Abstract
This in vitro study evaluated the activities of vancomycin, LY333328, and teicoplanin alone and in combination with gentamicin, rifampin, and RP59500 against Staphylococcus aureus isolates with intermediate susceptibilities to vancomycin. Ampicillin-sulbactam and trovafloxacin were also evaluated. LY333328 and ampicillin-sulbactam resulted in bactericidal activity against all isolates. The combination of gentamicin with glycopeptides showed synergistic activity, while rifampin had no added benefit.
Staphylococcus aureus is a virulent organism which continues to be a major pathogen in human infections. Virtually all isolates of S. aureus produce β-lactamase to penicillin G, making the semisynthetic penicillinase-resistant compounds the primary drugs of choice. However, over the past 3 decades, the increased prevalence of methicillin-resistant S. aureus (MRSA) has become a worldwide problem, and glycopeptides such as vancomycin continue to be the only recommended and consistently active agents.
Recently there have been reports of S. aureus clinical isolates with intermediate susceptibilities to vancomycin (MIC = 8 μg/ml) in Japan and the United States (2, 4). The recent isolation of these clinical strains of vancomycin-intermediate S. aureus (VISA) has heightened the need for effective alternative antimicrobial treatments, such as combination therapy or the use of investigational agents. To assess the potential impact of these VISA strains, we evaluated the activities of various glycopeptides and other antimicrobial agents, alone and in combination with gentamicin or rifampin, against three different VISA strains.
The VISA strains tested include 14379 (William Beaumont Hospital, Royal Oak, Mich.), MU-50 (Juntendo Hospital, Tokyo, Japan), and HIP5836 (New Jersey strain) (Centers for Disease Control and Prevention, Atlanta, Georgia). A clinical strain of MRSA (MRSA-494) was used as a control for the comparison of activities. Antimicrobials evaluated include LY333328 (supplied by Eli Lilly and Company, Indianapolis, Ind.), vancomycin, gentamicin, trimethoprim, sulfamethoxazole, and tetracycline (commercially purchased from Sigma Chemicals, St. Louis, Mo.), teicoplanin and rifampin powder for injection (supplied by Marion-Merrell Dow Pharmaceuticals Inc., Kansas City, Mo.), oxazolidinone PNU766 (supplied by Pharmacia-Upjohn Laboratories, Kalamazoo, Mich.), quinupristin-dalfopristin (supplied by Rhone-Poulenc Rorer, Collegeville, Pa.), trovafloxacin and ampicillin-sulbactam (supplied by Pfizer, Inc., Groton, Conn.), clinafloxacin (supplied by Parke-Davis Pharmaceutical Research, Ann Arbor, Mich.), and levofloxacin (supplied by R. W. Johnson Pharmaceutical Research, Raritan, N.J.). Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (125 mg/liter) (SMHB) was used for all susceptibility testing and time-kill curve studies.
MICs and minimal bactericidal concentrations (MBCs) of various antibiotics were determined by broth microdilution in SMHB according to the guidelines of the National Committee for Clinical Laboratory Standards (6).
Initial time-kill curves were conducted with LY333328, vancomycin, teicoplanin, ampicillin-sulbactam, quinupristin-dalfopristin (RP59500), and trovafloxacin in SMHB at one-half, one, two, and four times the MIC obtained by microdilution susceptibility testing. Gentamicin, RP59500, and rifampin were tested in combination with LY333328, vancomycin, and teicoplanin. Combination time-kill curves were tested at one-half, one, and two times the MIC to detect for synergistic, antagonistic, or additive effects (3, 7). Synergy was defined as a >2-log-unit reduction in CFU/milliliter compared to the reduction produced by the most active agent used alone. Each time-kill curve experiment was performed in triplicate with a 24-well tissue culture plate by adding 0.2 ml of a 1:10 dilution of a 0.5 McFarland standard suspension of the organism (final concentration of 106 CFU/ml) to 1.7 ml of SMHB plus 0.1 ml of the antibiotic stock. Preliminary experiments in our laboratory showed no difference between this method and the traditional test tube methods. Samples (0.1 ml) were removed from each well at 0, 8, and 24 h and diluted appropriately in cold 0.9% sodium chloride. Samples were then plated on tryptic soy agar plates with an autoplate spiral dispenser (model 3000; Spiral Bioscience, Frederick, Md.). Plates were incubated at 35°C for 24 to 48 h, and colony counts (log10 CFU/milliliter) were determined with a laser bacteria colony counter (model 500A; Spiral System Instruments, Inc.). The limit of detection for this method is 2.5 log10 CFU/ml.
Time-kill curves were plotted graphically as log10 CFU/milliliter versus time. Residual bacterial inocula at 24 h were compared between regimens by using a two-way analysis of variance with Tukey’s Post-Hoc test. P values of <0.05 were indicative of statistically significant differences.
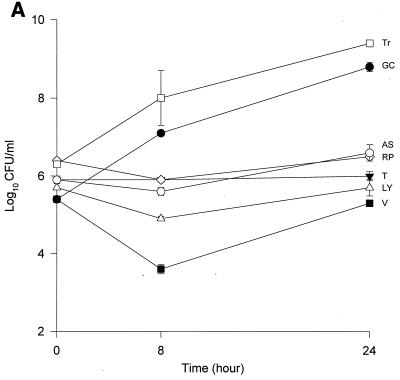
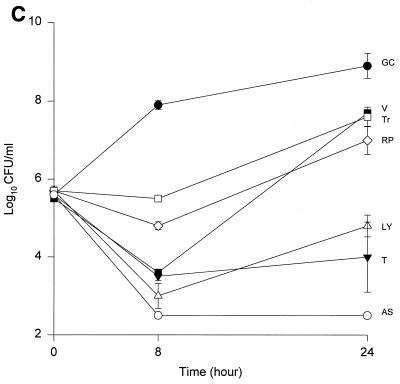
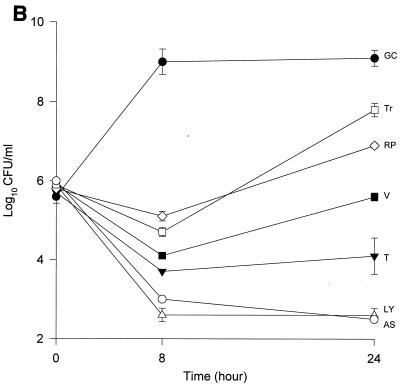
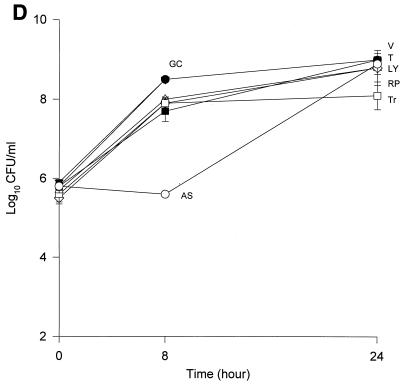
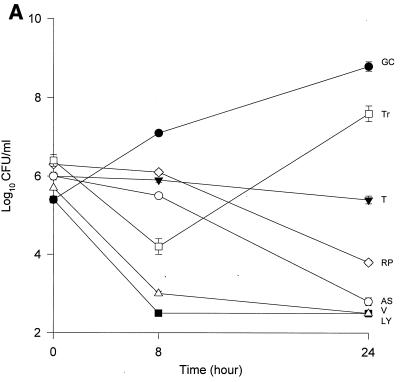
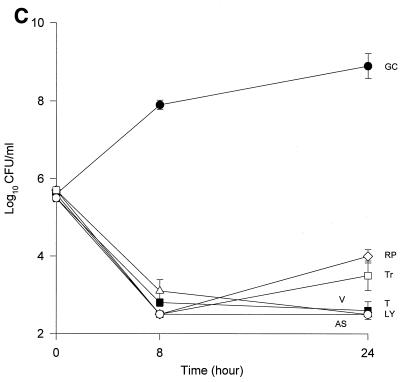
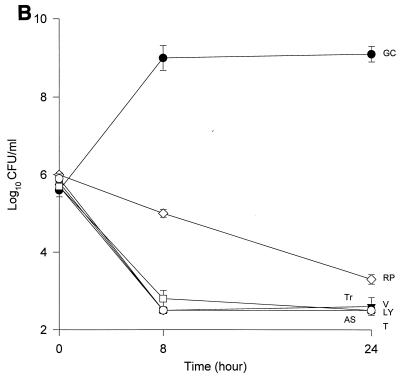
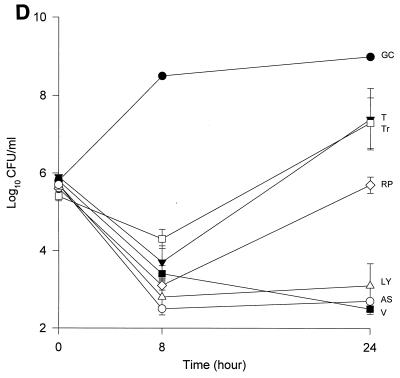
The MICs and MBCs for each isolate are shown in Table 1. Compared to the values for the other two isolates, the majority of the agents evaluated appear to have lower MICs and MBCs for HIP5836. The killing activities of the various glycopeptides, ampicillin-sulbactam, and trovafloxacin against the different strains of VISA and the control isolate are shown in Fig. 1 and 2. Against the VISA isolates, vancomycin alone at one-half times the MIC reduced the initial inoculum significantly at 8 h; however, regrowth was observed at 24 h for all the isolates. At two times the MIC, vancomycin reduced the starting inoculum by ≥3 log10 CFU/ml for all isolates and no regrowth was observed. The same concentration of vancomycin (one-half times the MIC) against HIP5836 resulted in greater regrowth at 24 h than against the other two isolates of VISA. A similar concentration ratio (concentration/MIC) of LY333328 produced killing comparable to that of vancomycin against 14379. However, substantial regrowth was seen at 24 h with HIP5836 when LY333328 and teicoplanin were used at concentrations of one-half times the MIC. Against MU-50, LY333328 at one-half times the MIC was the most active glycopeptide. Teicoplanin had no activity against 14379, even at higher concentrations of four times the MIC. At four times the MIC all antibiotics resulted in 99.9% killing by 24 h against all isolates.
TABLE 1.
Antibiotic susceptibilities of various VISA strains
| Antibiotic | MIC/MBC (μg/ml) for S. aureus
|
|||
|---|---|---|---|---|
| 14379 | MU-50 | HIP5836 | MRSA-494 | |
| Vancomycin | 8/8 | 8/12 | 6/6 | 0.75/1 |
| LY333328 | 1/2 | 2/8 | 1/1 | 0.5/2 |
| Teicoplanin | 8/16 | 16/32 | 2/4 | 0.25/0.25 |
| Ampicillin-sulbactam | 32/64 | 64/64 | 8/8 | 16/16 |
| Gentamicin | 64/>256 | 128/128 | 0.25/0.5 | 1/1 |
| Rifampin | 1,024/>2,048 | 2,048/>2,048 | 2,048/>4,096 | 0.0075/0.015 |
| RP59500 | 0.25/1 | 0.5/1 | 0.25/0.25 | 0.25/1 |
| Oxazolidinone PNU766 | 1/4 | 2/16 | 0.5/2 | 4/>16 |
| Trovafloxacin | 0.5/1 | 2/2 | 1/1 | 0.015/0.03 |
| Clinafloxacin | 1/2 | 1/1 | 0.5/0.5 | 0.03/0.06 |
| Tetracycline | 2/16 | 128/128 | 0.5/0.5 | 128/>512 |
| Levofloxacin | >16/>16 | 8/8 | 8/16 | 0.125/0.25 |
| TMP-SMXa | 4/>64 | 0.06/0.125 | 0.06/0.06 | 0.125/0.5 |
TMP-SMX, trimethoprim-sulfamethoxazole.
FIG. 1.
Time-kill curves of vancomycin (V) (■), LY333328 (LY) (▵), teicoplanin (T) (▾), RP59500 (RP) (◊), trovafloxacin (Tr) (□), and ampicillin-sulbactam (AS) (○) and growth control curve (GC) (●) versus S. aureus 14379 (A), MU-50 (B), HIP5836 (C), and MRSA-494 (D) at one-half times the MIC. Error bars indicate standard deviations.
FIG. 2.
Time-kill curves of vancomycin (V) (■), LY333328 (LY) (▵), teicoplanin (T) (▾), RP59500 (RP) (◊), trovafloxacin (Tr) (□), and ampicillin-sulbactam (AS) (○) and growth control curve (GC) (●) versus S. aureus 14379 (A), MU-50 (B), HIP5836 (C), and MRSA-494 (D) at two times the MIC. Error bars indicate standard deviations.
Bactericidal activity of ampicillin-sulbactam against HIP5836 was seen at concentrations of one or more times the MIC (8 μg/ml). Against MU-50 and 14379, concentrations of 16 and 32 μg/ml (one-half and one times the MIC) were required to achieve bactericidal activity. Interestingly, higher concentrations of 32 to 64 μg/ml (two to four times the MIC) were required to achieve bactericidal activity against MRSA-494.
RP59500 and trovafloxacin had bactericidal activities against HIP5836 only at higher concentrations of two to four times the MIC. RP59500, at one-half times the MIC, provided additive killing effects when combined with vancomycin, teicoplanin, and LY333328 but had no added benefit when concentrations greater than or equal to the MIC were used.
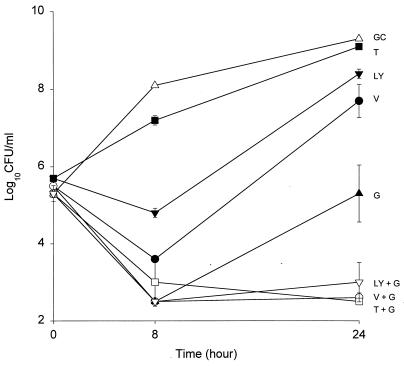
Gentamicin combined with vancomycin resulted in synergistic activity (a reduction of >2 log10 CFU/ml) against all three isolates of VISA. Gentamicin in combination with LY333328 also resulted in synergistic activity against 14379 and HIP5836. Due to the increased bactericidal activity (≥3-log-unit reduction in log10 CFU/milliliter) of LY333328 against MU-50, we were unable to detect synergism in combination with gentamicin. Subinhibitory concentrations of gentamicin added to teicoplanin resulted in synergy against HIP5836 and 14379 and added activity against MU-50 (data not shown). Data for gentamicin in combination with vancomycin and teicoplanin against HIP5836 is shown in Fig. 3. Rifampin (0.0038 μg/ml) in combination with vancomycin (0.375 μg/ml) had synergistic effects against the standard control; however, at concentrations up to 512 to 1,024 μg/ml it did not provide synergistic activities against any of the VISA isolates when it was combined with the glycopeptides.
FIG. 3.
Time-kill curves of growth control curve (GC) (▵); time-kill curves of vancomycin (V) (●), teicoplanin (T) (■), LY333328 (LY) (▾), and gentamicin (G) (▴) alone; and time-kill curves of LY + G (▿), V + G (○), and T + G (□) in combination versus S. aureus HIP5836 at one-half times the MIC. Error bars indicate standard deviations.
Our data suggests that vancomycin alone can produce significant (P < 0.05) killing against the VISA strains 14379 and MU-50 at one-half and one times the MIC, which may suggest the presence of subpopulations for which MICs are <8 μg/ml. Vancomycin concentrations of 3 μg/ml used against HIP5836 resulted in regrowth at 24 h, which may suggest a more homogeneous population for which the MIC was >3 μg/ml compared to the other two isolates. This data is consistent with the previous reports of heterogeneity of MU-50 (1). As demonstrated in our study, slightly higher concentrations of vancomycin can provide adequate killing activity against these VISA isolates. The newer glycopeptide LY333328 reduced the starting inocula by 99.9% at concentrations of two times the MIC, suggesting bactericidal activity for this agent.
Irrespective of the MIC of ampicillin-sulbactam, a significant reduction in log10 CFU/milliliter was observed for isolates 14379 and MU-50 after 24 h of incubation. Slightly higher concentrations (32 to 64 μg/ml) were required to achieve the same activity against the MRSA-494 strain evaluated in the study. The enhanced bactericidal activity of ampicillin-sulbactam against VISA strains may be attributed to the alteration in the expression of PBP 2 and 2′ or to other unknown mechanisms, since all VISA strains to date contain the mecA gene (8).
All of the VISA isolates examined in this study appeared to be sensitive to trovafloxacin and clinafloxacin but were resistant to levofloxacin. In time-kill studies trovafloxacin resulted in 99.9% killing at concentrations of 2 to 4 μg/ml, which are within achievable concentrations in serum (9). The combination of gentamicin with vancomycin or LY333328 resulted in synergistic activity, but gentamicin produced variable results in combination with teicoplanin. Previous work by Mulazimoglu et al. demonstrated that the combination of vancomycin and gentamicin did not result in synergistic activity against MRSA isolates with high-level gentamicin resistance (MIC > 500 μg/ml) (5). Although it was previously concluded that synergism is not predictable against the isolates evaluated, our study demonstrated synergistic activity against three of these VISA isolates. Furthermore, our results suggest that gentamicin (MIC ≤ 128 μg/ml) may be used in combination with vancomycin or LY333328 to enhance the bactericidal activities of these agents against VISA. No added benefit was seen when rifampin was used in combination with any of the glycopeptides.
Even though the development of VISA strains is alarming, it appears that these isolates remain susceptible to slightly higher doses of vancomycin. These isolates are also sensitive to a wide range of antibiotics, providing viable options in case of a poor response to vancomycin. Additionally, the use of gentamicin in combination with vancomycin may further improve killing. Ampicillin-sulbactam demonstrated bactericidal activity against these isolates and may play a role in the treatment of infections caused by VISA. Additional studies of VISA strains are warranted to further investigate these hypotheses.
Acknowledgments
This work was supported by a grant from Eli Lilly and Co.
We thank Mark J. Zervos, Keiichi Hiramatsu, and Fred C. Tenover for supplying us with the VISA isolates 14379, MU-50, and HIP5836.
REFERENCES
- 1.Boyce J M, Medeiros A A, Hiramatsu K. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) from the United States with subpopulations of cells with reduced susceptibility to vancomycin, abstr. LB-15. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. Morbid Mortal Weekly Rep. 1997;46:624–635. [PubMed] [Google Scholar]
- 3.Hackbarth C J, Chambers H F, Sande M A. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:611–613. doi: 10.1128/aac.29.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Mulazimoglu L, Drenning S D, Muder R R. Vancomycin-gentamicin synergism revisited: effect of gentamicin susceptibility of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1534–1535. doi: 10.1128/aac.40.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 7.Palmer S M, Rybak M J. Pharmacodynamics of once- or twice-daily levofloxacin versus vancomycin, with or without rifampin, against Staphylococcus aureus in an in vitro model with infected platelet-fibrin clots. Antimicrob Agents Chemother. 1996;40:701–705. doi: 10.1128/aac.40.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenover, F. C. (Centers for Disease Control and Prevention). 1998. Personal communication.
- 9.Vincent J, Venitz J, Teng R, Baris B A, Willavize S A, Polzer R J, Freidman H L. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J Antimicrob Chemother. 1997;39:75–80. doi: 10.1093/jac/39.suppl_2.75. [DOI] [PubMed] [Google Scholar]