahi Mohammad. Asian J Anim Vet Adv. 2012;7(11):1166–1174. [Google Scholar]
- 77.Narayan D.S., Patra V., Dinda S. Diabetes and indian traditional medicines an overview. Int J Pharm Pharmaceut Sci. 2012;4 [Google Scholar]
- 78.Alberti K.G.M.M., Zimmet P., Shaw J. International diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med. 2007;24(5):451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 79.Belsti Y., Akalu Y., Animut Y. Attitude, practice and its associated factors towards Diabetes complications among type 2 diabetic patients at Addis Zemen District hospital, Northwest Ethiopia. BMC Publ Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-08953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dinku T., Tadesse S., Asres K. Antidiabetic activity of the leaf extracts of Pentas schimperiana subsp. schimperiana (A. Rich) Vatke on alloxan induced diabetic mice. Ethiop Pharmaceut J. 2010;28(1) [Google Scholar]
- 81.Pearson E. Personalized medicine in diabetes: the role of ‘omics’ and biomarkers. Diabet Med. 2016;33(6):712–717. doi: 10.1111/dme.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohki T., et al. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Invest. 2016;36(4):313–319. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 83.Rajalakshmi M., et al. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats. Afr J.Pharm. Pharmacol. 2009;3(5):171–180. [Google Scholar]
- 84.Begashaw B., et al. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Compl Alternative Med. 2017;17(1):1–11. doi: 10.1186/s12906-017-1841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bekele T., et al. Department of Pharmaceutical Chemistry, School of Pharmacy, Addis Ababa University April; 2008. Antidiabetic activity and phytochemical screening of crude extracts of Stevia rebaudiana Bertoni and Ajuga remota Benth grown in Ethiopia on alloxan-induced diabetic mice. [Google Scholar]
- 86.Holstein A., et al. Impact of clinical factors and CYP2C9 variants for the risk of severe sulfonylurea-induced hypoglycemia. Eur J Clin Pharmacol. 2011;67(5):471–476. doi: 10.1007/s00228-010-0976-1. [DOI] [PubMed] [Google Scholar]
- 87.Nabi S.A., et al. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Compl Alternative Med. 2013;13(1):1–9. doi: 10.1186/1472-6882-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schloot N.C., et al. Risk of severe hypoglycemia in sulfonylurea‐treated patients from diabetes centers in Germany/Austria: how big is the problem? Which patients are at risk? Diabetes Metabol Res Rev. 2016;32(3):316–324. doi: 10.1002/dmrr.2722. [DOI] [PubMed] [Google Scholar]
- 89.Meresa A., et al. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. AJADD. 2017;5(1):40–58. [Google Scholar]
- 90.Ogurtsova K., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 91.Alene M., et al. Evaluation of antidiabetic activity of Ajuga integrifolia (Lamiaceae) root extract and solvent fractions in mice. Evid base Compl Alternative Med. 2020:2020. doi: 10.1155/2020/6642588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tafesse T.B., et al. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Compl Alternative Med. 2017;17(1):1–9. doi: 10.1186/s12906-017-1757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gebreyohannis T., Shibeshi W., Asres K. Effects of solvent fractions of caylusea abyssinica (fresen.) fisch. and mey. on blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Nat Remedies. 2013;14(1):67–75. [Google Scholar]
- 94.Ifesan B., et al. European J Med Plants; 2013. Antioxidant and Antimicrobial properties of selected plant leaves. [Google Scholar]
- 95.Oboh G., Rocha J.B.T. Polyphenols in red pepper [Capsicum annuum var. aviculare (Tepin)] and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver. Eur Food Res Tech. 2007;225(2):239–247. [Google Scholar]
- 96.Salehi B., et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):551. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Ishaq R.K., et al. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9(9):430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chadt A., Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflueg Arch Eur J Physiol. 2020;472(9):1273–1298. doi: 10.1007/s00424-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moradi B., et al. The most useful medicinal herbs to treat diabetes. Biomed Res Ther. 2018;5(8):2538–2551. [Google Scholar]
- 100.Gebremeskel L., Tuem K.B., Teklu T. Evaluation of antidiabetic effect of ethanolic leaves extract of Becium grandiflorum Lam.(Lamiaceae) in streptozotocin-induced diabetic mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:1481. doi: 10.2147/DMSO.S246996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belayneh Y.M., Birru E.M., Ambikar D. Evaluation of hypoglycemic, antihyperglycemic and antihyperlipidemic activities of 80% methanolic seed extract of Calpurnia aurea (Ait.) Benth.(Fabaceae) in mice. J Exp Pharmacol. 2019;11:73. doi: 10.2147/JEP.S212206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marrelli M., et al. Effects of saponins on lipid metabolism: a review of potential health benefits in the treatment of obesity. Molecules. 2016;21(10):1404. doi: 10.3390/molecules21101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar A., et al. Role of plant-derived alkaloids against diabetes and diabetes-related complications: a mechanism-based approach. Phytochemistry Rev. 2019;18(5):1277–1298. [Google Scholar]
- 104.Yikna B.B., Yehualashet A.S. Medicinal plant extracts evaluated in vitro and in vivo for antidiabetic activities in Ethiopia: Bases for future clinical trials and related investigations. Evid base Compl Alternative Med. 2021:2021. doi: 10.1155/2021/9108499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erejuwa O.O. Oxidative stress in diabetes mellitus: is there a role for hypoglycemic drugs and/or antioxidants. Oxidative stress and diseases. 2012;217:246. [Google Scholar]
- 106.Nazaruk J., Borzym-Kluczyk M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochemistry Rev. 2015;14(4):675–690. doi: 10.1007/s11101-014-9369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Assefa F., Seifu D., Makonnen E. Antihyperglycemic and antihyperlipidemic activities of ethanol extract of Ajuga remota Benth (Harmegusa) leaves in streptozotocin induced diabetic rats. Afr J Pharm Pharmacol. 2017;11(2):17–24. [Google Scholar]
- 108.Kifle Z.D., Yesuf J.S., Atnafie S.A. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae) J Exp Pharmacol. 2020;12:151–167. doi: 10.2147/JEP.S249964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Belayneh Y.M., Birru E.M. Antidiabetic activities of hydromethanolic leaf extract of Calpurnia aurea (ait.) Benth. Subspecies aurea (Fabaceae) in mice. Evid base Compl Alternative Med. 2018 doi: 10.1155/2018/3509073. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Melaku B.C., Amare G.G. Evaluation of antidiabetic and antioxidant potential of hydromethanolic seed extract of Datura stramonium Linn (solanaceae) J Exp Pharmacol. 2020;12:181. doi: 10.2147/JEP.S258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dinku T., Tadesse S., Asres K. Antidiabetic activity of the leafextracts of Pentas schimperiana subsp. schimperiana (A. Rich) Vatke on alloxaninduced diabetic mice. Ethiop Pharmaceut J. 2010;28:22–26. [Google Scholar]
- 112.Tesfaye A., Makonnen E., Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113. [Google Scholar]
- 113.Alema N.M., et al. Antidiabetic activity of extracts of terminalia brownii fresen. stem bark in mice. J Exp Pharmacol. 2020;12:61. doi: 10.2147/JEP.S240266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gebeyehu E. Antidiabetic activity of hydroalcoholic extract of the root of Croton macrostachys in Streptozotocin induced diabetic mice. World J Pharm Sci. 2015;3(2):185–191. [Google Scholar]
- 115.Birru E.M., Abdelwuhab M., Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Compl Alternative Med. 2015;15(1):321. doi: 10.1186/s12906-015-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shewamene Z., Abdelwuhab M., Birhanu Z. Methanolic leaf exctract of Otostegia integrifolia Benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Compl Alternative Med. 2015;15(1):19. doi: 10.1186/s12906-015-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tamiru W., Engidawork E., Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Compl Alternative Med. 2012;12(1):151. doi: 10.1186/1472-6882-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gorems A. Evaluation of antihyperglycemic and hypoglycemic activities of the aqueous leaf extract of Rubus Erlangeri Engl. (Rosacea) in Mice. 2019 doi: 10.1016/j.metop.2021.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tefera M.M., et al. Antidiabetic effect of germinated Lens culinaris medik seed extract in streptozotocin-induced diabetic mice. J Exp Pharmacol. 2020;12:39. doi: 10.2147/JEP.S228834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Demoz M.S., et al. Evaluation of the anti-diabetic potential of the methanol extracts of aloe camperi, meriandra dianthera and a polyherb. J Diabetes Mellitus. 2015;5(4):267. [Google Scholar]
- 121.Chinsembu K., Syakalima M., Semenya S. Ethnomedicinal plants used by traditional healers in the management of HIV/AIDS opportunistic diseases in Lusaka, Zambia. South Afr J Bot. 2019;122:369–384. [Google Scholar]
- 122.Abebe, Y., D. Seifu, and T. Tolessa, Effect of crude extract of Khat (Catha Edulis) on the plasma glucose level of normoglycemic and STZ induced type 2 diabetic rats.
- 123.Mantovaneli I., et al. The effect of temperature and flow rate on the clarification of the aqueous Stevia-extract in a fixed-bed column with zeolites. Braz J Chem Eng. 2004;21(3):449–458. [Google Scholar]
- 124.Bekeri D., Adane L., Mamo F. Phytochemical investigation and isolation of compounds from Ajuga integrifolia root extract. World J Chem. 2018;13(1):1–13. [Google Scholar]
- 125.Abdissa D., et al. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beshir K., et al. In-vivo wound healing activity of 70% ethanol leaf extract of Beciumgrandiflorum Lam.(Lamiaceae) in mice. Ethiopian Pharmaceut J. 2016;32:117–130. [Google Scholar]
- 127.Zekeya N., et al. Analysis of phytochemical composition of Bersama abyssinica by gas chromatography-mass spectrometry. J Pharmacogn Phytochem. 2014;3(4):246–252. [Google Scholar]
- 128.Anza M., et al. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica. J. Adv. Bot. Zool. 2015;3(2):1–5. [Google Scholar]
- 129.Sileshi T., et al. Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. J Coastal Life Med. 2014;2:214–221. [Google Scholar]
- 130.Abdela J., Engidawork E., Shibeshi W. In vivo antimalarial activity of solvent fractions of the leaves of justicia schimperiana hochst. Ex Nees against Plasmodium berghei in Mice. Ethiop Pharmaceut J. 2014;30(2):95–108. [Google Scholar]
- 131.Tarekegne, W., et al., World J Pharm Pharmaceut Sci.
- 132.Shobha G., et al. Phytochemical profile, antibacterial and antidiabetic effects of crude aqueous leaf extract of Datura stramonium. Pharmacophore. 2014;5(2):273–278. [Google Scholar]
- 133.Otsuka H. Natural products isolation. Springer; 2006. Purification by solvent extraction using partition coefficient; pp. 269–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the data is included in the manuscript. Additional can be found from the corresponding author based on reasonable request.